Section 5 5Intermolecular Forces Intra versus Intermolecular Forces


- Slides: 13

Section 5. 5—Intermolecular Forces

Intra- versus Inter-molecular Forces l So far this chapter has been discussing intramolecular forces ¡ Intramolecular forces = forces within the molecule (chemical bonds) l Now let’s talk about intermolecular forces ¡ Intermolecular forces = forces between separate molecules

Breaking Intramolecular forces l Breaking of intramolecular forces (within the molecule) is a chemical change ¡ 2 H 2 + O 2 2 H 2 O ¡ Bonds are broken within the molecules and new bonds are formed to form new molecules

Breaking Intermolecular forces l Breaking of intermolecular forces (between separate molecules) is a physical change ¡ Breaking glass is breaking the intermolecular connections between the glass molecules to separate it into multiple pieces. ¡ Boiling water is breaking the intermolecular forces in liquid water to allow the molecules to separate and be individual gas molecules.

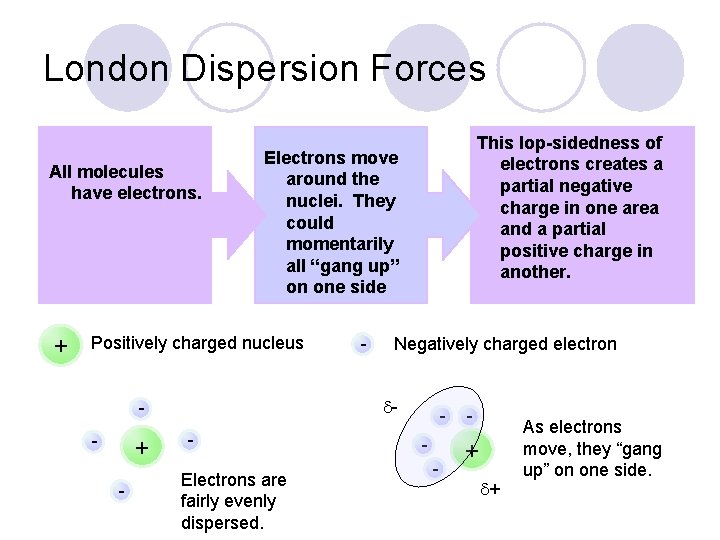
London Dispersion Forces All molecules have electrons. + Positively charged nucleus + - - Negatively charged electron - - This lop-sidedness of electrons creates a partial negative charge in one area and a partial positive charge in another. Electrons move around the nuclei. They could momentarily all “gang up” on one side Electrons are fairly evenly dispersed. - - + + As electrons move, they “gang up” on one side.

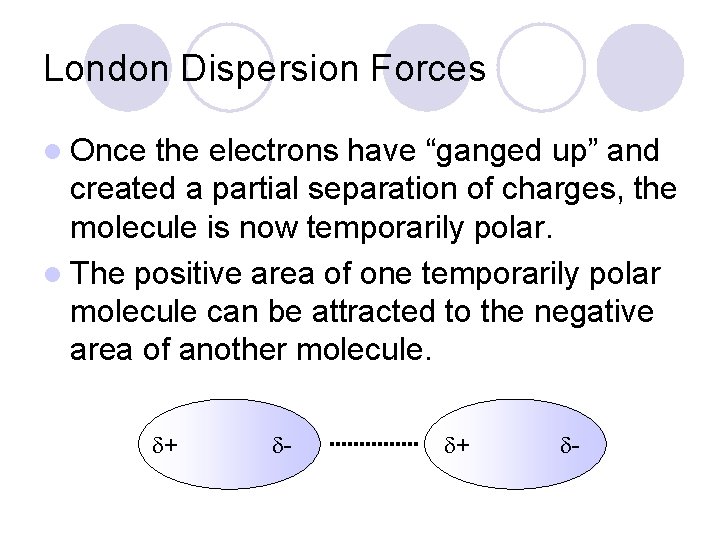
London Dispersion Forces l Once the electrons have “ganged up” and created a partial separation of charges, the molecule is now temporarily polar. l The positive area of one temporarily polar molecule can be attracted to the negative area of another molecule. + -

Strength of London Dispersion Forces Electrons can gang-up and cause a nonpolar molecule to be temporarily polar The electrons will move again, returning the molecule back to non-polar The polarity was temporary, therefore the molecule cannot always form LDF. London Dispersion Forces are the weakest of the intermolecular forces because molecules can’t form it all the time.

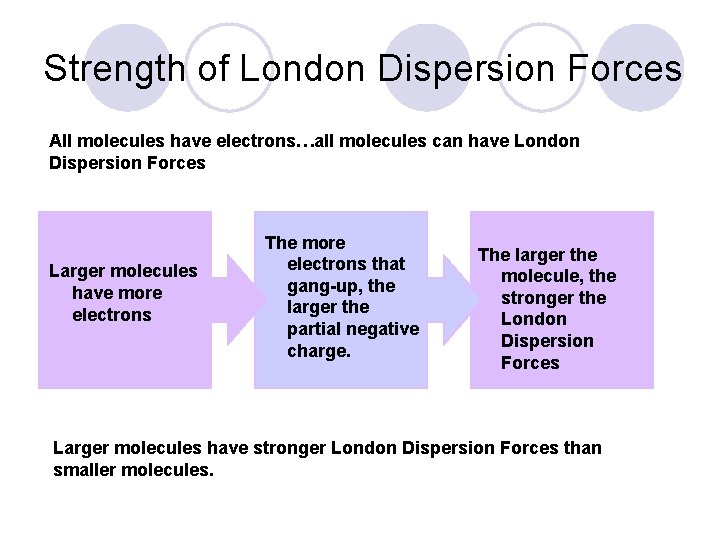
Strength of London Dispersion Forces All molecules have electrons…all molecules can have London Dispersion Forces Larger molecules have more electrons The more electrons that gang-up, the larger the partial negative charge. The larger the molecule, the stronger the London Dispersion Forces Larger molecules have stronger London Dispersion Forces than smaller molecules.

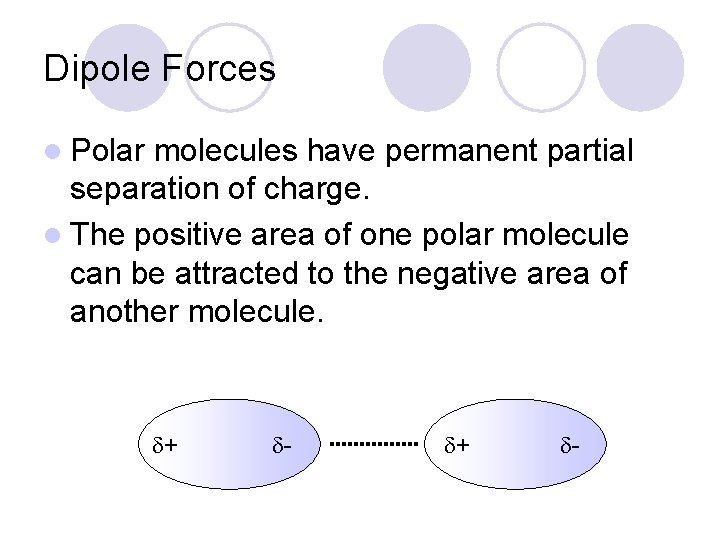
Dipole Forces l Polar molecules have permanent partial separation of charge. l The positive area of one polar molecule can be attracted to the negative area of another molecule. + -

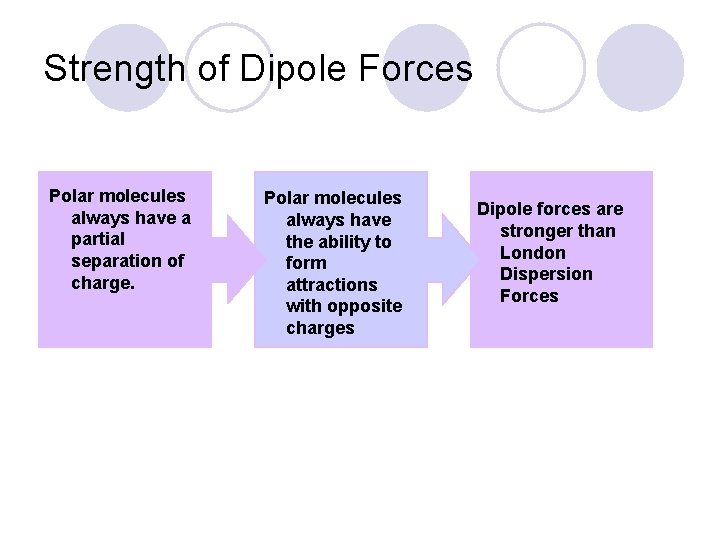
Strength of Dipole Forces Polar molecules always have a partial separation of charge. Polar molecules always have the ability to form attractions with opposite charges Dipole forces are stronger than London Dispersion Forces

Hydrogen Bonding l Hydrogen has 1 proton and 1 electron. ¡ l When that electron is shared unevenly (a polar bond) with another atom, the electron is farther from the hydrogen proton than usual. ¡ l There are no “inner” electrons. It bonds with the only one it has. This happens when Hydrogen bonds with Nitrogen, Oxygen or Fluorine This creates a very strong dipole (separation of charges) since there’s no other electrons around the hydrogen proton to counter-act the proton’s positive charge.

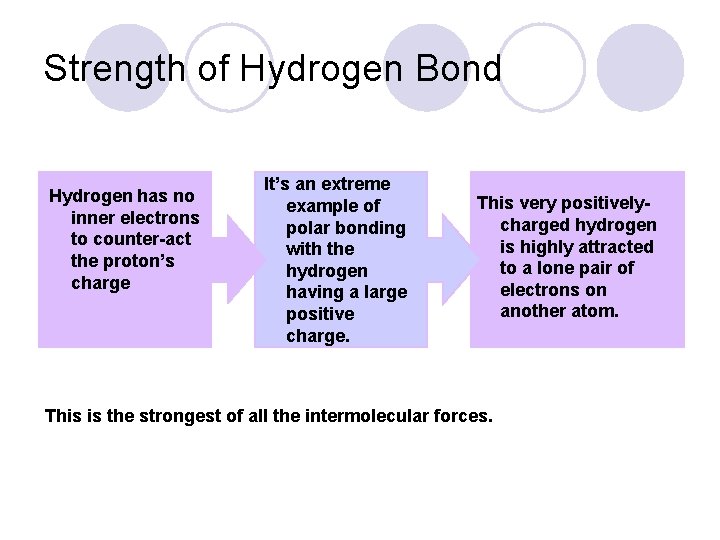
Strength of Hydrogen Bond Hydrogen has no inner electrons to counter-act the proton’s charge It’s an extreme example of polar bonding with the hydrogen having a large positive charge. This very positivelycharged hydrogen is highly attracted to a lone pair of electrons on another atom. This is the strongest of all the intermolecular forces.

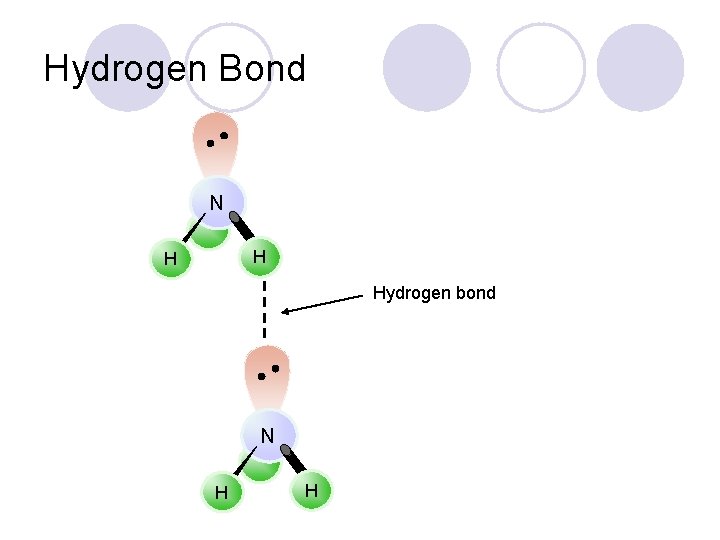
Hydrogen Bond N H H Hydrogen bond N H H