Alcohols n Alcohols contain an OH group connected





- Slides: 13

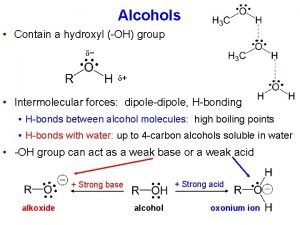
Alcohols

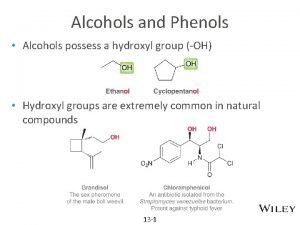
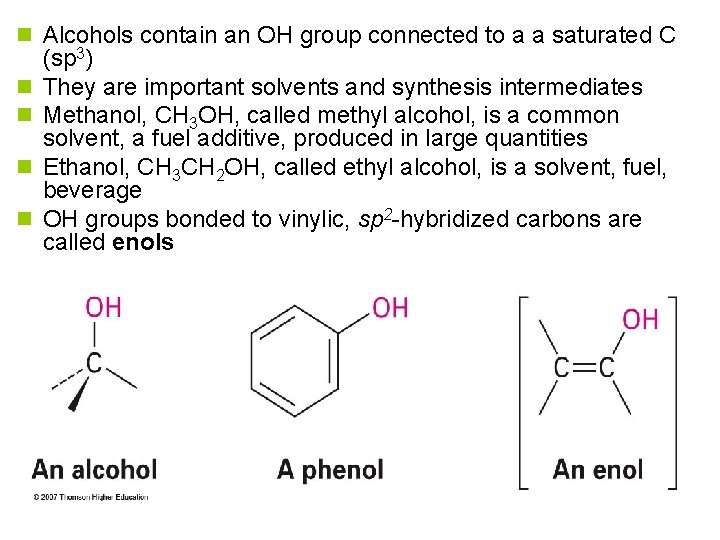
n Alcohols contain an OH group connected to a a saturated C n n (sp 3) They are important solvents and synthesis intermediates Methanol, CH 3 OH, called methyl alcohol, is a common solvent, a fuel additive, produced in large quantities Ethanol, CH 3 CH 2 OH, called ethyl alcohol, is a solvent, fuel, beverage OH groups bonded to vinylic, sp 2 -hybridized carbons are called enols

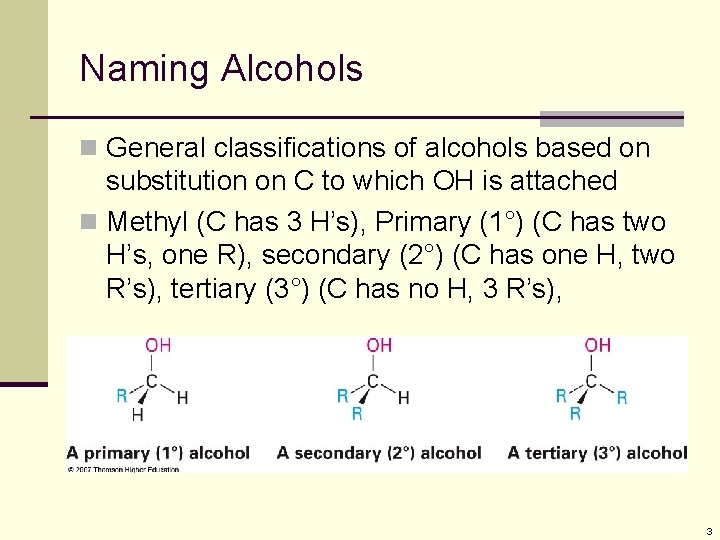
Naming Alcohols n General classifications of alcohols based on substitution on C to which OH is attached n Methyl (C has 3 H’s), Primary (1°) (C has two H’s, one R), secondary (2°) (C has one H, two R’s), tertiary (3°) (C has no H, 3 R’s), 3

IUPAC Rules for Naming Alcohols n Select the longest carbon chain containing the hydroxyl group, and derive the parent name by replacing the -e ending of the corresponding alkane with -ol n Number the chain from the end nearer the hydroxyl group n Number substituents according to position on chain, listing the substituents in alphabetical order 4

Examples

Assigning Priority Halogens < alkanes < alkenes (alkynes) < amines < OH < ketone < aldehyde < acid < ester


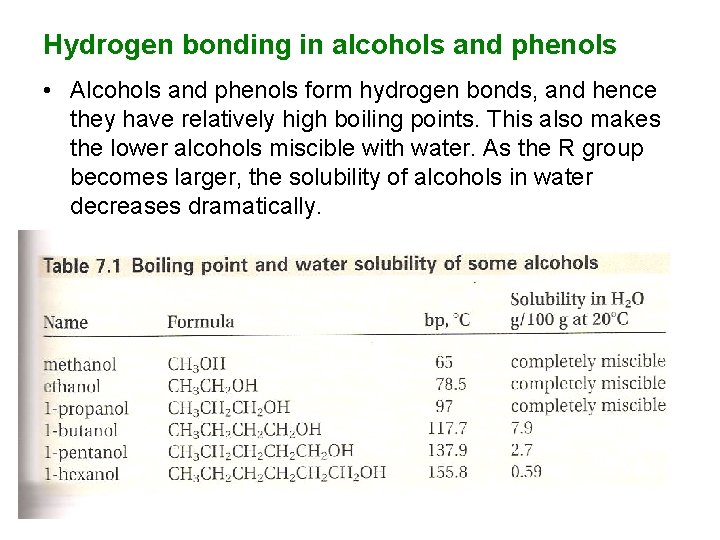
Hydrogen bonding in alcohols and phenols • Alcohols and phenols form hydrogen bonds, and hence they have relatively high boiling points. This also makes the lower alcohols miscible with water. As the R group becomes larger, the solubility of alcohols in water decreases dramatically.

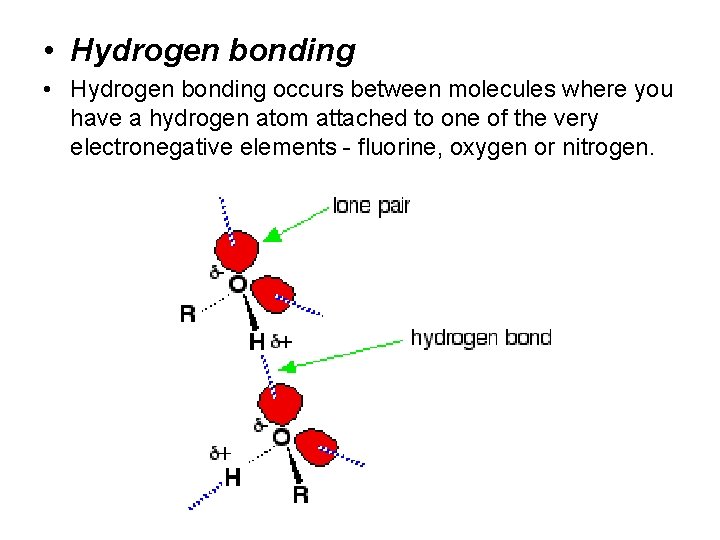
• Hydrogen bonding occurs between molecules where you have a hydrogen atom attached to one of the very electronegative elements - fluorine, oxygen or nitrogen.

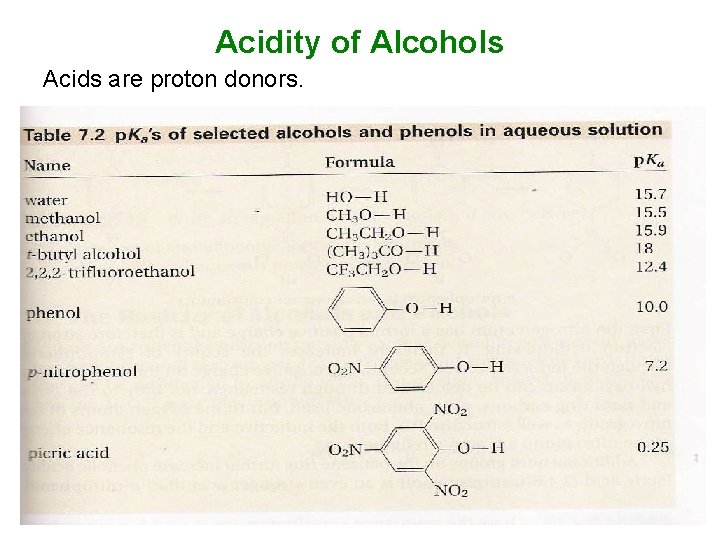
Acidity of Alcohols Acids are proton donors.

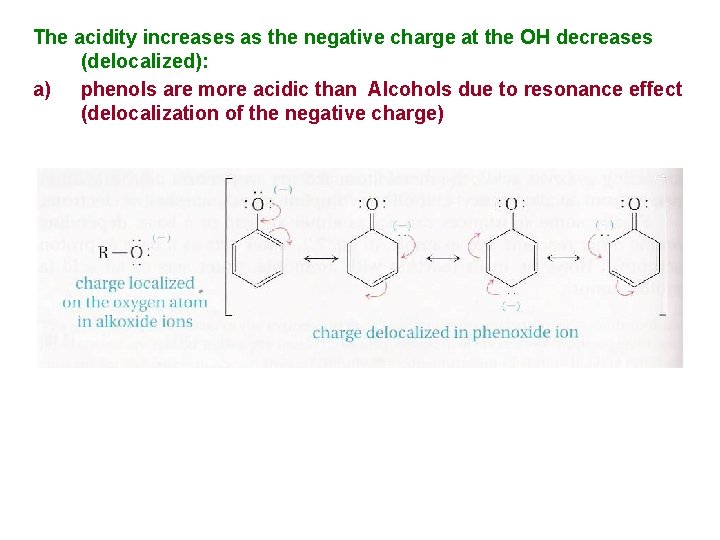
The acidity increases as the negative charge at the OH decreases (delocalized): a) phenols are more acidic than Alcohols due to resonance effect (delocalization of the negative charge)

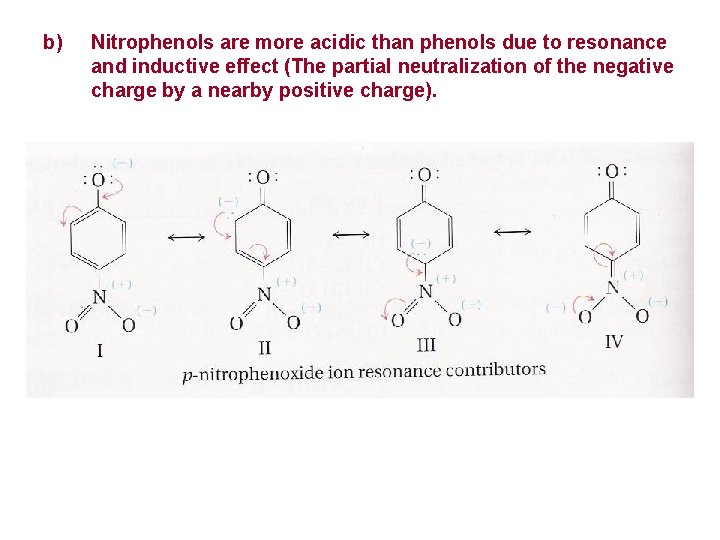
b) Nitrophenols are more acidic than phenols due to resonance and inductive effect (The partial neutralization of the negative charge by a nearby positive charge).

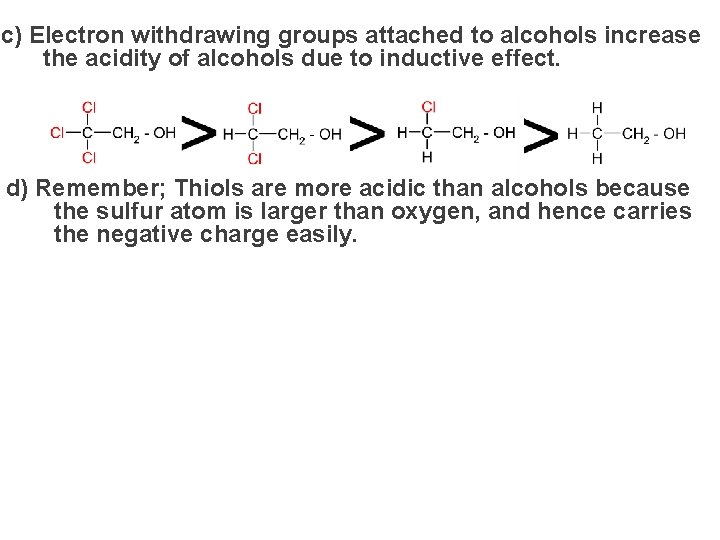
c) Electron withdrawing groups attached to alcohols increase the acidity of alcohols due to inductive effect. d) Remember; Thiols are more acidic than alcohols because the sulfur atom is larger than oxygen, and hence carries the negative charge easily.
 Wye delta diagram
Wye delta diagram In a triangle connected source feeding a y connected load
In a triangle connected source feeding a y connected load Phase to phase voltage
Phase to phase voltage What is lucas reagent
What is lucas reagent Nomenclature
Nomenclature Alcohols containing cppp nucleus
Alcohols containing cppp nucleus Alcohols nomenclature
Alcohols nomenclature Chlorination
Chlorination Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Acidity of alcohols
Acidity of alcohols High boiling point alcohols
High boiling point alcohols Butanone isomers
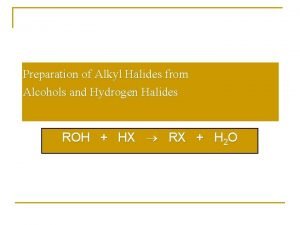
Butanone isomers Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Names of sugar alcohols
Names of sugar alcohols