Hydrogen Bonding Intermolecular forces of attraction between molecules
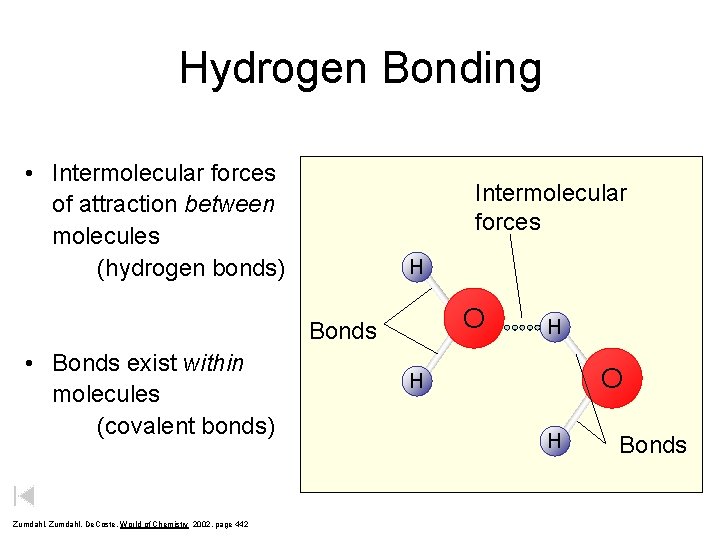
Hydrogen Bonding • Intermolecular forces of attraction between molecules (hydrogen bonds) Intermolecular forces H O Bonds • Bonds exist within molecules (covalent bonds) Zumdahl, De. Coste, World of Chemistry 2002, page 442 H O H H Bonds
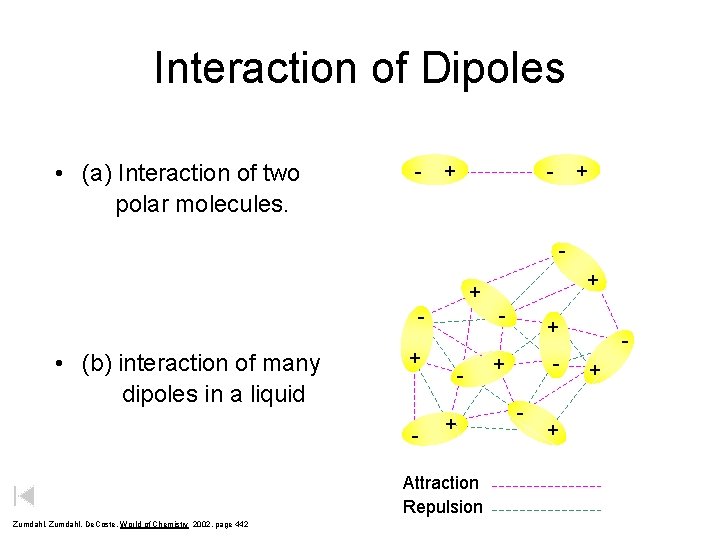
Interaction of Dipoles • (a) Interaction of two polar molecules. - + + + - - • (b) interaction of many dipoles in a liquid + - + Attraction Repulsion Zumdahl, De. Coste, World of Chemistry 2002, page 442 + - + +
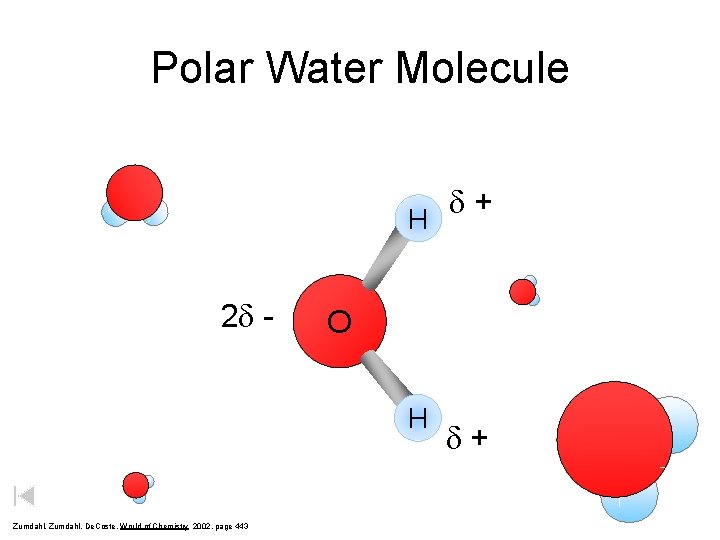
Polar Water Molecule d + H 2 d - O H Zumdahl, De. Coste, World of Chemistry 2002, page 443 d+

Hydrogen Bonding among water Molecules
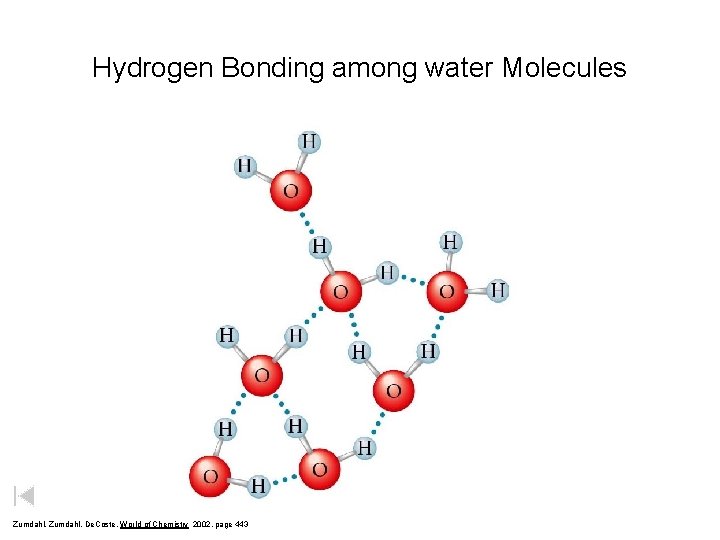
Hydrogen Bonding among water Molecules Zumdahl, De. Coste, World of Chemistry 2002, page 443
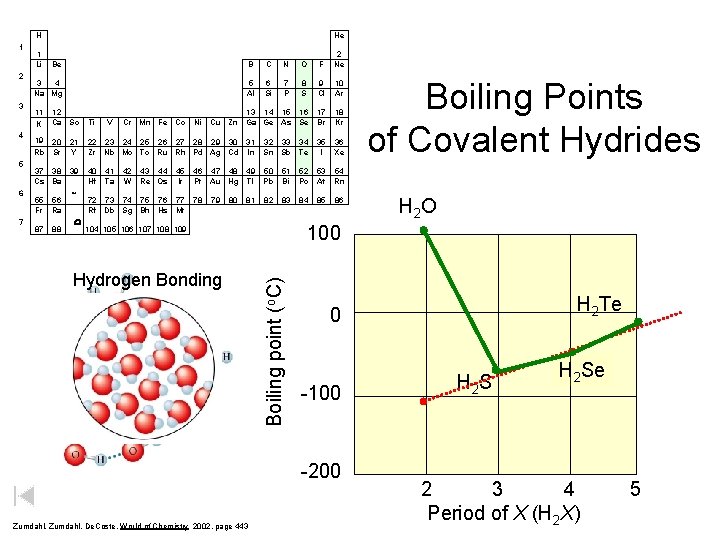
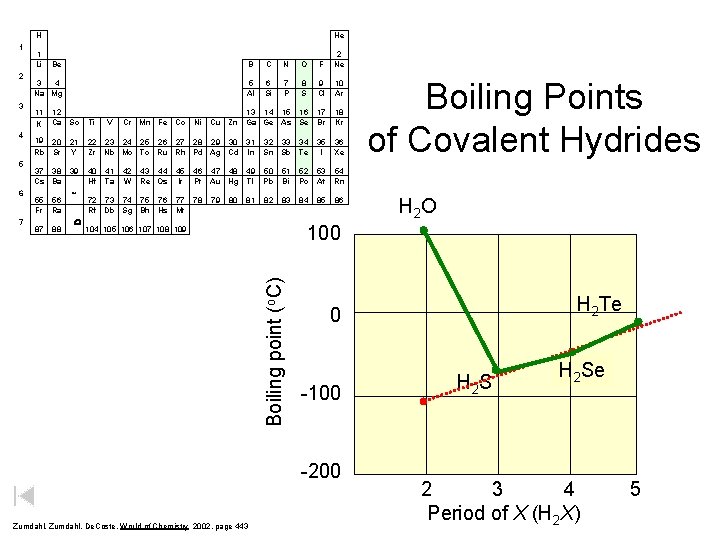
H 2 3 4 5 6 7 1 Li He Be B C N O F 2 Ne 3 4 Na Mg 5 Al 6 Si 7 P 8 S 9 Cl 10 Ar 13 14 15 16 Cu Zn Ga Ge As Se 17 Br 18 Kr 36 Xe 11 K 12 Ca Sc Ti V Cr Mn Fe Co Ni 19 20 Rb Sr 21 Y 22 23 24 25 26 27 28 29 30 31 Zr Nb Mo Tc Ru Rh Pd Ag Cd In 32 33 34 Sn Sb Te 35 I 37 38 Cs Ba 39 40 Hf 46 47 48 49 Pt Au Hg Tl 50 Pb 51 Bi 52 Po 53 54 At Rn 78 82 83 84 85 55 Fr 87 56 Ra 88 * W 41 Ta 42 W 43 44 45 Re Os Ir 72 73 74 75 76 77 Rf Db Sg Bh Hs Mt 79 80 81 Hydrogen Bonding 86 H 2 O H 2 Te 0 -100 -200 Zumdahl, De. Coste, World of Chemistry 2002, page 443 Boiling Points of Covalent Hydrides 100 104 105 106 107 108 109 Boiling point (o. C) 1 H 2 S H 2 Se 2 3 4 Period of X (H 2 X) 5
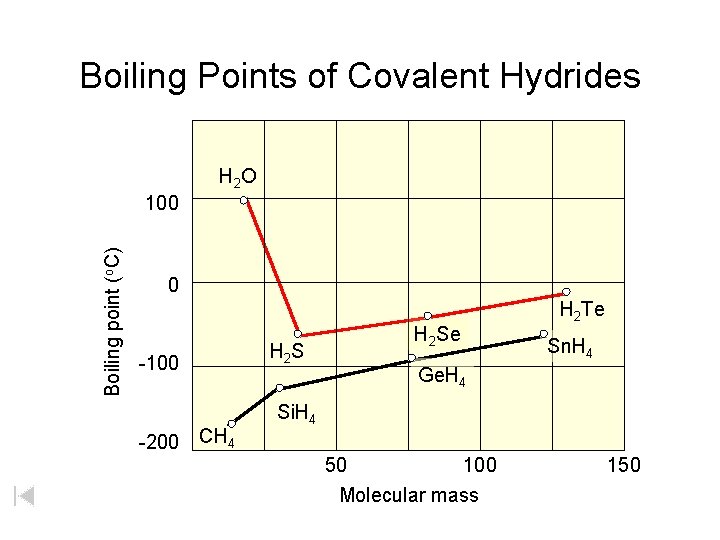
Boiling Points of Covalent Hydrides H 2 O Boiling point (o. C) 100 0 -100 -200 CH 4 H 2 S H 2 Se H 2 Te Sn. H 4 Ge. H 4 Si. H 4 50 100 Molecular mass 150
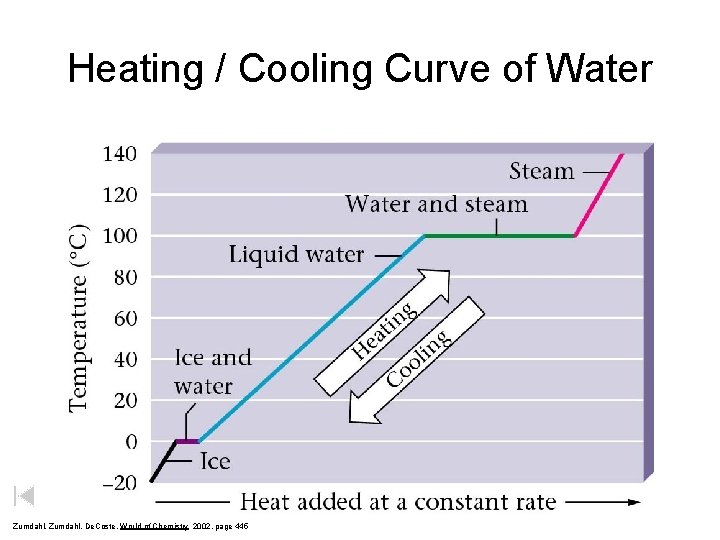
Heating / Cooling Curve of Water Zumdahl, De. Coste, World of Chemistry 2002, page 445
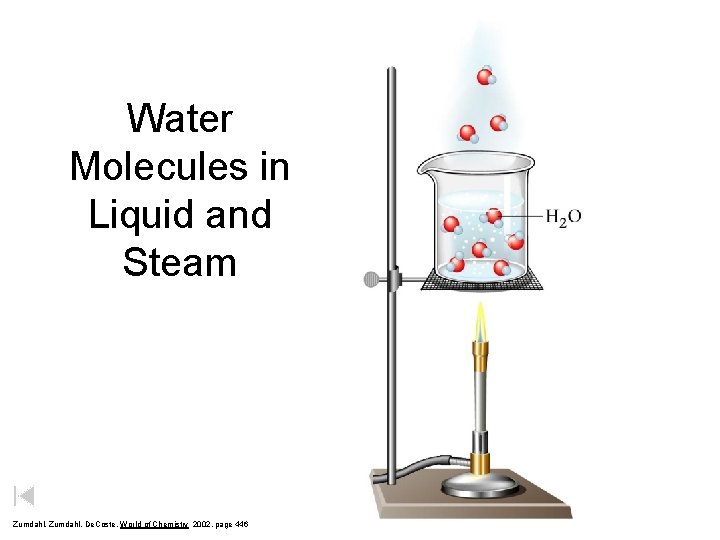
Water Molecules in Liquid and Steam Zumdahl, De. Coste, World of Chemistry 2002, page 446
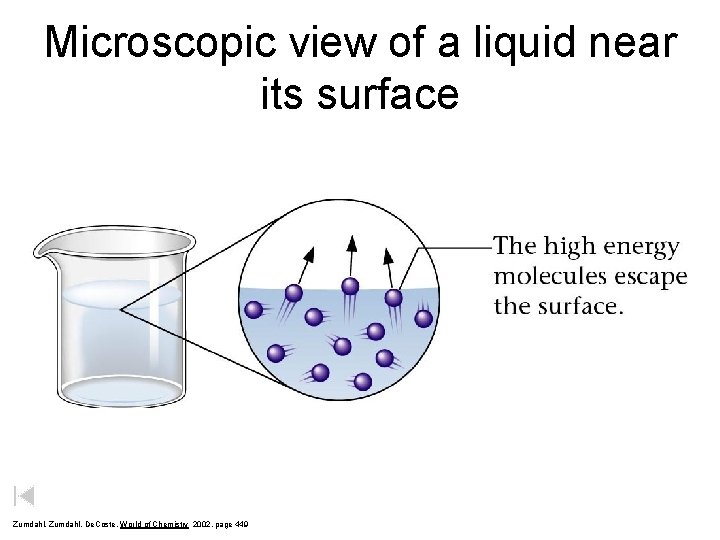
Microscopic view of a liquid near its surface Zumdahl, De. Coste, World of Chemistry 2002, page 449
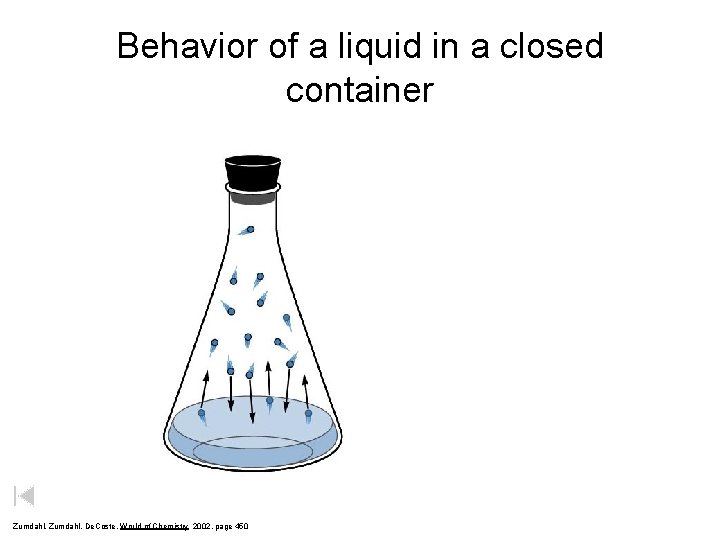
Behavior of a liquid in a closed container Zumdahl, De. Coste, World of Chemistry 2002, page 450

Water rapidly boiling on a stove
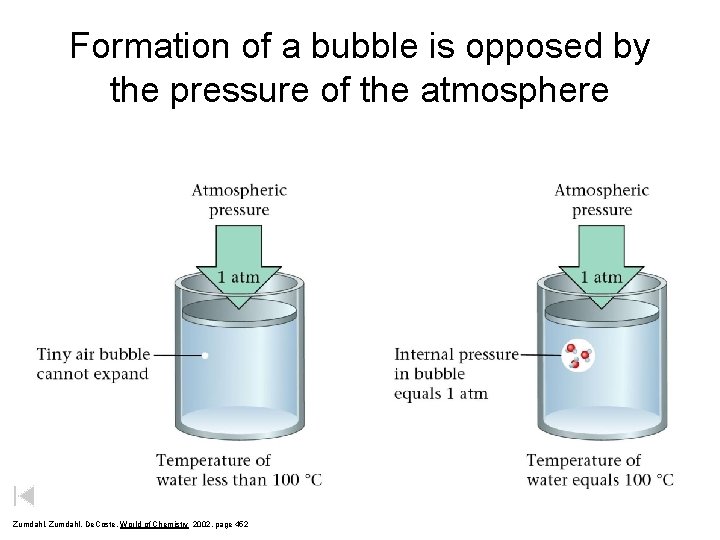
Formation of a bubble is opposed by the pressure of the atmosphere Zumdahl, De. Coste, World of Chemistry 2002, page 452

Boiling Points of Covalent Hydrides H 2 O Boiling point (o. C) 100 H 2 Te 0 -100 -200 Zumdahl, De. Coste, World of Chemistry 2002, page 443 H 2 S H 2 Se 2 3 4 Period of X (H 2 X) 5
H 1 2 3 4 5 6 Be B C N O F 2 Ne 3 4 Na Mg 5 Al 6 Si 7 P 8 S 9 Cl 10 Ar 13 14 15 16 Cu Zn Ga Ge As Se 17 Br 18 Kr 36 Xe 11 K 12 Ca Sc Ti V Cr Mn Fe Co Ni 19 20 Rb Sr 21 Y 22 23 24 25 26 27 28 29 30 31 Zr Nb Mo Tc Ru Rh Pd Ag Cd In 32 33 34 Sn Sb Te 35 I 37 38 Cs Ba 39 40 Hf 46 47 48 49 Pt Au Hg Tl 50 Pb 51 Bi 52 Po 53 54 At Rn 78 82 83 84 85 55 Fr 87 56 Ra 88 * W 41 Ta 42 W 43 44 45 Re Os Ir 72 73 74 75 76 77 Rf Db Sg Bh Hs Mt 79 80 81 86 H 2 O H 2 Te 0 -100 -200 Zumdahl, De. Coste, World of Chemistry 2002, page 443 Boiling Points of Covalent Hydrides 100 104 105 106 107 108 109 Boiling point (o. C) 7 1 Li He H 2 S H 2 Se 2 3 4 Period of X (H 2 X) 5
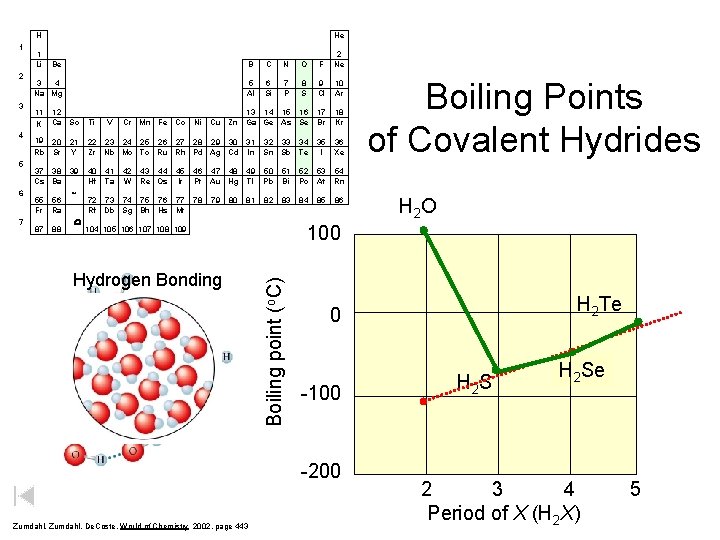
H 2 3 4 5 6 7 1 Li He Be B C N O F 2 Ne 3 4 Na Mg 5 Al 6 Si 7 P 8 S 9 Cl 10 Ar 13 14 15 16 Cu Zn Ga Ge As Se 17 Br 18 Kr 36 Xe 11 K 12 Ca Sc Ti V Cr Mn Fe Co Ni 19 20 Rb Sr 21 Y 22 23 24 25 26 27 28 29 30 31 Zr Nb Mo Tc Ru Rh Pd Ag Cd In 32 33 34 Sn Sb Te 35 I 37 38 Cs Ba 39 40 Hf 46 47 48 49 Pt Au Hg Tl 50 Pb 51 Bi 52 Po 53 54 At Rn 78 82 83 84 85 55 Fr 87 56 Ra 88 * W 41 Ta 42 W 43 44 45 Re Os Ir 72 73 74 75 76 77 Rf Db Sg Bh Hs Mt 79 80 81 Hydrogen Bonding 86 H 2 O H 2 Te 0 -100 -200 Zumdahl, De. Coste, World of Chemistry 2002, page 443 Boiling Points of Covalent Hydrides 100 104 105 106 107 108 109 Boiling point (o. C) 1 H 2 S H 2 Se 2 3 4 Period of X (H 2 X) 5

Boiling Points of Covalent Hydrides H 2 O Boiling point (o. C) 100 0 -100 -200 CH 4 H 2 S H 2 Se H 2 Te Sn. H 4 Ge. H 4 Si. H 4 50 100 Molecular mass 150
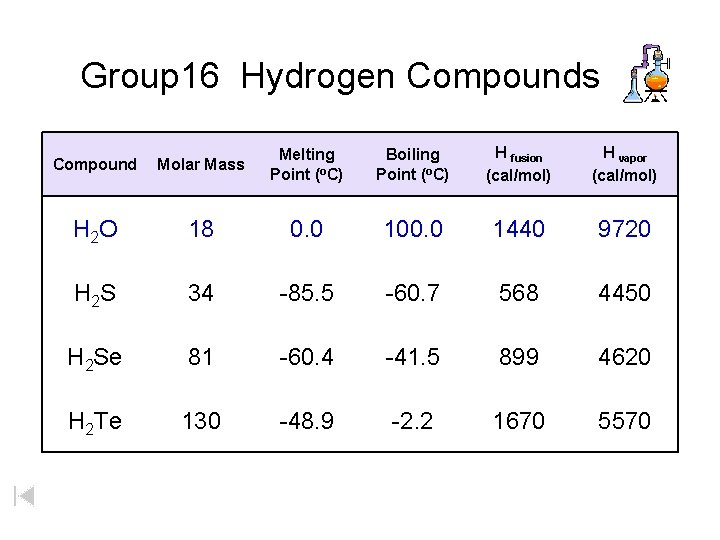
Group 16 Hydrogen Compounds Compound Molar Mass Melting Point (o. C) Boiling Point (o. C) H fusion (cal/mol) H vapor (cal/mol) H 2 O 18 0. 0 100. 0 1440 9720 H 2 S 34 -85. 5 -60. 7 568 4450 H 2 Se 81 -60. 4 -41. 5 899 4620 H 2 Te 130 -48. 9 -2. 2 1670 5570
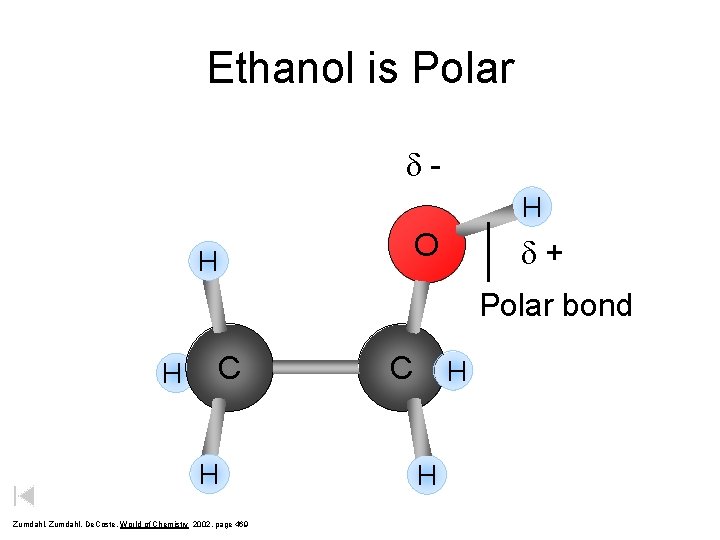
Ethanol is Polar d. H O H d+ Polar bond H C H Zumdahl, De. Coste, World of Chemistry 2002, page 469 C H H
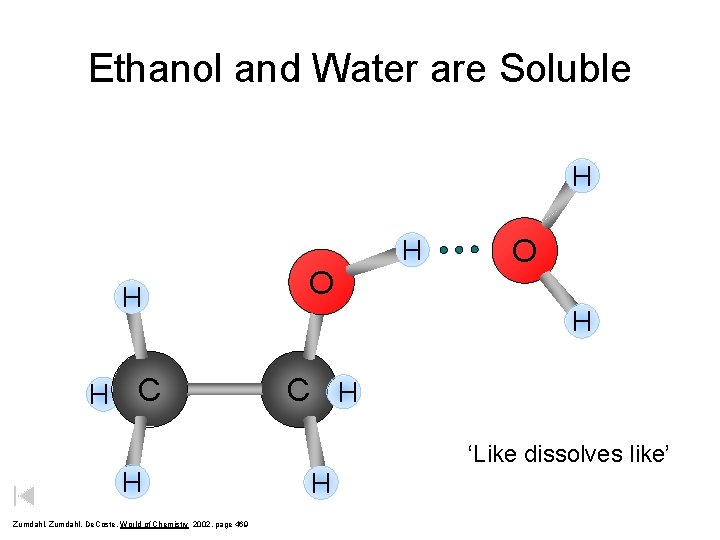
Ethanol and Water are Soluble H H H C H Zumdahl, De. Coste, World of Chemistry 2002, page 469 O H C H ‘Like dissolves like’ H
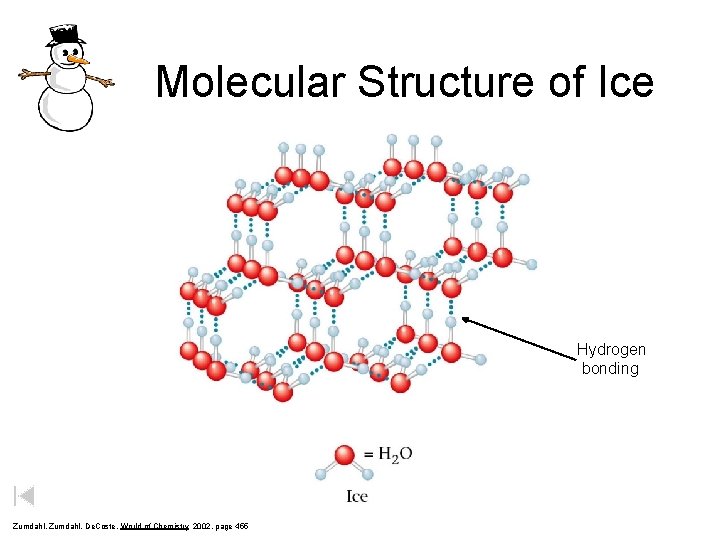
Molecular Structure of Ice Hydrogen bonding Zumdahl, De. Coste, World of Chemistry 2002, page 455
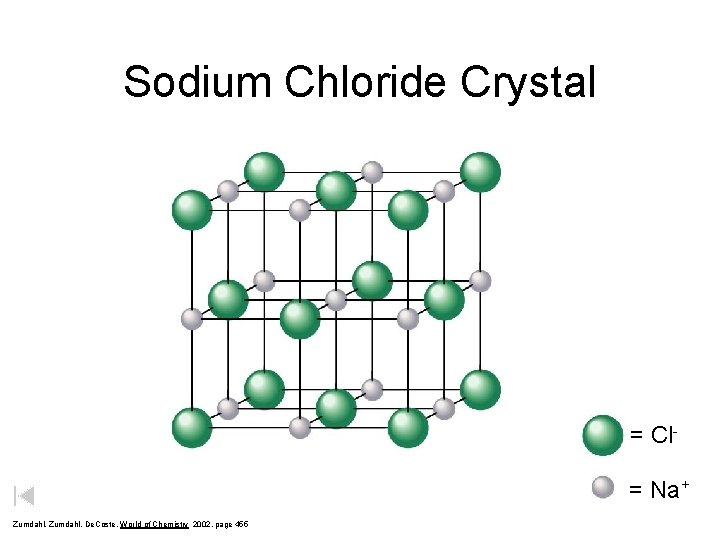
Sodium Chloride Crystal = Cl= Na+ Zumdahl, De. Coste, World of Chemistry 2002, page 455

Packing of Na. Cl Ions = Cl 1 Zumdahl, De. Coste, World of Chemistry 2002, page 456 = Na 1+

Packing of Na. Cl Ions Electron Microscope Photograph of Na. Cl
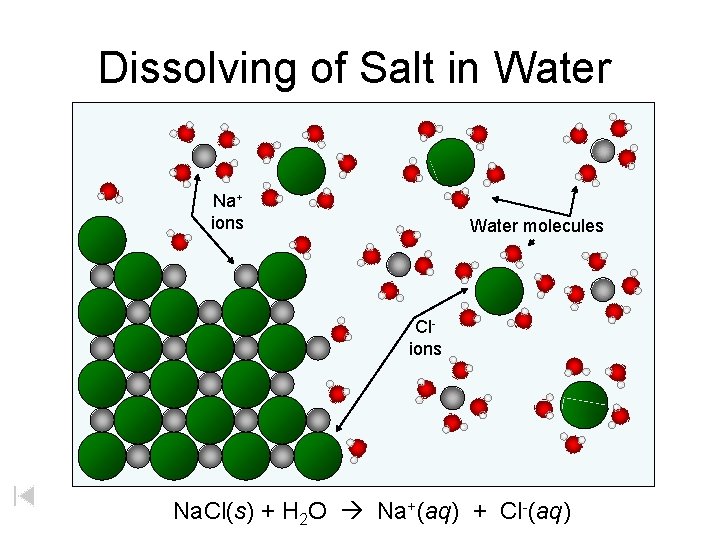
Dissolving of Salt in Water Na+ ions Water molecules Clions Na. Cl(s) + H 2 O Na+(aq) + Cl-(aq)
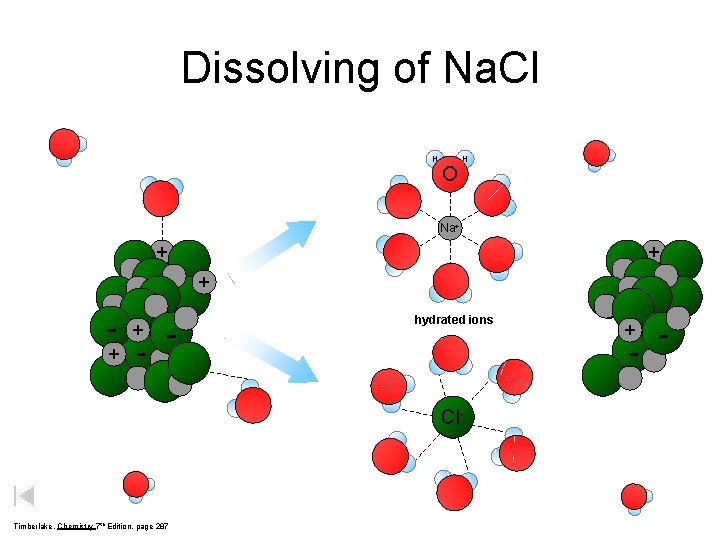
Dissolving of Na. Cl H O H Na+ + - - hydrated ions - Cl- Timberlake, Chemistry 7 th Edition, page 287 + -
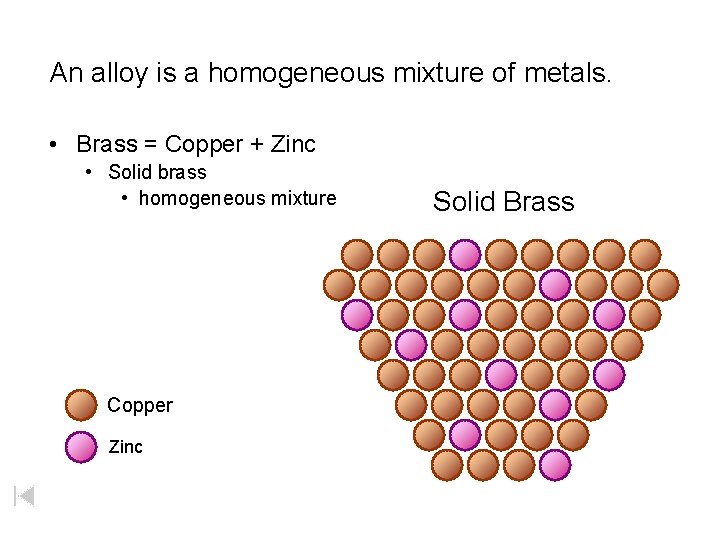
An alloy is a homogeneous mixture of metals. • Brass = Copper + Zinc • Solid brass • homogeneous mixture Copper Zinc Solid Brass

• Brass = Copper + Zinc • Brass plated • heterogeneous mixture • Only brass on outside Copper Zinc Brass Plated
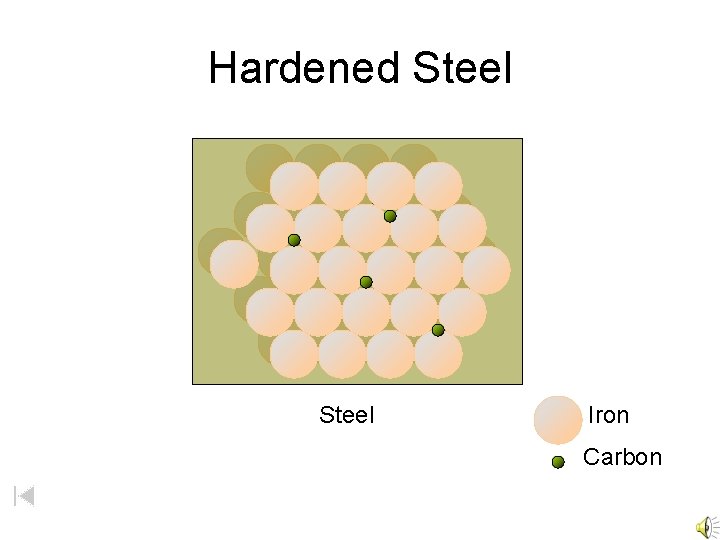
Hardened Steel Iron Carbon

Gold Copper Silver 24 karat gold 24/ 24 atoms Au 18 karat gold 18/ 24 atoms Au 14 karat gold 14/ 24 atoms Au
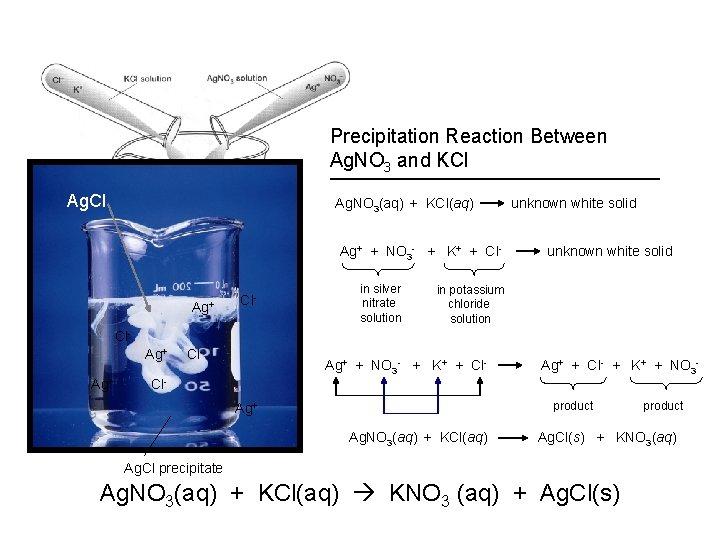
Precipitation Reaction Between Ag. NO 3 and KCl Ag. NO 3(aq) + KCl(aq) Ag+ + NO 3 - + K+ + Cl- K+ Cl- NO 3 - + NO 3 - Ag Ag+ ? Cl. K+ Cl- Cl. K+ NO 3 - in silver nitrate solution unknown white solid in potassium chloride solution Ag+ + NO 3 - + K+ + Cl- Ag+ + Cl- + K+ + NO 3 product Ag+ Ag. NO 3(aq) + KCl(aq) product Ag. Cl(s) + KNO 3(aq) Ag. Cl precipitate Ag. NO 3(aq) + KCl(aq) KNO 3 (aq) + Ag. Cl(s)
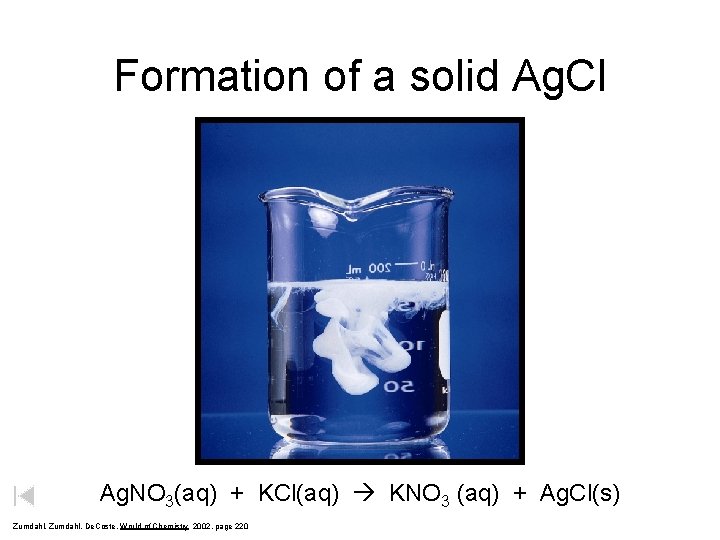
Formation of a solid Ag. Cl Ag. NO 3(aq) + KCl(aq) KNO 3 (aq) + Ag. Cl(s) Zumdahl, De. Coste, World of Chemistry 2002, page 220
- Slides: 32