Part 2 8 Coordination Chemistry 1 Outline Coordination



- Slides: 120

Part 2. 8: Coordination Chemistry 1

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field Theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry 2

History of Inorganic Chemistry • Ancient times through Alchemy: – Descriptive chemistry, techniques, minerals (Cu compounds), glasses, glazes, gunpowder • 17 th Century – Mineral acids (HCl, HNO 3, H 2 SO 4), salts and their reactions, acid and bases – Quantitative work became important, molar mass, gases, volumes – 1869: The periodic table • Late 1800 s: Chemical Industry – Isolate, refine, purify metals and compounds • 1896: Discovery of Radioactivity – Atomic structure, quantum mechanics, nuclear chemistry (through early 20 th century) 3

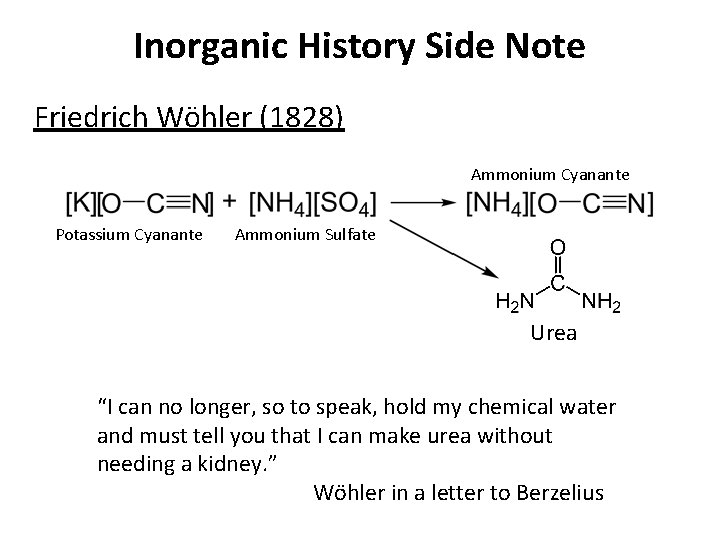
Inorganic History Side Note Friedrich Wöhler (1828) Ammonium Cyanante Potassium Cyanante Ammonium Sulfate Urea “I can no longer, so to speak, hold my chemical water and must tell you that I can make urea without needing a kidney. ” Wöhler in a letter to Berzelius

History of Inorganic Chemistry • 20 th Century – Coordination chemistry, organometallic chemistry – WWII & Military projects: Manhattan project, jet fuels (boron compounds) • 1950 s – Crystal field theory, ligand field theory, molecular orbital theory • 1955 – Organometallic catalysis of organic reaction (polymerization of ethylene) 5

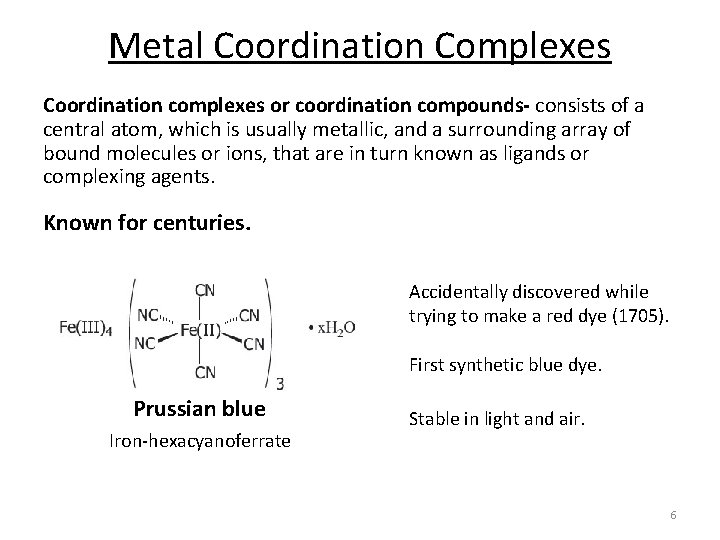
Metal Coordination Complexes Coordination complexes or coordination compounds- consists of a central atom, which is usually metallic, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Known for centuries. Accidentally discovered while trying to make a red dye (1705). First synthetic blue dye. Prussian blue Iron‐hexacyanoferrate Stable in light and air. 6

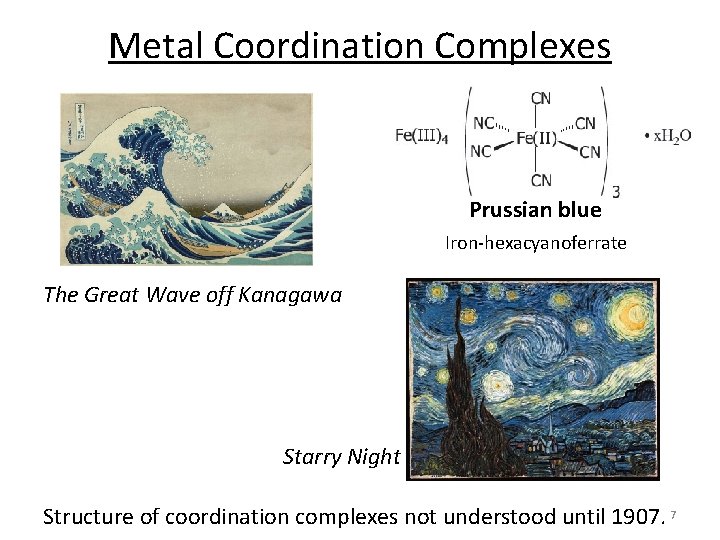
Metal Coordination Complexes Prussian blue Iron‐hexacyanoferrate The Great Wave off Kanagawa Starry Night Structure of coordination complexes not understood until 1907. 7

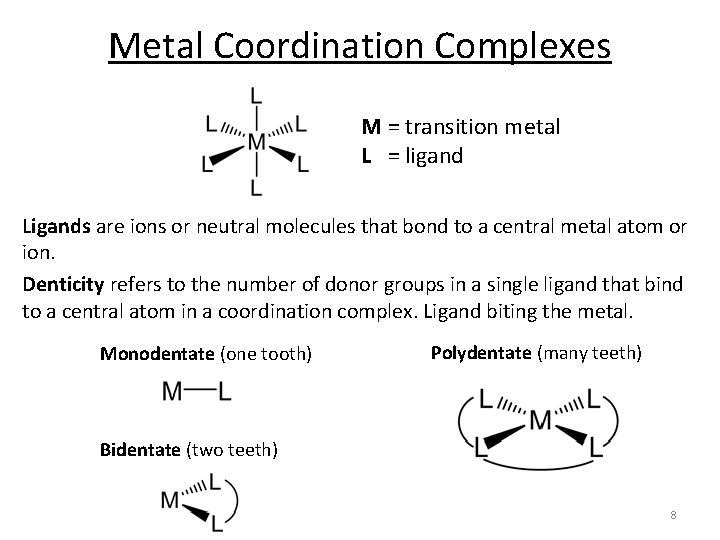
Metal Coordination Complexes M = transition metal L = ligand Ligands are ions or neutral molecules that bond to a central metal atom or ion. Denticity refers to the number of donor groups in a single ligand that bind to a central atom in a coordination complex. Ligand biting the metal. Monodentate (one tooth) Polydentate (many teeth) Bidentate (two teeth) 8

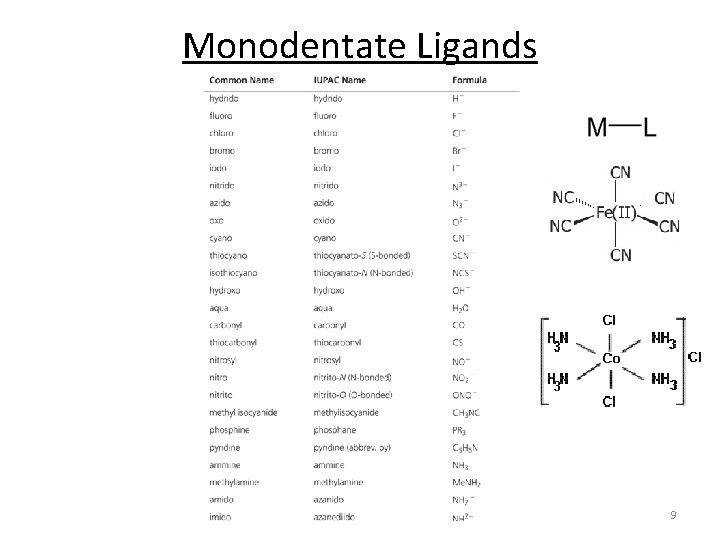
Monodentate Ligands 9

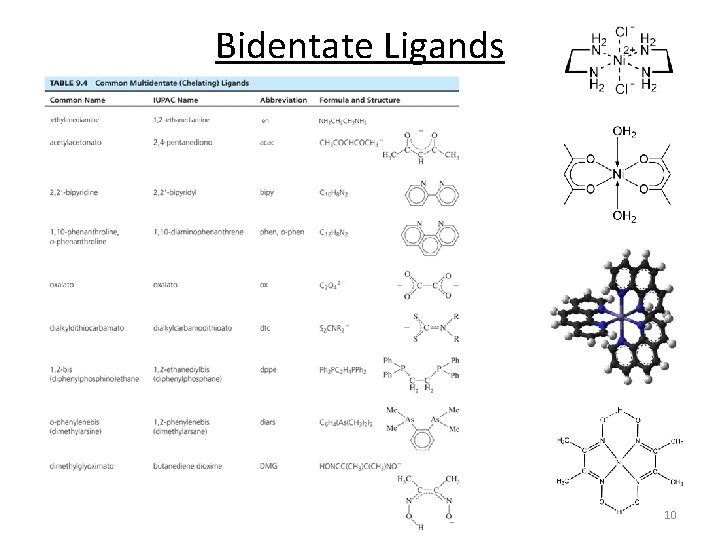
Bidentate Ligands 10

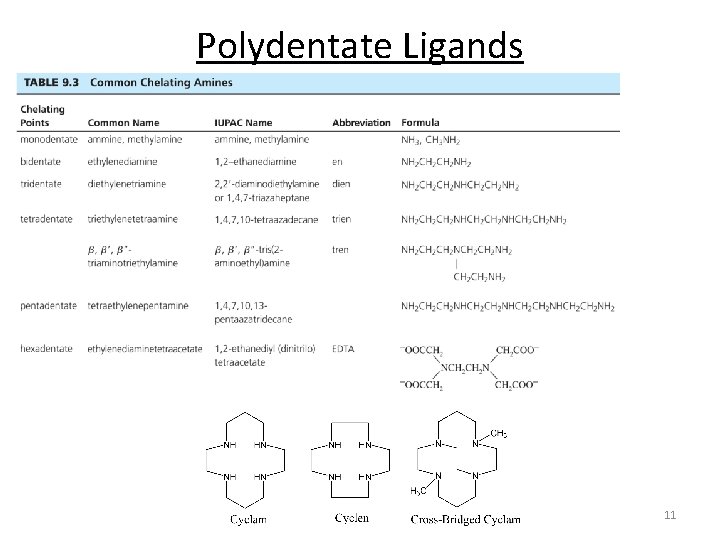
Polydentate Ligands 11

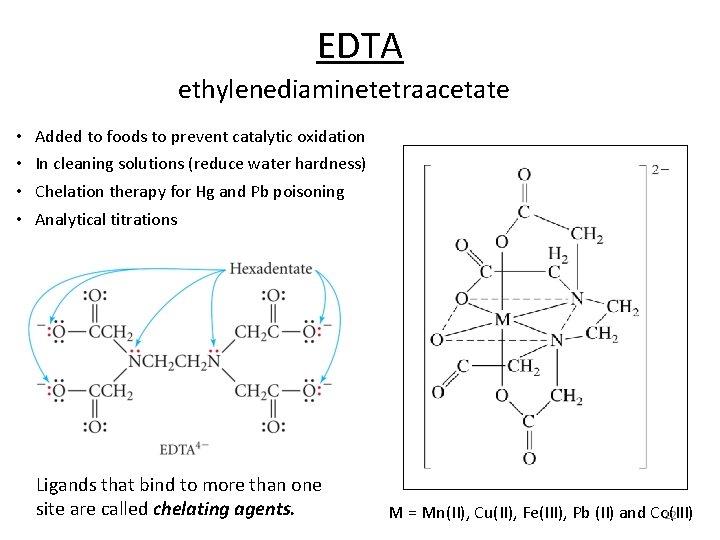
EDTA ethylenediaminetetraacetate • • Added to foods to prevent catalytic oxidation In cleaning solutions (reduce water hardness) Chelation therapy for Hg and Pb poisoning Analytical titrations Ligands that bind to more than one site are called chelating agents. M = Mn(II), Cu(II), Fe(III), Pb (II) and Co(III) 12

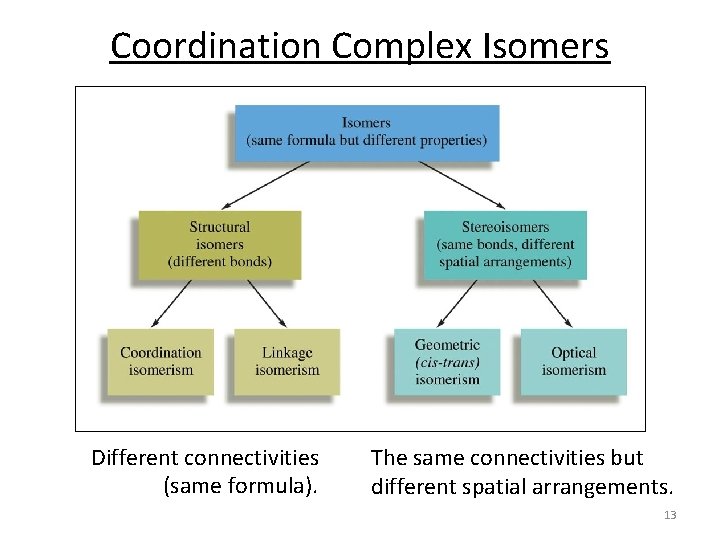
Coordination Complex Isomers Different connectivities (same formula). The same connectivities but different spatial arrangements. 13

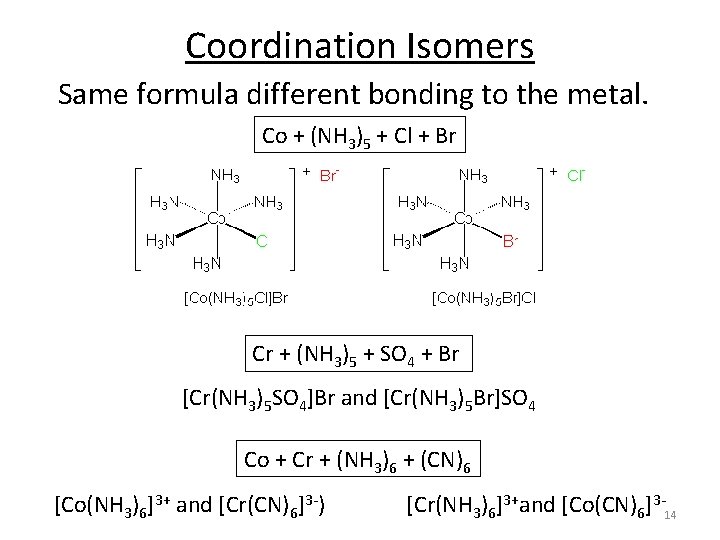
Coordination Isomers Same formula different bonding to the metal. Co + (NH 3)5 + Cl + Br Cr + (NH 3)5 + SO 4 + Br [Cr(NH 3)5 SO 4]Br and [Cr(NH 3)5 Br]SO 4 Co + Cr + (NH 3)6 + (CN)6 [Co(NH 3)6]3+ and [Cr(CN)6]3‐) [Cr(NH 3)6]3+and [Co(CN)6]3‐ 14

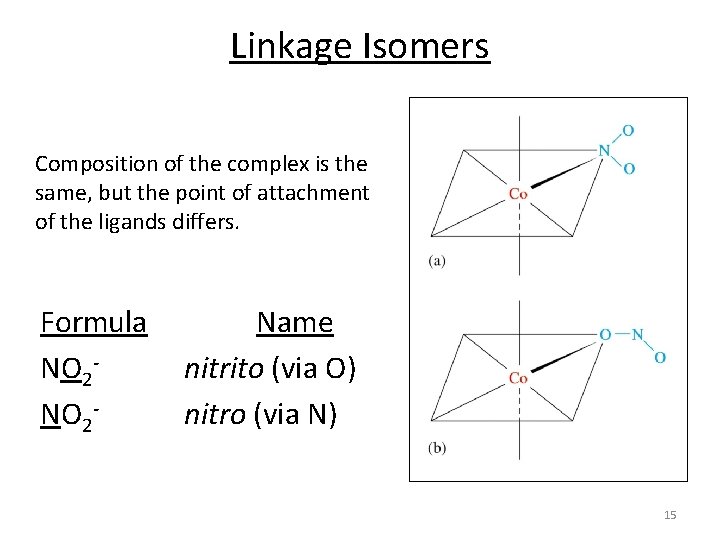
Linkage Isomers Composition of the complex is the same, but the point of attachment of the ligands differs. Formula NO 2‐ Name nitrito (via O) nitro (via N) 15

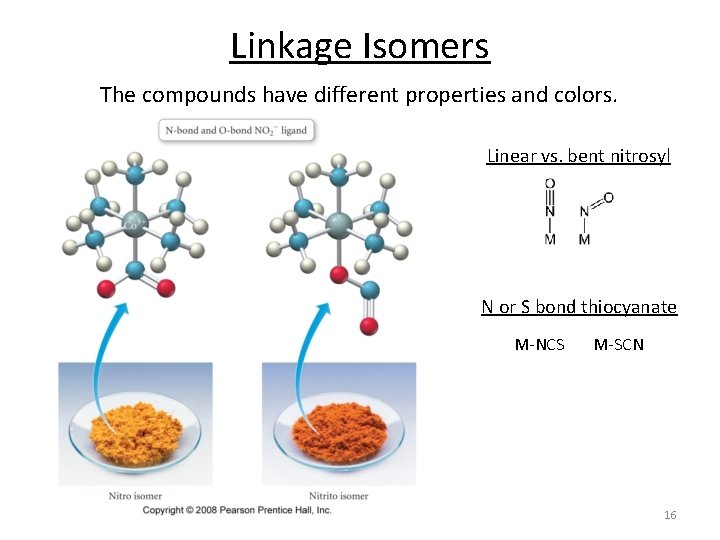
Linkage Isomers The compounds have different properties and colors. Linear vs. bent nitrosyl N or S bond thiocyanate M‐NCS M‐SCN 16

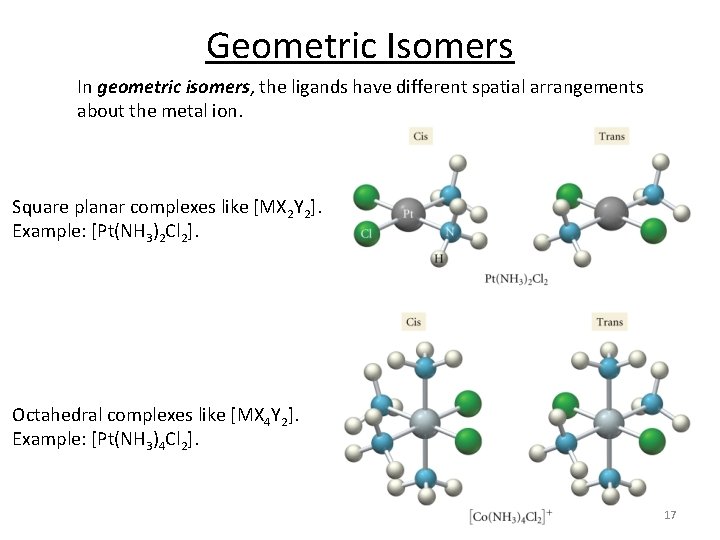
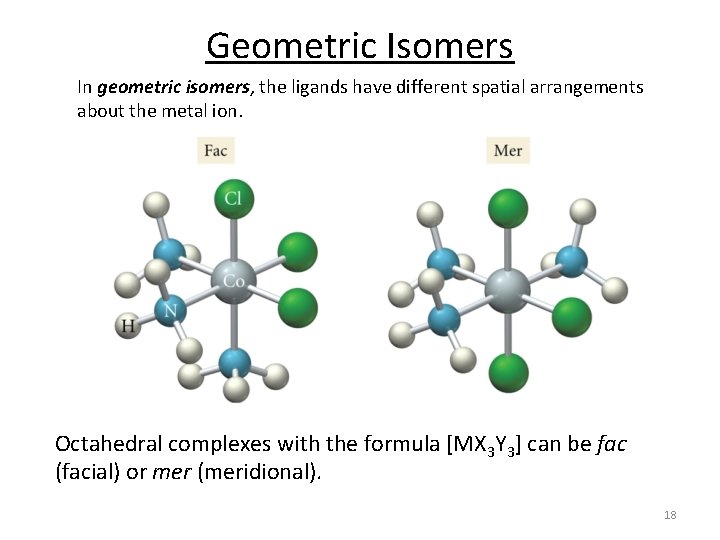
Geometric Isomers In geometric isomers, the ligands have different spatial arrangements about the metal ion. Square planar complexes like [MX 2 Y 2]. Example: [Pt(NH 3)2 Cl 2]. Octahedral complexes like [MX 4 Y 2]. Example: [Pt(NH 3)4 Cl 2]. 17

Geometric Isomers In geometric isomers, the ligands have different spatial arrangements about the metal ion. Octahedral complexes with the formula [MX 3 Y 3] can be fac (facial) or mer (meridional). 18

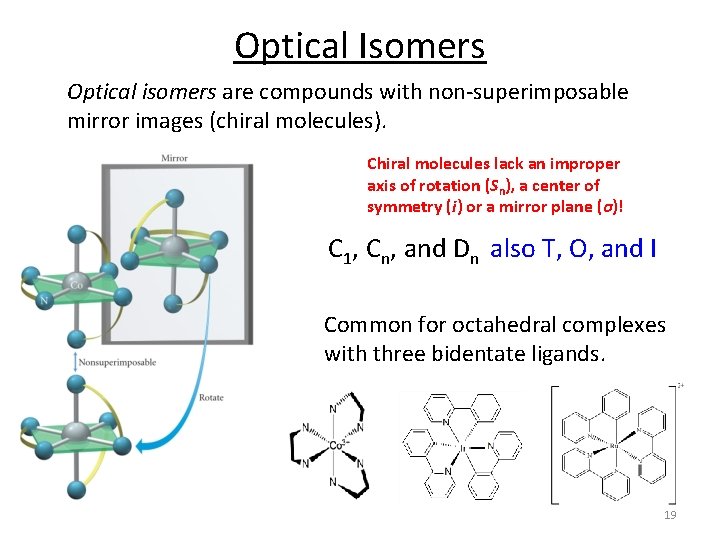
Optical Isomers Optical isomers are compounds with non‐superimposable mirror images (chiral molecules). Chiral molecules lack an improper axis of rotation (Sn), a center of symmetry (i) or a mirror plane (σ)! C 1, Cn, and Dn also T, O, and I Common for octahedral complexes with three bidentate ligands. 19

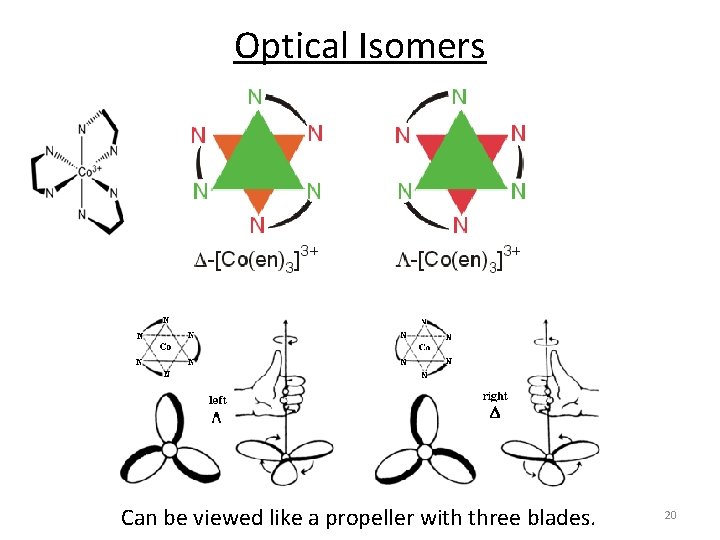
Optical Isomers Can be viewed like a propeller with three blades. 20

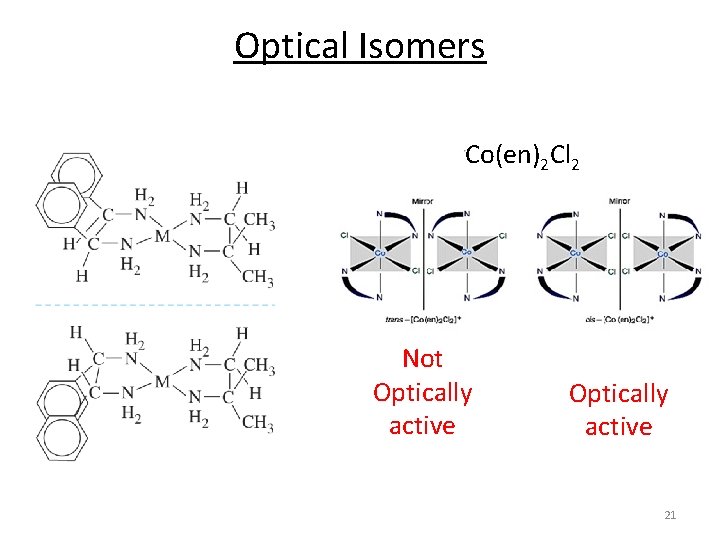
Optical Isomers Co(en)2 Cl 2 Not Optically active 21

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field Theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry 22

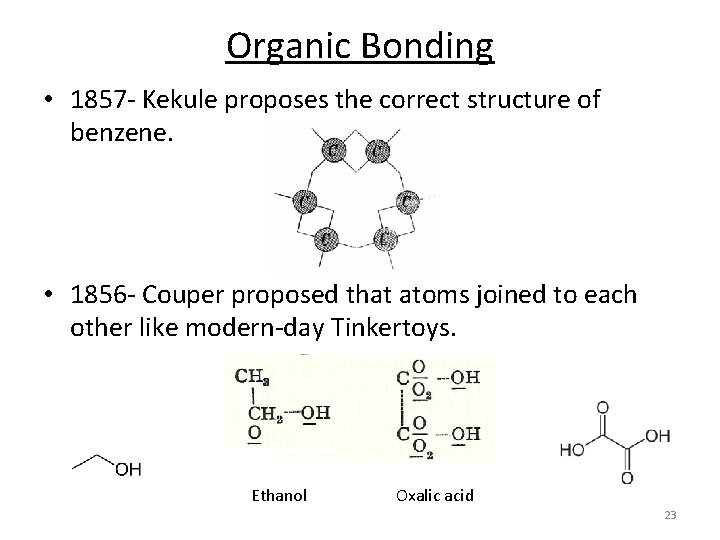
Organic Bonding • 1857‐ Kekule proposes the correct structure of benzene. • 1856‐ Couper proposed that atoms joined to each other like modern‐day Tinkertoys. Ethanol Oxalic acid 23

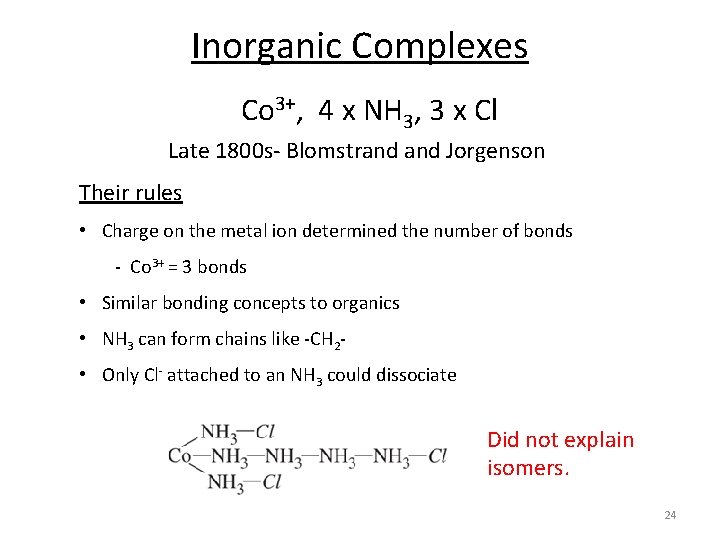
Inorganic Complexes Co 3+, 4 x NH 3, 3 x Cl Late 1800 s‐ Blomstrand Jorgenson Their rules • Charge on the metal ion determined the number of bonds ‐ Co 3+ = 3 bonds • Similar bonding concepts to organics • NH 3 can form chains like ‐CH 2‐ • Only Cl‐ attached to an NH 3 could dissociate Did not explain isomers. 24

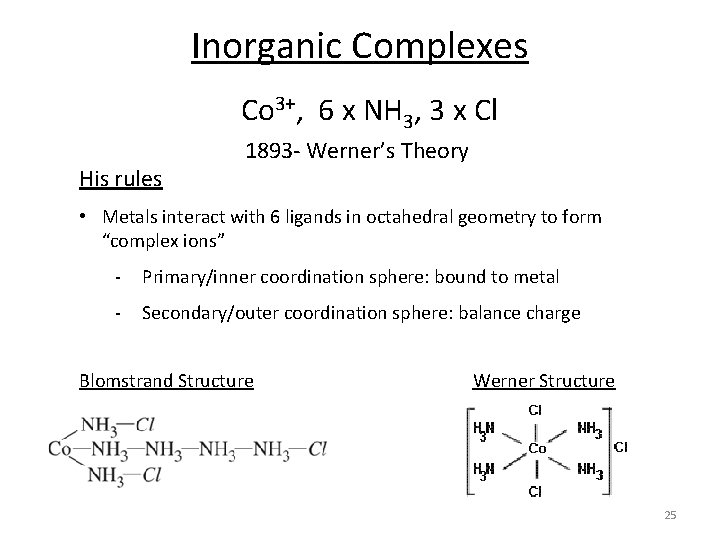
Inorganic Complexes Co 3+, 6 x NH 3, 3 x Cl His rules 1893‐ Werner’s Theory • Metals interact with 6 ligands in octahedral geometry to form “complex ions” ‐ Primary/inner coordination sphere: bound to metal ‐ Secondary/outer coordination sphere: balance charge Blomstrand Structure Werner Structure 25

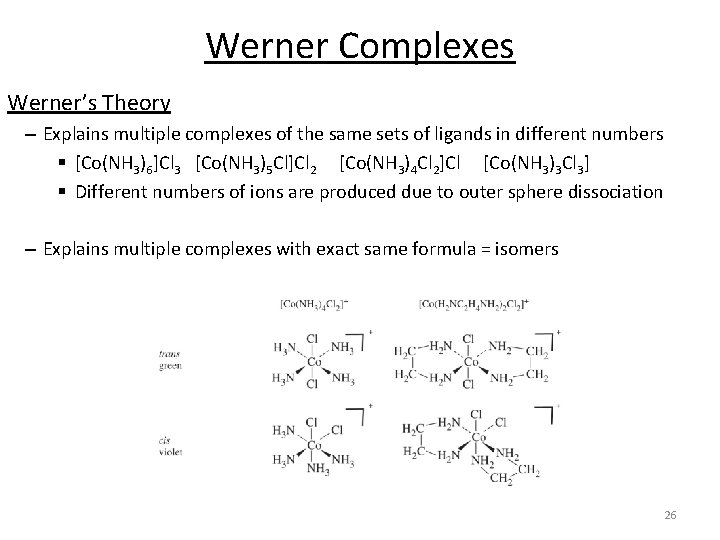
Werner Complexes Werner’s Theory – Explains multiple complexes of the same sets of ligands in different numbers § [Co(NH 3)6]Cl 3 [Co(NH 3)5 Cl]Cl 2 [Co(NH 3)4 Cl 2]Cl [Co(NH 3)3 Cl 3] § Different numbers of ions are produced due to outer sphere dissociation – Explains multiple complexes with exact same formula = isomers 26

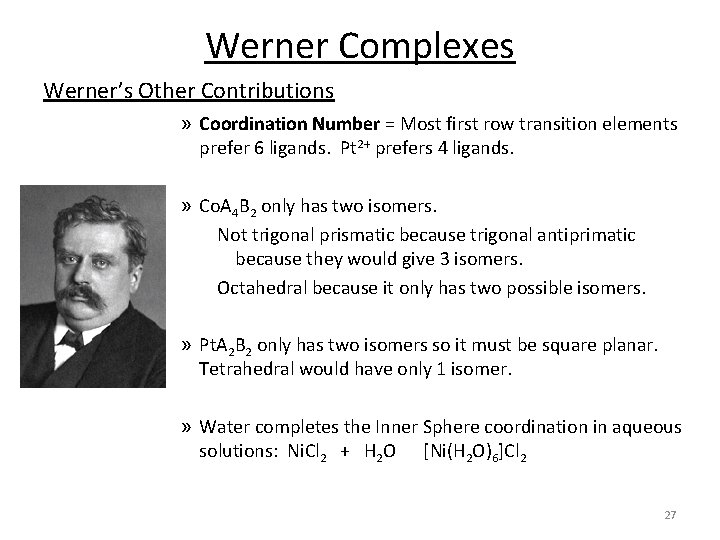
Werner Complexes Werner’s Other Contributions » Coordination Number = Most first row transition elements prefer 6 ligands. Pt 2+ prefers 4 ligands. » Co. A 4 B 2 only has two isomers. Not trigonal prismatic because trigonal antiprimatic because they would give 3 isomers. Octahedral because it only has two possible isomers. » Pt. A 2 B 2 only has two isomers so it must be square planar. Tetrahedral would have only 1 isomer. » Water completes the Inner Sphere coordination in aqueous solutions: Ni. Cl 2 + H 2 O [Ni(H 2 O)6]Cl 2 27

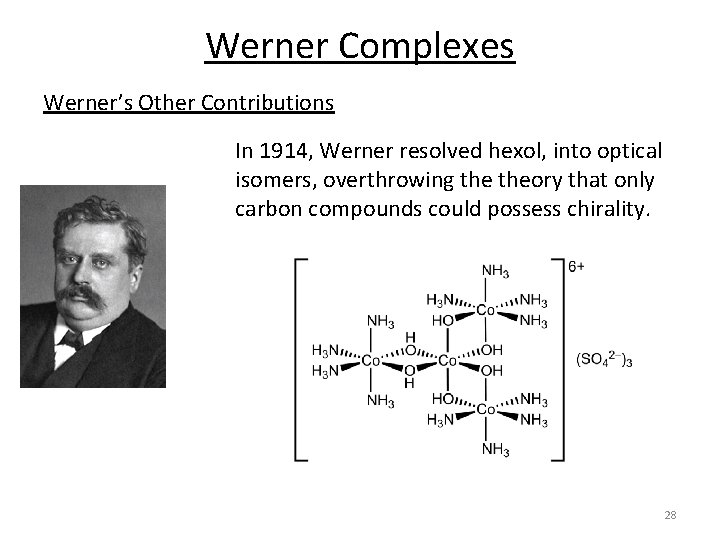
Werner Complexes Werner’s Other Contributions In 1914, Werner resolved hexol, into optical isomers, overthrowing theory that only carbon compounds could possess chirality. 28

Werner Complexes Werner was awarded the Nobel Prize in 1913 (only inorg. up until 1973) 29

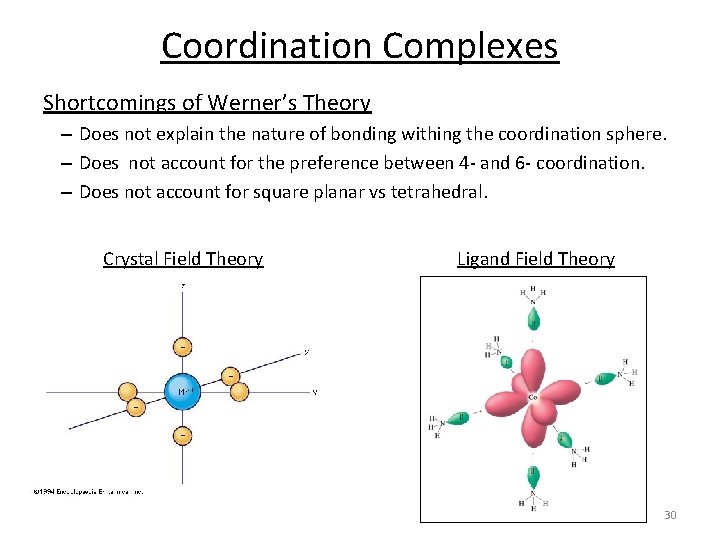
Coordination Complexes Shortcomings of Werner’s Theory – Does not explain the nature of bonding withing the coordination sphere. – Does not account for the preference between 4‐ and 6‐ coordination. – Does not account for square planar vs tetrahedral. Crystal Field Theory Ligand Field Theory 30

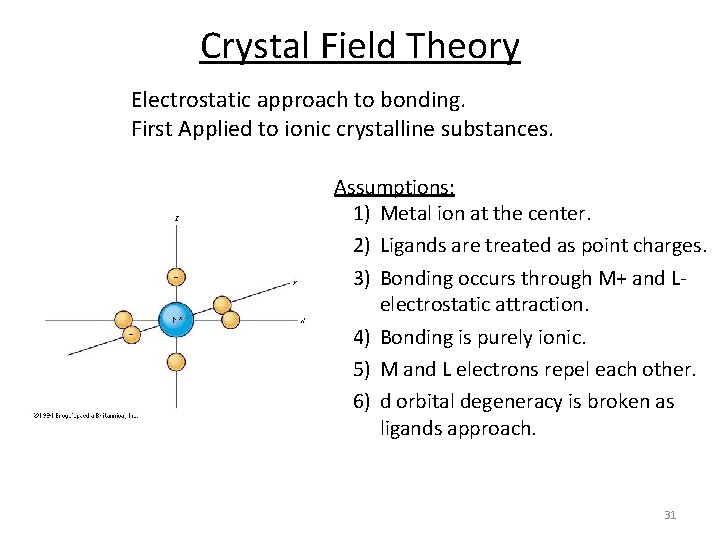
Crystal Field Theory Electrostatic approach to bonding. First Applied to ionic crystalline substances. Assumptions: 1) Metal ion at the center. 2) Ligands are treated as point charges. 3) Bonding occurs through M+ and L‐ electrostatic attraction. 4) Bonding is purely ionic. 5) M and L electrons repel each other. 6) d orbital degeneracy is broken as ligands approach. 31

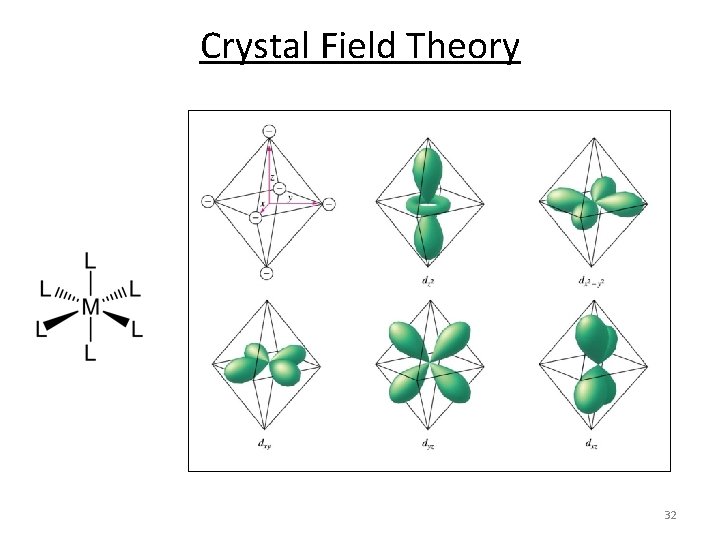
Crystal Field Theory 32

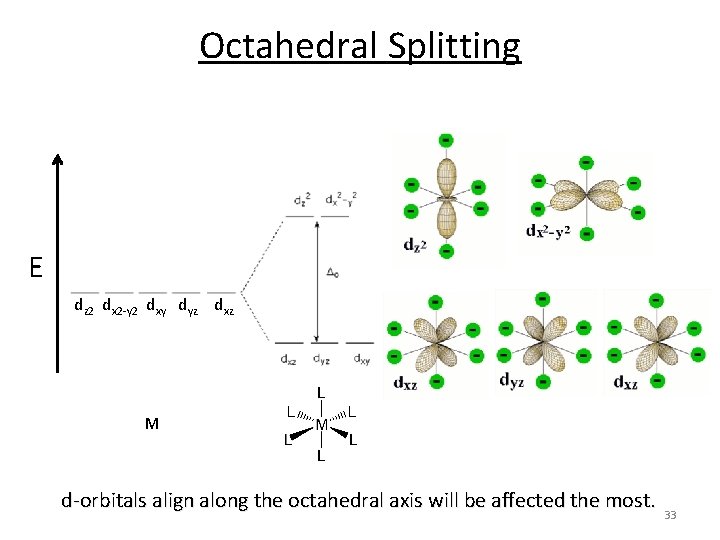
Octahedral Splitting E dz 2 dx 2‐y 2 dxy dyz dxz M d‐orbitals align along the octahedral axis will be affected the most. 33

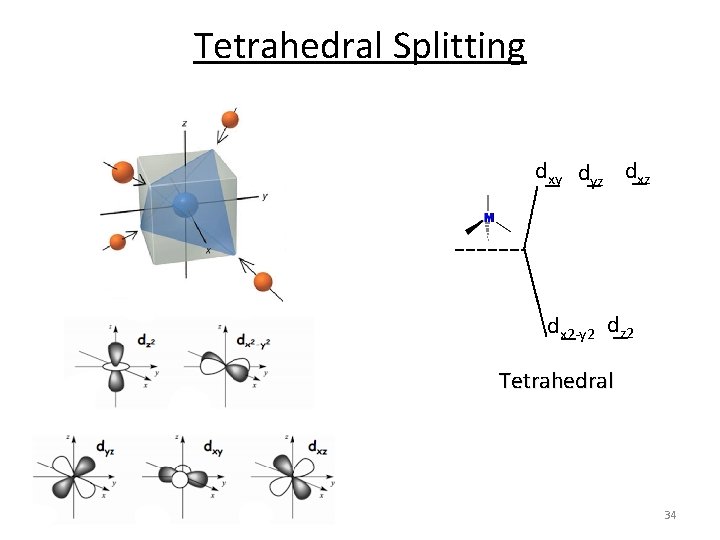
Tetrahedral Splitting dxy dyz dx 2‐y 2 dz 2 Tetrahedral 34

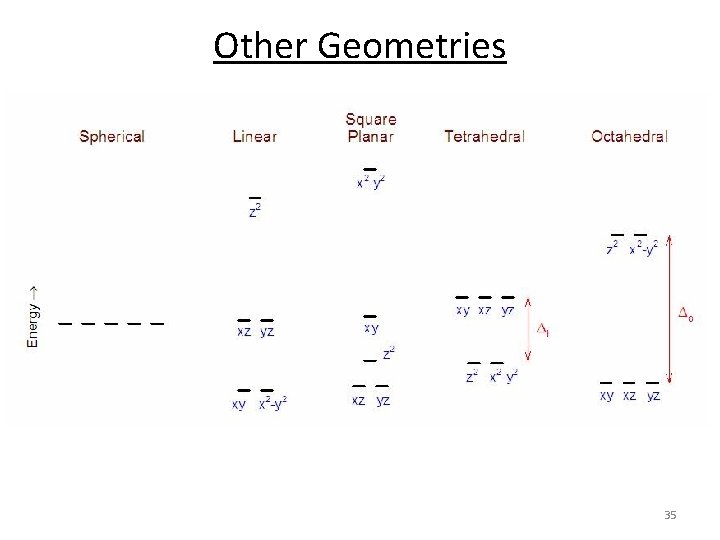
Other Geometries 35

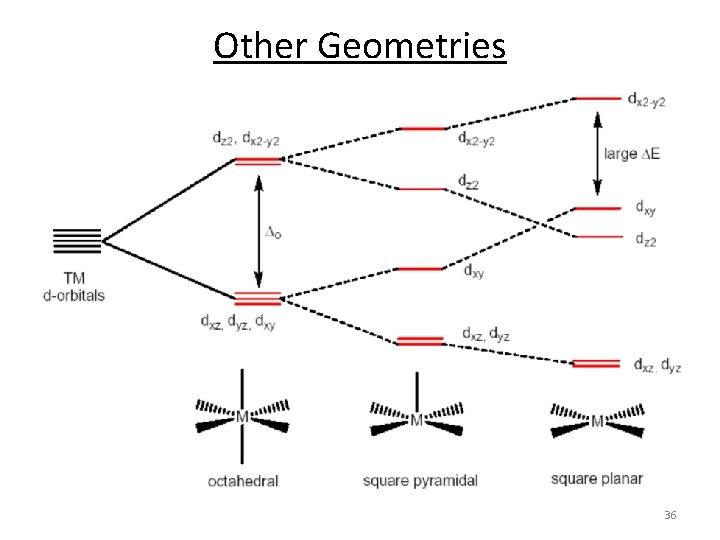
Other Geometries 36

Crystal Field Theory Merits of crystal field theory: 1) Can be used to predict the most favorable geometry for the complex. 2) Can account for why some complexes are tetrahedral and others square planar. 3) Usefull in interpreting magnetic properties. 4) The colors of many transition metal complexes can be rationalized. Limitations of crystal field theory: 1) Becomes less accurate as delocalization increases (more covalent character). 2) Point charge does not accurately represent complexes. 3) Does not account for pi bonding interactions. 4) Does not account for the relative strengths of the ligands. 37

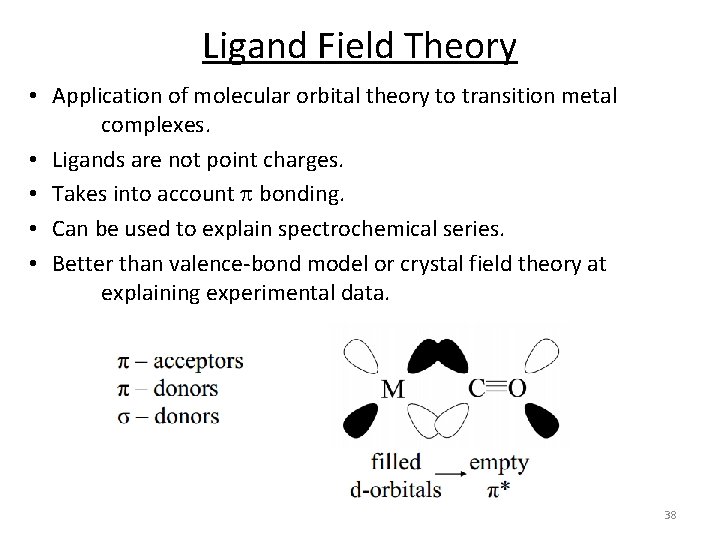
Ligand Field Theory • Application of molecular orbital theory to transition metal complexes. • Ligands are not point charges. • Takes into account p bonding. • Can be used to explain spectrochemical series. • Better than valence‐bond model or crystal field theory at explaining experimental data. 38

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field Theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry 39

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field Theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry • Octahedral s bonding p bonding ‐ Ligand Field Strength • Square Planar s bonding p bonding • Tetrahedral • Organometallics 40

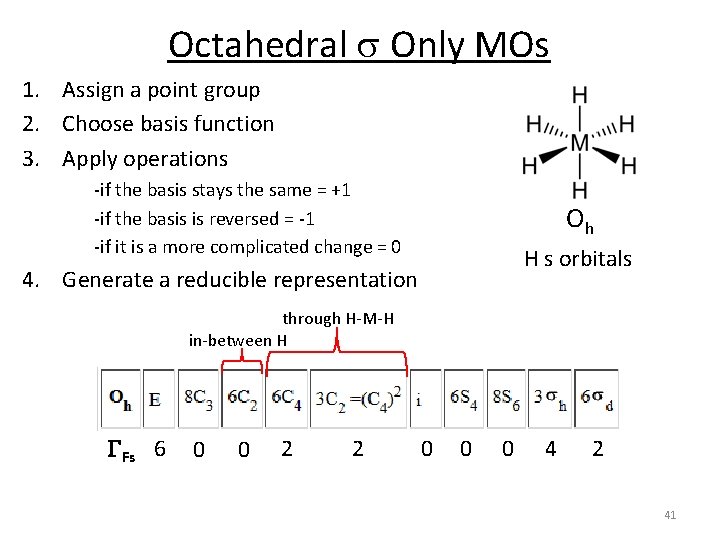
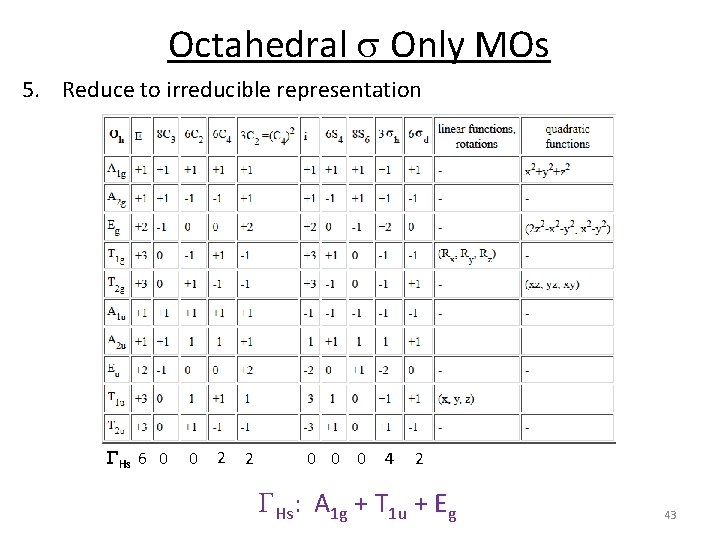
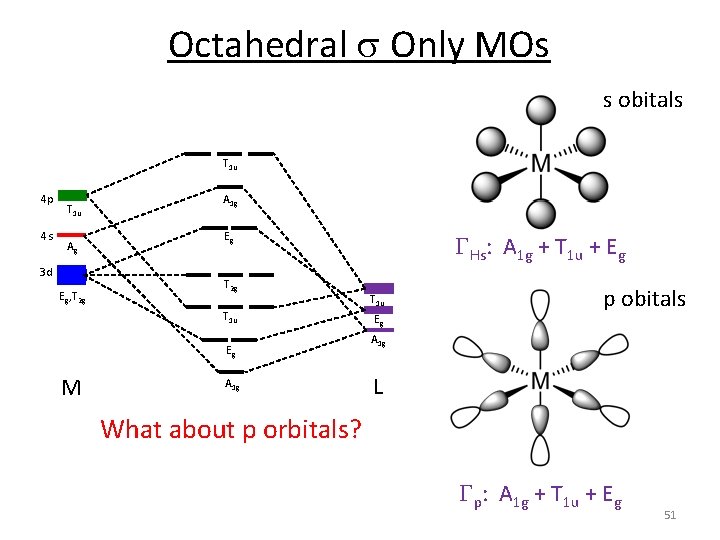
Octahedral s Only MOs 1. Assign a point group 2. Choose basis function 3. Apply operations ‐if the basis stays the same = +1 ‐if the basis is reversed = ‐ 1 ‐if it is a more complicated change = 0 Oh H s orbitals 4. Generate a reducible representation through H‐M‐H in‐between H GFs 6 0 0 2 2 0 0 0 4 2 41

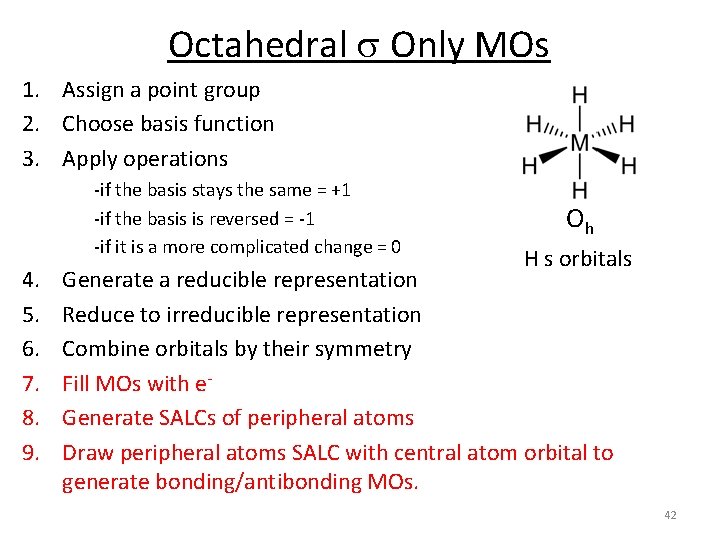
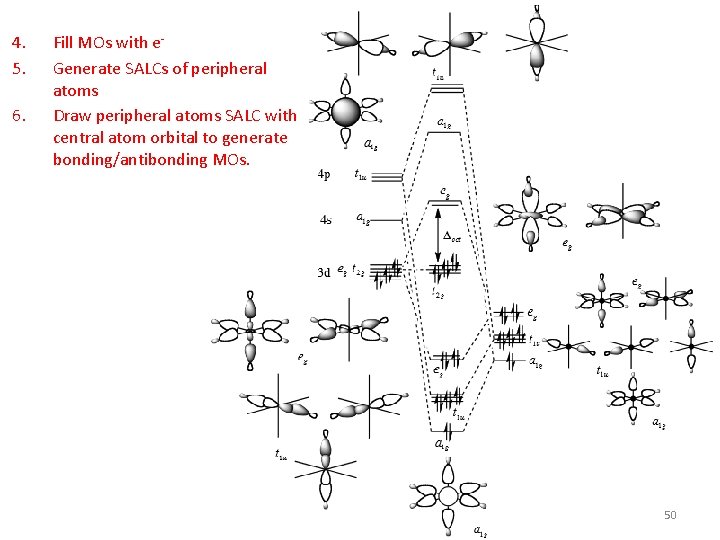
Octahedral s Only MOs 1. Assign a point group 2. Choose basis function 3. Apply operations ‐if the basis stays the same = +1 ‐if the basis is reversed = ‐ 1 ‐if it is a more complicated change = 0 4. 5. 6. 7. 8. 9. Oh H s orbitals Generate a reducible representation Reduce to irreducible representation Combine orbitals by their symmetry Fill MOs with e‐ Generate SALCs of peripheral atoms Draw peripheral atoms SALC with central atom orbital to generate bonding/antibonding MOs. 42

Octahedral s Only MOs 5. Reduce to irreducible representation GHs 6 0 0 2 2 0 0 0 4 2 GHs: A 1 g + T 1 u + Eg 43

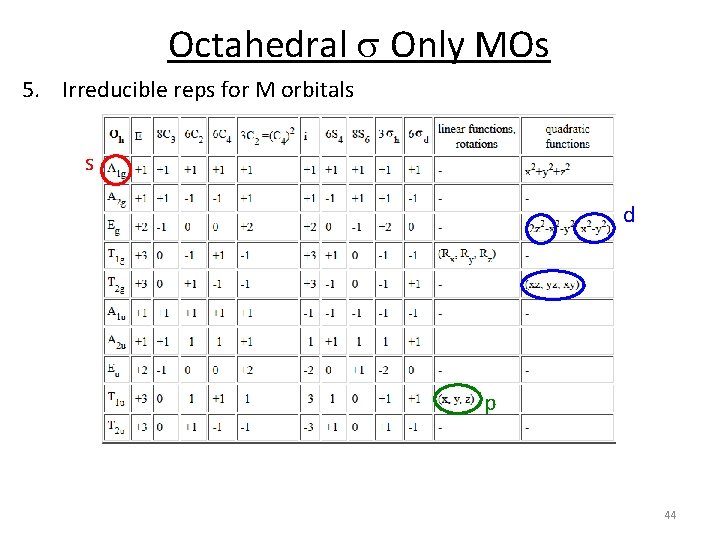
Octahedral s Only MOs 5. Irreducible reps for M orbitals s d p 44

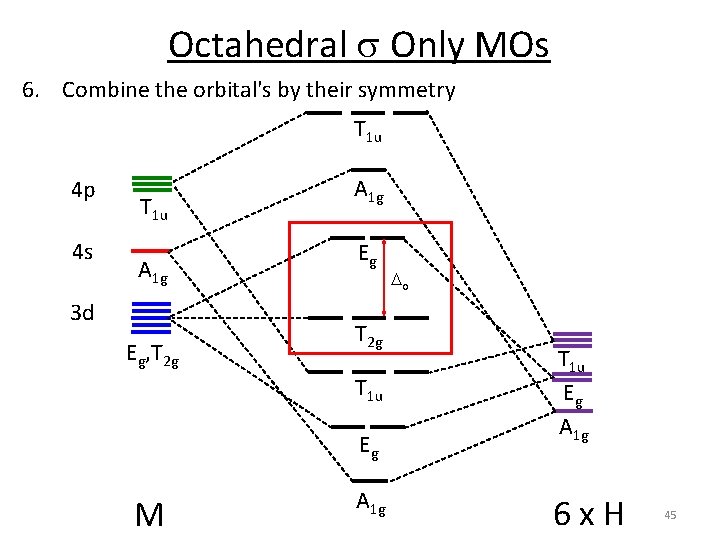
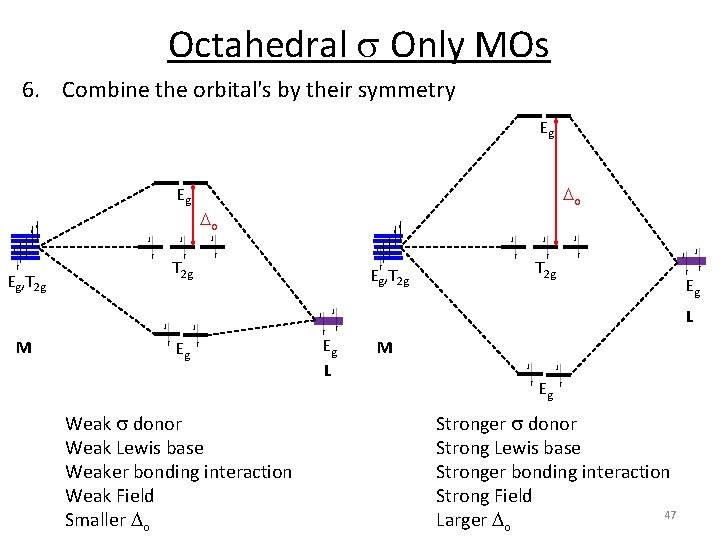
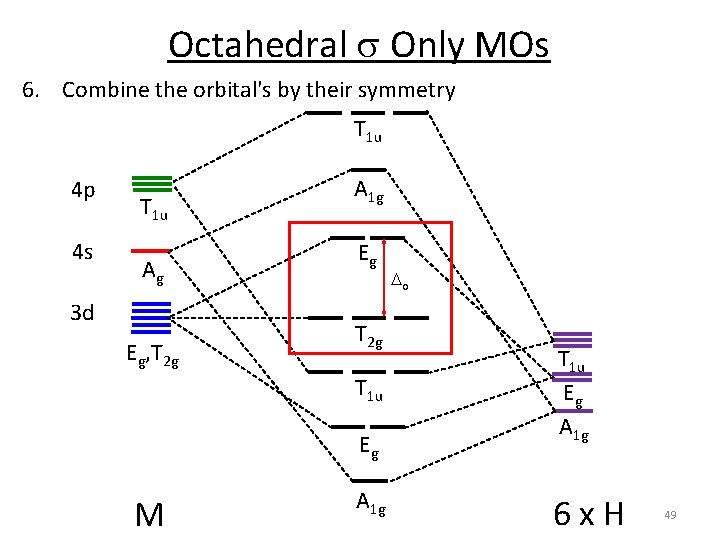
Octahedral s Only MOs 6. Combine the orbital's by their symmetry T 1 u 4 p 4 s T 1 u A 1 g 3 d Eg, T 2 g A 1 g Eg T 2 g T 1 u Eg M A 1 g o T 1 u Eg A 1 g 6 x H 45

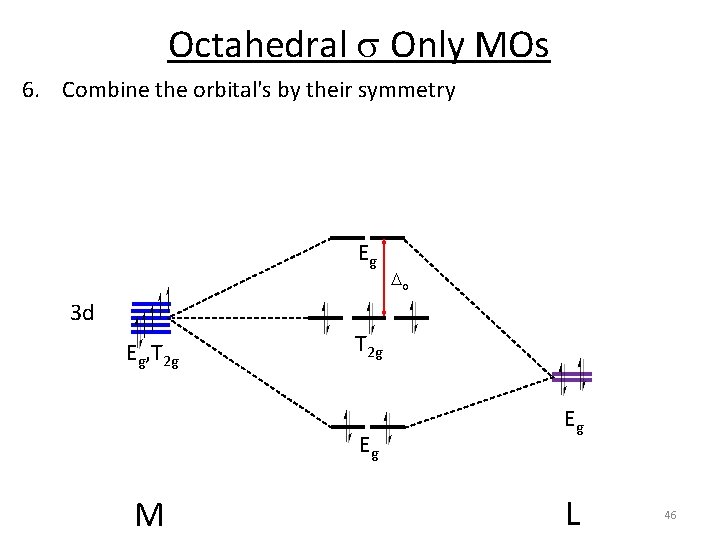
Octahedral s Only MOs 6. Combine the orbital's by their symmetry Eg o 3 d Eg, T 2 g Eg M Eg L 46

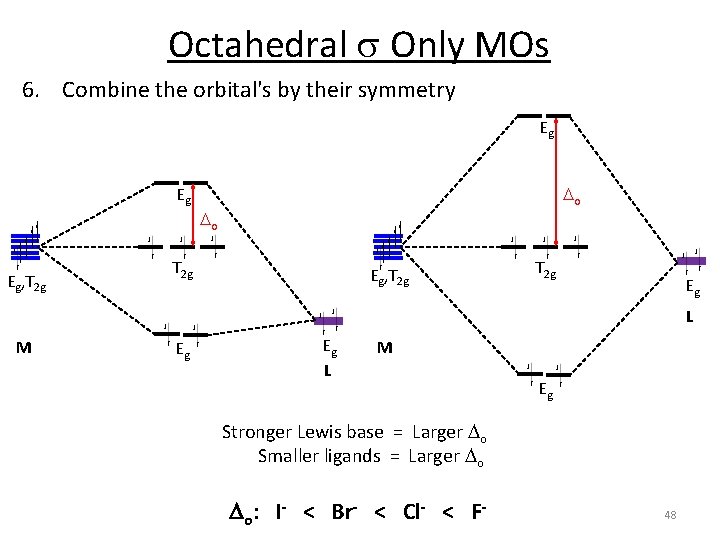
Octahedral s Only MOs 6. Combine the orbital's by their symmetry Eg Eg Eg, T 2 g o o T 2 g Eg, T 2 g Eg L M Eg Weak s donor Weak Lewis base Weaker bonding interaction Weak Field Smaller o Eg L M Eg Stronger s donor Strong Lewis base Stronger bonding interaction Strong Field 47 Larger o

Octahedral s Only MOs 6. Combine the orbital's by their symmetry Eg Eg Eg, T 2 g o o T 2 g Eg, T 2 g Eg L M Eg Stronger Lewis base = Larger o Smaller ligands = Larger o Do: I- < Br- < Cl- < F- 48

Octahedral s Only MOs 6. Combine the orbital's by their symmetry T 1 u 4 p 4 s T 1 u Ag 3 d Eg, T 2 g A 1 g Eg T 2 g T 1 u Eg M A 1 g o T 1 u Eg A 1 g 6 x H 49

4. 5. 6. Fill MOs with e‐ Generate SALCs of peripheral atoms Draw peripheral atoms SALC with central atom orbital to generate bonding/antibonding MOs. 50

Octahedral s Only MOs s obitals T 1 u 4 p 4 s T 1 u Ag 3 d Eg, T 2 g A 1 g Eg T 2 g T 1 u Eg M A 1 g GHs: A 1 g + T 1 u + Eg T 1 u Eg p obitals A 1 g L What about p orbitals? Gp: A 1 g + T 1 u + Eg 51

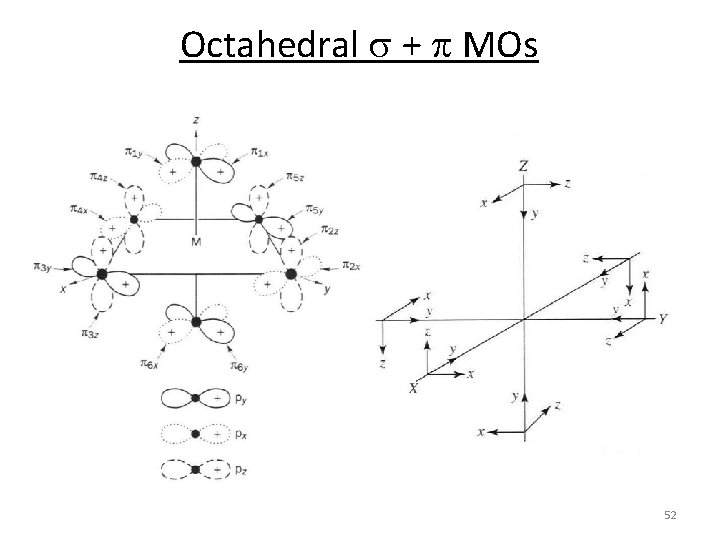
Octahedral s + p MOs 52

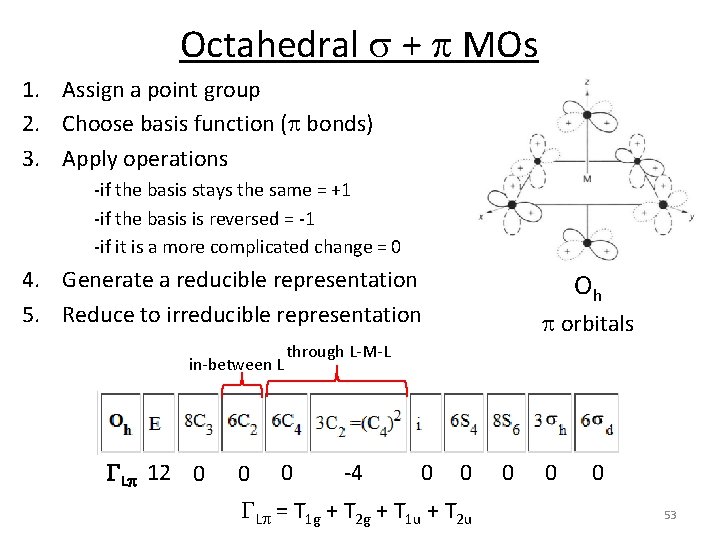
Octahedral s + p MOs 1. Assign a point group 2. Choose basis function (p bonds) 3. Apply operations ‐if the basis stays the same = +1 ‐if the basis is reversed = ‐ 1 ‐if it is a more complicated change = 0 4. Generate a reducible representation 5. Reduce to irreducible representation in‐between L GLp 12 0 0 Oh p orbitals through L‐M‐L 0 ‐ 4 0 0 GLp = T 1 g + T 2 g + T 1 u + T 2 u 0 0 0 53

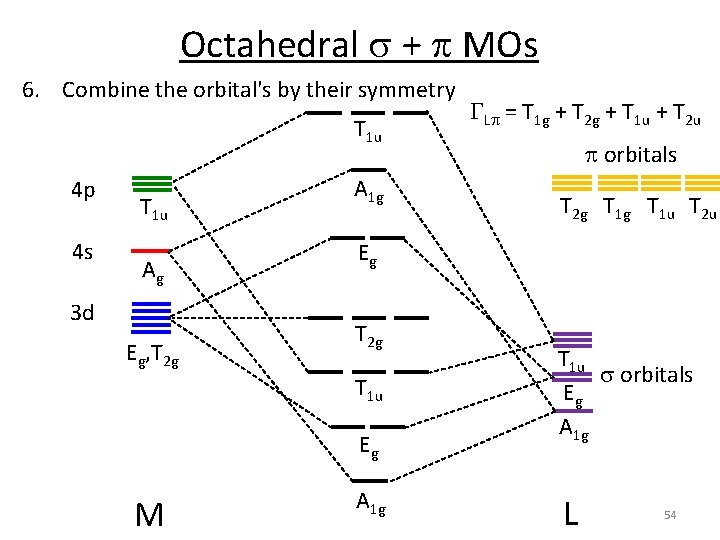
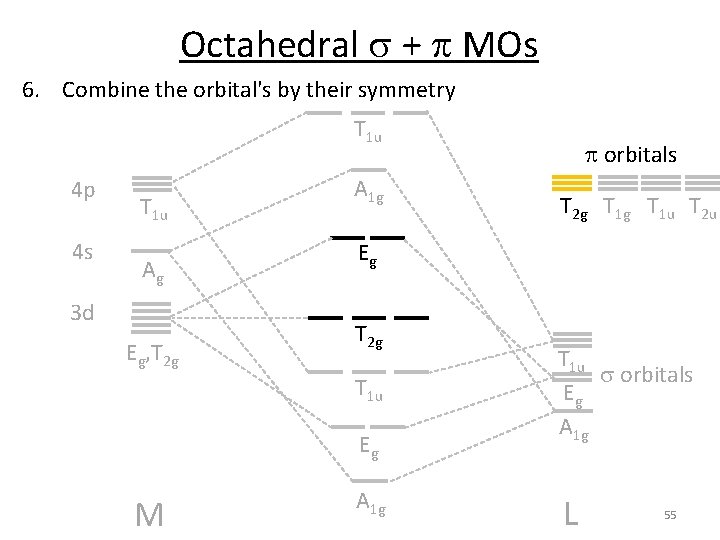
Octahedral s + p MOs 6. Combine the orbital's by their symmetry T 1 u 4 p 4 s T 1 u Ag 3 d Eg, T 2 g A 1 g p orbitals T 2 g T 1 u T 2 u Eg T 2 g T 1 u Eg M GLp = T 1 g + T 2 g + T 1 u + T 2 u A 1 g T 1 u s orbitals Eg A 1 g L 54

Octahedral s + p MOs 6. Combine the orbital's by their symmetry T 1 u 4 p 4 s T 1 u Ag 3 d Eg, T 2 g A 1 g T 2 g T 1 u T 2 u Eg T 2 g T 1 u Eg M p orbitals A 1 g T 1 u s orbitals Eg A 1 g L 55

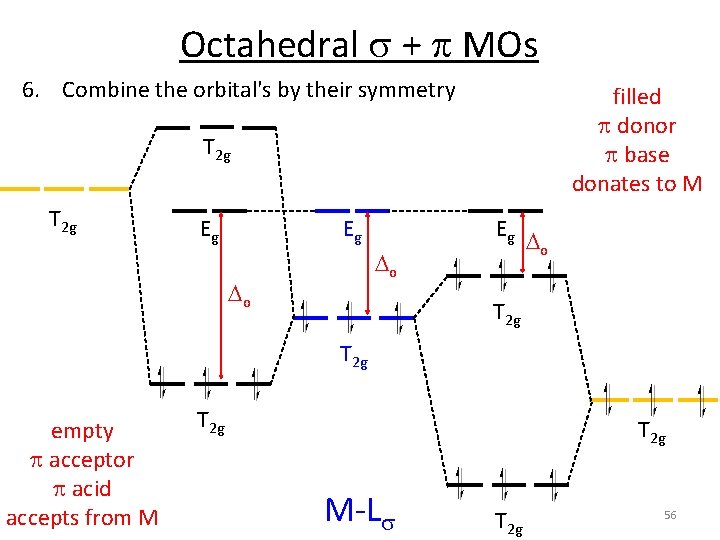
Octahedral s + p MOs 6. Combine the orbital's by their symmetry filled p donor p base donates to M T 2 g Eg Eg Eg o o o T 2 g empty p acceptor p acid accepts from M T 2 g M‐Ls T 2 g 56

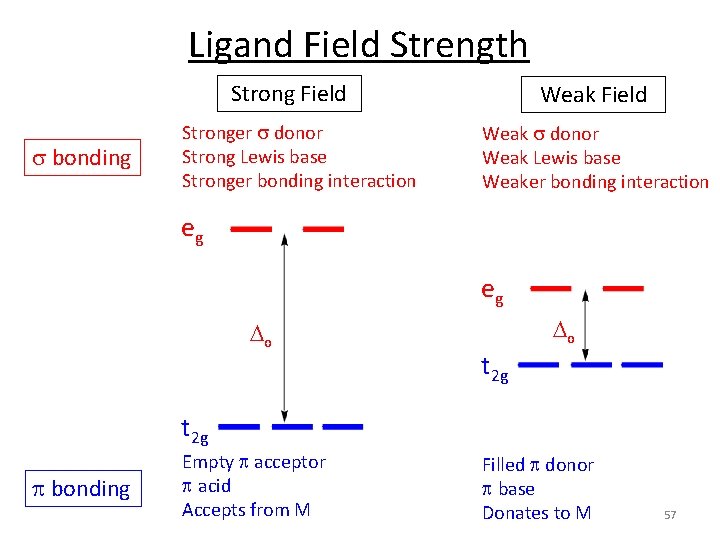
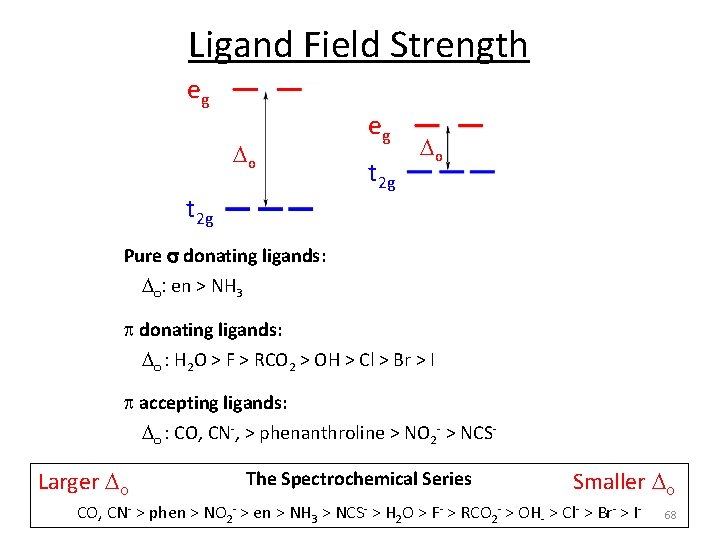
Ligand Field Strength Strong Field s bonding Stronger s donor Strong Lewis base Stronger bonding interaction Weak Field Weak s donor Weak Lewis base Weaker bonding interaction eg eg o o t 2 g p bonding Empty p acceptor p acid Accepts from M Filled p donor p base Donates to M 57

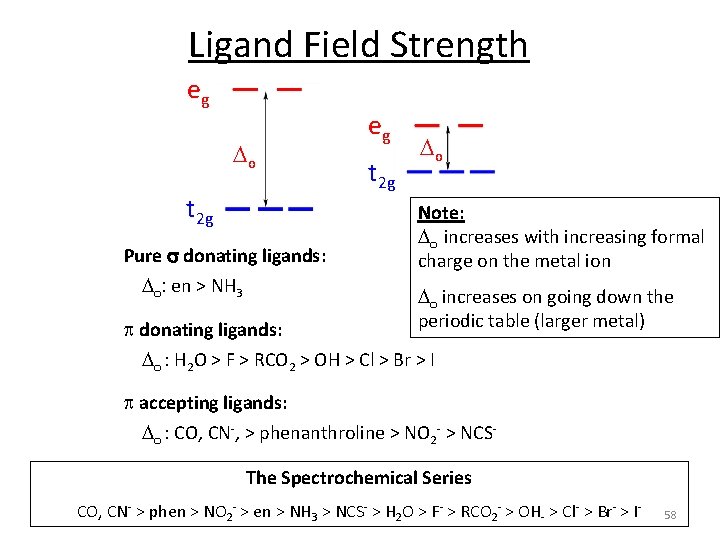
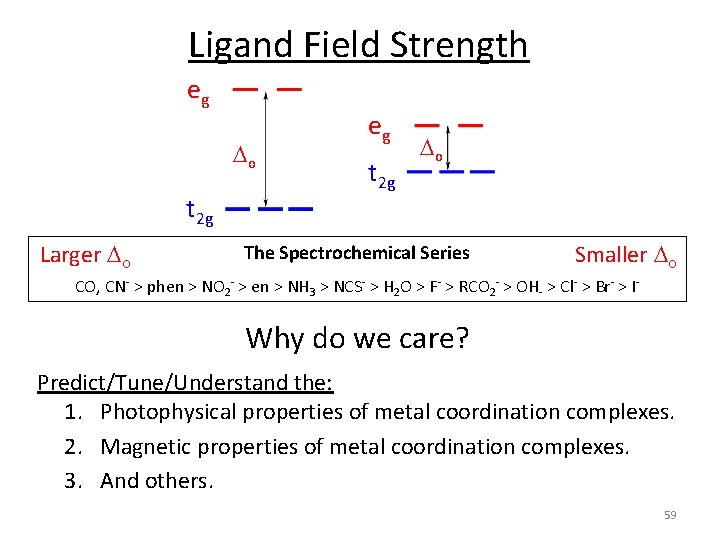
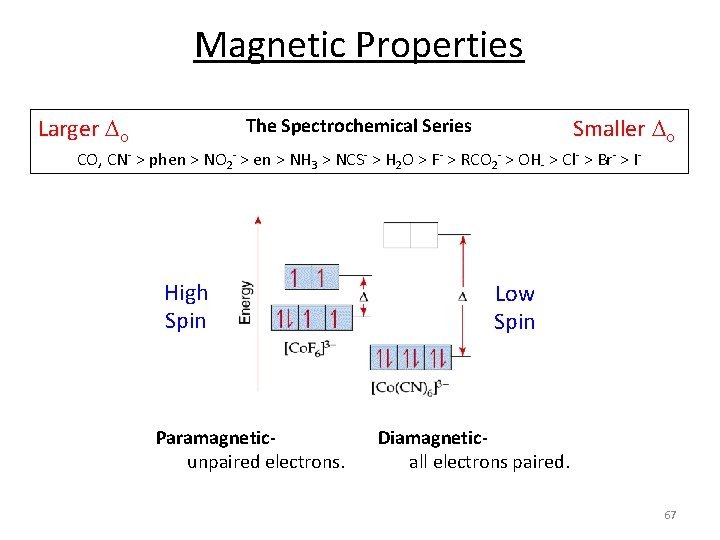
Ligand Field Strength eg o t 2 g Pure s donating ligands: o: en > NH 3 eg t 2 g o Note: o increases with increasing formal charge on the metal ion o increases on going down the periodic table (larger metal) p donating ligands: o : H 2 O > F > RCO 2 > OH > Cl > Br > I p accepting ligands: o : CO, CN‐, > phenanthroline > NO 2‐ > NCS‐ The Spectrochemical Series CO, CN‐ > phen > NO 2‐ > en > NH 3 > NCS‐ > H 2 O > F‐ > RCO 2‐ > OH‐ > Cl‐ > Br‐ > I‐ 58

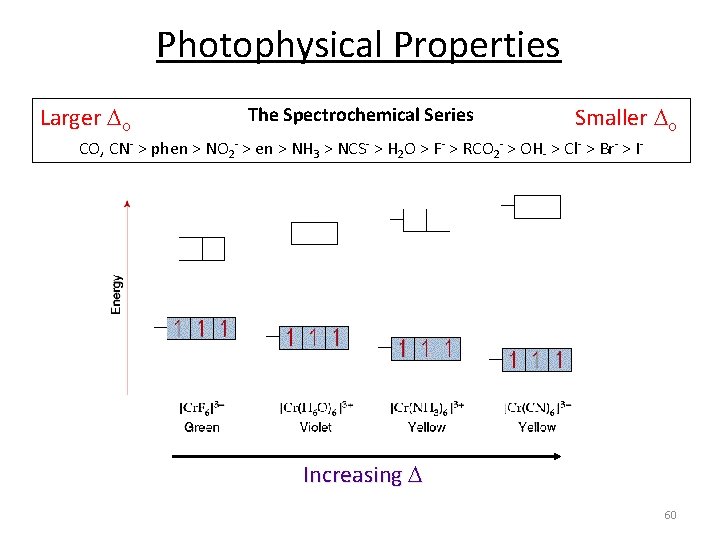
Ligand Field Strength eg o t 2 g Larger o eg t 2 g o The Spectrochemical Series Smaller o CO, CN‐ > phen > NO 2‐ > en > NH 3 > NCS‐ > H 2 O > F‐ > RCO 2‐ > OH‐ > Cl‐ > Br‐ > I‐ Why do we care? Predict/Tune/Understand the: 1. Photophysical properties of metal coordination complexes. 2. Magnetic properties of metal coordination complexes. 3. And others. 59

Photophysical Properties Larger o The Spectrochemical Series Smaller o CO, CN‐ > phen > NO 2‐ > en > NH 3 > NCS‐ > H 2 O > F‐ > RCO 2‐ > OH‐ > Cl‐ > Br‐ > I‐ Increasing 60

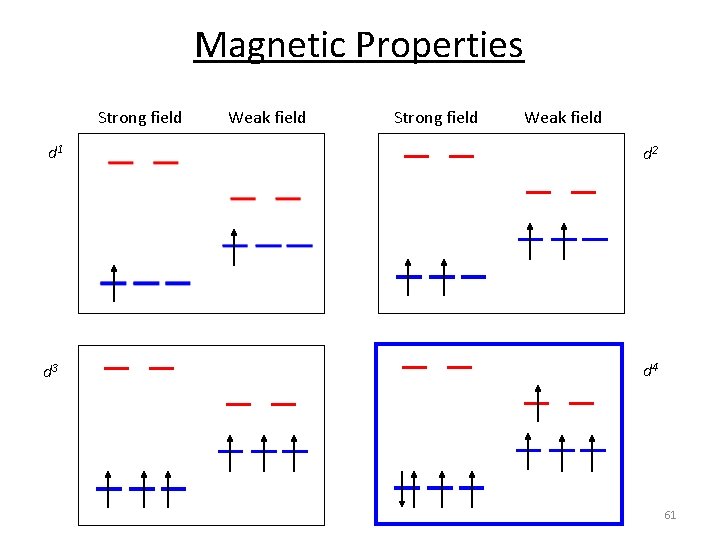
Magnetic Properties Strong field Weak field d 1 d 2 d 3 d 4 61

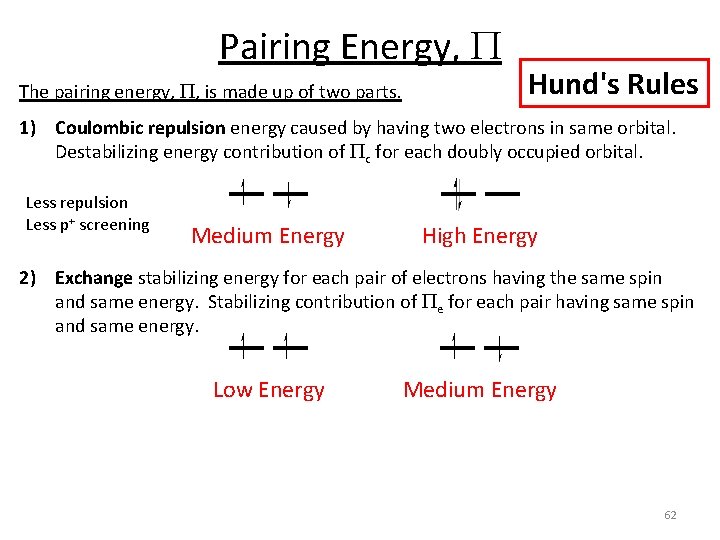
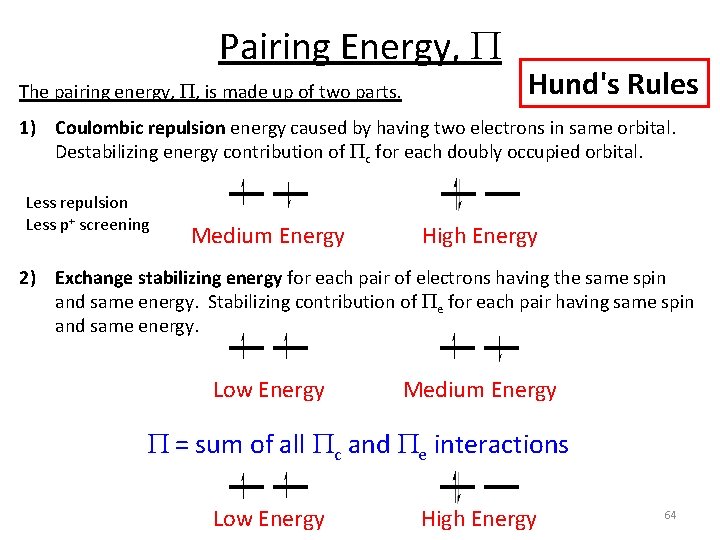
Pairing Energy, P The pairing energy, P, is made up of two parts. Hund's Rules 1) Coulombic repulsion energy caused by having two electrons in same orbital. Destabilizing energy contribution of Pc for each doubly occupied orbital. Less repulsion Less p+ screening Medium Energy High Energy 2) Exchange stabilizing energy for each pair of electrons having the same spin and same energy. Stabilizing contribution of Pe for each pair having same spin and same energy. Low Energy Medium Energy 62

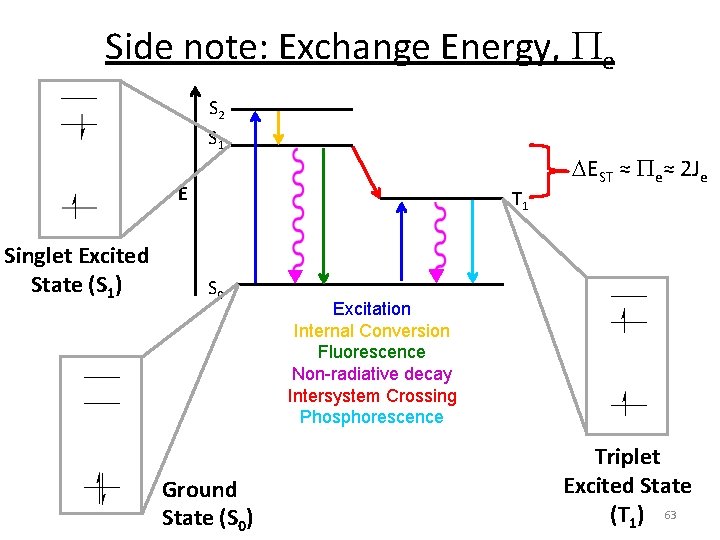
Side note: Exchange Energy, Pe S 2 S 1 E Singlet Excited State (S 1) T 1 S 0 Ground State (S 0) EST ≈ Pe≈ 2 Je Excitation Internal Conversion Fluorescence Non-radiative decay Intersystem Crossing Phosphorescence Triplet Excited State (T 1) 63

Pairing Energy, P The pairing energy, P, is made up of two parts. Hund's Rules 1) Coulombic repulsion energy caused by having two electrons in same orbital. Destabilizing energy contribution of Pc for each doubly occupied orbital. Less repulsion Less p+ screening Medium Energy High Energy 2) Exchange stabilizing energy for each pair of electrons having the same spin and same energy. Stabilizing contribution of Pe for each pair having same spin and same energy. Low Energy Medium Energy P = sum of all Pc and Pe interactions Low Energy High Energy 64

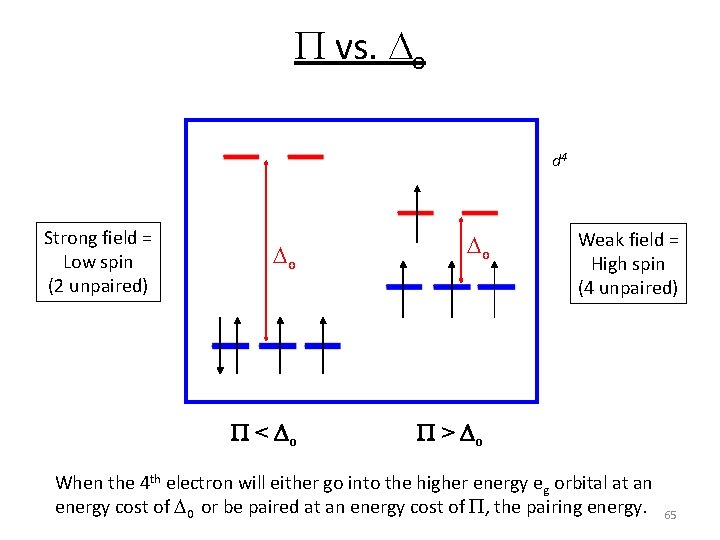
P vs. o d 4 Strong field = Low spin (2 unpaired) o o P < Do P > Do Weak field = High spin (4 unpaired) When the 4 th electron will either go into the higher energy eg orbital at an energy cost of 0 or be paired at an energy cost of P, the pairing energy. 65

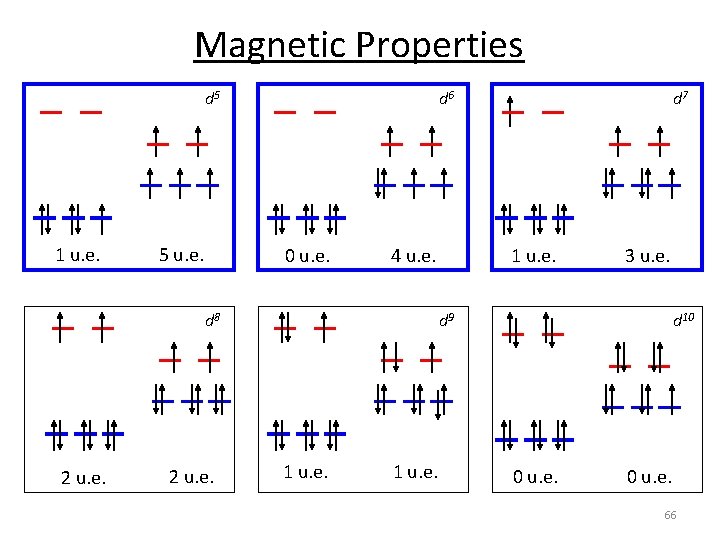
Magnetic Properties d 5 1 u. e. 5 u. e. d 6 0 u. e. 4 u. e. d 8 2 u. e. d 7 1 u. e. 3 u. e. d 9 1 u. e. d 10 0 u. e. 66

Magnetic Properties Larger o Smaller o The Spectrochemical Series CO, CN‐ > phen > NO 2‐ > en > NH 3 > NCS‐ > H 2 O > F‐ > RCO 2‐ > OH‐ > Cl‐ > Br‐ > I‐ High Spin Paramagnetic‐ unpaired electrons. Low Spin Diamagnetic‐ all electrons paired. 67

Ligand Field Strength eg o t 2 g eg t 2 g o Pure s donating ligands: o: en > NH 3 p donating ligands: o : H 2 O > F > RCO 2 > OH > Cl > Br > I p accepting ligands: o : CO, CN‐, > phenanthroline > NO 2‐ > NCS‐ Larger o The Spectrochemical Series Smaller o CO, CN‐ > phen > NO 2‐ > en > NH 3 > NCS‐ > H 2 O > F‐ > RCO 2‐ > OH‐ > Cl‐ > Br‐ > I‐ 68

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field Theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry • Octahedral s bonding p bonding ‐ Ligand Field Strength • Square Planar s bonding p bonding • Tetrahedral • Organometallics 69

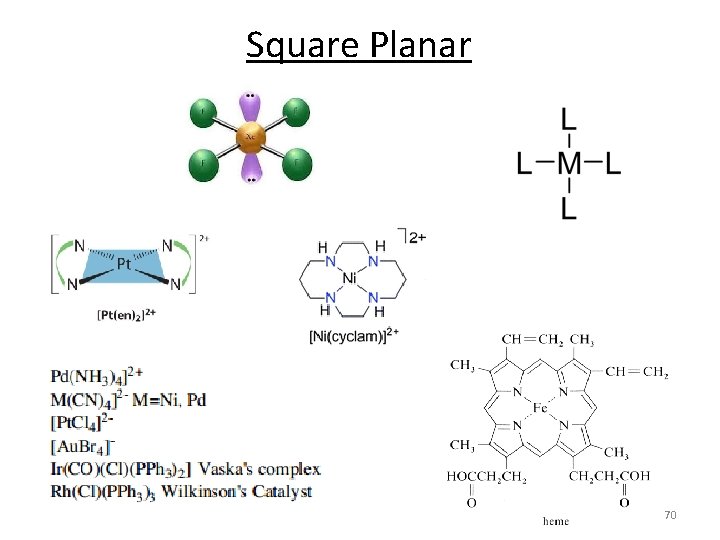
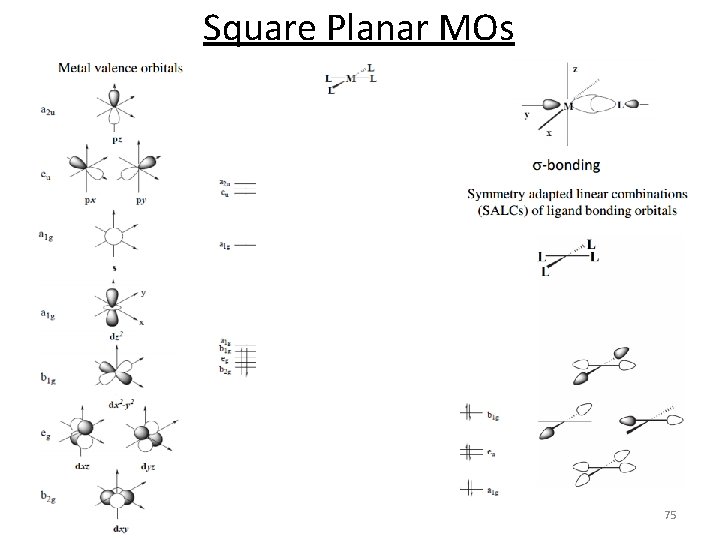
Square Planar 70

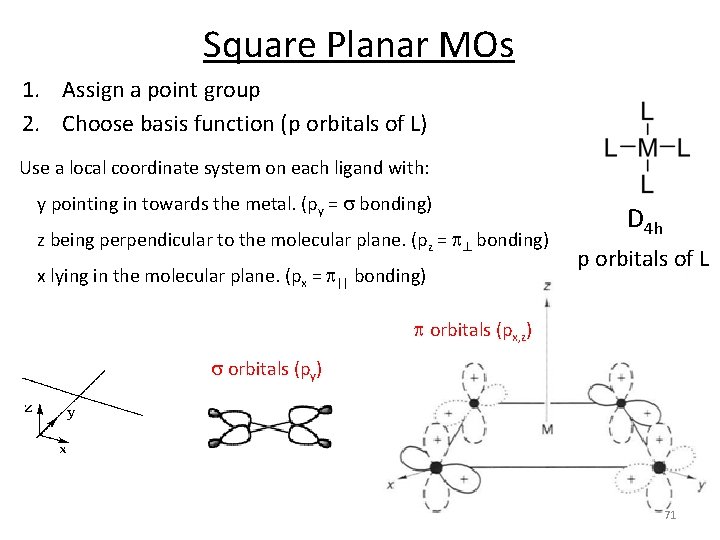
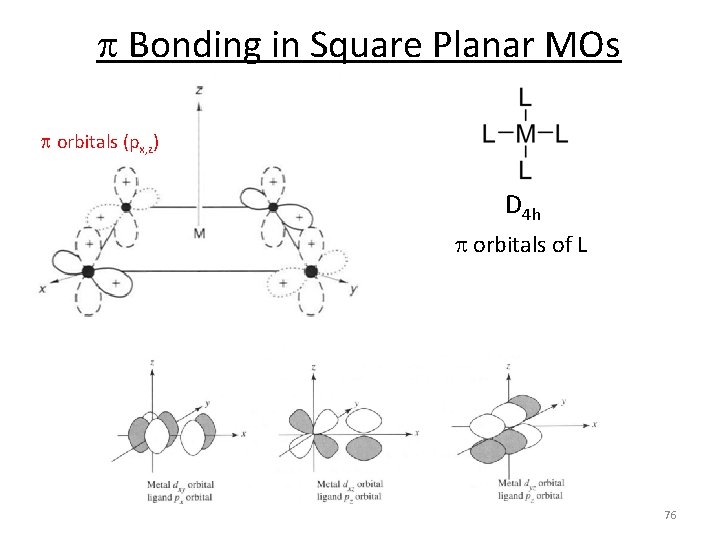
Square Planar MOs 1. Assign a point group 2. Choose basis function (p orbitals of L) Use a local coordinate system on each ligand with: y pointing in towards the metal. (py = s bonding) z being perpendicular to the molecular plane. (pz = p^ bonding) x lying in the molecular plane. (px = p|| bonding) D 4 h p orbitals of L p orbitals (px, z) s orbitals (py) 71

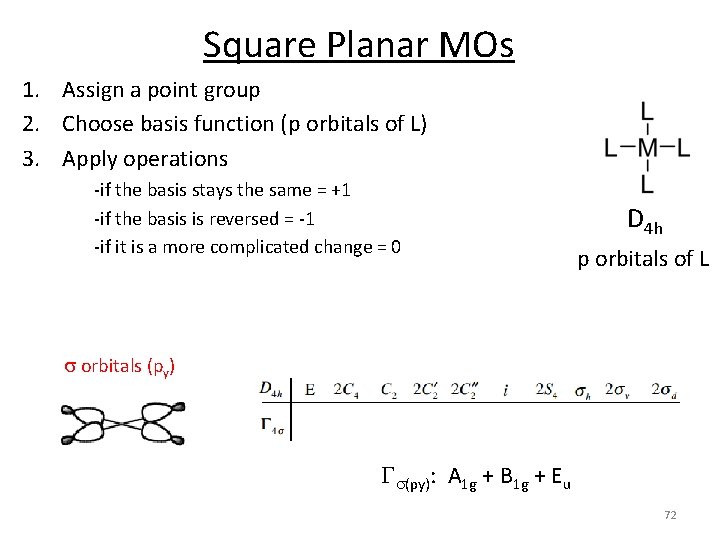
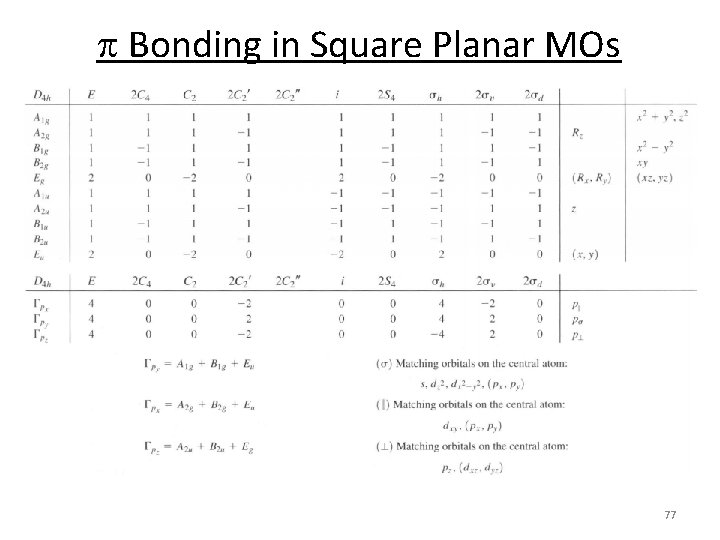
Square Planar MOs 1. Assign a point group 2. Choose basis function (p orbitals of L) 3. Apply operations ‐if the basis stays the same = +1 ‐if the basis is reversed = ‐ 1 ‐if it is a more complicated change = 0 D 4 h p orbitals of L s orbitals (py) Gs(py): A 1 g + B 1 g + Eu 72

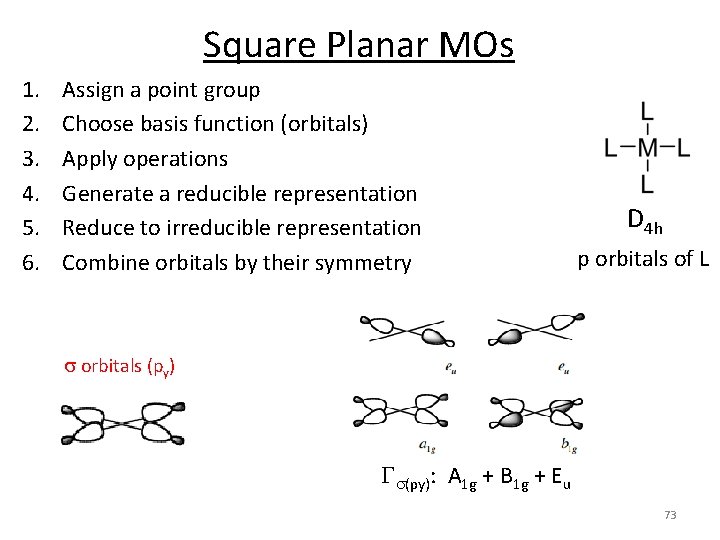
Square Planar MOs 1. 2. 3. 4. 5. 6. Assign a point group Choose basis function (orbitals) Apply operations Generate a reducible representation Reduce to irreducible representation Combine orbitals by their symmetry D 4 h p orbitals of L s orbitals (py) Gs(py): A 1 g + B 1 g + Eu 73

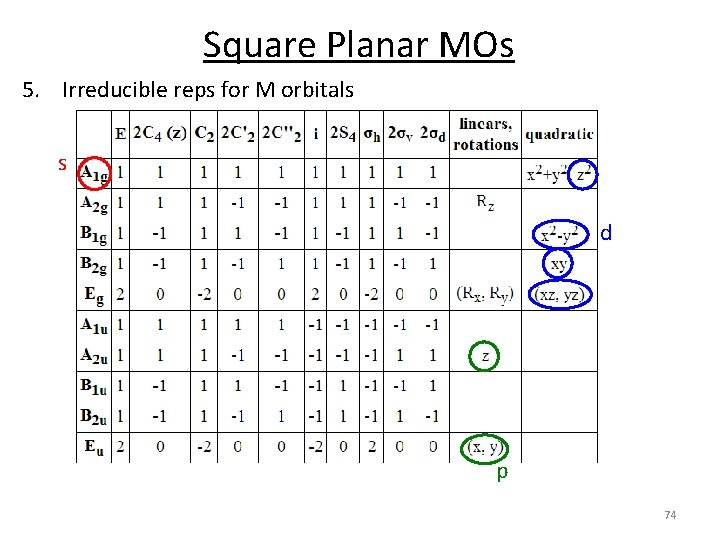
Square Planar MOs 5. Irreducible reps for M orbitals s d p 74

Square Planar MOs 75

p Bonding in Square Planar MOs p orbitals (px, z) D 4 h p orbitals of L 76

p Bonding in Square Planar MOs 77

Complete Square Planar MOs 78

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field Theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry • Octahedral s bonding p bonding ‐ Ligand Field Strength • Square Planar s bonding p bonding • Tetrahedral • Organometallics 79

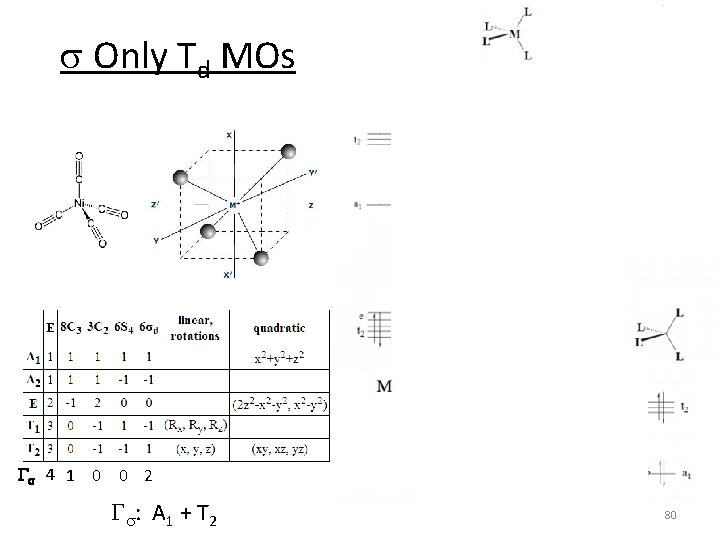
s Only Td MOs Gs 4 1 0 0 2 Gs: A 1 + T 2 80

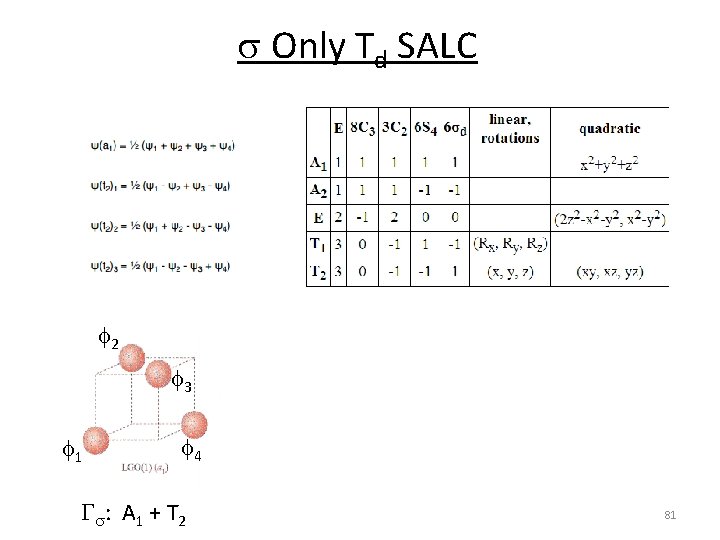
s Only Td SALC f 2 f 3 f 1 f 4 Gs: A 1 + T 2 81

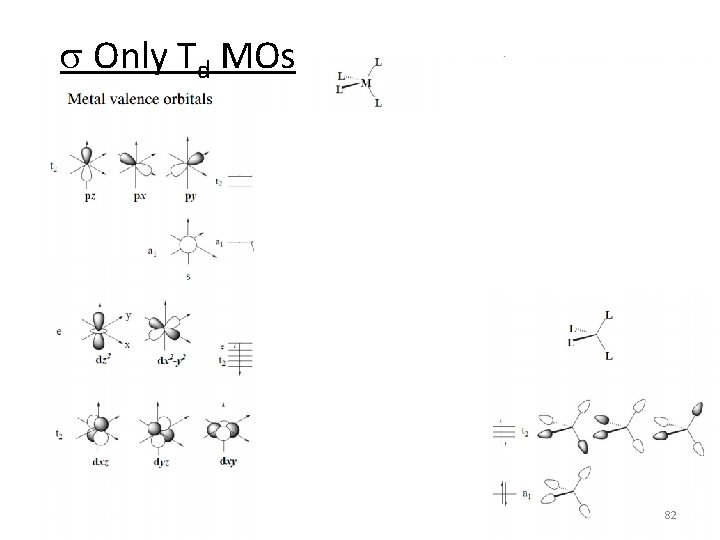
s Only Td MOs 82

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field Theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry • Octahedral s bonding p bonding ‐ Ligand Field Strength • Square Planar s bonding p bonding • Tetrahedral • Organometallics 83

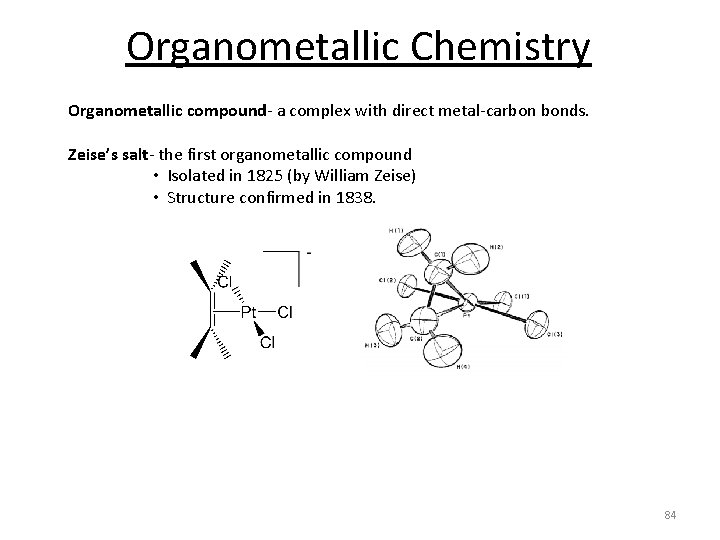
Organometallic Chemistry Organometallic compound‐ a complex with direct metal‐carbon bonds. Zeise’s salt‐ the first organometallic compound • Isolated in 1825 (by William Zeise) • Structure confirmed in 1838. 84

p‐bonding Ligands 85

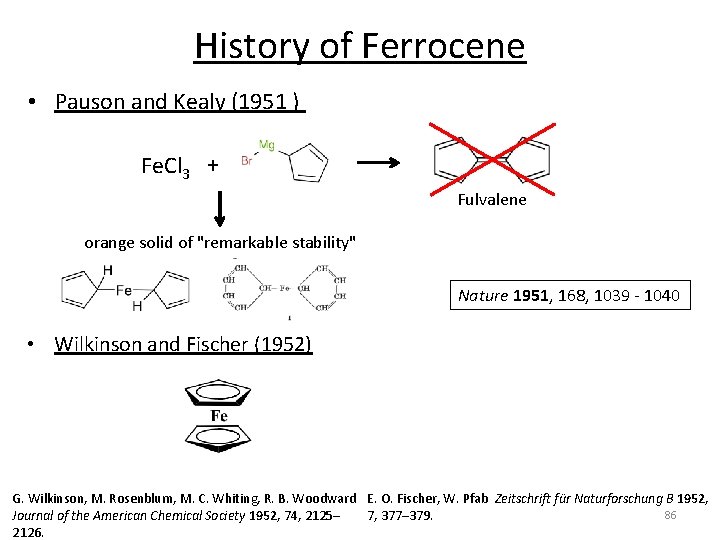
History of Ferrocene • Pauson and Kealy (1951 ) Fe. Cl 3 + Fulvalene orange solid of "remarkable stability" Nature 1951, 168, 1039 ‐ 1040 • Wilkinson and Fischer (1952) G. Wilkinson, M. Rosenblum, M. C. Whiting, R. B. Woodward E. O. Fischer, W. Pfab Zeitschrift für Naturforschung B 1952, 86 Journal of the American Chemical Society 1952, 74, 2125– 7, 377– 379. 2126.

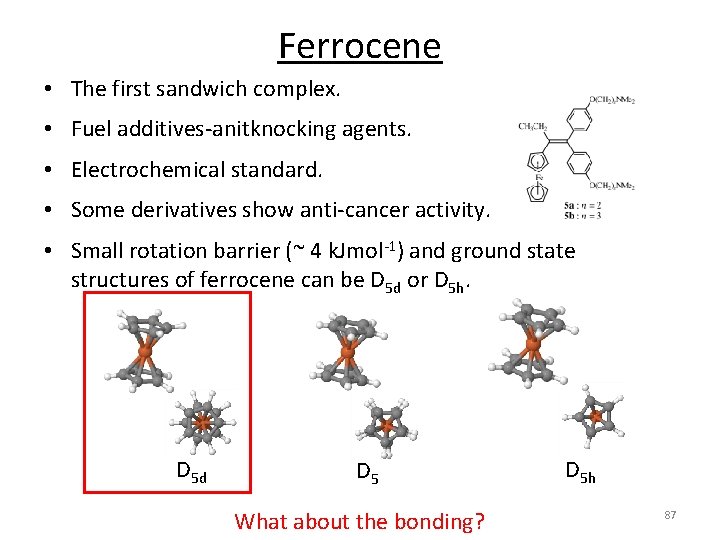
Ferrocene • The first sandwich complex. • Fuel additives‐anitknocking agents. • Electrochemical standard. • Some derivatives show anti‐cancer activity. • Small rotation barrier (~ 4 k. Jmol‐ 1) and ground state structures of ferrocene can be D 5 d or D 5 h. D 5 d D 5 What about the bonding? D 5 h 87

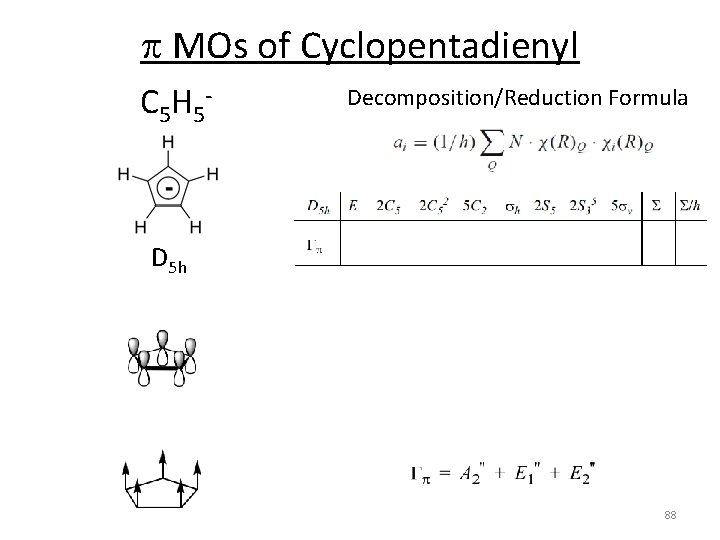
p MOs of Cyclopentadienyl C 5 H 5 ‐ Decomposition/Reduction Formula D 5 h 88

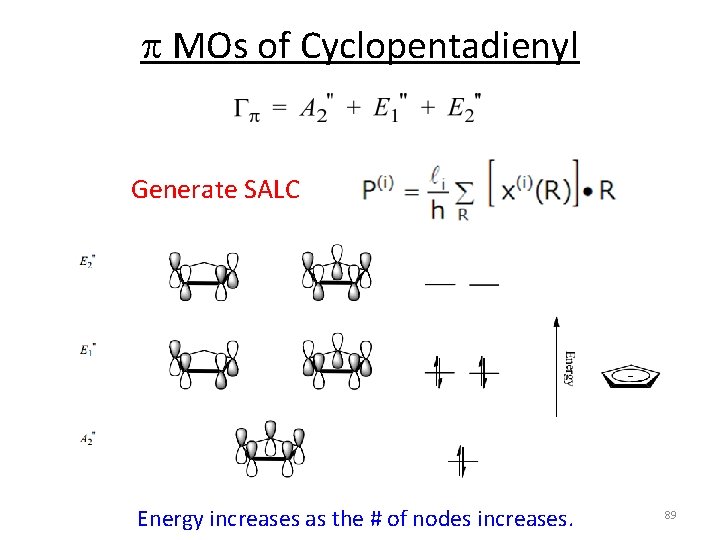
p MOs of Cyclopentadienyl Generate SALC Energy increases as the # of nodes increases. 89

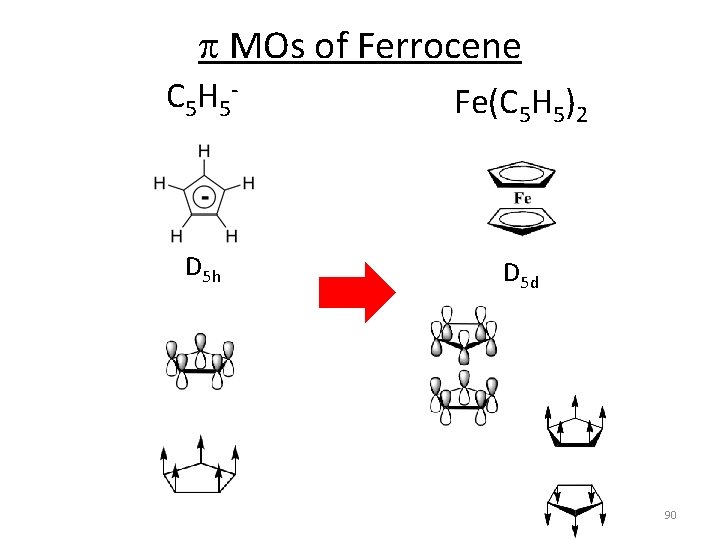
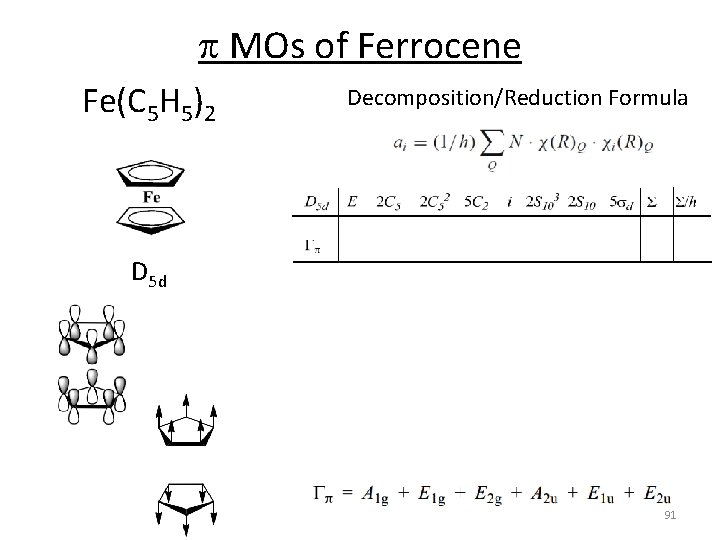
p MOs of Ferrocene C 5 H 5 ‐ Fe(C 5 H 5)2 D 5 h D 5 d 90

p MOs of Ferrocene Fe(C 5 H 5)2 Decomposition/Reduction Formula D 5 d 91

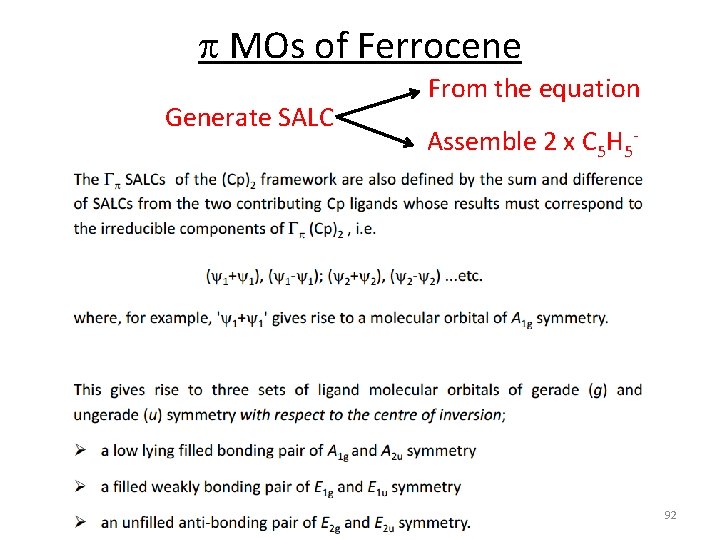
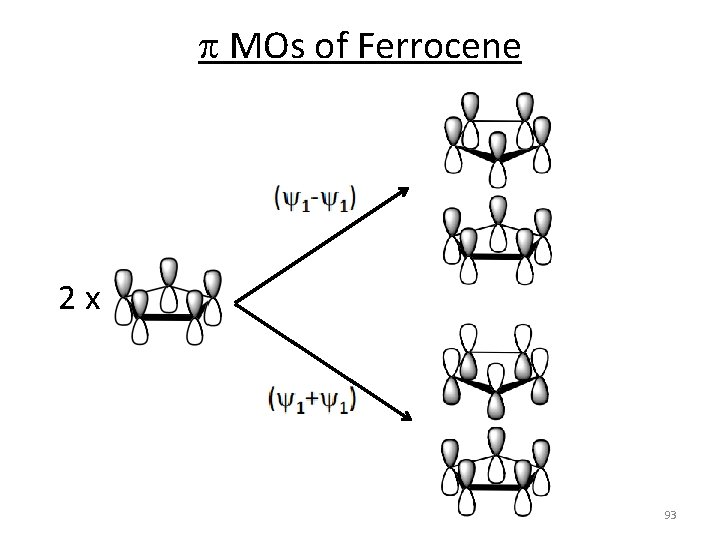
p MOs of Ferrocene Generate SALC From the equation Assemble 2 x C 5 H 5‐ 92

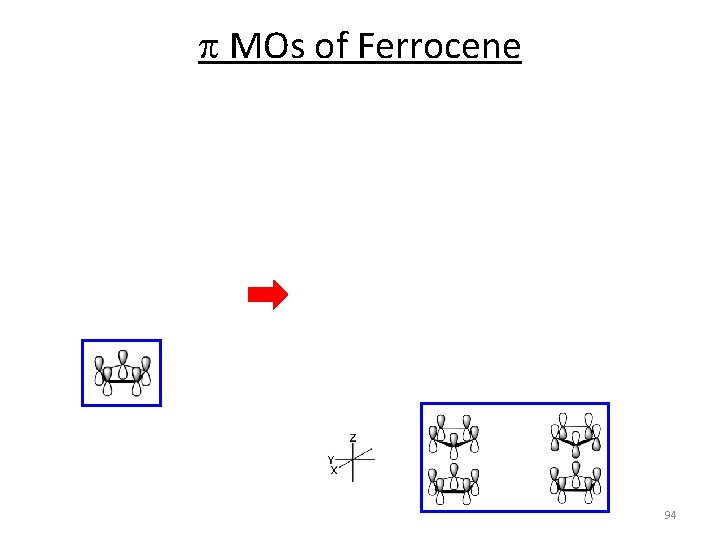
p MOs of Ferrocene 2 x 93

p MOs of Ferrocene 94

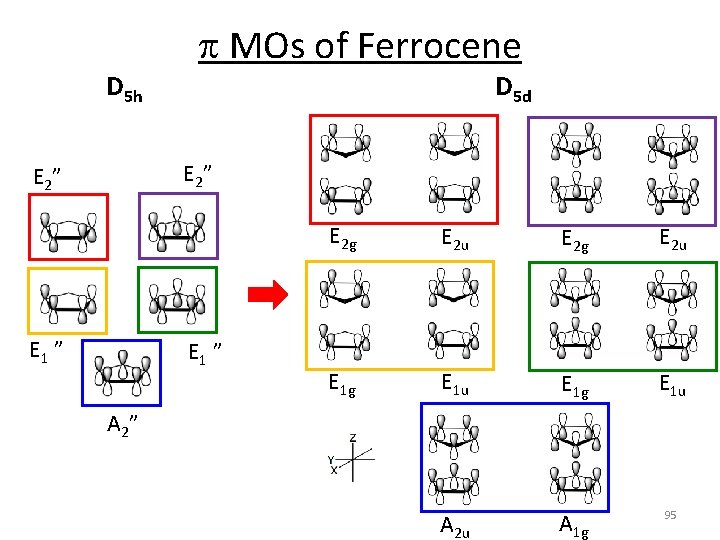
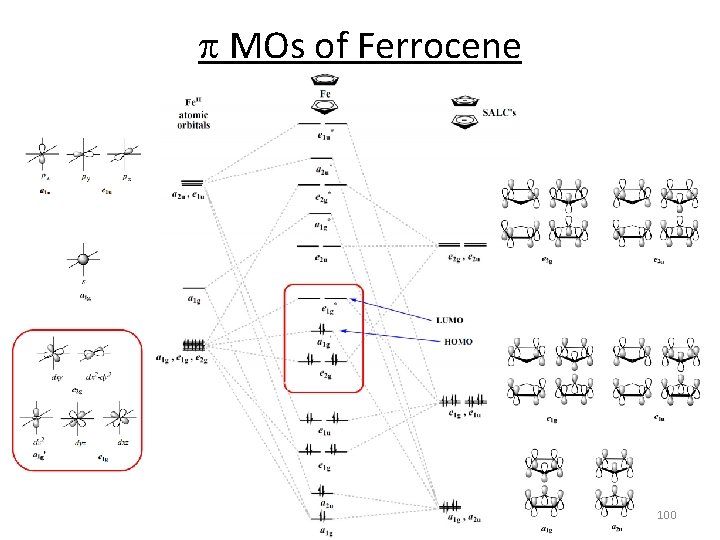
p MOs of Ferrocene D 5 h D 5 d E 2 ” E 1 ” E 2 g E 2 u E 1 g E 1 u A 2 u A 1 g 95 A 2”

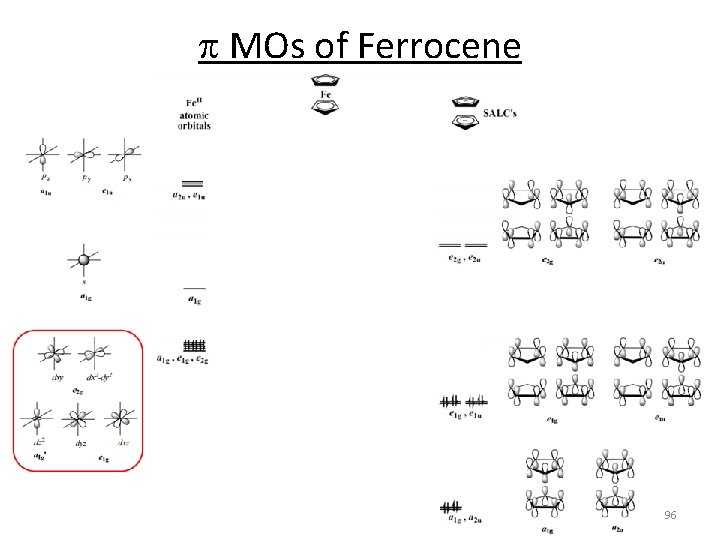
p MOs of Ferrocene 96

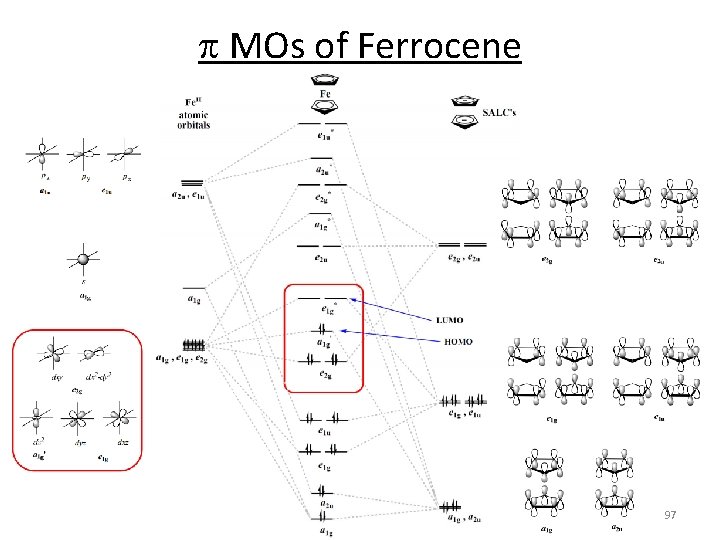
p MOs of Ferrocene 97

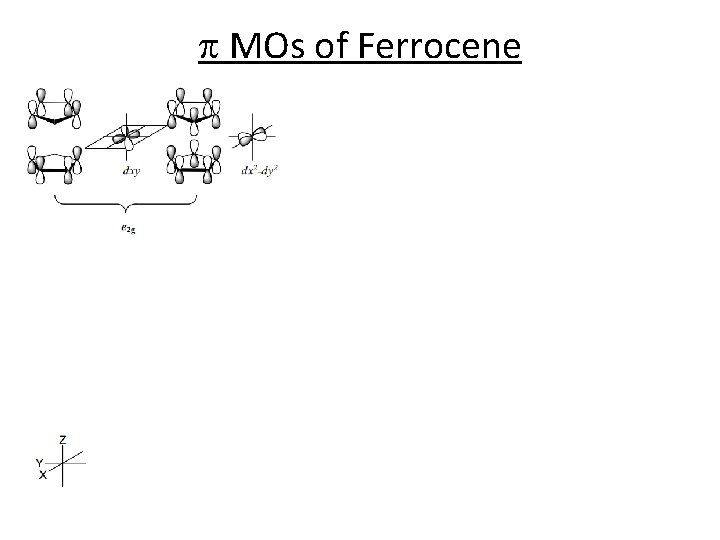
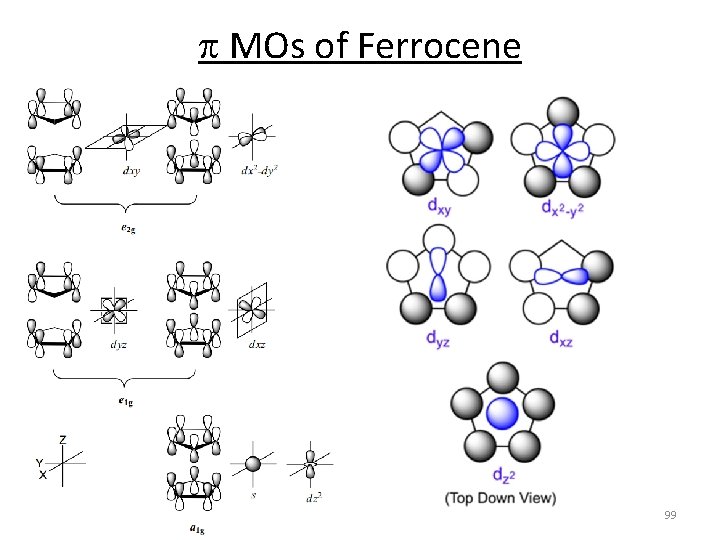
p MOs of Ferrocene 98

p MOs of Ferrocene 99

p MOs of Ferrocene 100

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry 101

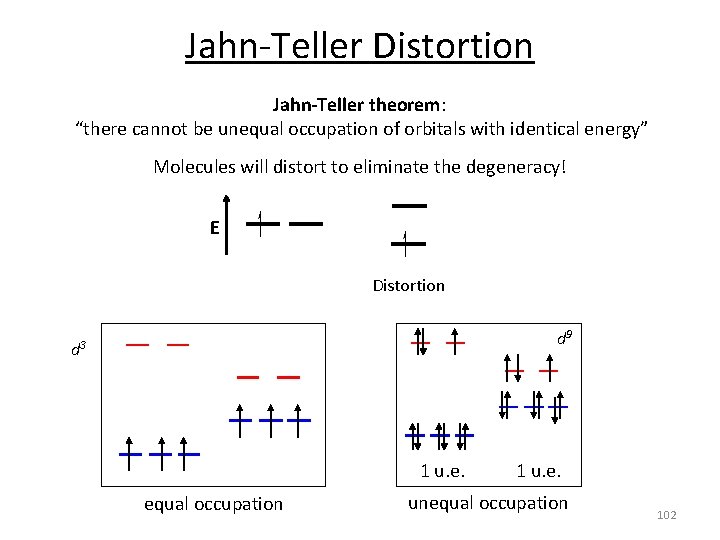
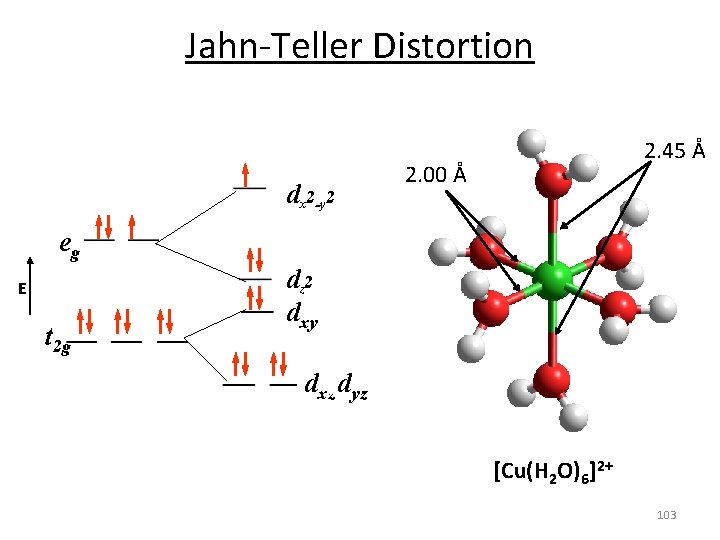
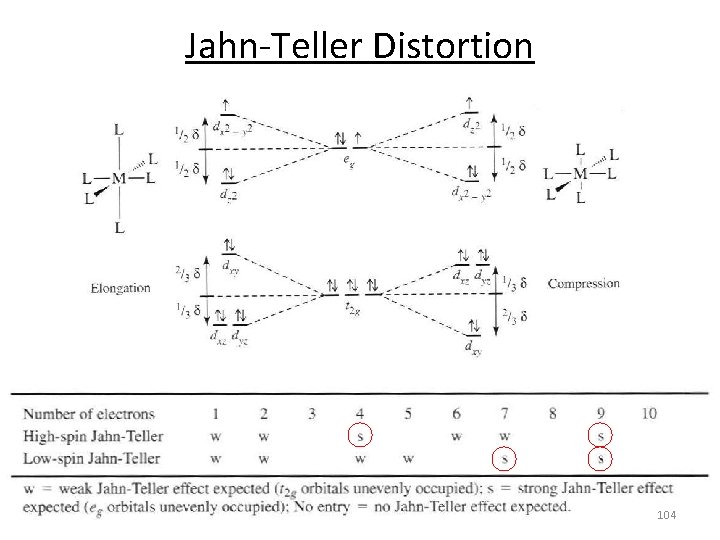
Jahn‐Teller Distortion Jahn-Teller theorem: “there cannot be unequal occupation of orbitals with identical energy” Molecules will distort to eliminate the degeneracy! E Distortion d 9 d 3 1 u. e. equal occupation 1 u. e. unequal occupation 102

Jahn‐Teller Distortion dx 2 -y 2 2. 45 Å 2. 00 Å eg E t 2 g dz 2 dxy dxz dyz [Cu(H 2 O)6]2+ 103

Jahn‐Teller Distortion 104

Jahn‐Teller Distortion 105

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry 106

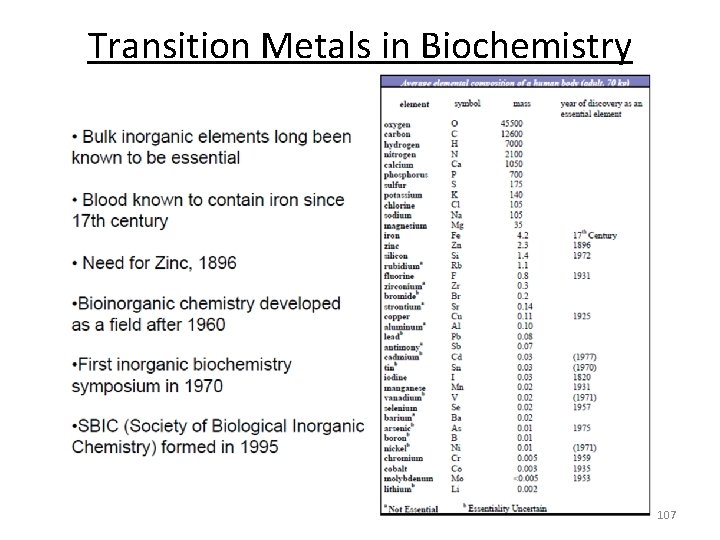
Transition Metals in Biochemistry 107

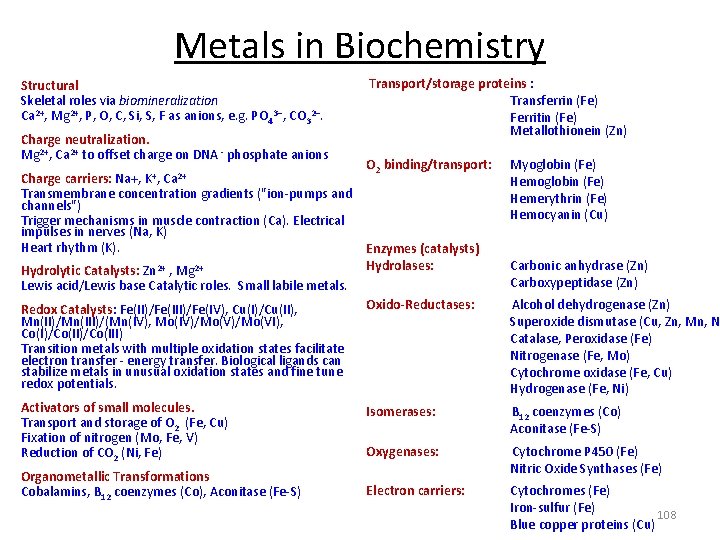
Metals in Biochemistry Structural Skeletal roles via biomineralization Ca 2+, Mg 2+, P, O, C, Si, S, F as anions, e. g. PO 43 , CO 32. Transport/storage proteins : Charge neutralization. Mg 2+, Ca 2+ to offset charge on DNA ‐ phosphate anions O 2 binding/transport: Transferrin (Fe) Ferritin (Fe) Metallothionein (Zn) Myoglobin (Fe) Charge carriers: Na+, K+, Ca 2+ Hemoglobin (Fe) Transmembrane concentration gradients ("ion‐pumps and Hemerythrin (Fe) channels") Hemocyanin (Cu) Trigger mechanisms in muscle contraction (Ca). Electrical impulses in nerves (Na, K) Heart rhythm (K). Enzymes (catalysts) Hydrolases: Carbonic anhydrase (Zn) Hydrolytic Catalysts: Zn 2+ , Mg 2+ Carboxypeptidase (Zn) Lewis acid/Lewis base Catalytic roles. Small labile metals. Oxido-Reductases: Alcohol dehydrogenase (Zn) Redox Catalysts: Fe(II)/Fe(IV), Cu(I)/Cu(II), Mn(II)/Mn(III)/(Mn(IV), Mo(IV)/Mo(VI), Superoxide dismutase (Cu, Zn, Mn, N Co(I)/Co(III) Catalase, Peroxidase (Fe) Transition metals with multiple oxidation states facilitate Nitrogenase (Fe, Mo) electron transfer ‐ energy transfer. Biological ligands can stabilize metals in unusual oxidation states and fine tune Cytochrome oxidase (Fe, Cu) redox potentials. Hydrogenase (Fe, Ni) Activators of small molecules. Isomerases: B 12 coenzymes (Co) Transport and storage of O 2 (Fe, Cu) Aconitase (Fe‐S) Fixation of nitrogen (Mo, Fe, V) Oxygenases: Cytochrome P 450 (Fe) Reduction of CO 2 (Ni, Fe) Nitric Oxide Synthases (Fe) Organometallic Transformations. Electron carriers: Cytochromes (Fe) Cobalamins, B 12 coenzymes (Co), Aconitase (Fe‐S) Iron‐sulfur (Fe) 108 Blue copper proteins (Cu)

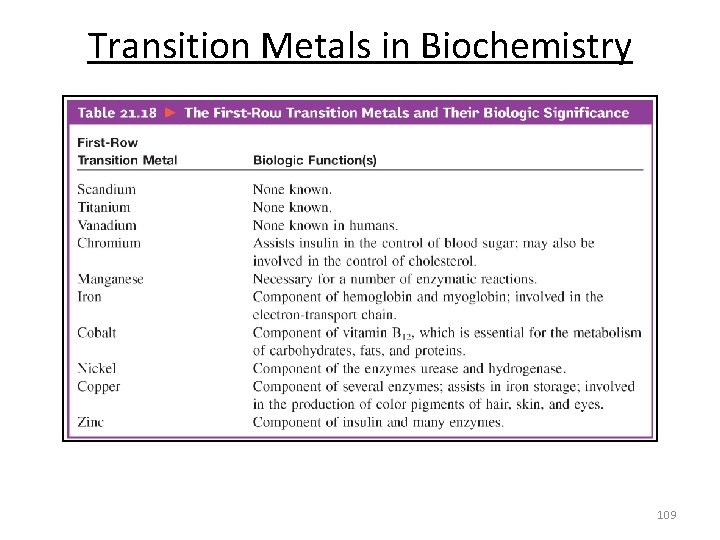
Transition Metals in Biochemistry 109

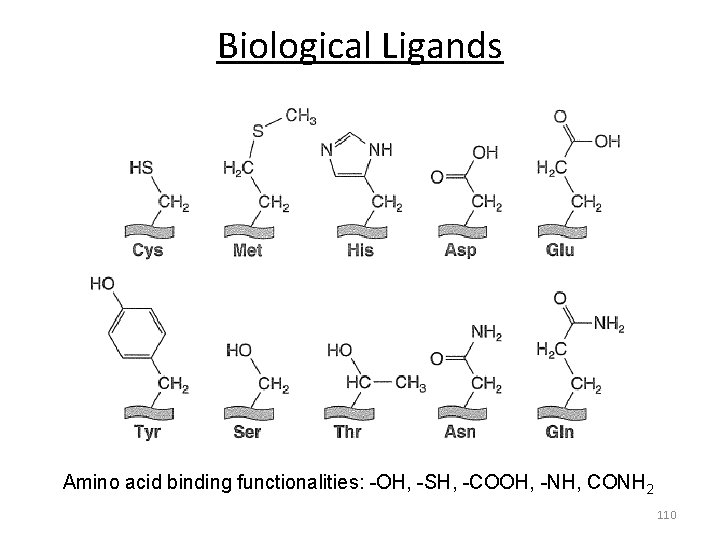
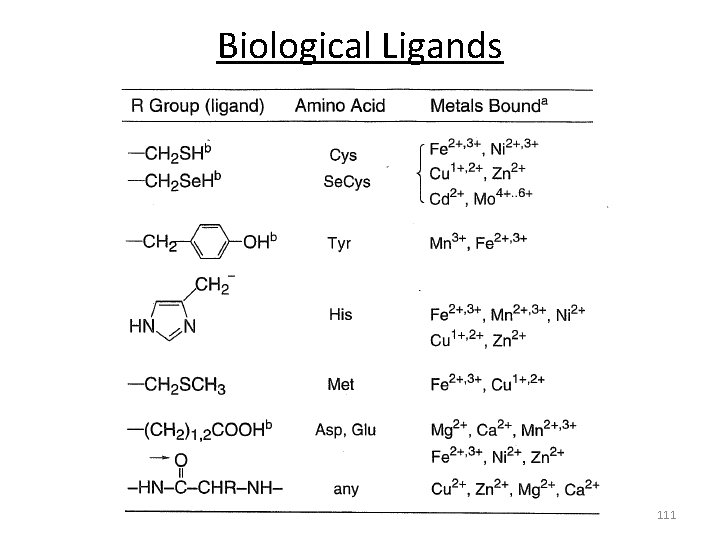
Biological Ligands Amino acid binding functionalities: -OH, -SH, -COOH, -NH, CONH 2 110

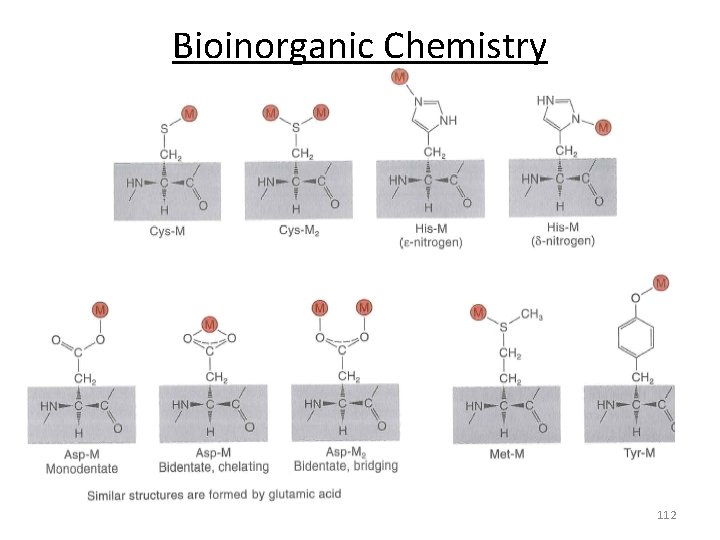
Biological Ligands 111

Bioinorganic Chemistry 112

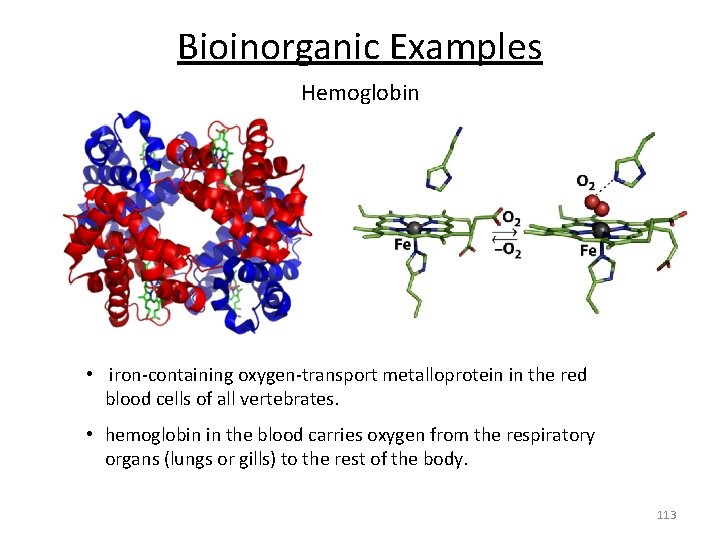
Bioinorganic Examples Hemoglobin • iron‐containing oxygen‐transport metalloprotein in the red blood cells of all vertebrates. • hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the rest of the body. 113

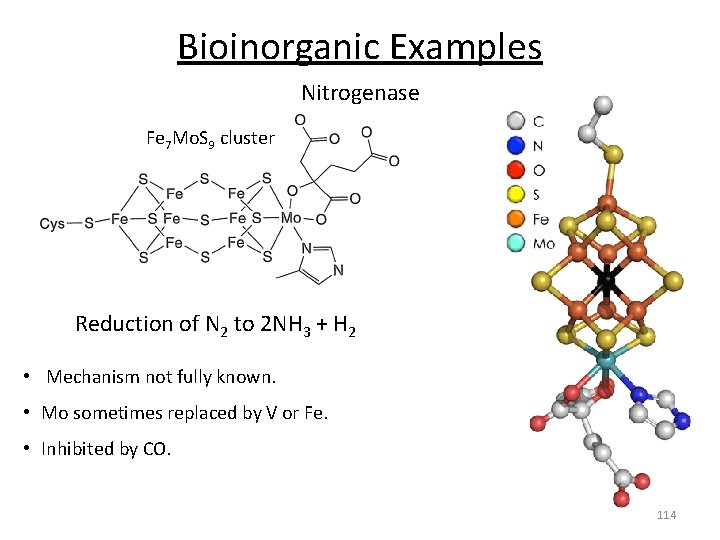
Bioinorganic Examples Nitrogenase Fe 7 Mo. S 9 cluster Reduction of N 2 to 2 NH 3 + H 2 • Mechanism not fully known. • Mo sometimes replaced by V or Fe. • Inhibited by CO. 114

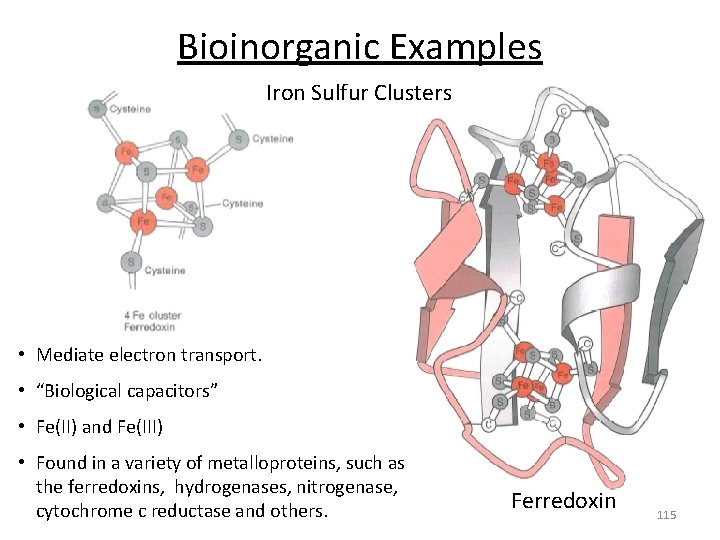
Bioinorganic Examples Iron Sulfur Clusters • Mediate electron transport. • “Biological capacitors” • Fe(II) and Fe(III) • Found in a variety of metalloproteins, such as the ferredoxins, hydrogenases, nitrogenase, cytochrome c reductase and others. Ferredoxin 115

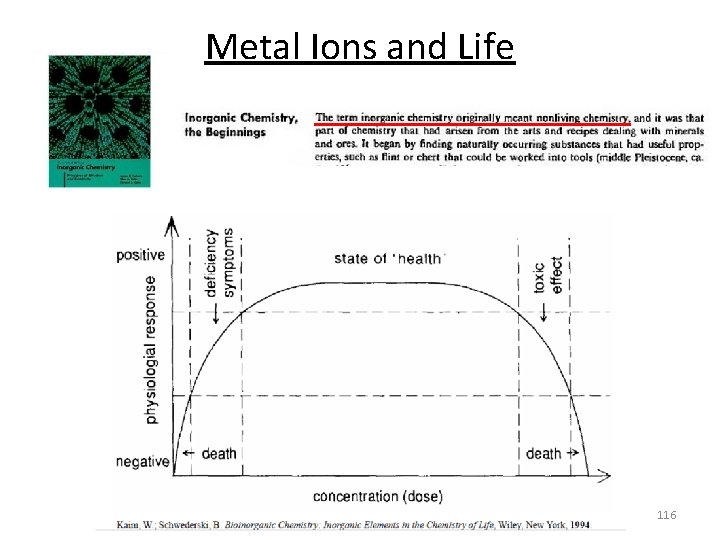
Metal Ions and Life 116

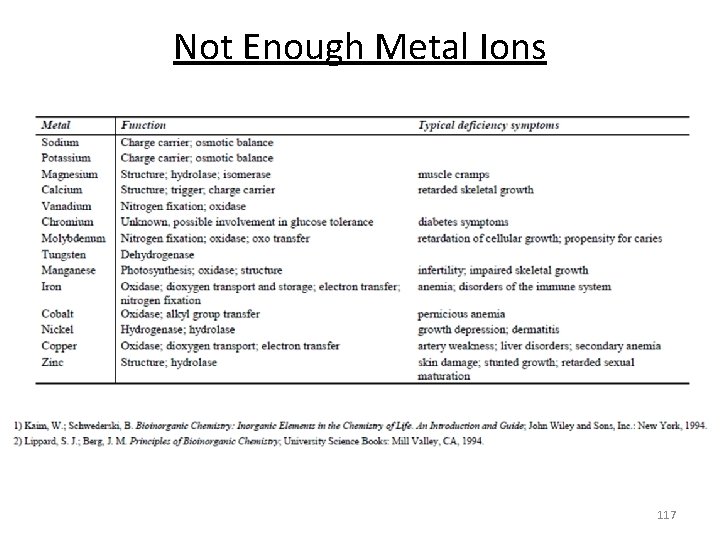
Not Enough Metal Ions 117

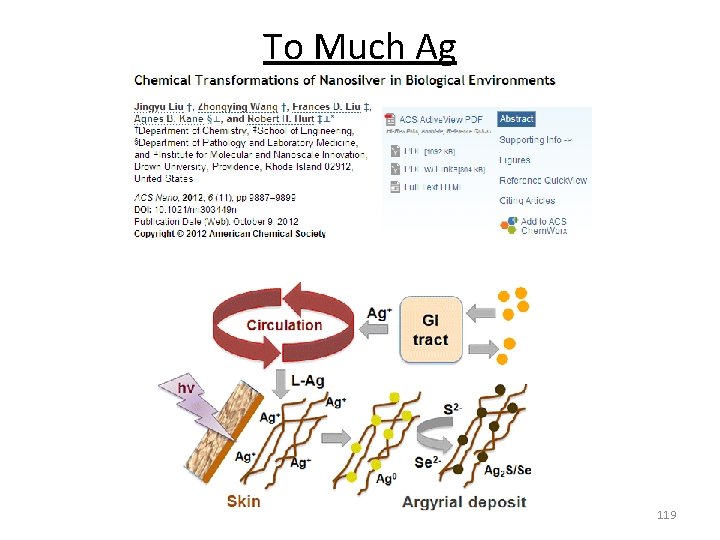
Excess Metal Ions Paul Karason‐ Used silver to “treat” dermatitis, acid reflux and other issues. Colloidal Silver Argyria or argyrosis: a condition caused by inappropriate exposure to chemical compounds of the element silver. Food and Drug Administration (FDA) doesn't approve of colloidal silver as a medical treatment! 118

To Much Ag 119

Outline • Coordination Complexes – History – Ligands – Isomers • • Inorganic Bonding Crystal Field Theory Ligand Field theory Orbital Diagrams Ligand Field Jahn‐Teller Distortion Bioinorganic Chemistry 120