Alkenes Alkynes Mr Shields Regents Chemistry U 16










- Slides: 19

- Alkenes - Alkynes Mr. Shields Regents Chemistry U 16 L 03 1

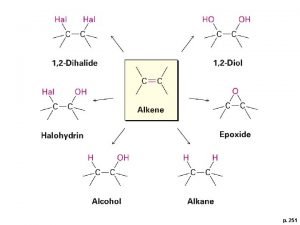
Alkenes Ø Like the alkanes, the Alkenes form another homologous hydrocarbon series Ø Each member contains one double covalent bond between two C atoms. l So alkenes are unsaturated hydrocarbons. Ø General formula = Cn. H 2 n (2 H less than the Alkanes) l What was the general formula for the Alkanes? 2

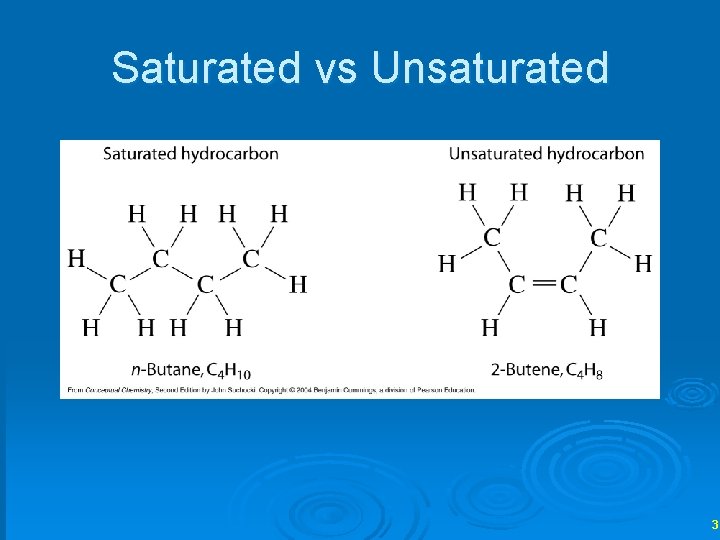
Saturated vs Unsaturated 3

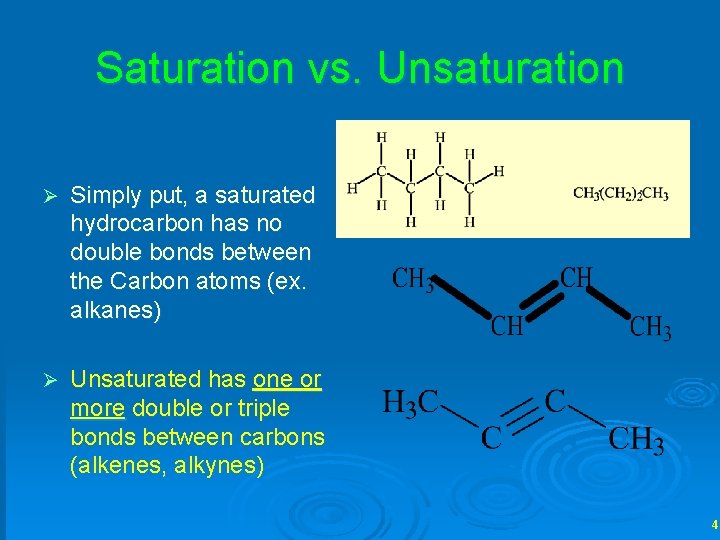
Saturation vs. Unsaturation Ø Simply put, a saturated hydrocarbon has no double bonds between the Carbon atoms (ex. alkanes) Ø Unsaturated has one or more double or triple bonds between carbons (alkenes, alkynes) 4

Physical properties of the Alkenes -Alkenes have properties similar to the alkanes - Insoluble in water - But very soluble in organic solvents - Less dense than water (density of water = 1. 0) - B. P. and M. P increase w/ increasing carbon # - Slightly lower than the corresponding alkane - As with alkanes B. P rises about 20 -30 C per carbon - Hexane b. p. 69 C; 1 -Hexene b. p. 63. 5 C - Heptane b. p. 98 C; 1 -Heptene b. p. 93 C 5

Naming Alkenes Ø Names are derived using the same prefix used to name the alkane chain with the same number of C atoms. Ø Replace the –ane ending of the alkane name with –ene. 1 st member is C 2 H 4, ethene. H H Why 1 st ? C=C H H 6

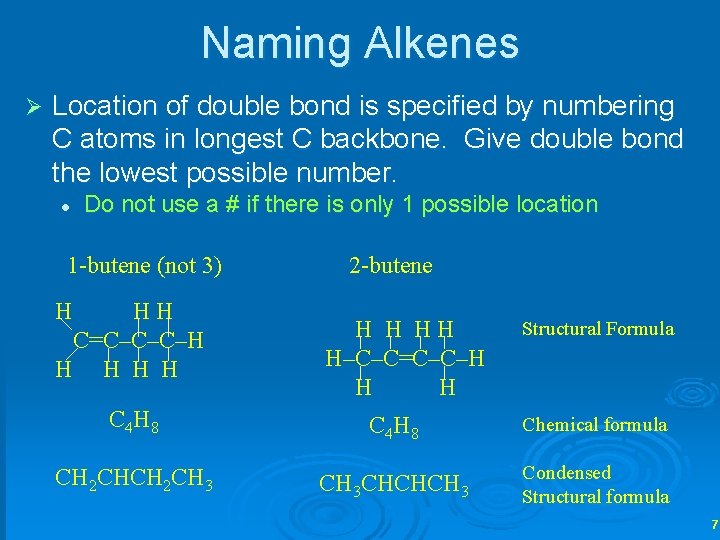
Naming Alkenes Ø Location of double bond is specified by numbering C atoms in longest C backbone. Give double bond the lowest possible number. l Do not use a # if there is only 1 possible location 1 -butene (not 3) H HH C=C–C–C–H H H 2 -butene H H HH H–C–C=C–C–H H H Structural Formula C 4 H 8 Chemical formula CH 2 CH 3 CHCHCH 3 Condensed Structural formula 7

Alkene Homologous Series (w/ C 1 -C 2 double bond) Ethene Ø Propene Ø 1 -Butene Ø 1 -Pentene Ø 1 -Hexene Ø 1 -Heptene Ø 1 -Octene Ø 1 -Nonene Ø 1 -Decene Ø C=C C-C-C=C C-C-C-C-C=C C-C-C-C-C-C-C=C What is the name of this alkene? C-C-C=C-C-C-C 8

Naming Alkenes Ø Once double bond is numbered specify substituents alphabetically by carbon number l Do not use a number if there is only 1 possible location Ø Use di, tri and tetra for multiple substituents of the same group 2, 3 -dimethyl-1 -butene C=C–C–C C C Structural Formula (w/o the H) C 6 H 12 Chemical formula CH 2 C(CH 3)CH 3 Condensed Structural formula 9

Problems: Ø Draw the following alkenes: l l 2 -Butene methylpropene 4–Methyl– 2 -pentene 3, 3 -Dimethyl-1 -butene 10

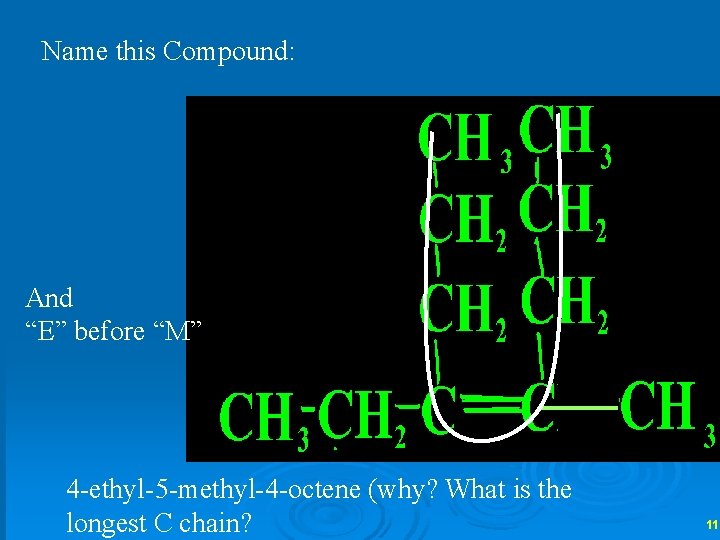
Name this Compound: And “E” before “M” 4 -ethyl-5 -methyl-4 -octene (why? What is the longest C chain? 11

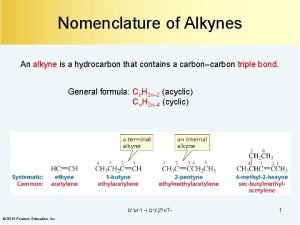
Alkynes Ø Homologous series of unsaturated hydrocarbons that contain one triple bond. Ø Each member contains one triple carbon-carbon bond. l Ø Alkynes are unsaturated. General formula = Cn. H 2 n-2 12

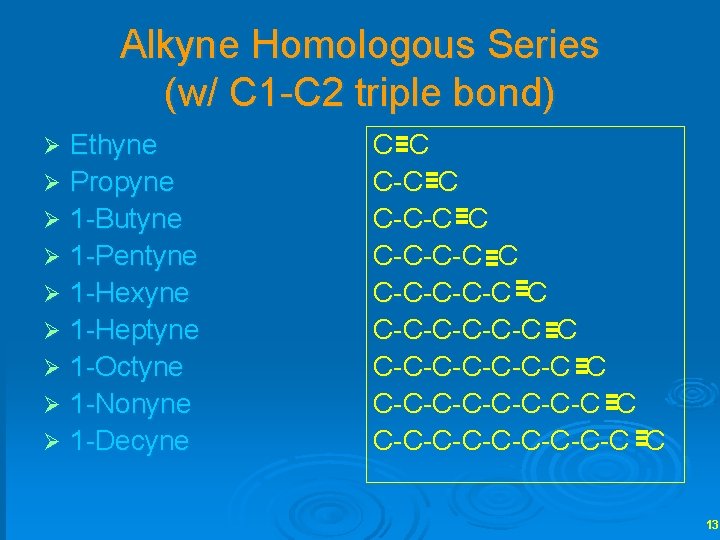
Alkyne Homologous Series (w/ C 1 -C 2 triple bond) Ethyne Ø Propyne Ø 1 -Butyne Ø 1 -Pentyne Ø 1 -Hexyne Ø 1 -Heptyne Ø 1 -Octyne Ø 1 -Nonyne Ø 1 -Decyne Ø C C C-C-C-C-C C C-C-C-C-C-C-C C 13

Ethyne (Common name) A common use of one of the alkynes: The Acetylene torch What is the IUPAC name for this compound? 14

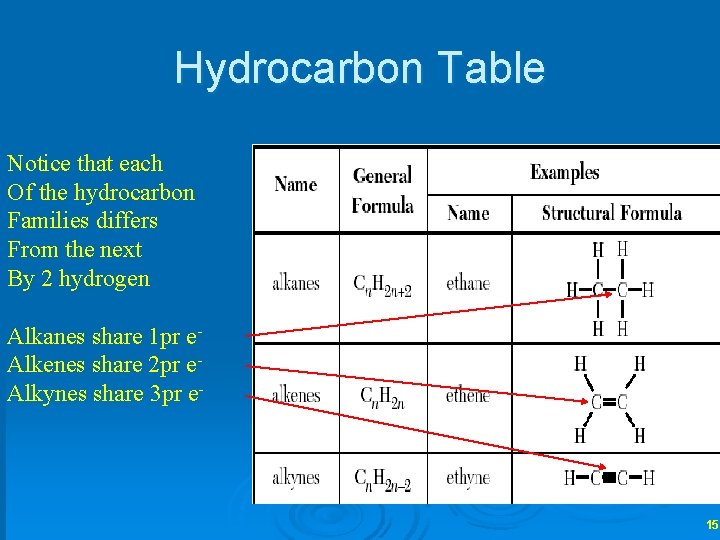
Hydrocarbon Table Notice that each Of the hydrocarbon Families differs From the next By 2 hydrogen Alkanes share 1 pr e. Alkenes share 2 pr e. Alkynes share 3 pr e- 15

Naming Alkynes Ø Use the corresponding name from the alkane series and change the –ane to –yne. Ø If necessary, number the carbon atom at which the triple bond occurs with the lowest number. Ø Use the same naming process you used for naming Alkenes 16

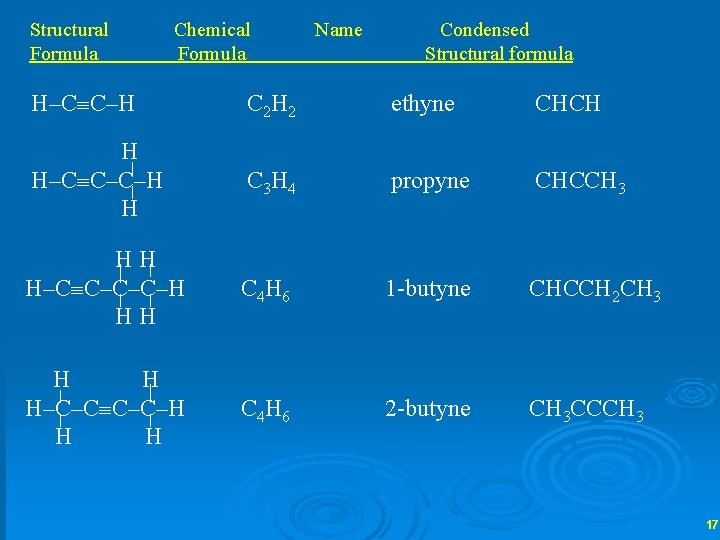
Structural Formula Chemical Formula Name Condensed Structural formula H–C C–H C 2 H 2 ethyne CHCH H H–C C–C–H H C 3 H 4 propyne CHCCH 3 HH H–C C–C–C–H HH C 4 H 6 1 -butyne CHCCH 2 CH 3 H H H–C–C C–C–H H H C 4 H 6 2 -butyne CH 3 CCCH 3 17

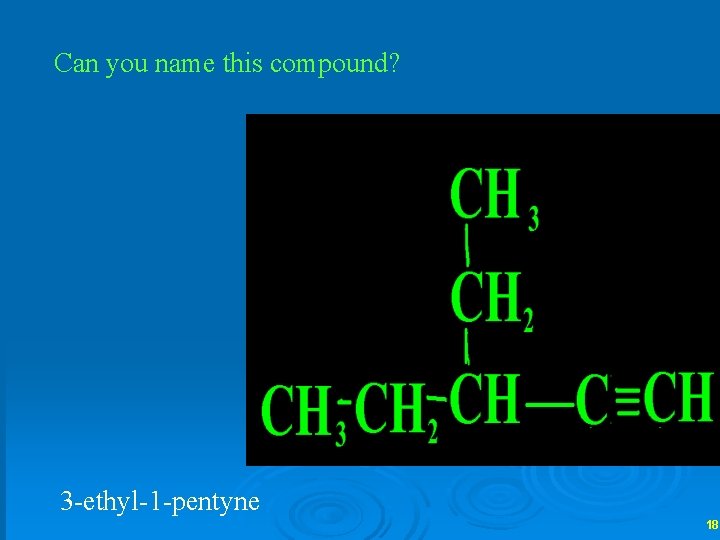
Can you name this compound? 3 -ethyl-1 -pentyne 18

Problems Name this alkyne: C C-C-C=C-C-C C C C 5, 7 -diethyl-3 -methyl-4 -decyne 19
 Alkanes alkenes alkynes
Alkanes alkenes alkynes Nysedregents
Nysedregents Mole conversion chart
Mole conversion chart Nysedregents
Nysedregents June 2010 chemistry regents answers
June 2010 chemistry regents answers Regents chemistry midterm
Regents chemistry midterm Regents
Regents January 2018 chemistry regents answers
January 2018 chemistry regents answers Chemistry regents bonding questions
Chemistry regents bonding questions Chemistry regents 2011
Chemistry regents 2011 Alkynes
Alkynes Halogenation of alkynes
Halogenation of alkynes Filter plates for aqueous filtration
Filter plates for aqueous filtration Hybridization of alkynes
Hybridization of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Alkynes
Alkynes Triple bond nomenclature
Triple bond nomenclature Combustion reaction of alkanes
Combustion reaction of alkanes Alkynes structural formula
Alkynes structural formula Chemical reactions of alkynes
Chemical reactions of alkynes