Chemistry Midterm Review Regents Chemistry Sig Figs Round









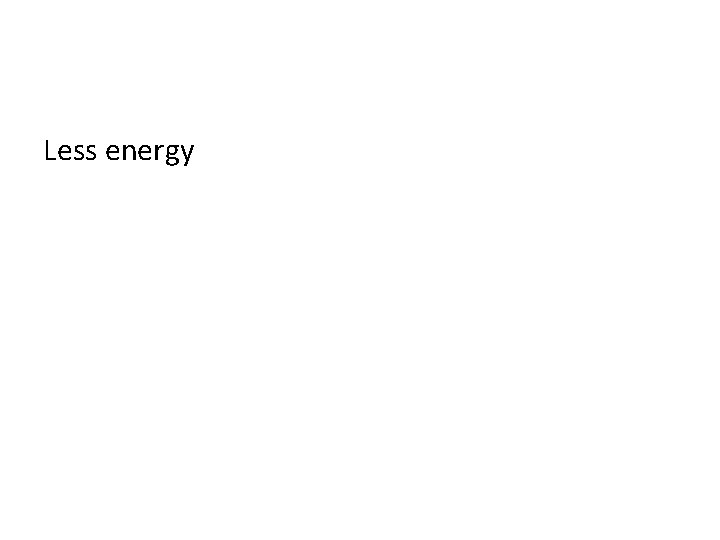

















































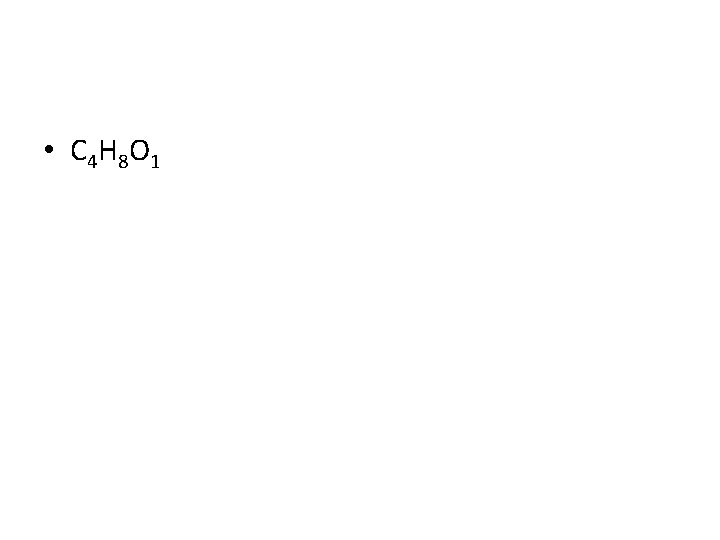



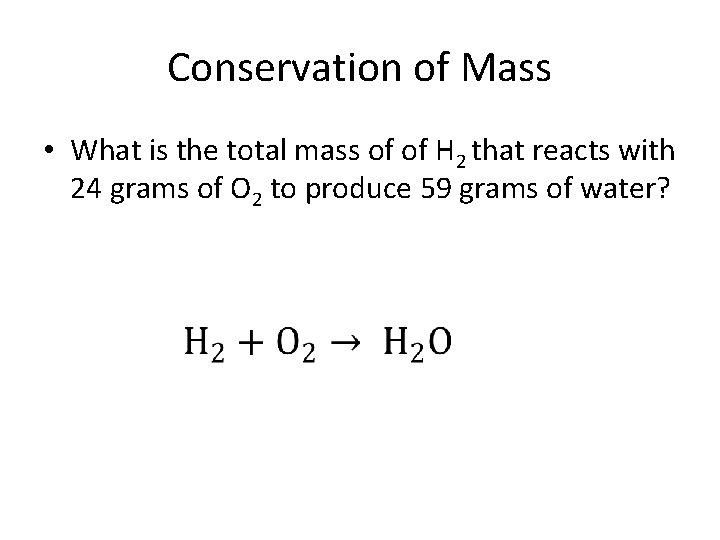

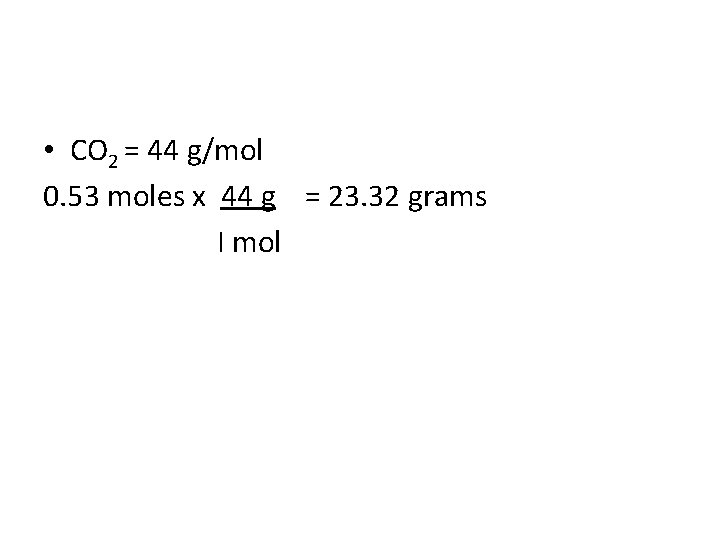

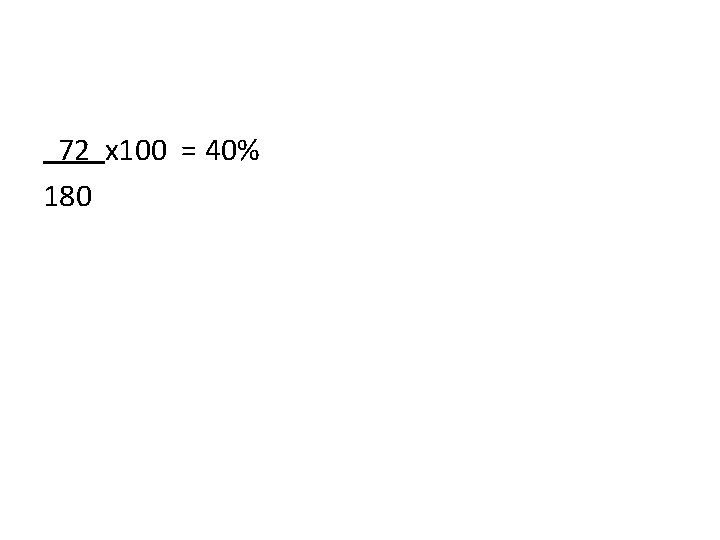
- Slides: 100

Chemistry Midterm Review Regents Chemistry

Sig Figs Round the following number to 3 sig figs: 0. 0067894

0. 00679

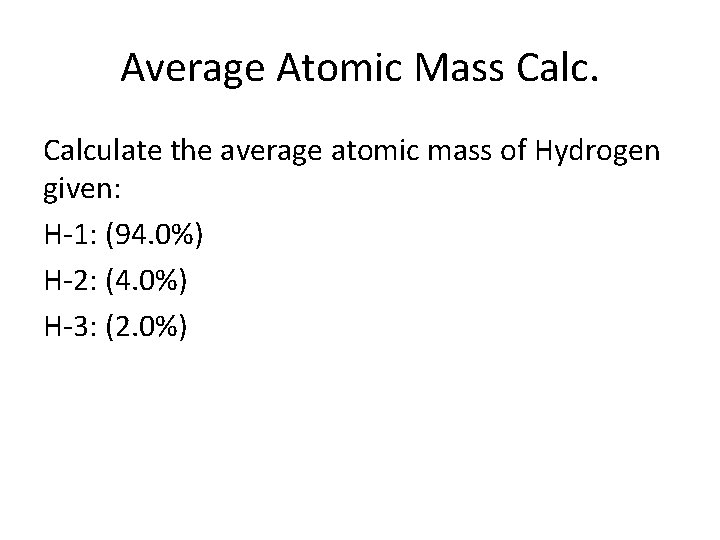
Average Atomic Mass Calculate the average atomic mass of Hydrogen given: H-1: (94. 0%) H-2: (4. 0%) H-3: (2. 0%)

(1)(. 94) + (2)(. 04) + (3)(. 02) = 1. 08 amu

Rutherford’s Gold Foil • What two conclusions did Rutherford make about the structure of an atom from his gold foil experiment?

1. Dense, positively charged nucleus 2. Atom is made mostly of empty space

Excited Electron Config. Write an excited electron configuration for an atom of chlorine.

2 -7 -8 OR 1 -8 -8 OR 2 -8 -6 -1

Energy of Electrons found in the first shell (compared to electrons found in the last shell) have _________ (more/less) energy?
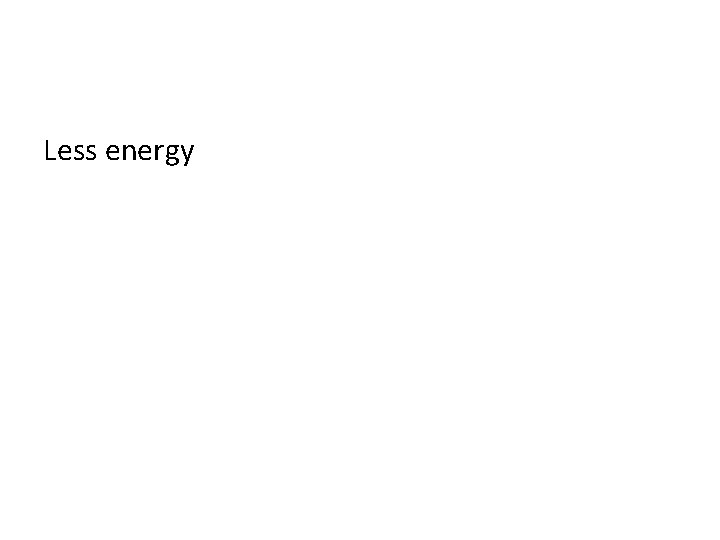
Less energy

Mass of Subatomic Particles • The mass of a proton and neutron is…….

1 amu

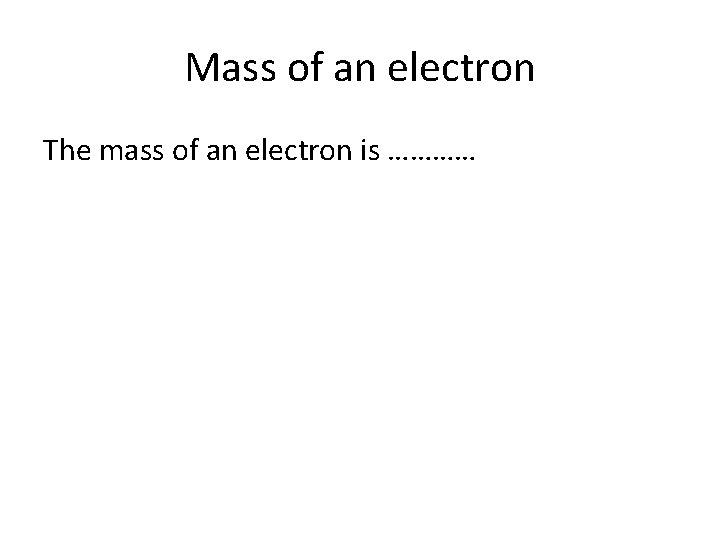
Mass of an electron The mass of an electron is …………

0 amu

Charge of subatomic particles What are the charges of a proton, neutron and electron?

Proton = +1 Neutron = 0 Electron = -1

Finding # of p+, e- and n 0 (neutral atom) • How many protons, neutrons and electrons are there in a neutral atom of potassium?

Protons = 19 Neutrons = 20 (39 amu- 19 = 20) Electrons = 19

Finding # of p+, e- and n 0 (ion) How many protons, neutrons and electrons are there in an ion of bromine?

Protons = 35 Neutrons = 45 (80 - 35 = 45) Electrons = 36

Finding # of p+, e- and n 0 (isotope) How many protons, neutrons and electrons are in an isotope of carbon-14?

Protons = 6 Neutrons = 8 (14 -6 = 8) Electrons = 6

Occupied Principal Energy Levels If the electron configuration of an atom is 2 -8 -3, how many occupied principal energy levels are there?

3

Energy of Excited Electrons When an excited electron returns back down to ground state, energy is _________.

Released

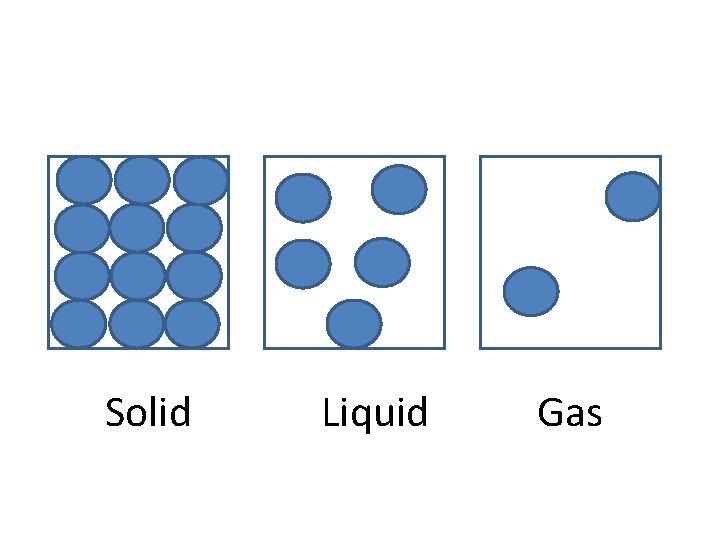
Particle Diagrams Draw a particle diagram for a solid, liquid and gas.

Solid Liquid Gas

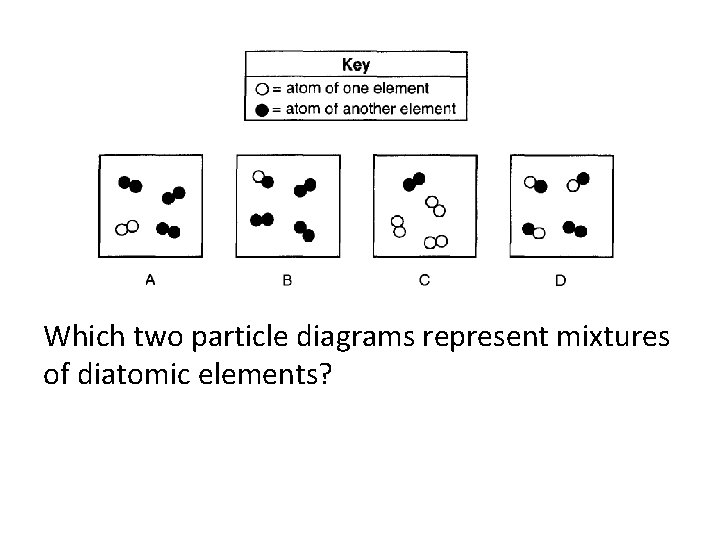
Which two particle diagrams represent mixtures of diatomic elements?
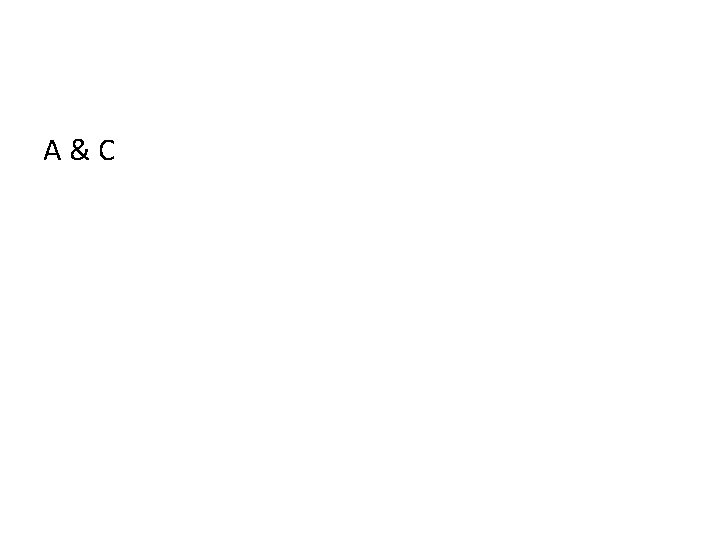
A&C

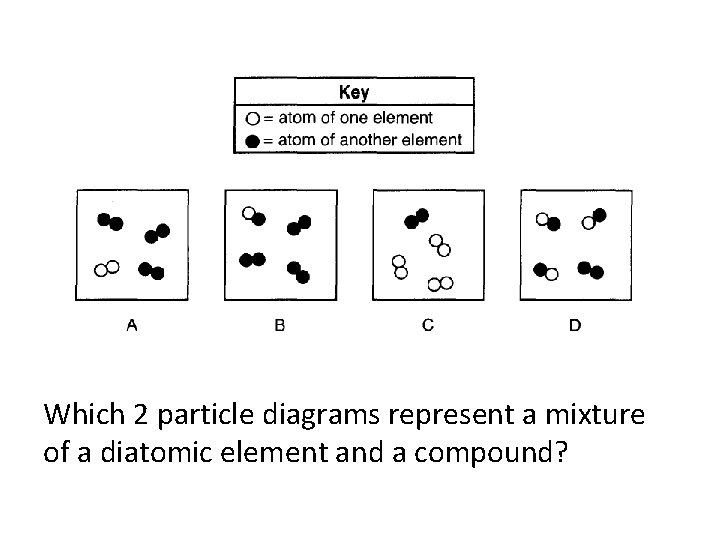
Which 2 particle diagrams represent a mixture of a diatomic element and a compound?

B&D

Mixtures Which of the following represents a mixture? (1) Li. Cl (s) (2) Li. Cl (g) (3) Li. Cl (l) (4) Li. Cl (aq)

Li. Cl (aq)

Distillation A mixture is separated by distillation. What is distillation?

When a mixture is separated by difference in boiling points.

Metalloid Which element has both properties of metals and non metals? (1) Germanium (2) Sodium (3) Carbon (4) Aluminum

Germanium

Name That Category • • The following properties describe what? Good conductor of heat & electricity Malleable Ductile

• Metals

Name That Category • These group forms colored ions in solution…

• Transition Metals

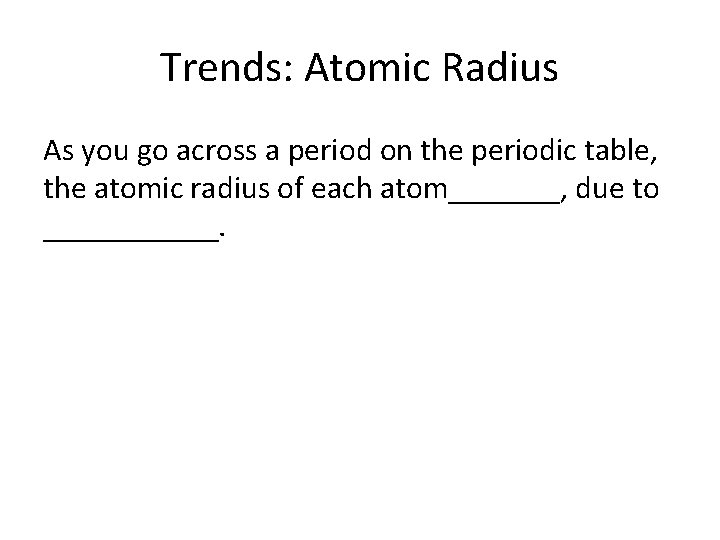
Trends: Atomic Radius As you go across a period on the periodic table, the atomic radius of each atom_______, due to ______.

Decreases, due to increased nuclear charge

Trends: Atomic Radius

As you go down a group on the periodic table, the atomic radius of each atom _______, due to _____.

Increases, increases of shells/orbitals

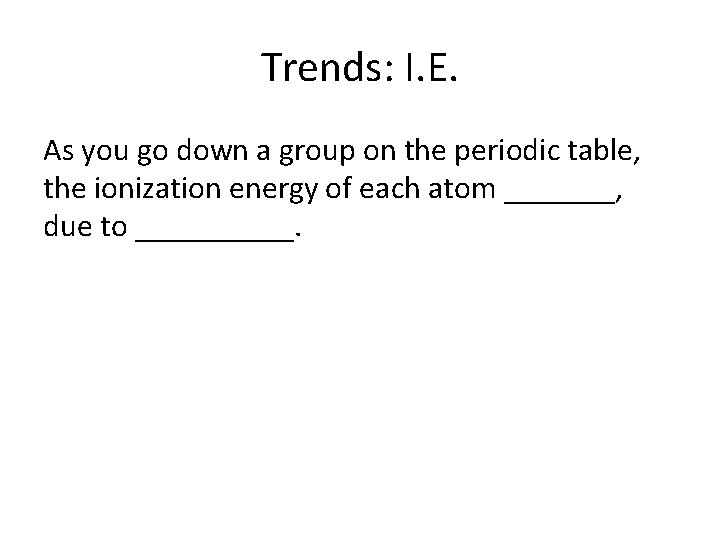
Trends: I. E. As you go down a group on the periodic table, the ionization energy of each atom _______, due to _____.

Decreases, increased shielding effect

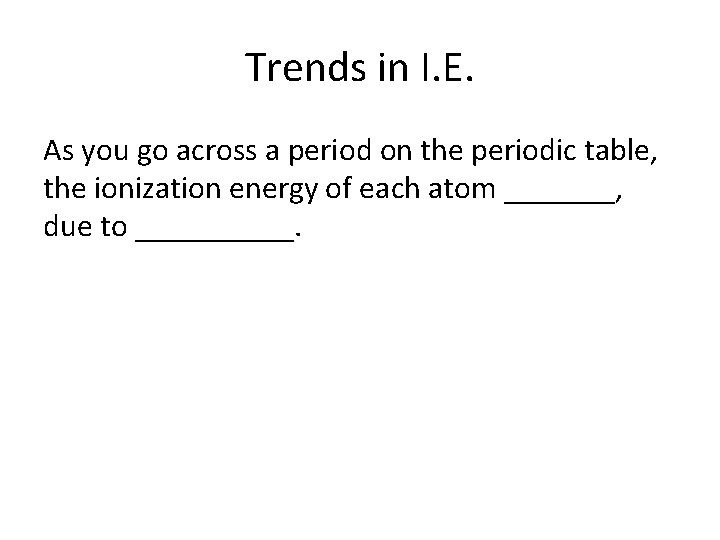
Trends in I. E. As you go across a period on the periodic table, the ionization energy of each atom _______, due to _____.

Increases, increased nuclear charge

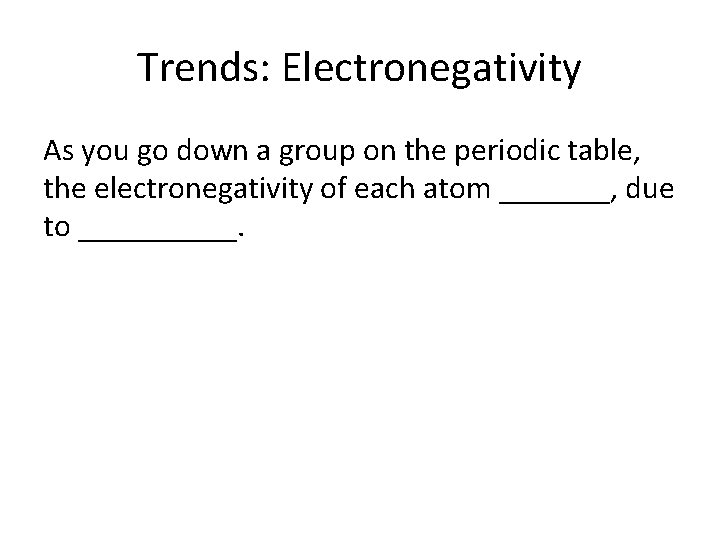
Trends: Electronegativity As you go down a group on the periodic table, the electronegativity of each atom _______, due to _____.

Decreases (think in relation to F!)

Trends: Electronegativity As you go across a period on the periodic table, the atomic radius of each atom _______, due to _____.

Increases (in relation to F!)

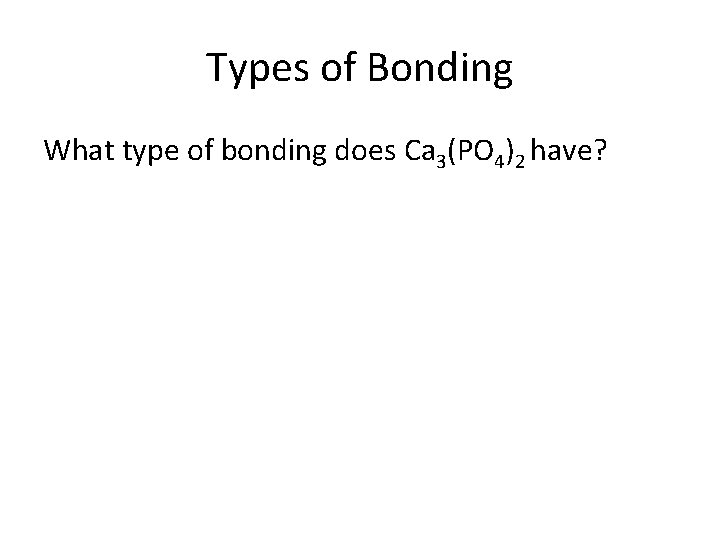
Types of Bonding What type of bonding does Ca 3(PO 4)2 have?

• Both ionic and covalent

Types of Bonding What type of bonding does Strontium have?

• Metallic

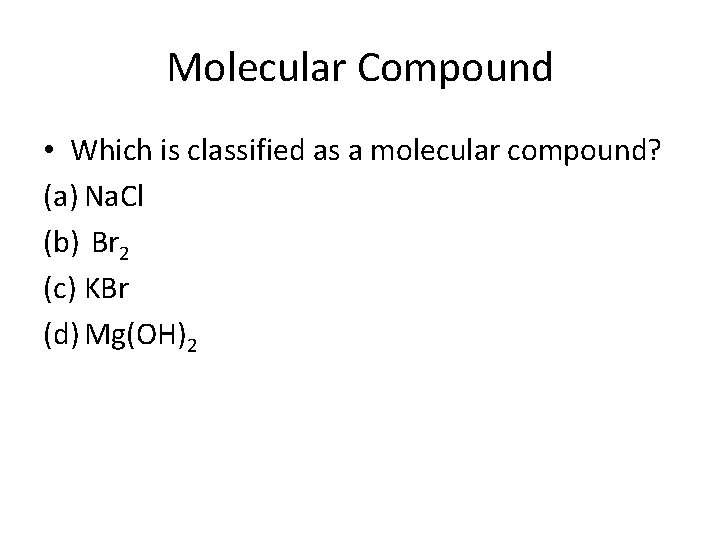
Molecular Compound • Which is classified as a molecular compound? (a) Na. Cl (b) Br 2 (c) KBr (d) Mg(OH)2


Name That Category • What kind of compound has the following properties: • Good conductor of electricity in aqueous solutions • Crystalline structure • High melting point

• Ionic Compounds

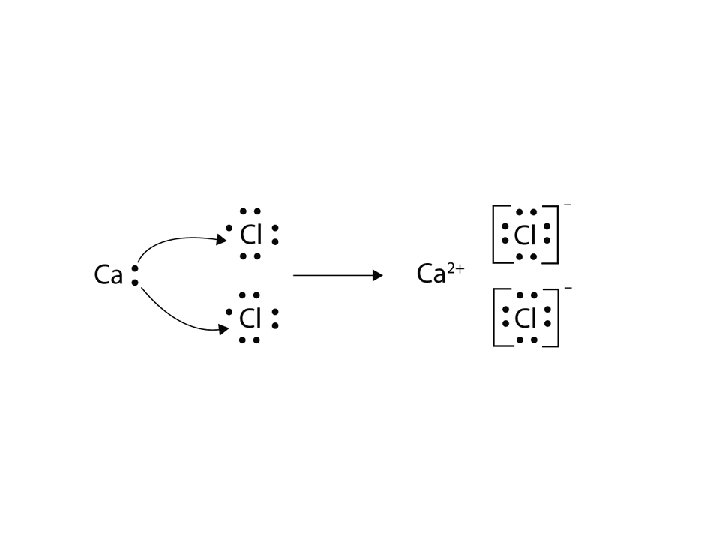
Draw a Dot Diagram • Draw an electron dot diagram for Ca. Cl 2


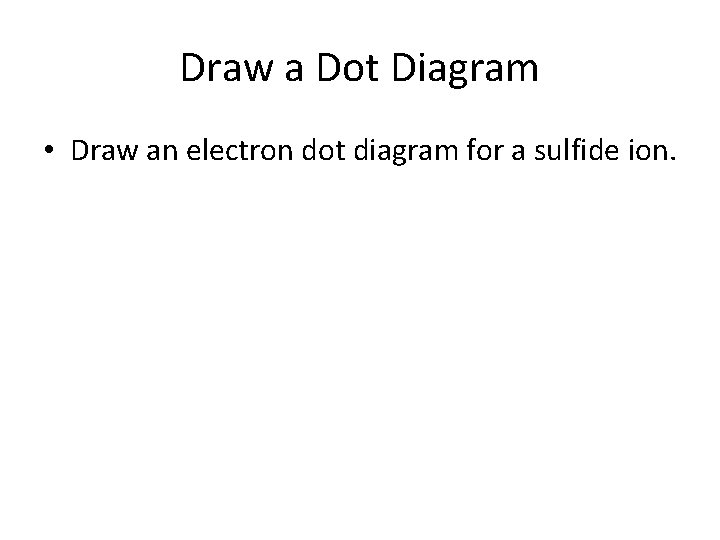
Draw a Dot Diagram • Draw an electron dot diagram for a sulfide ion.


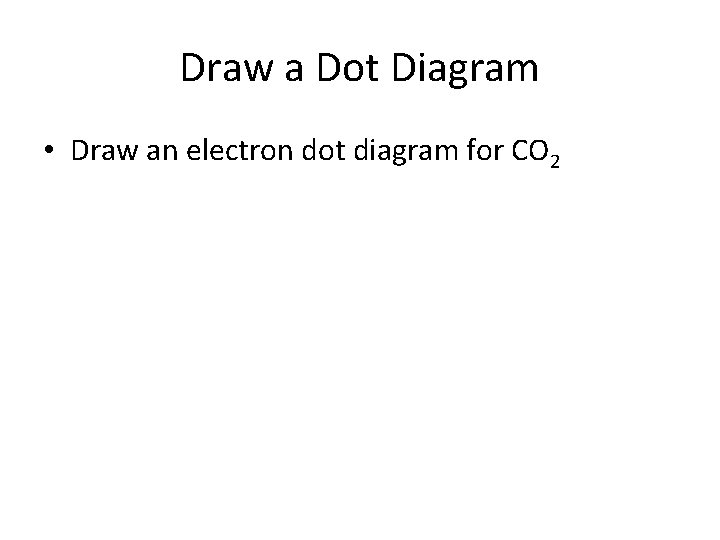
Draw a Dot Diagram • Draw an electron dot diagram for CO 2


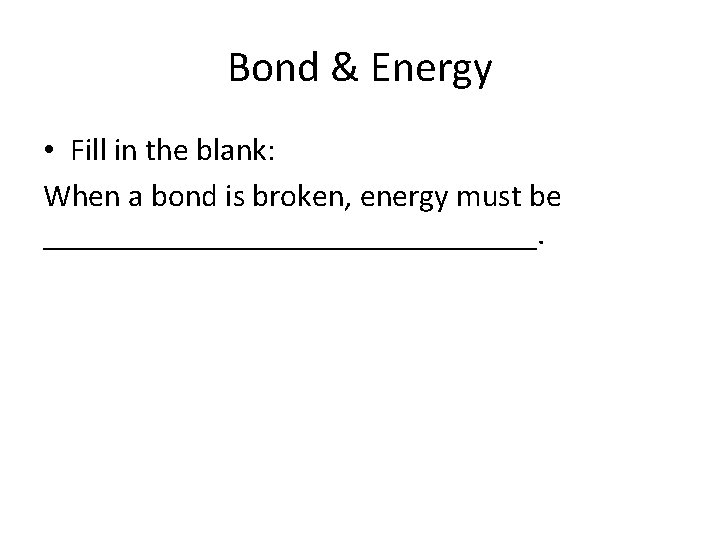
Bond & Energy • Fill in the blank: When a bond is broken, energy must be ________________.

• Absorbed (BARF)

IMF’s A Van der Waals force in responsible of the different phases of matter in group 17. Fill in the blank: When size decreases, Van der Waal force ___________.

• Decreases (think of your gases at the top of the group).

Bond Polarity • Does H 2 O have polar or nonpolar bonds? • Why?

• Polar bonds because the EN difference between H and O is greater than 0. 4.

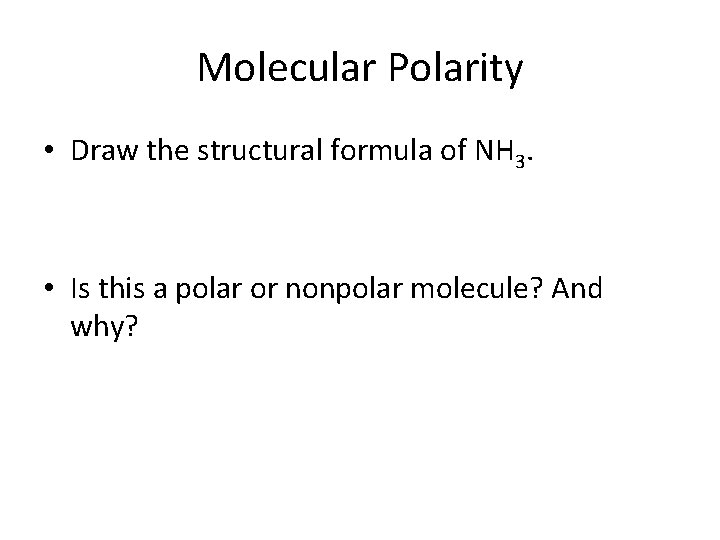
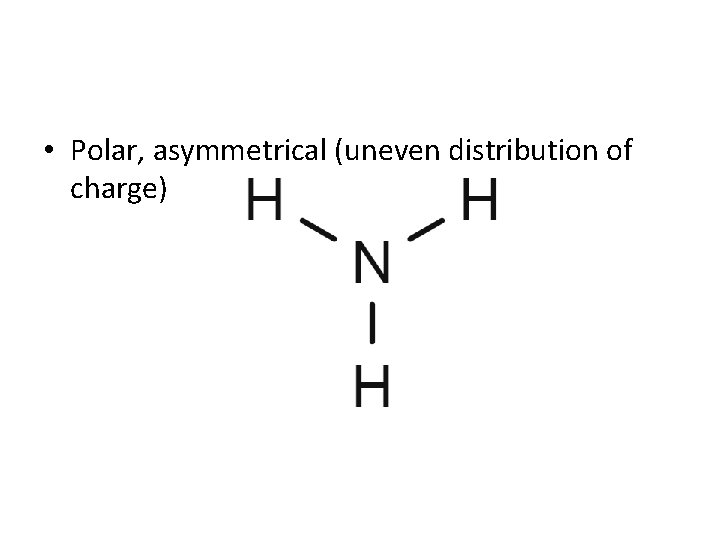
Molecular Polarity • Draw the structural formula of NH 3. • Is this a polar or nonpolar molecule? And why?

• Polar, asymmetrical (uneven distribution of charge)

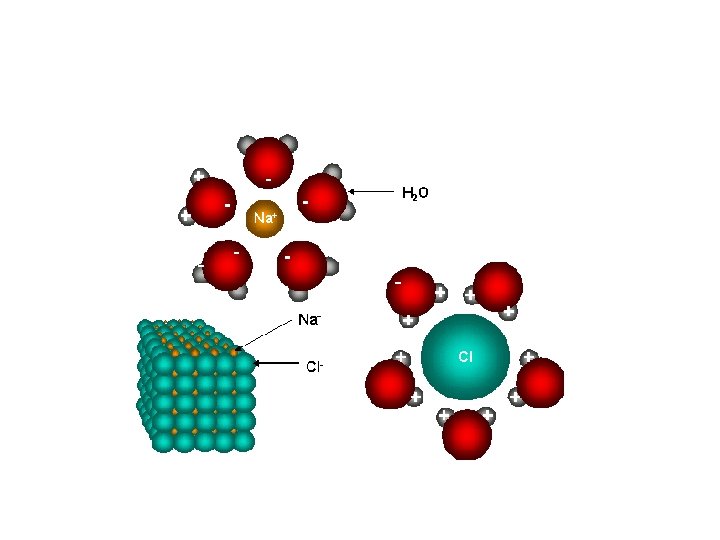
Draw a Picture • Draw a picture of a molecule ion attraction between Na. Cl and H 2 O.


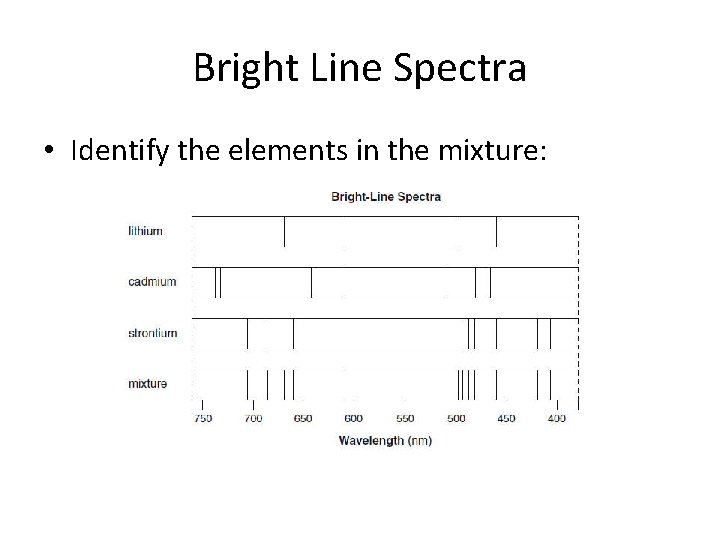
Bright Line Spectra • Identify the elements in the mixture:

• Strontium & Lithium

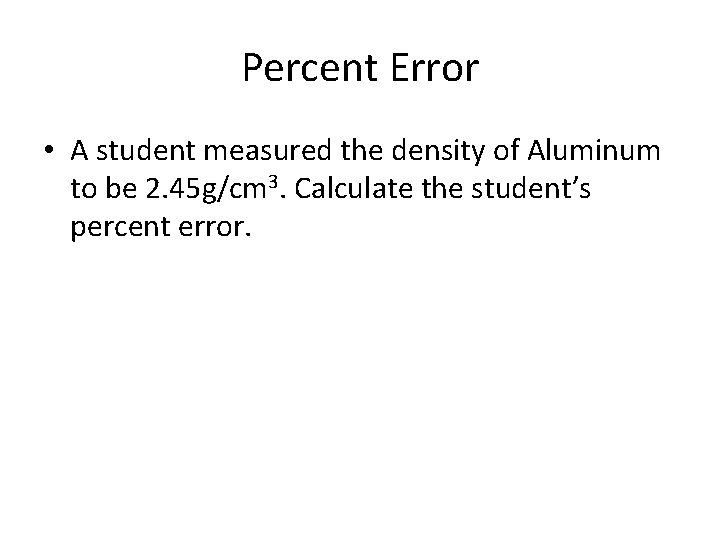
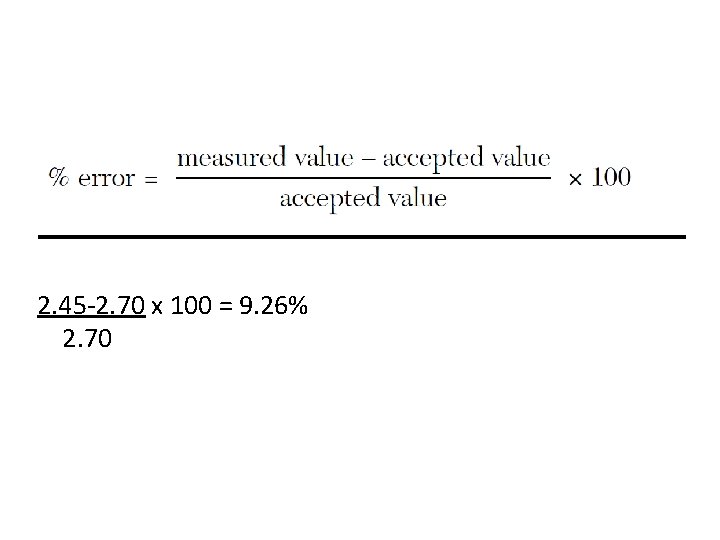
Percent Error • A student measured the density of Aluminum to be 2. 45 g/cm 3. Calculate the student’s percent error.

2. 45 -2. 70 x 100 = 9. 26% 2. 70

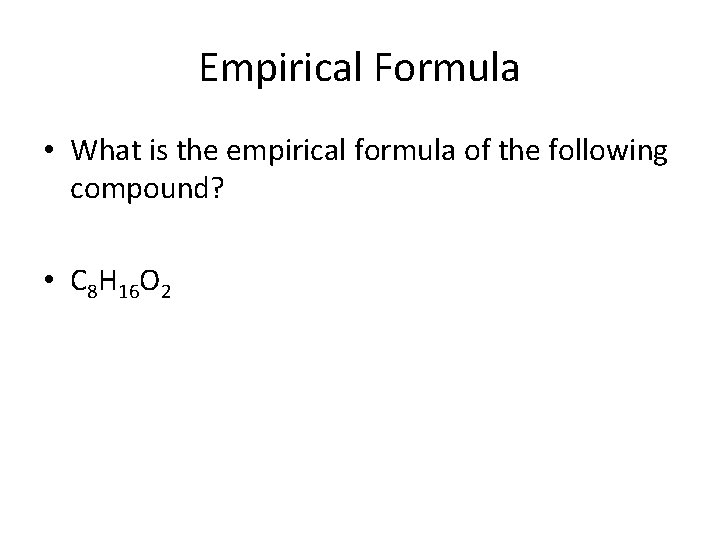
Empirical Formula • What is the empirical formula of the following compound? • C 8 H 16 O 2
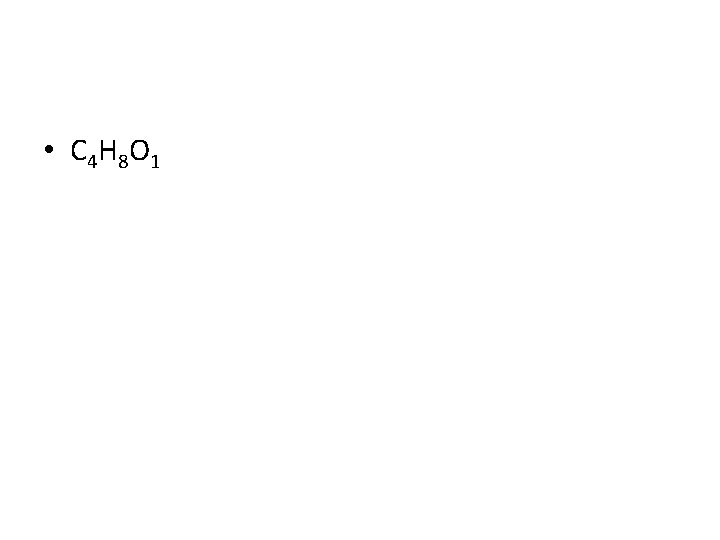
• C 4 H 8 O 1

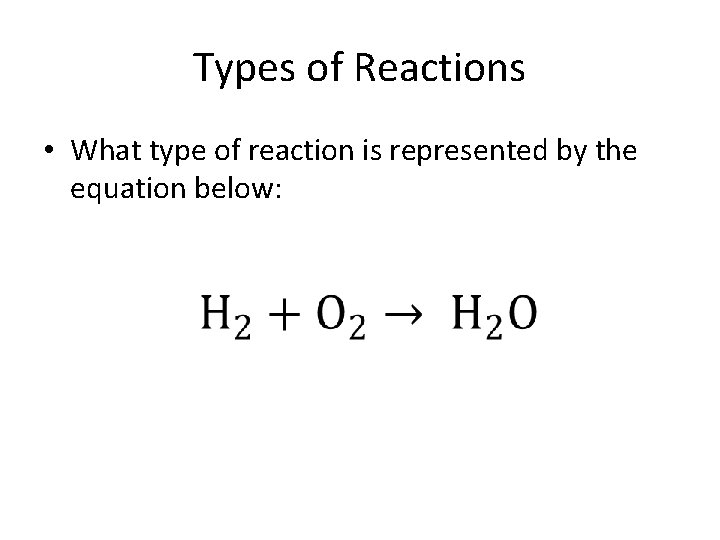
Types of Reactions • What type of reaction is represented by the equation below:

• Synthesis

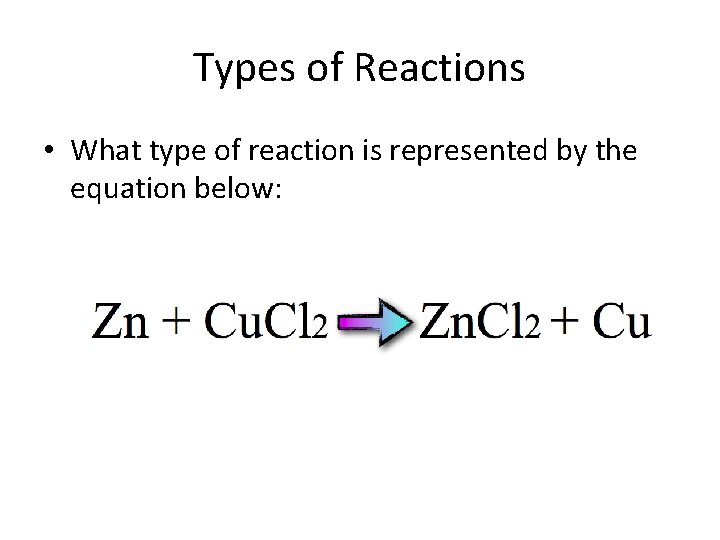
Types of Reactions • What type of reaction is represented by the equation below:

• Single Replacement

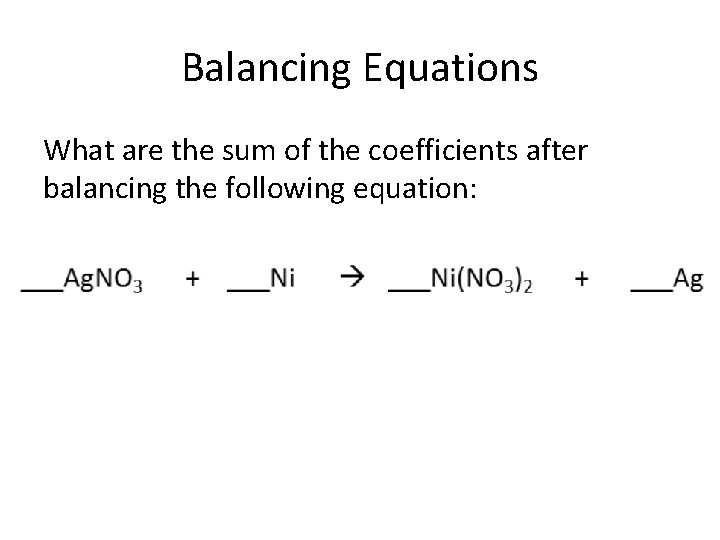
Balancing Equations What are the sum of the coefficients after balancing the following equation:

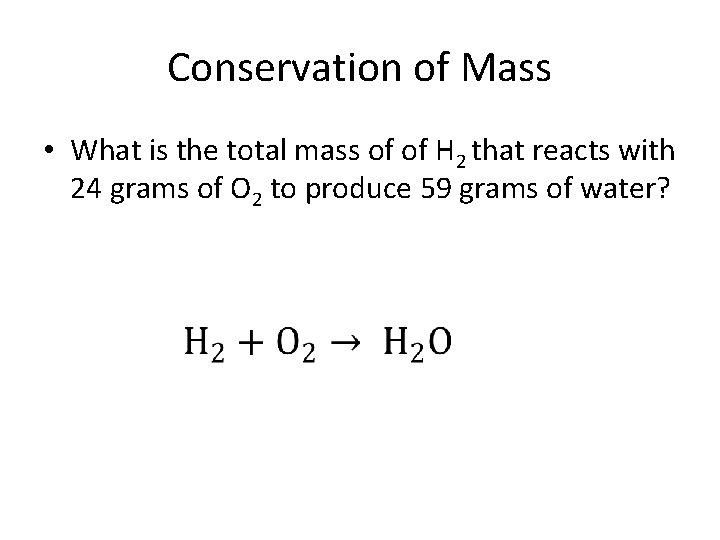
Conservation of Mass • What is the total mass of of H 2 that reacts with 24 grams of O 2 to produce 59 grams of water?

• 35 grams

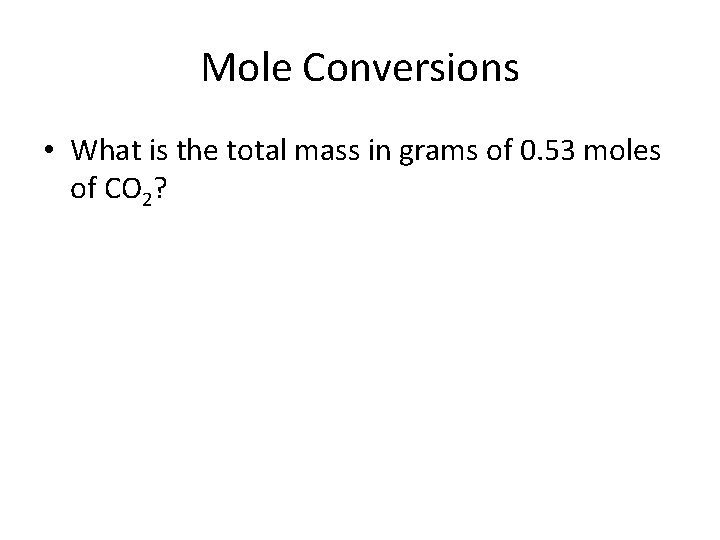
Mole Conversions • What is the total mass in grams of 0. 53 moles of CO 2?
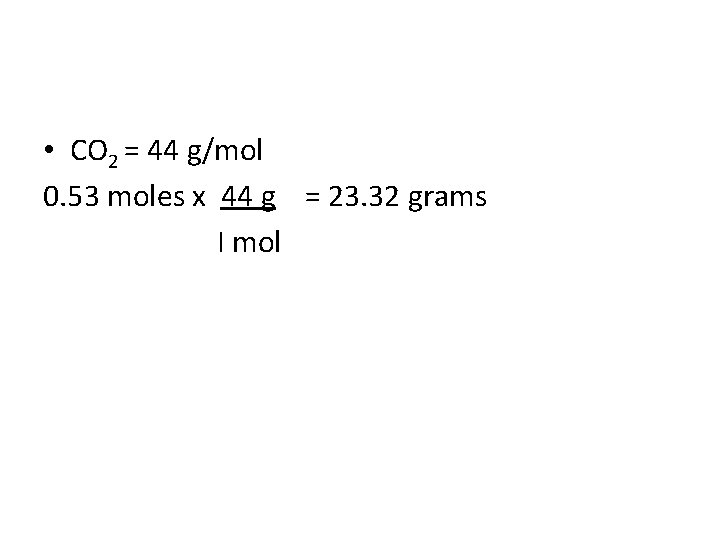
• CO 2 = 44 g/mol 0. 53 moles x 44 g = 23. 32 grams I mol

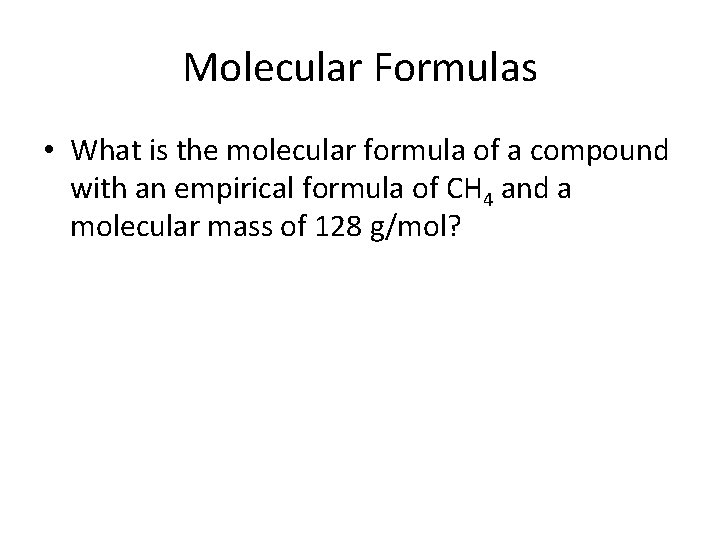
Molecular Formulas • What is the molecular formula of a compound with an empirical formula of CH 4 and a molecular mass of 128 g/mol?

• CH 4 = 16 g/mol • 128 = 8 16 C 8 H 32

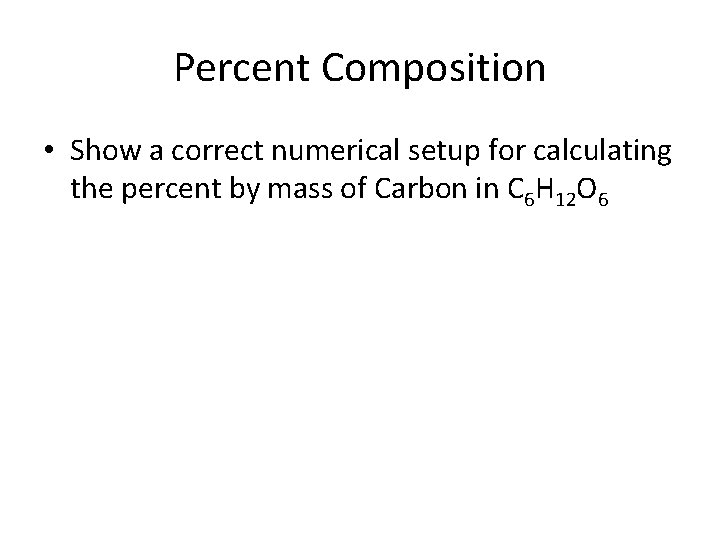
Percent Composition • Show a correct numerical setup for calculating the percent by mass of Carbon in C 6 H 12 O 6
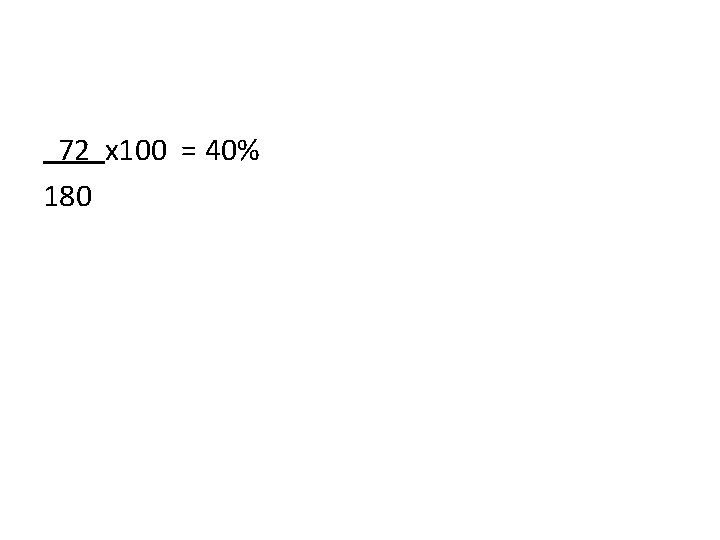
72 x 100 = 40% 180
 Significant figures rules with examples
Significant figures rules with examples How to round sig figs when multiplying
How to round sig figs when multiplying How to round sig figs when multiplying
How to round sig figs when multiplying Regents chemistry midterm
Regents chemistry midterm What is a significant figure in chemistry
What is a significant figure in chemistry What counts as a significant figure
What counts as a significant figure When to use sig figs in chemistry
When to use sig figs in chemistry Buret sig figs
Buret sig figs Significant figures rules addition
Significant figures rules addition Significant figures in measurements
Significant figures in measurements Sig fig rules for antilog
Sig fig rules for antilog How many significant figures are in the number 176 cm?
How many significant figures are in the number 176 cm? Half reaction at anode
Half reaction at anode Addition of significant figures
Addition of significant figures Percent error
Percent error Log sig fig
Log sig fig Hf acid or base
Hf acid or base Standard deviation chemistry
Standard deviation chemistry What are sig
What are sig Sig figs game
Sig figs game How many sig figs in 320
How many sig figs in 320 Sig up
Sig up How many sig figs in 5200
How many sig figs in 5200 How many sig figs are in 7080
How many sig figs are in 7080 Atlantic pacific rule sig figs
Atlantic pacific rule sig figs How many sig figs are in .000077?
How many sig figs are in .000077? Rules for counting significant figures
Rules for counting significant figures How many sig figs in 1250
How many sig figs in 1250 How many sig figs are in 5000
How many sig figs are in 5000 Where do sig figs come from
Where do sig figs come from Are trailing zeros significant
Are trailing zeros significant How to count significant figures
How to count significant figures Sig figs division
Sig figs division How to tell significant figures
How to tell significant figures Sig fig rule
Sig fig rule What is 3 significant figures
What is 3 significant figures A24mx
A24mx Stoichiometry sig figs
Stoichiometry sig figs Sig figs pacific atlantic rule
Sig figs pacific atlantic rule Chemistry regents bonding questions
Chemistry regents bonding questions Which substance can be decomposed chemically
Which substance can be decomposed chemically Ap chemistry midterm review
Ap chemistry midterm review Us history regents review
Us history regents review Earth science regents lab practical review
Earth science regents lab practical review American history regents review
American history regents review Michael britt brain mnemonics
Michael britt brain mnemonics Algebra 1 midterm exam
Algebra 1 midterm exam Whap midterm review
Whap midterm review Marketing midterm review
Marketing midterm review Global 9 midterm review
Global 9 midterm review Trig midterm review
Trig midterm review Business law midterm review
Business law midterm review Apes midterm exam
Apes midterm exam Algebra 2 midterm exam answers
Algebra 2 midterm exam answers Spanish 2 midterm practice test
Spanish 2 midterm practice test English 10 midterm exam answers
English 10 midterm exam answers World history semester 2 final exam review
World history semester 2 final exam review Biology midterm review
Biology midterm review Bisexts
Bisexts Solutions chemistry regents questions
Solutions chemistry regents questions Chemistry regents 2011
Chemistry regents 2011 Chemistry regents conversion chart
Chemistry regents conversion chart June 2010 chemistry regents
June 2010 chemistry regents January 2006 chemistry regents answers
January 2006 chemistry regents answers January 2012 chemistry regents
January 2012 chemistry regents January 2012 chemistry regents answers
January 2012 chemistry regents answers December round the world
December round the world 3 little figs
3 little figs Grapes and figs
Grapes and figs Figs compression socks
Figs compression socks Which map shows the most probable areas of precipitation
Which map shows the most probable areas of precipitation Periodic table of elements regents
Periodic table of elements regents Tristan denley louisiana
Tristan denley louisiana Beaks of finches lab answers
Beaks of finches lab answers Ohm's law worksheet regents physics answer key
Ohm's law worksheet regents physics answer key Regents physics work power energy
Regents physics work power energy June 2007 physics regents
June 2007 physics regents Ela common core regents
Ela common core regents Louisiana board of regents
Louisiana board of regents Part d earth science regents
Part d earth science regents Earth science lab practical
Earth science lab practical Regents policy 5402
Regents policy 5402 What were two indirect results of the crusades
What were two indirect results of the crusades The justinian code is considered a milestone because it
The justinian code is considered a milestone because it June 2018 geometry regents answers
June 2018 geometry regents answers Decolonization regents questions
Decolonization regents questions Crq global regents
Crq global regents Energy flow trophic levels
Energy flow trophic levels 282 ways to pass the earth science regents
282 ways to pass the earth science regents Thematic essay human rights
Thematic essay human rights Regents physics work power energy
Regents physics work power energy Projectile motion regents questions
Projectile motion regents questions Table g chemistry
Table g chemistry United states history and government regents
United states history and government regents Enduring issues posters
Enduring issues posters The diagram below represents a 155 newton box on a ramp
The diagram below represents a 155 newton box on a ramp Characteristics of life regents questions
Characteristics of life regents questions Comprehensive english regents
Comprehensive english regents La board of regents
La board of regents Skyward regents
Skyward regents Global 9 regents
Global 9 regents