Metal Complexes Chapter 24 Metal Complex consists of
![Answer 1. a) diiodoargentate(I) ion [I—Ag—I]– b) tris(ethylenediamine)cobalt(III) ion c) cis-[Co. Cl 2(en)2]Cl c) Answer 1. a) diiodoargentate(I) ion [I—Ag—I]– b) tris(ethylenediamine)cobalt(III) ion c) cis-[Co. Cl 2(en)2]Cl c)](https://slidetodoc.com/presentation_image/52d35daca694752e72bc4152cd309d5b/image-9.jpg)
![Sample Questions A complex [Co. A 6]3+ is red. The complex [Co. B 6]3+ Sample Questions A complex [Co. A 6]3+ is red. The complex [Co. B 6]3+](https://slidetodoc.com/presentation_image/52d35daca694752e72bc4152cd309d5b/image-20.jpg)
![Sample Questions A complex [Co. A 6]3+ is red. The complex [Co. B 6]3+ Sample Questions A complex [Co. A 6]3+ is red. The complex [Co. B 6]3+](https://slidetodoc.com/presentation_image/52d35daca694752e72bc4152cd309d5b/image-21.jpg)
- Slides: 21

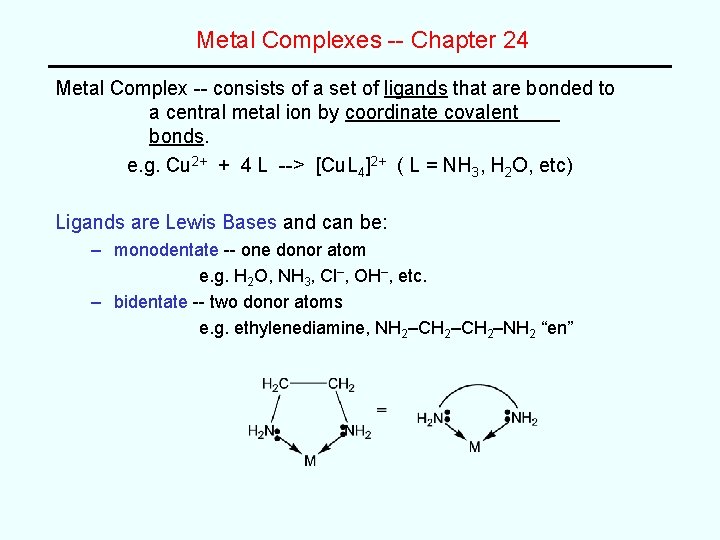
Metal Complexes -- Chapter 24 Metal Complex -- consists of a set of ligands that are bonded to a central metal ion by coordinate covalent bonds. e. g. Cu 2+ + 4 L --> [Cu. L 4]2+ ( L = NH 3, H 2 O, etc) Ligands are Lewis Bases and can be: – monodentate -- one donor atom e. g. H 2 O, NH 3, Cl–, OH–, etc. – bidentate -- two donor atoms e. g. ethylenediamine, NH 2–CH 2–NH 2 “en”

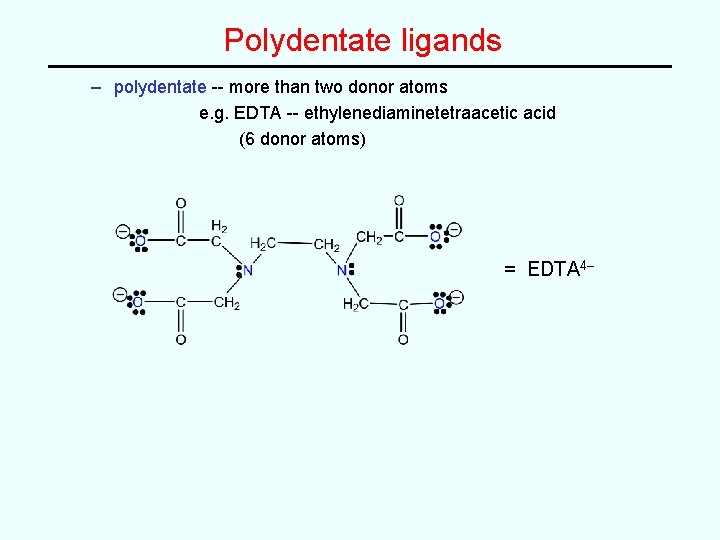
Polydentate ligands – polydentate -- more than two donor atoms e. g. EDTA -- ethylenediaminetetraacetic acid (6 donor atoms) = EDTA 4–

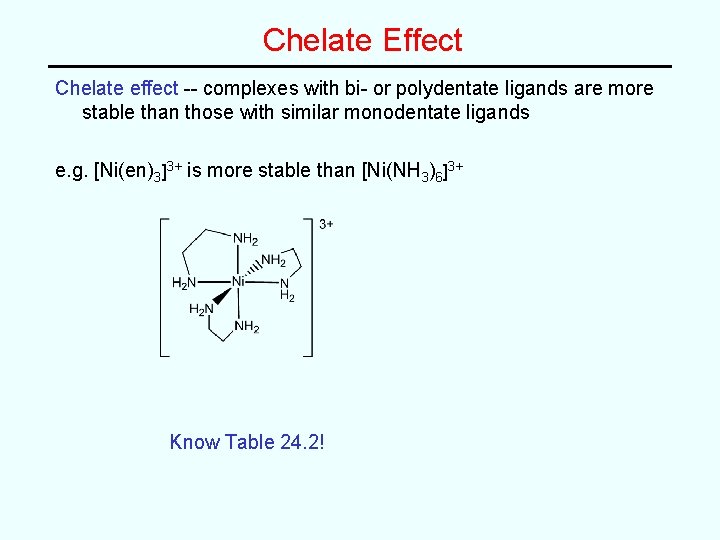
Chelate Effect Chelate effect -- complexes with bi- or polydentate ligands are more stable than those with similar monodentate ligands e. g. [Ni(en)3]3+ is more stable than [Ni(NH 3)6]3+ Know Table 24. 2!

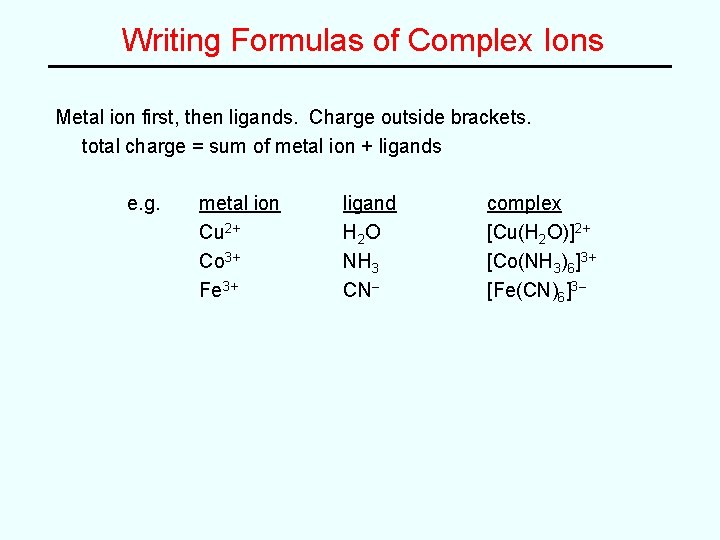
Writing Formulas of Complex Ions Metal ion first, then ligands. Charge outside brackets. total charge = sum of metal ion + ligands e. g. metal ion Cu 2+ Co 3+ Fe 3+ ligand H 2 O NH 3 CN– complex [Cu(H 2 O)]2+ [Co(NH 3)6]3+ [Fe(CN)6]3–

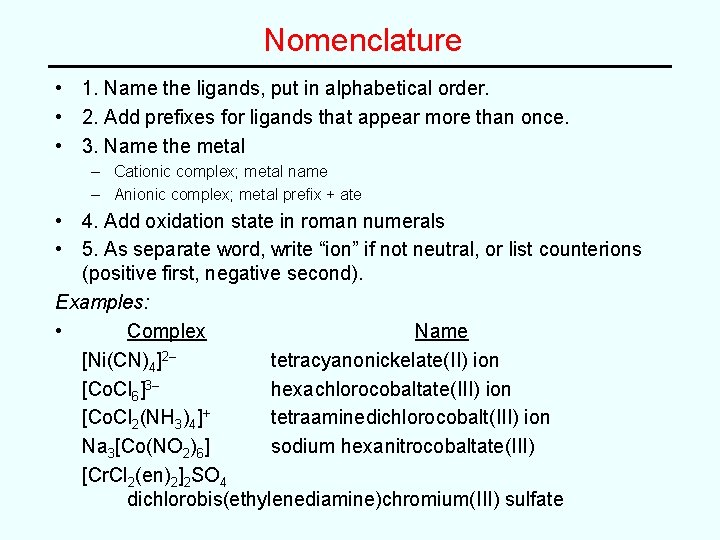
Nomenclature • 1. Name the ligands, put in alphabetical order. • 2. Add prefixes for ligands that appear more than once. • 3. Name the metal – Cationic complex; metal name – Anionic complex; metal prefix + ate • 4. Add oxidation state in roman numerals • 5. As separate word, write “ion” if not neutral, or list counterions (positive first, negative second). Examples: • Complex Name [Ni(CN)4]2– tetracyanonickelate(II) ion [Co. Cl 6]3– hexachlorocobaltate(III) ion [Co. Cl 2(NH 3)4]+ tetraaminedichlorocobalt(III) ion Na 3[Co(NO 2)6] sodium hexanitrocobaltate(III) [Cr. Cl 2(en)2]2 SO 4 dichlorobis(ethylenediamine)chromium(III) sulfate

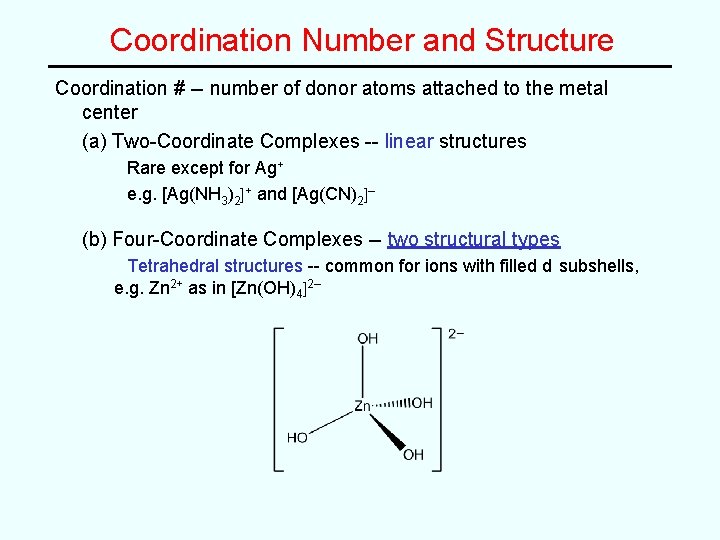
Coordination Number and Structure Coordination # -- number of donor atoms attached to the metal center (a) Two-Coordinate Complexes -- linear structures Rare except for Ag+ e. g. [Ag(NH 3)2]+ and [Ag(CN)2]– (b) Four-Coordinate Complexes -- two structural types Tetrahedral structures -- common for ions with filled d subshells, e. g. Zn 2+ as in [Zn(OH)4]2–

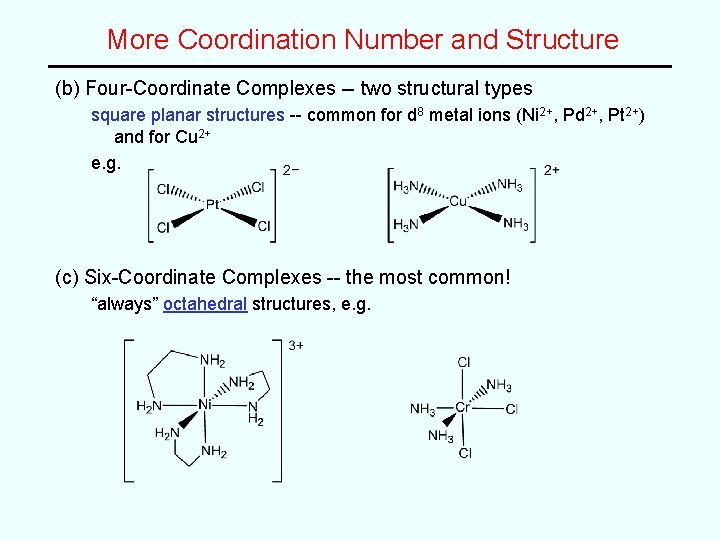
More Coordination Number and Structure (b) Four-Coordinate Complexes -- two structural types square planar structures -- common for d 8 metal ions (Ni 2+, Pd 2+, Pt 2+) and for Cu 2+ e. g. (c) Six-Coordinate Complexes -- the most common! “always” octahedral structures, e. g.

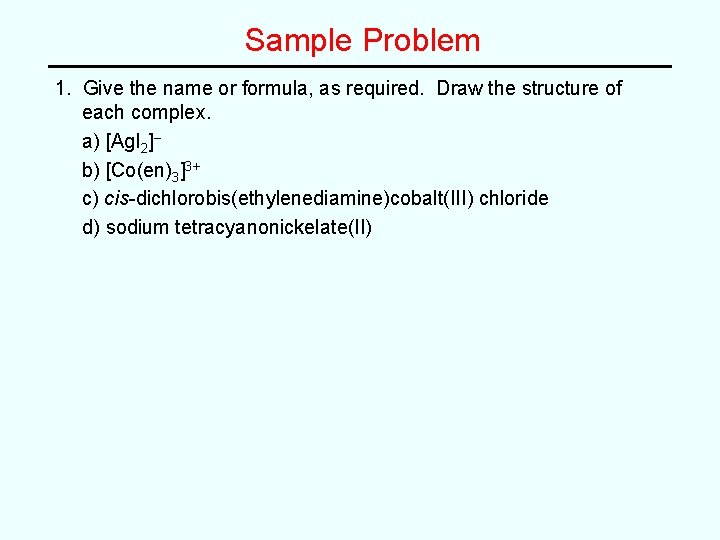
Sample Problem 1. Give the name or formula, as required. Draw the structure of each complex. a) [Ag. I 2]– b) [Co(en)3]3+ c) cis-dichlorobis(ethylenediamine)cobalt(III) chloride d) sodium tetracyanonickelate(II)
![Answer 1 a diiodoargentateI ion IAgI b trisethylenediaminecobaltIII ion c cisCo Cl 2en2Cl c Answer 1. a) diiodoargentate(I) ion [I—Ag—I]– b) tris(ethylenediamine)cobalt(III) ion c) cis-[Co. Cl 2(en)2]Cl c)](https://slidetodoc.com/presentation_image/52d35daca694752e72bc4152cd309d5b/image-9.jpg)
Answer 1. a) diiodoargentate(I) ion [I—Ag—I]– b) tris(ethylenediamine)cobalt(III) ion c) cis-[Co. Cl 2(en)2]Cl c) Na 2[Ni(CN)4]

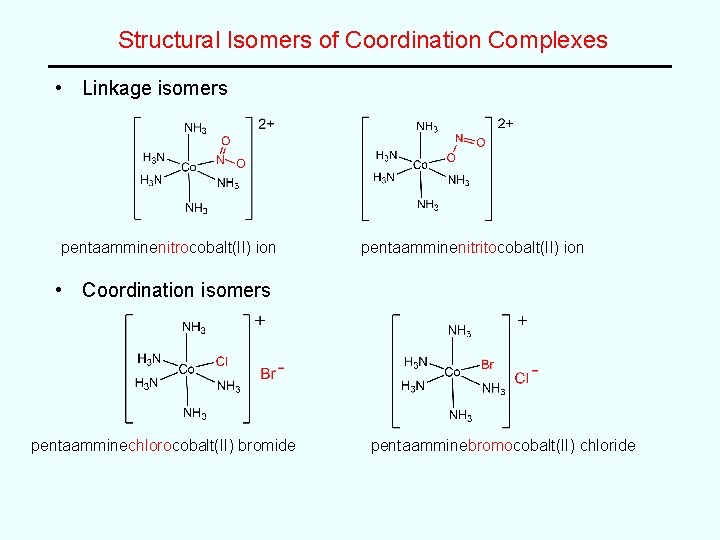
Structural Isomers of Coordination Complexes • Linkage isomers pentaamminenitrocobalt(II) ion pentaamminenitritocobalt(II) ion • Coordination isomers pentaamminechlorocobalt(II) bromide pentaamminebromocobalt(II) chloride

Isomerism; Overview 11

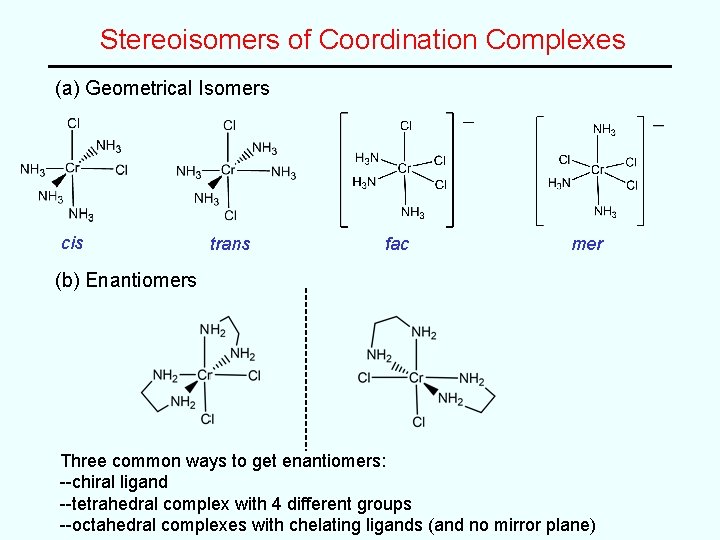
Stereoisomers of Coordination Complexes (a) Geometrical Isomers cis trans fac mer (b) Enantiomers Three common ways to get enantiomers: --chiral ligand --tetrahedral complex with 4 different groups --octahedral complexes with chelating ligands (and no mirror plane)

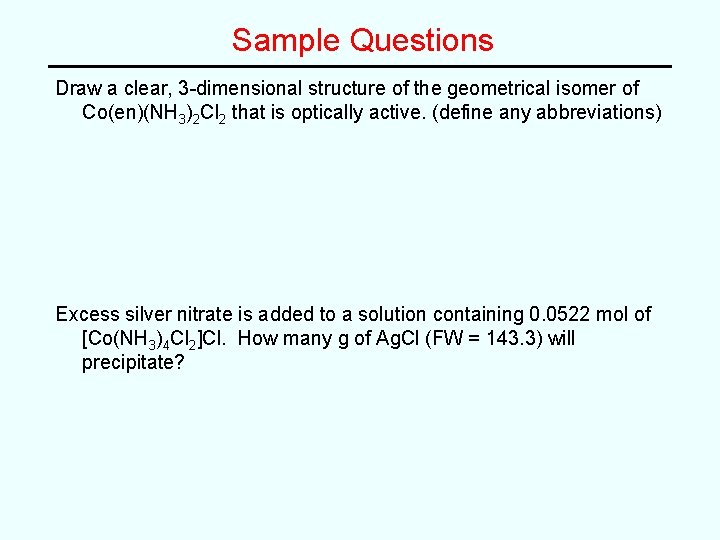
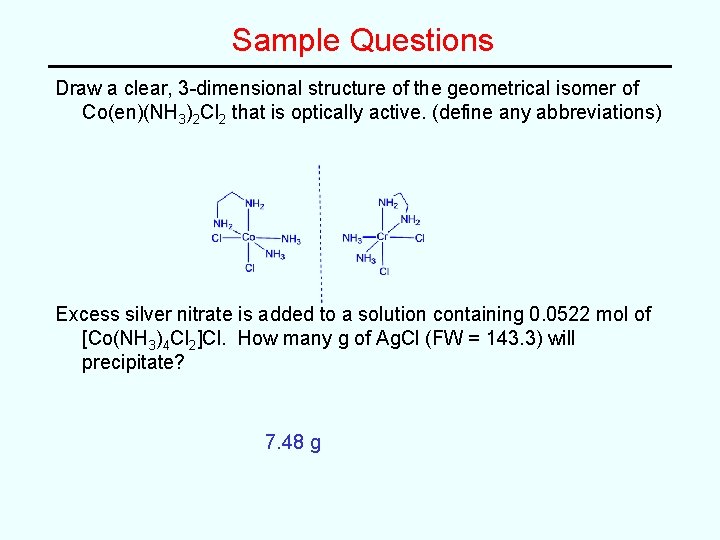
Sample Questions Draw a clear, 3 -dimensional structure of the geometrical isomer of Co(en)(NH 3)2 Cl 2 that is optically active. (define any abbreviations) Excess silver nitrate is added to a solution containing 0. 0522 mol of [Co(NH 3)4 Cl 2]Cl. How many g of Ag. Cl (FW = 143. 3) will precipitate?

Sample Questions Draw a clear, 3 -dimensional structure of the geometrical isomer of Co(en)(NH 3)2 Cl 2 that is optically active. (define any abbreviations) Excess silver nitrate is added to a solution containing 0. 0522 mol of [Co(NH 3)4 Cl 2]Cl. How many g of Ag. Cl (FW = 143. 3) will precipitate? 7. 48 g

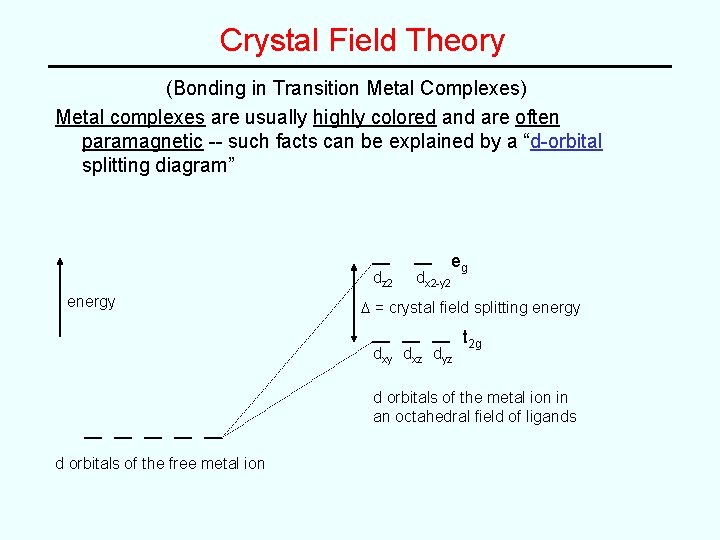
Crystal Field Theory (Bonding in Transition Metal Complexes) Metal complexes are usually highly colored and are often paramagnetic -- such facts can be explained by a “d-orbital splitting diagram” dz 2 energy dx 2 -y 2 eg D = crystal field splitting energy dxz dyz t 2 g d orbitals of the metal ion in an octahedral field of ligands d orbitals of the free metal ion

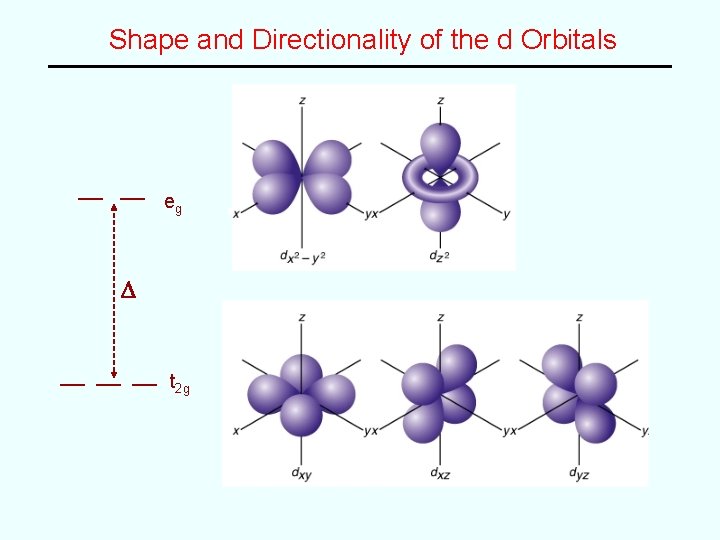
Shape and Directionality of the d Orbitals eg D t 2 g

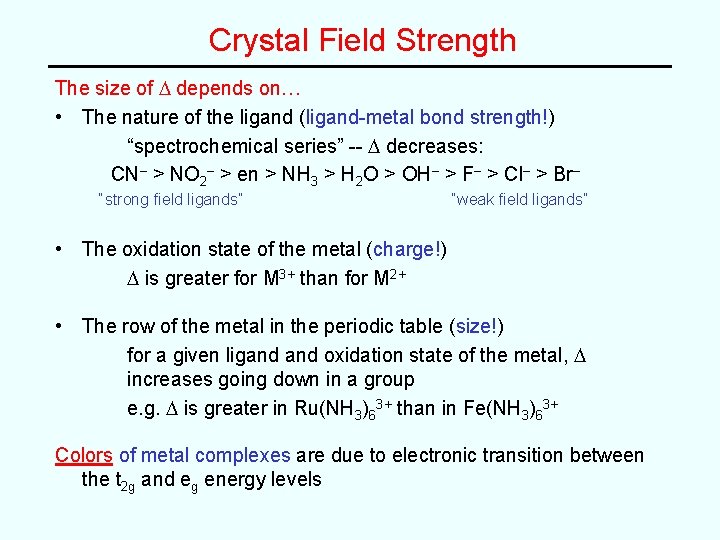
Crystal Field Strength The size of D depends on… • The nature of the ligand (ligand-metal bond strength!) “spectrochemical series” -- D decreases: CN– > NO 2– > en > NH 3 > H 2 O > OH– > F– > Cl– > Br– “strong field ligands” “weak field ligands” • The oxidation state of the metal (charge!) D is greater for M 3+ than for M 2+ • The row of the metal in the periodic table (size!) for a given ligand oxidation state of the metal, D increases going down in a group e. g. D is greater in Ru(NH 3)63+ than in Fe(NH 3)63+ Colors of metal complexes are due to electronic transition between the t 2 g and eg energy levels

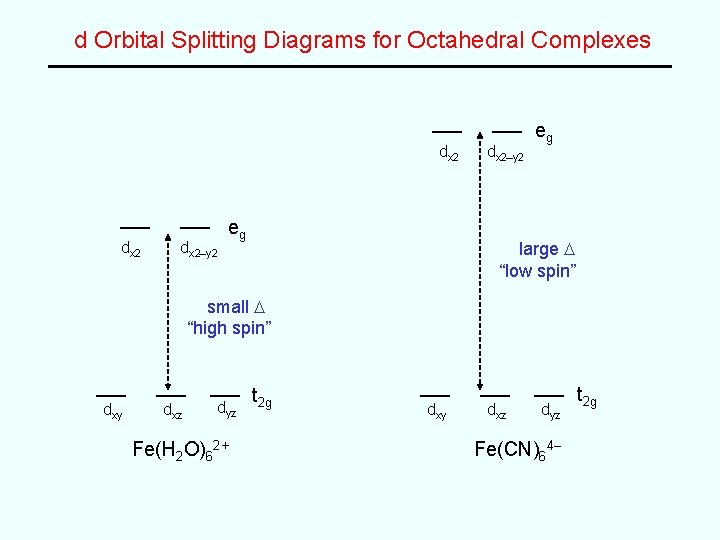
d Orbital Splitting Diagrams for Octahedral Complexes dx 2–y 2 eg large D “low spin” small D “high spin” dxy dxz dyz Fe(H 2 O)62+ t 2 g dxy dxz dyz Fe(CN)64– t 2 g

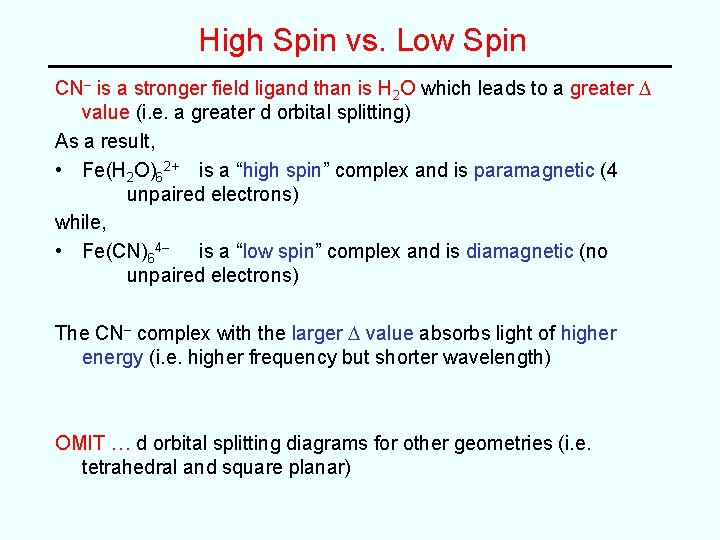
High Spin vs. Low Spin CN– is a stronger field ligand than is H 2 O which leads to a greater D value (i. e. a greater d orbital splitting) As a result, • Fe(H 2 O)62+ is a “high spin” complex and is paramagnetic (4 unpaired electrons) while, • Fe(CN)64– is a “low spin” complex and is diamagnetic (no unpaired electrons) The CN– complex with the larger D value absorbs light of higher energy (i. e. higher frequency but shorter wavelength) OMIT … d orbital splitting diagrams for other geometries (i. e. tetrahedral and square planar)
![Sample Questions A complex Co A 63 is red The complex Co B 63 Sample Questions A complex [Co. A 6]3+ is red. The complex [Co. B 6]3+](https://slidetodoc.com/presentation_image/52d35daca694752e72bc4152cd309d5b/image-20.jpg)
Sample Questions A complex [Co. A 6]3+ is red. The complex [Co. B 6]3+ is green. a) Which ligand, A or B, produces the larger crystal field splitting D? b) If the two ligands are ammonia and water, which is A and which is B? c) Draw the d orbital diagram of either complex, assuming it is “high spin. ” How many unpaired electrons will it have? Give the order of increase of D in the following sets: a) Cr(NH 3)63+, Cr. Cl 63–, Cr(CN)63– b) Co(H 2 O)62+, Co(H 2 O)63+, Rh(H 2 O)63+
![Sample Questions A complex Co A 63 is red The complex Co B 63 Sample Questions A complex [Co. A 6]3+ is red. The complex [Co. B 6]3+](https://slidetodoc.com/presentation_image/52d35daca694752e72bc4152cd309d5b/image-21.jpg)
Sample Questions A complex [Co. A 6]3+ is red. The complex [Co. B 6]3+ is green. a) Which ligand, A or B, produces the larger crystal field splitting D? b) If the two ligands are ammonia and water, which is A and which is B? c) Draw the d orbital diagram of either complex, assuming it is “high spin. ” How many unpaired electrons will it have? eg a) A dz 2 dx 2 -y 2 b) A = NH 3, B = H 2 O c) 4 unpaired electrons dxy dxz dyz t 2 g Give the order of increase of D in the following sets: a) Cr(NH 3)63+, Cr. Cl 63–, Cr(CN)63– b) Co(H 2 O)62+, Co(H 2 O)63+, Rh(H 2 O)63+ a) Cr(CN)63– > Cr(NH 3)63+ > Cr. Cl 63– b) Rh(H 2 O)63+ > Co(H 2 O)62+