Organic Compounds are divided in to two Organic







- Slides: 17

Organic Compounds are divided in to two – Organic and Inorganic Organic Compounds – • Originate from living things • They are easily decomposed • They are the main component of most of our fuels such as gasoline, oil and natural gas

Organic Compounds • They are also the active ingredient in pharmaceuticals such as aspirin and ibuprofen • Organic compounds are composed of C and H and also include a few other elements such as N, S and O. • The simplest organic compound is CH 4

Chemistry of Carbon The chemistry of Carbon is unique and complex because carbon frequently bond to itself to form chain branched and ring structures Carbon also forms double and triple bonds with itself and other elements

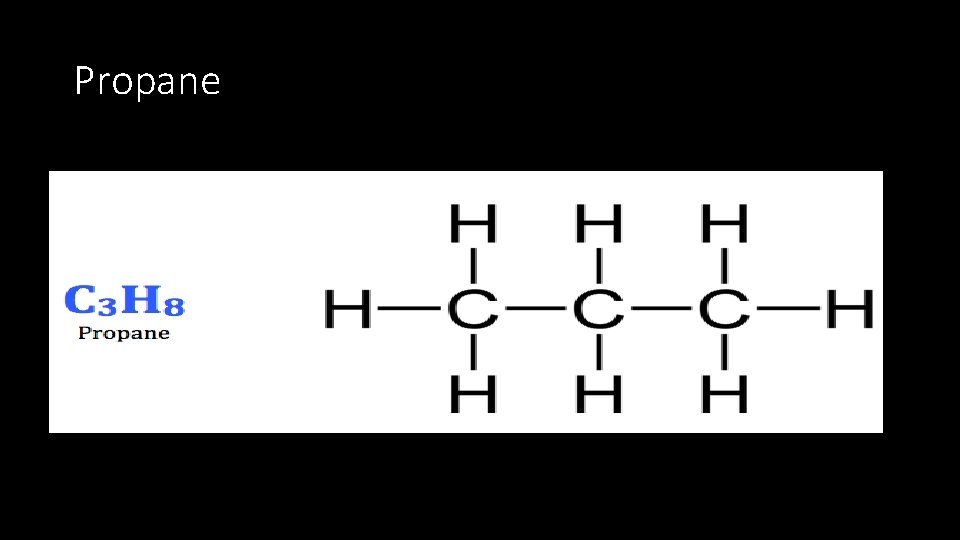
Propane

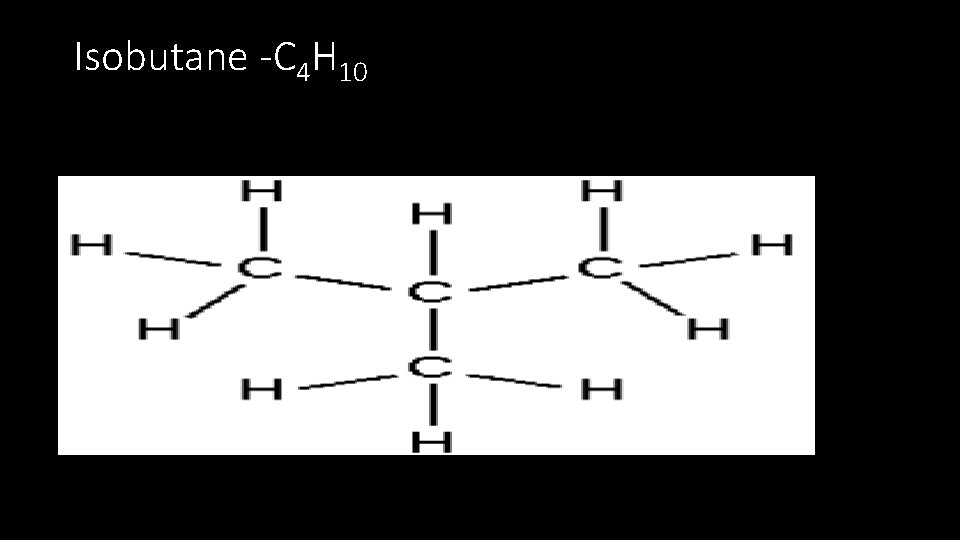
Isobutane -C 4 H 10

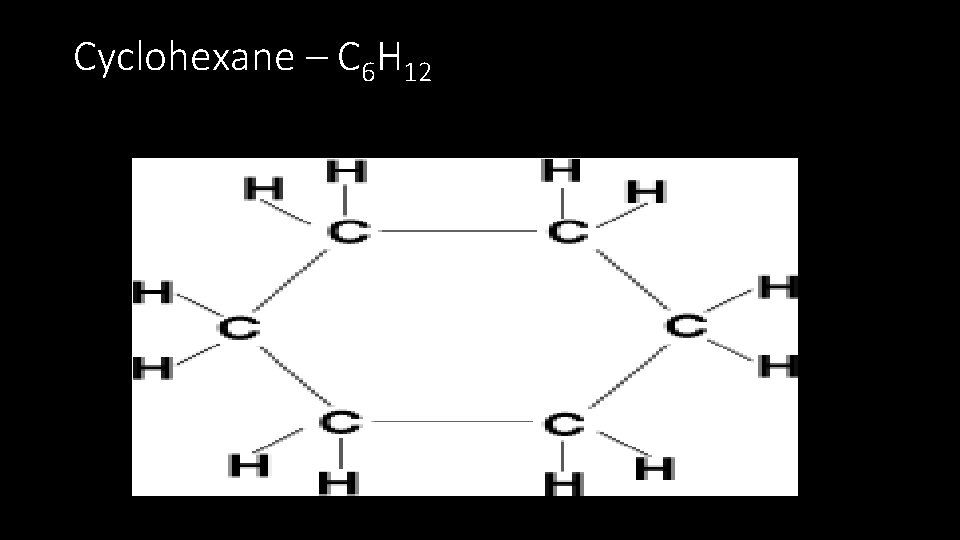
Cyclohexane – C 6 H 12

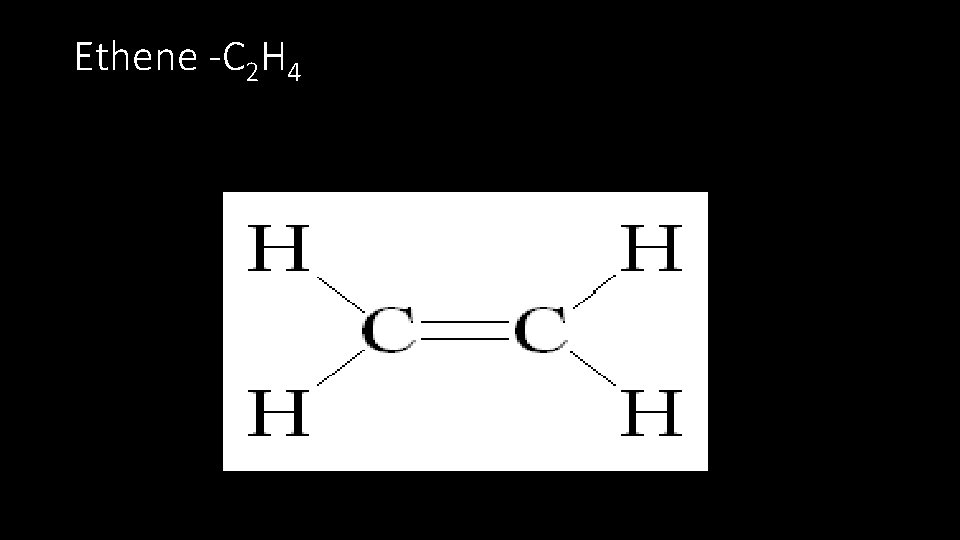
Ethene -C 2 H 4

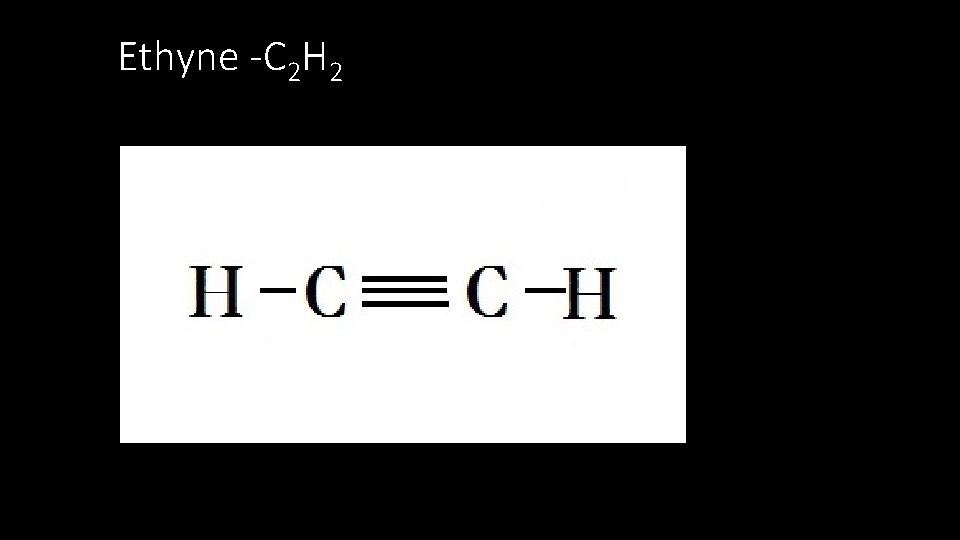
Ethyne -C 2 H 2

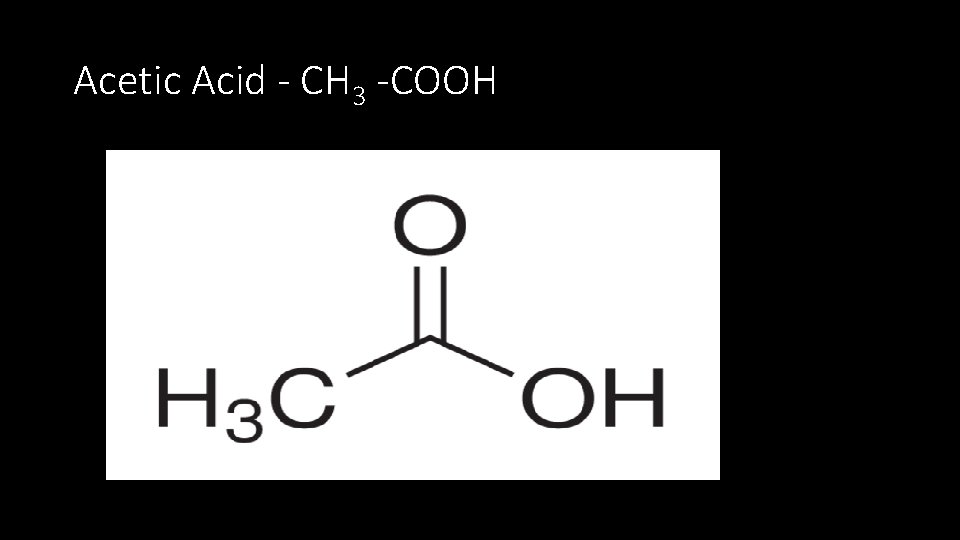
Acetic Acid - CH 3 -COOH

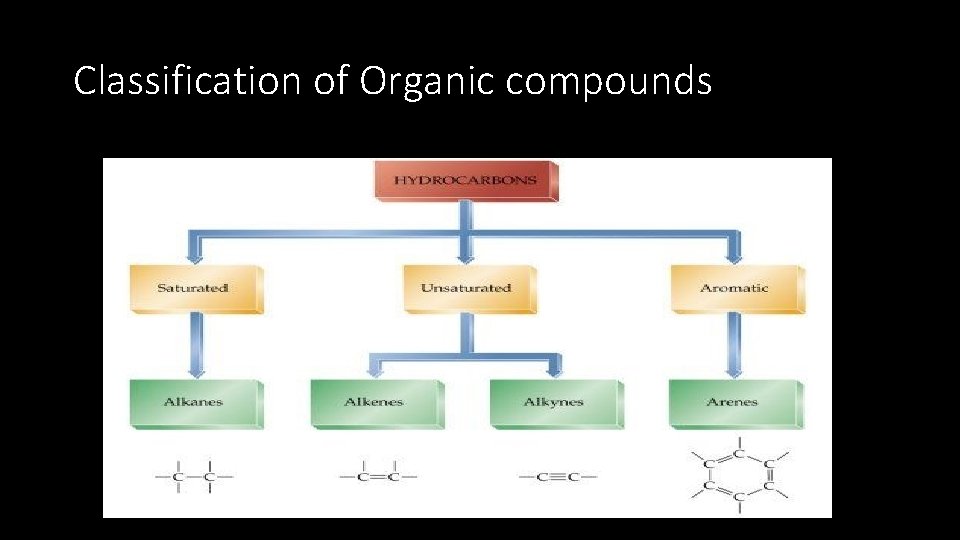
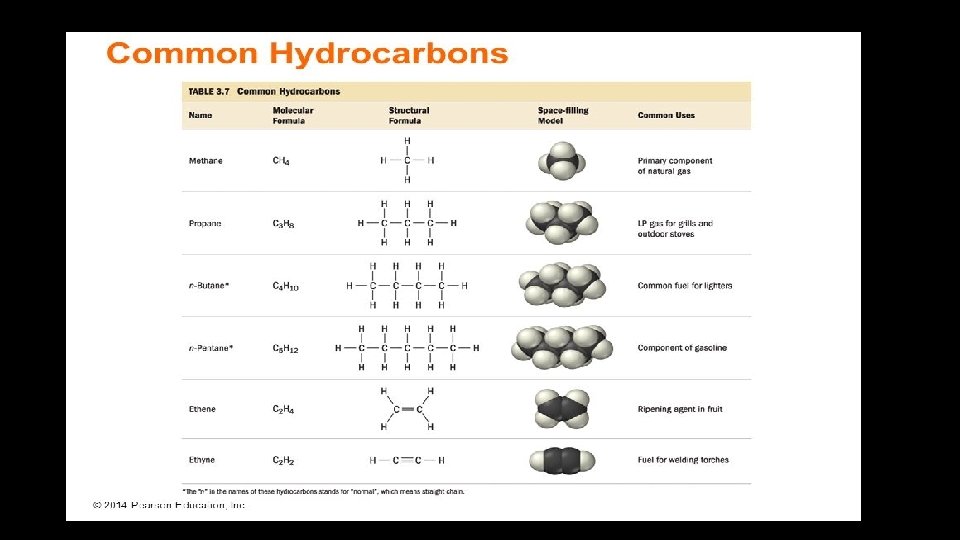
Organic compounds - Classification • Organic compounds are classified in to Hydrocarbons and functionalized hydrocarbons • Hydrocarbons are organic compounds that contains only carbon and hydrogen. Hydrocarbon containing only single bond are called alkanes while those containing double or triple bonds are alkenes are alkynes

Classification of Organic compounds

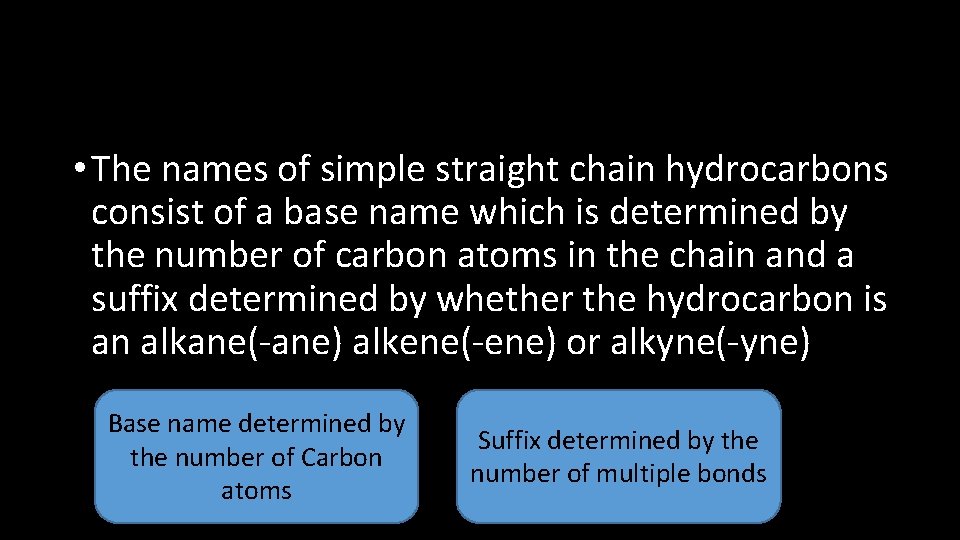
• The names of simple straight chain hydrocarbons consist of a base name which is determined by the number of carbon atoms in the chain and a suffix determined by whether the hydrocarbon is an alkane(-ane) alkene(-ene) or alkyne(-yne) Base name determined by the number of Carbon atoms Suffix determined by the number of multiple bonds

The base names for a number of hydrocarbons are listed Meth = 1 Eth = 2 Prop = 3 But = 4 Pent = 5 Hex = 6 Hept = 7 Oct = 8 non = 9 dec = 10


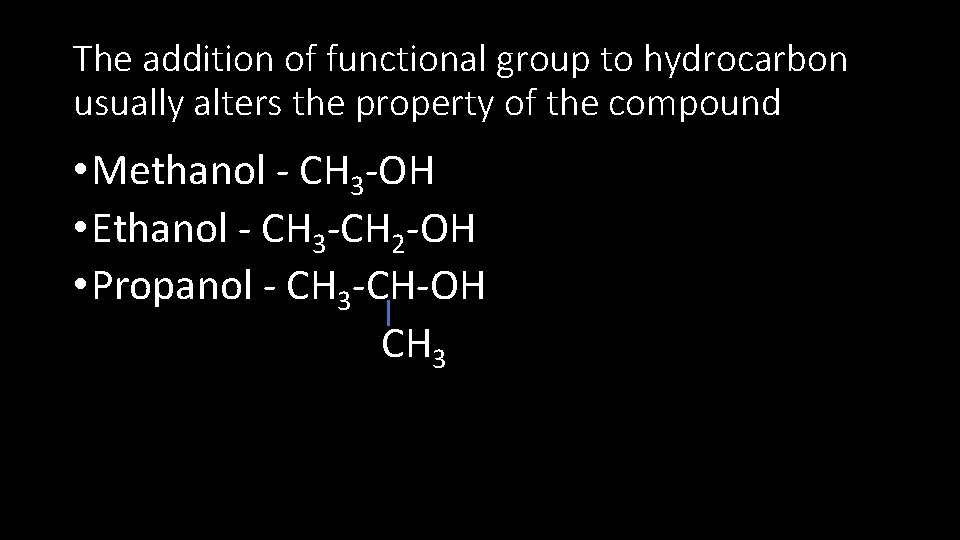
Functionalized Hydrocarbons • They are hydrocarbons with functional groups • Example: Alcohols have –OH as functional group • They have the general formula R-OH • Where R represents the hydrocarbon portion of the molecule • Methanol, Ethanol, and propanol belongs to the same family.

The addition of functional group to hydrocarbon usually alters the property of the compound • Methanol - CH 3 -OH • Ethanol - CH 3 -CH 2 -OH • Propanol - CH 3 -CH-OH CH 3
