Regents Chemistry Properties of Solutions Properties of Solutions















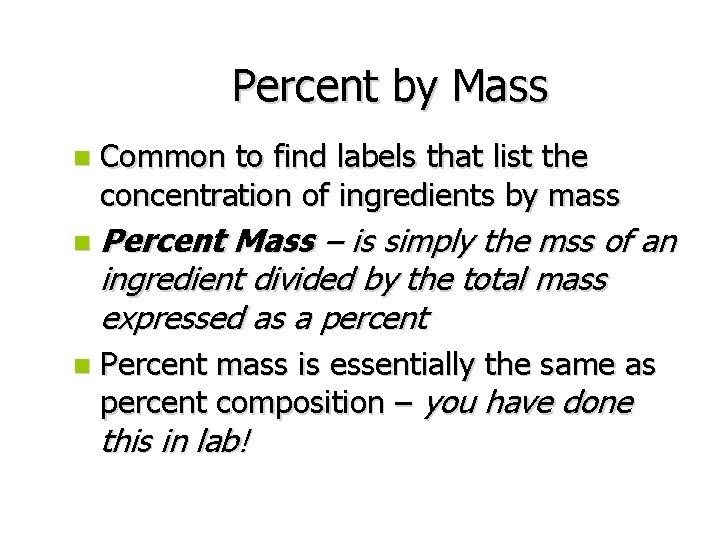












- Slides: 45

Regents Chemistry • Properties of Solutions

Properties of Solutions • Review - What’s a solution – a solution is a homogeneous mixture of substance in the same physical state • Most chemical reactions take place in solutions • We will learn the nature and properties of solutions and ways to express the concentration of solutions

What do Solutions Contain? • Solutions contain atoms, ions or molecules in which one substance spread uniformly throughout a second substance • Ex: Salt water

Types of Solutions • Solutions exist in all three states! • A solid may be dissolved in another solid – ex: Brass is a mixture if zinc and copper • A Metal solution is called an alloy • Air is a gaseous solution and can vary depending on the conditions – ex: amount of water vapor varies daily

Liquid Solutions • We will mostly focus on solutions containing a liquid • We identify parts of a liquid solution by how it is made • Solute - is the substance that is being dissolved, and it is the substance present in the smaller amount • Solvent - substance that dissolves the solute - most common is water

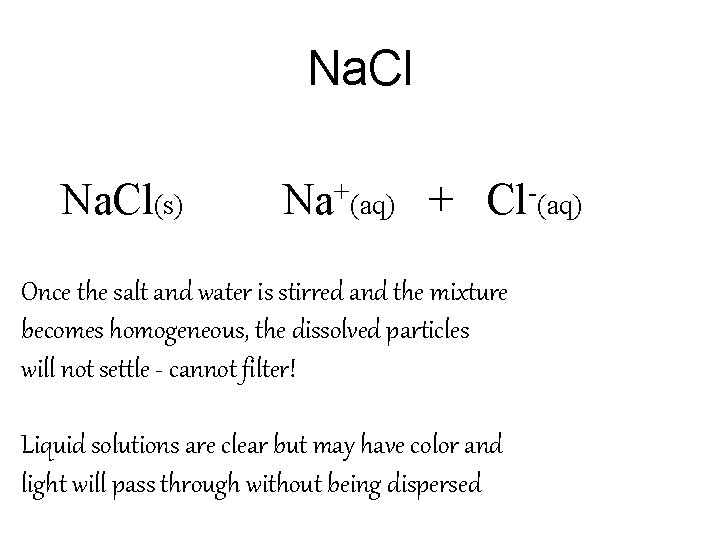
Na. Cl(s) Na+(aq) + Cl-(aq) Once the salt and water is stirred and the mixture becomes homogeneous, the dissolved particles will not settle - cannot filter! Liquid solutions are clear but may have color and light will pass through without being dispersed

Liquid Solution Summary • 1. Solutions are homogeneous mixtures • 2. Solutions are clear and do not disperse light • 3. Solutions can have a color • 4. Solutions will pass through a filter

Solubility Factors • Some things dissolve in solvents and some don’t, so. . • Solubility - is how much of a solute will dissolve in a certain amount of solvent at a certain temperature • Materials with high solubility are said to be soluble • Materials with a low solubility are said to be insoluble

Nature of Solute and Solvent • Na. Cl dissolves in water because its positively and negatively charged ions are attracted to oppositely charged ends of the polar water molecule • The attractive forces between the water molecules and sodium ions are greater than the attractive forces between the sodium and chloride ions • Same goes for the chloride ions and positive end of water molecule

Like dissolves Like • Ionic substance dissolve in ionic solvents • Nonpolar substances, such as fats, dissolve in nonpolar solvents • So fats do not dissolve in water! No strong attractive forces between water molecules and fat molecules - must be dissolved in a nonpolar solvent • Why. . because the forces are weak and they simply mix together

Table summary

Effect of Temperature • As temperature increases, most solids become more soluble in water • A few exceptions exist: – Gases react in the opposite manner – As temperature increases, the solubility of all gases in liquids decreases

Effect of Pressure • Pressure has little or no effect on the solubility of solid or liquid solutes • Pressure does affect the solubility of gases in liquids • As pressure increases, the solubility of gases in liquids increases • Ex: opening a can of soda - the pressure decreases – CO 2 is no longer as soluble at the lowered pressure and escapes as bubbles

Regents Chemistry • Solubility Graphs and saturated and unsaturated solutions

Solubility Information • Solubility information may be presented in different ways • Table G in your Reference Tables shows the relationship between grams of solute that can be dissolved at various temperatures • Table F in Reference Tables provides some general guidelines about the solubility of ionic substances

Using Table G • Shows the maximum number of grams that can be dissolved in 100 g H 2 O at specific temperatures • Most show increasing solubility as temp increases, but a few don’t – these are gaseous NH 3, HCl and SO 2 – gases decrease in solubility as temp increases

Using Table G • Any point that is below the curve of a substance is considered unsaturated • Any point that is on the curve of a substance is considered saturated • Any point that is above the curve of a substance is considered supersaturated

Saturation • Unsaturated solutions hold less solute than maximum and no solid should be present • Saturated solutions hold the max amount and any additional will simply stay as a solid • Supersaturated solutions occur when the temperature is reduced but no crystals (solid) form out of solution - any additional solute added will cause crystals to form and solution will return to saturated state

Recognizing Degree of Saturation • Because solutions are clear, it is difficult to simply look at a solution and determine whether it is saturated, unsaturated or supersaturated – So how can we tell? • 1. We can compare the number of grams dissolved in a given volume to table G • 2. Add additional solute and see what happens!

Using Table F • Contains some guidelines for the solubility of common ionic compounds • YOU HAVE USED THIS TABLE BEFORE! • Explains if a reaction will form

Table G Practice Problem • Which substance on table G (solubility curve) is saturated with 20 g at 49 C? • How many grams of HCl would have to be added to a 70 g in solution to make it saturated at 10 C?

Regents Chemistry n Concentrations of Solutions – Molarity

What’s Molarity Let’s first review a mole…video clip n We sometimes refer to solutions as concentrated or dilute…but these are not scientifically precise terms. . n We need to know specific strengths to run reactions. . n – This is the purpose of molarity!

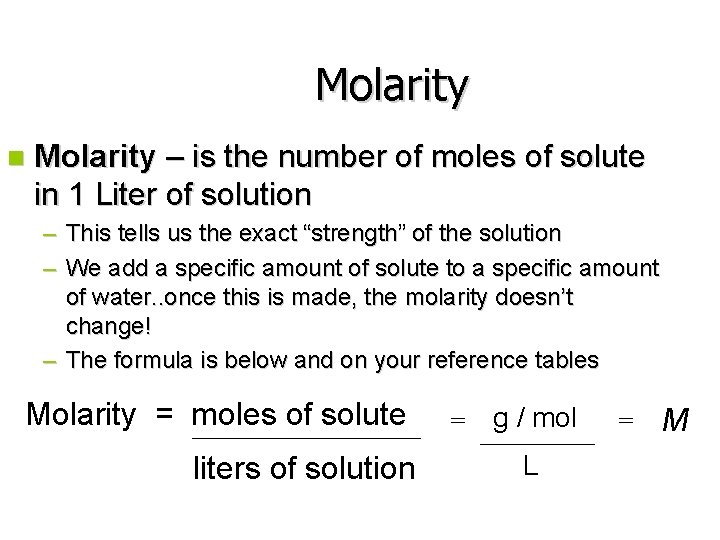
Molarity n Molarity – is the number of moles of solute in 1 Liter of solution – This tells us the exact “strength” of the solution – We add a specific amount of solute to a specific amount of water. . once this is made, the molarity doesn’t change! – The formula is below and on your reference tables Molarity = moles of solute liters of solution = g / mol L = M

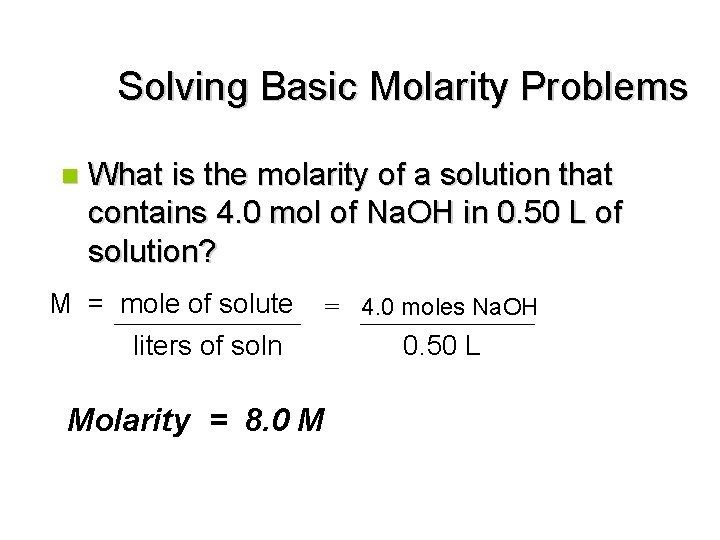
Solving Basic Molarity Problems n What is the molarity of a solution that contains 4. 0 mol of Na. OH in 0. 50 L of solution? M = mole of solute liters of soln Molarity = 8. 0 M = 4. 0 moles Na. OH 0. 50 L

Molarity w / no moles given… n What is we are given a gram amount instead of mole amount…can we still solve for molarity? – Yes! Practice Problem What is the molarity of a solution containing 82. 0 g of Ca(NO 3)3 in 2. 0 L of solution? 1. Convert 82. 0 grams to moles by using molar mass 2. Plug into Molarity equation and solve!

Additional Practice Problem n What is the molarity of a solution containing 26. 0 g KCl in 750 m. L of solution?

Rearranging the Equation We can rearrange the equation to solve for mole amount or liters of solution Example n How many moles of Ba. SO 4 are in a 2. 0 M solution originally made with 1. 5 L of solution? n

Regents Chemistry n % by mass, % by volume and ppm
Percent by Mass n Common to find labels that list the concentration of ingredients by mass n Percent Mass – is simply the mss of an ingredient divided by the total mass expressed as a percent n Percent mass is essentially the same as percent composition – you have done this in lab!

Percent by Mass Percent mass= mass of part X 100% mass of whole What is the percent mass of sodium hyd If 2. 50 g of Na. OH are added to 50. 00 g o

Percent by Volume When two liquids are mixed to form a solution, it is common to express the concentration of the solute as a percent by volume n For example, a solution of isopropyl alcohol contains 70% alcohol by volume n Percent by volume = Volume of solute X 100% Volume of solution

Practice Problem n What is the percent by volume of alcohol if 50. 0 m. L of ethanol is dilluted with water to form a total volume of 300 m. L?

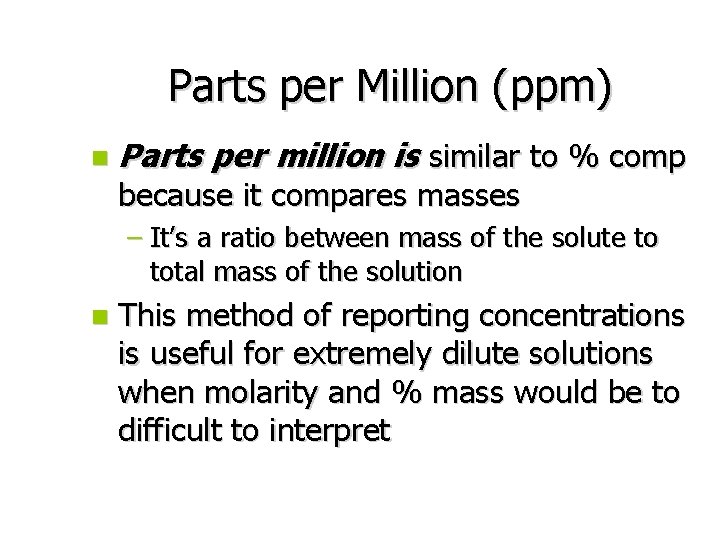
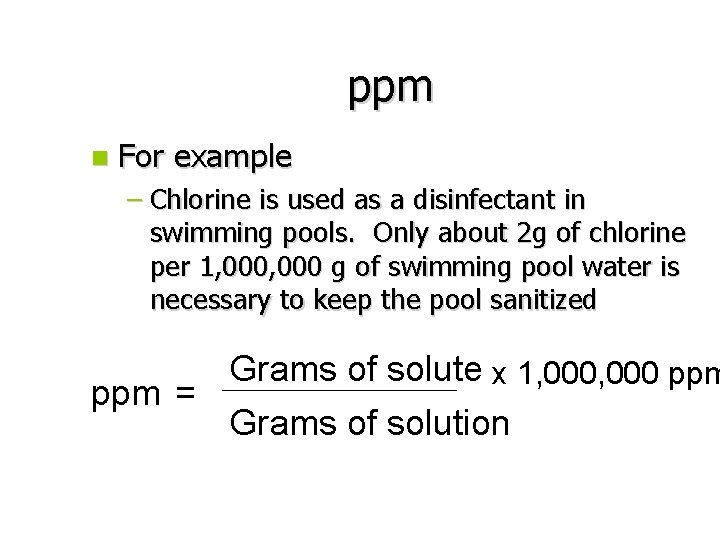
Parts per Million (ppm) n Parts per million is similar to % comp because it compares masses – It’s a ratio between mass of the solute to total mass of the solution n This method of reporting concentrations is useful for extremely dilute solutions when molarity and % mass would be to difficult to interpret

ppm n For example – Chlorine is used as a disinfectant in swimming pools. Only about 2 g of chlorine per 1, 000 g of swimming pool water is necessary to keep the pool sanitized Grams of solute x 1, 000 ppm = Grams of solution

Practice Problem Approximately 0. 0043 g of oxygen can be dissolved in 100 m. L of water at 20 degrees Celsius. Express this in terms of ppm n (assume 1 m. L water = 1. 0 g water n

Regents Chemistry • Colligative Properties

What are Colligative Properties? • Colligative properties are properties of a substance that are affected by the nature of a solute added to it • In terms of water: – Freezing and boiling points are colligative properties that are affected by the nature of the solute. . as we shall see…

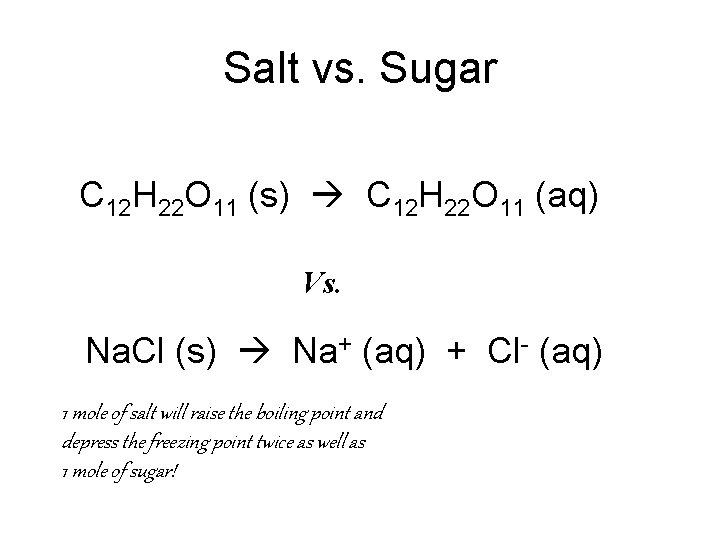
Molecular vs. Ionic • Molecular substances affect the freezing and boiling points of water different than ionic substances. . • Why? ? • Because ionic substance break apart into ions and molecules do not! – Ex: Salt vs. sugar

Salt vs. Sugar C 12 H 22 O 11 (s) C 12 H 22 O 11 (aq) Vs. Na. Cl (s) Na+ (aq) + Cl- (aq) 1 mole of salt will raise the boiling point and depress the freezing point twice as well as 1 mole of sugar!

Vapor Pressure and Boiling Point • When a substance that is normally a liquid enters a vapor phase, it is called a vapor • A liquid normally has molecules that escape its surface • The pressure that these molecules exert in the surrounding atmosphere is called vapor pressure

Vapor Pressure • Why do these molecules escape? • Liquids are held together by rather weak intermolecular forces • These forces are called dipole-dipole forces • As temperature increases, these forces become less effective and more molecules escape…thus VP increases!

Water is different… • Water is different than most liquids. . • It participates in hydrogen bonding in addition to dipole-dipole interactions. . • Thus it has a high boiling point and requires more energy to break the intermolecular forces. . • This is seen by observing the relationship between molecular weights and vapor pressure

Table H • Table H on your reference tables shows us the vapor pressure at various temperatures. . • Notice the boiling point for each liquid • Boiling Point – is when the vapor pressure of a liquid is equal to the atmospheric pressure… • This occurs when we see bubbles!

Using Table H • Find the Vapor Pressure of water at 75 degrees Celsius. • Which of the substances has the weakest intermolecular forces? Why? • Which has the strongest intermolecular forces? Why?
 Solutions chemistry regents questions
Solutions chemistry regents questions Chemistry regents 2011
Chemistry regents 2011 Mole conversion chart
Mole conversion chart Danswer
Danswer January 2009 chemistry regents answers
January 2009 chemistry regents answers Kent chemistry reference table
Kent chemistry reference table Nysedregents
Nysedregents Regents chemistry midterm
Regents chemistry midterm Chemistry regents bonding questions
Chemistry regents bonding questions Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Modern chemistry chapter 12 test
Modern chemistry chapter 12 test Chapter 13 solutions chemistry
Chapter 13 solutions chemistry Modern chemistry solutions
Modern chemistry solutions Ap chemistry solutions
Ap chemistry solutions Living by chemistry solutions
Living by chemistry solutions Chemistry dimensions 2 worksheet solutions
Chemistry dimensions 2 worksheet solutions Dimensional analysis worksheet
Dimensional analysis worksheet Living by chemistry solutions
Living by chemistry solutions Chapter 6 section 3 water and solutions
Chapter 6 section 3 water and solutions Us history regents review
Us history regents review On which station model would the present weather symbol
On which station model would the present weather symbol Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Tristan denley
Tristan denley Beaks of finches lab answer key
Beaks of finches lab answer key Internal resistance
Internal resistance Regents physics work power energy
Regents physics work power energy June 2007 physics regents
June 2007 physics regents Lab practical review earth science
Lab practical review earth science Ela regents text analysis rubric
Ela regents text analysis rubric Louisiana board of regents
Louisiana board of regents Part d earth science regents
Part d earth science regents Earth science lab practical
Earth science lab practical Uc regents policy 5402
Uc regents policy 5402 Middle ages regents questions
Middle ages regents questions Historians value the writings of ibn battuta because he –
Historians value the writings of ibn battuta because he – Geometry regents 2019
Geometry regents 2019 Decolonization regents questions
Decolonization regents questions Crq global regents
Crq global regents Nutrient chain foldable
Nutrient chain foldable 282 ways to pass the earth science regents
282 ways to pass the earth science regents American history regents review
American history regents review Thematic essay human rights
Thematic essay human rights Joule units
Joule units Regents physics projectile motion questions
Regents physics projectile motion questions Table g chemistry
Table g chemistry