terms Jeopardy Organic compounds Organic Compounds Organic compounds


















































- Slides: 53

terms Jeopardy Organic compounds Organic Compounds Organic compounds continued Flashback to chemistry Q $100 Q $100 Q $200 Q $200 Q $300 Q $300 Q $400 Q $400 Q $500 Q $500 Final Jeopardy

$100 Question from H 1 smallest unit of matter that cannot be broken down by ordinary chemical means

$100 Answer from H 1 What is: Atom

$200 Question from H 1 Atoms of an element that have a different number of neutrons

$200 Answer from H 1 What is: isotope

$300 Question from H 1 Bond formed by sharing electrons

$300 Answer from H 1 What is: covalent bonding

$400 Question from H 1 Define a compound

$400 Answer from H 1 Composed of 2 or more elements that are chemically combined

$500 Question from H 1 What is the difference between a monomer and a polymer? Provide an example of each

$500 Answer from H 1 Monomers are single units. Polymers are made of many monomers. Example: Monomer: nucleotide Polymer: DNA

$100 Question from H 2 Lipids are composed of

$100 Answer from H 2 What is: glycerol and 3 fatty acids

$200 Question from H 2 • What is the function of a carbohydrate?

$200 Answer from H 2 What is: quick source of energy

$300 Question from H 2 Amino acids are monomers of

$300 Answer from H 2 What is: protein

$400 Question from H 2 Why are enzymes important?

$400 Answer from H 2 What is: help mediate chemical reactions in body (ex: function in digestion)

$500 Question from H 2 What is the difference between carbohydrate, protein, lipids, and nucleic acids in terms of the elements that they contain?

$500 Answer from H 2 Carbohydrate: Carbon, hydrogen, oxygen Lipids: carbon and hydrogen Proteins: carbon hydrogen, oxygen, and nitrogen Nucleic acids: carbon, oxygen, hydrogen, nitrogen, phosphorous

$100 Question from H 3 What are the four organic compounds?

$100 Answer from H 3 What are: carbohydrates, proteins, lipids And nucleic acids

$200 Question from H 3 Distinguish between monosaccharide, Disaccharide, and polysaccharide

$200 Answer from H 3 What is: • monosaccharide 1 sugar • Disaccharide 2 sugars • Polysaccharide many sugars

$300 Question from H 3 The monomer that makes up the polysaccharide Starch is:

$300 Answer from H 3 What is: glucose

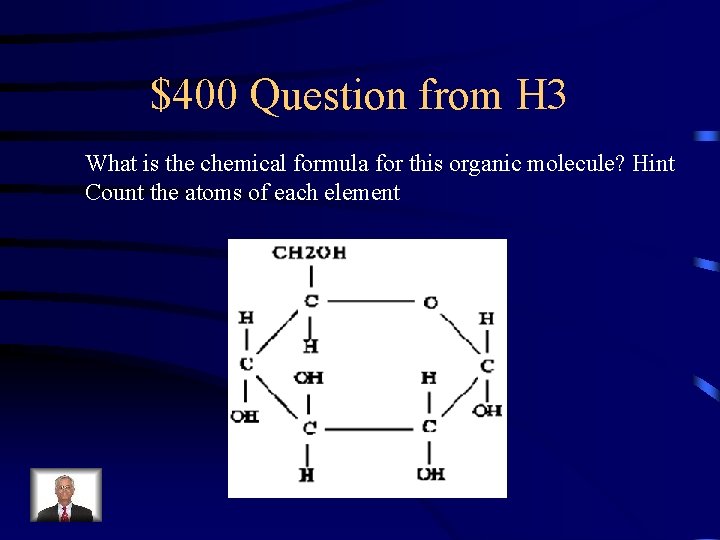
$400 Question from H 3 What is the chemical formula for this organic molecule? Hint Count the atoms of each element

$400 Answer from H 3 What is: C 6 H 12 O 6

$500 Question from H 3 What is the difference between saturated And unsaturated fats?

$500 Answer from H 3 What are: Saturated fat: single bond between carbons Unsaturated fats: double bonds between carbons

$100 Question from H 4 Two examples of nucleic acids are:

$100 Answer from H 4 What are: DNA and RNA

$200 Question from H 4 All organic compounds contain the elements

$200 Answer from H 4 What are: carbon and hydrogen

$300 Question from H 4 A sugar, a phosphate and a nitrogenous base make up a

$300 Answer from H 4 What is: nucleotide

$400 Question from H 4 Proteins contain all of the following elements except: (A) carbon (B) hydrogen (C) oxygen (D) sodium (E) nitrogen

$400 Answer from H 4 What is: D (sodium)

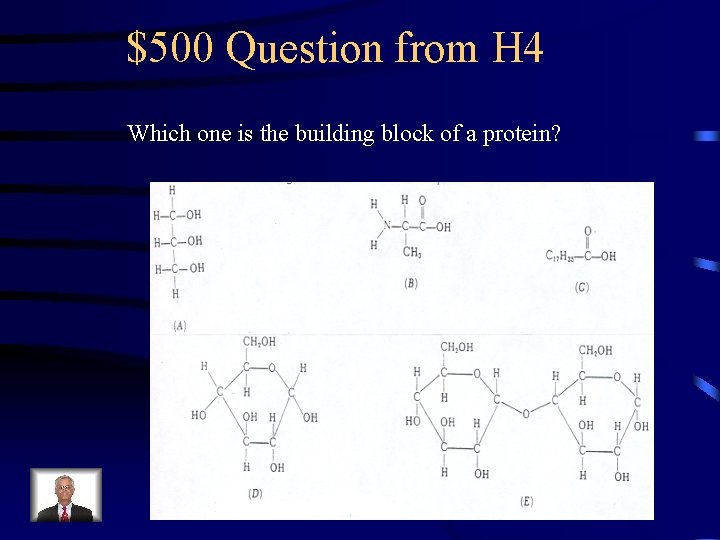
$500 Question from H 4 Which one is the building block of a protein?

$500 Answer from H 4 B

$100 Question from H 5 1. Atomic number = _____

$100 Answer from H 5 What is: # of protons

$200 Question from H 5 Acids have a p. H from ______ to _____

$200 Answer from H 5 What is: 1 -6

$300 Question from H 5 Distinguish between solution, solvent, and Solute

$300 Answer from H 5 What is: • Solvent substance in which the solute dissolves • Solute the substance that is dissolved • Solution solute + solvent

$400 Question from H 5 Determine how many protons, neutrons, and Electrons are in the following ion: Na+

$400 Answer from H 5 What is: • Protons: 11 • Neutrons: 23 -11 = 12 • Electrons 11 (but + charge so one less) = 10

$500 Question from H 5 Differentiate between neutral atoms, ions, and isotopes

$500 Answer from H 5 Ions different number of electrons Isotopes different number of neutrons Neutral atoms same number of protons and electrons

Final Jeopardy Describe the processes of photosynthesis and cellular respiration. How are they similar? Different?

Final Jeopardy Answer