Chapter 5 Alkenes and Alkynes II Reactions Elimination






- Slides: 34

Chapter 5. Alkenes and Alkynes II: Reactions

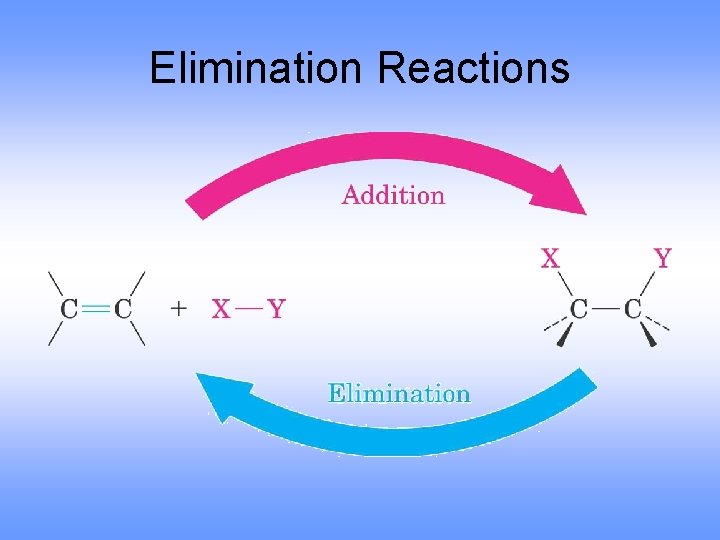
Elimination Reactions

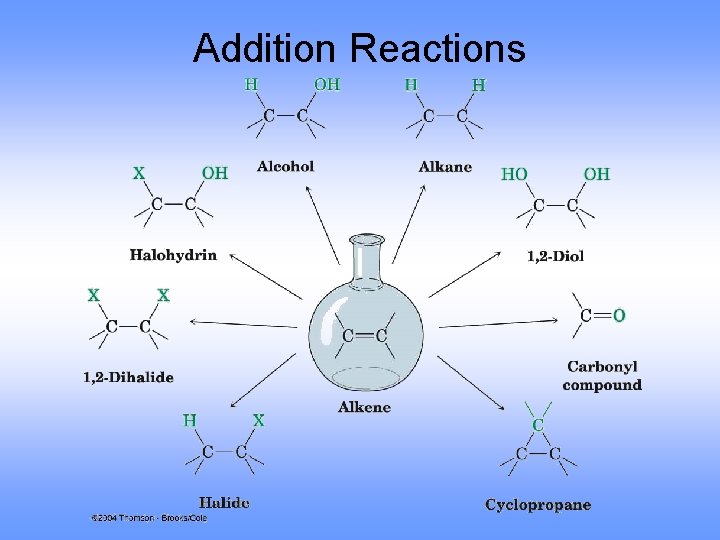
Addition Reactions

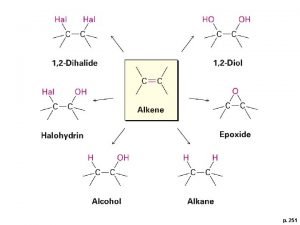
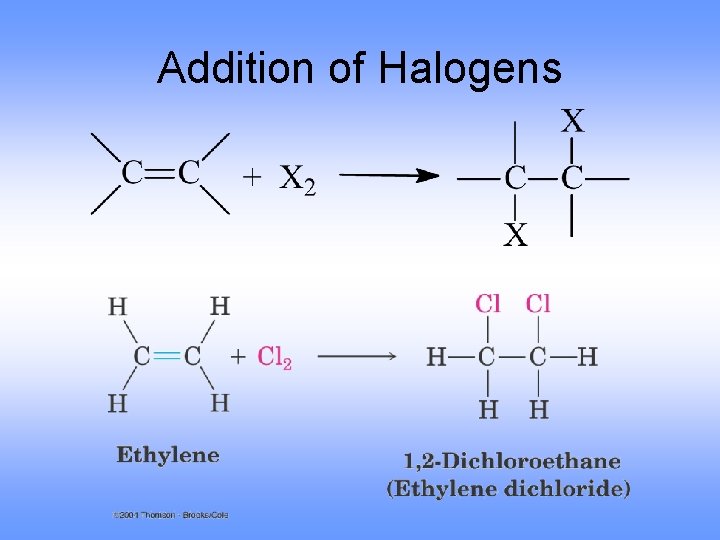
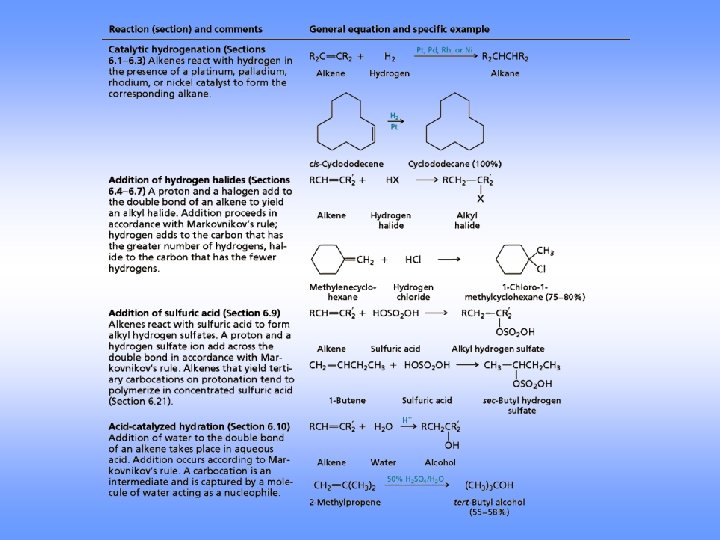
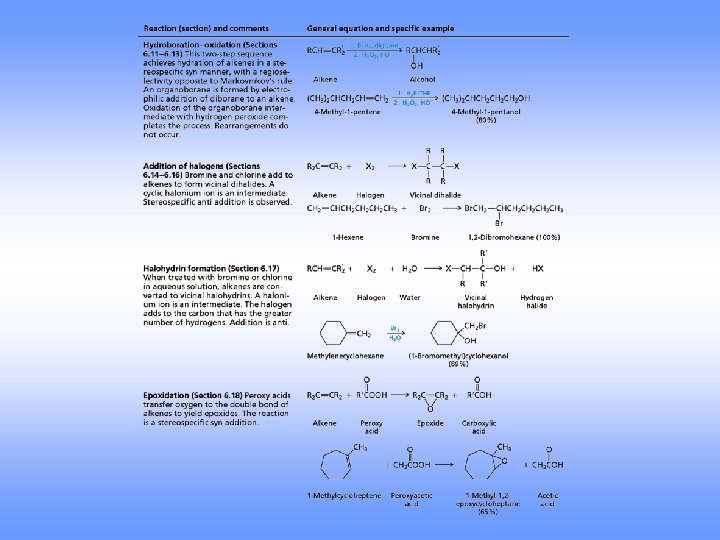
Addition of Halogens

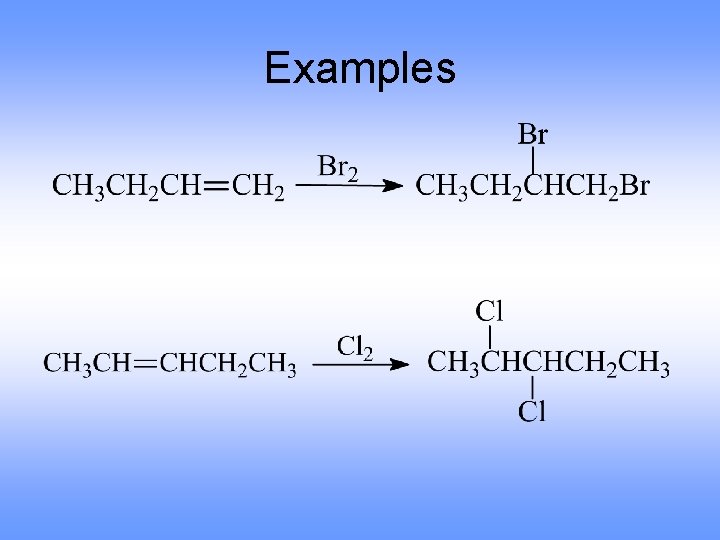
Examples

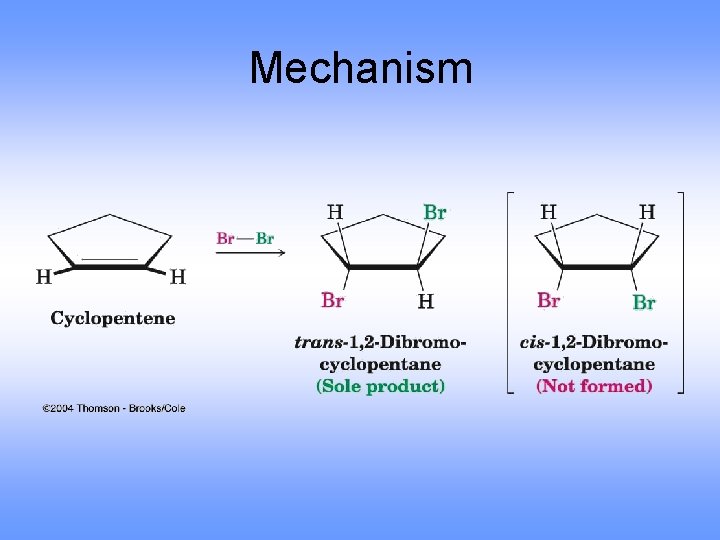
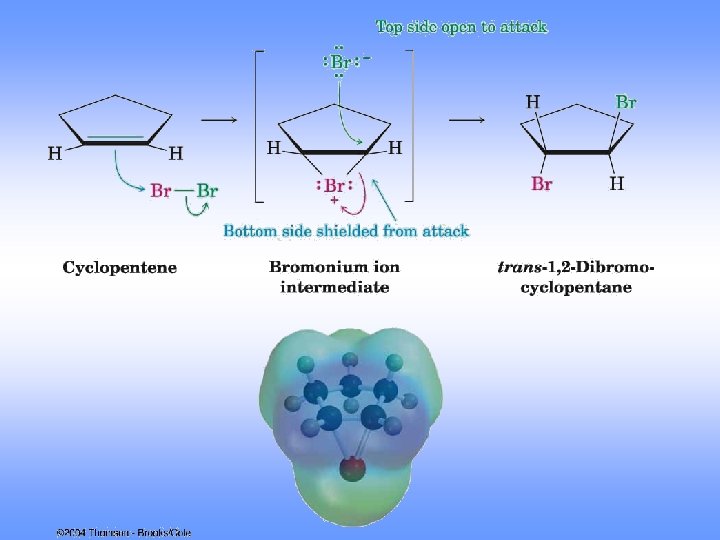
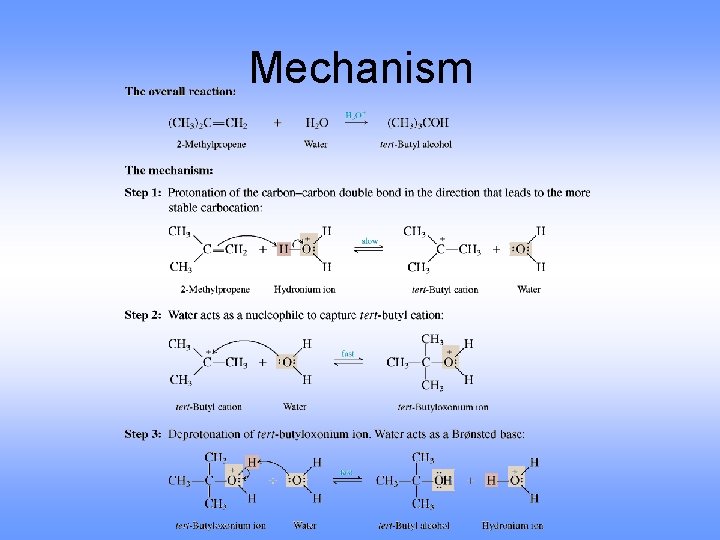
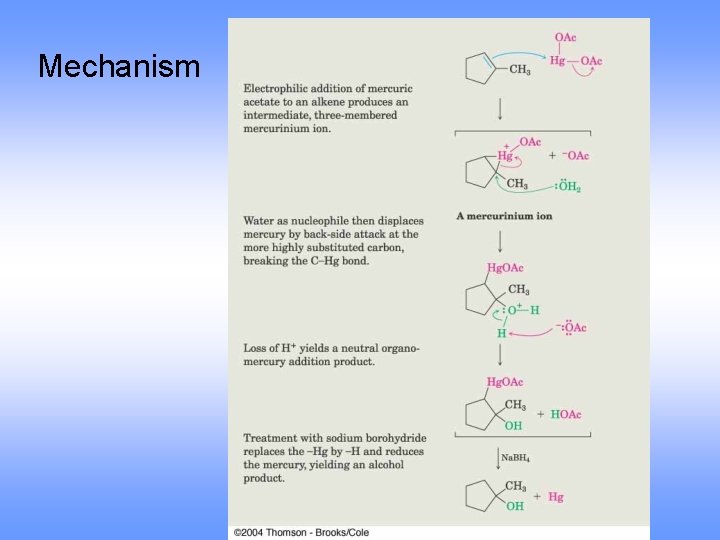
Mechanism


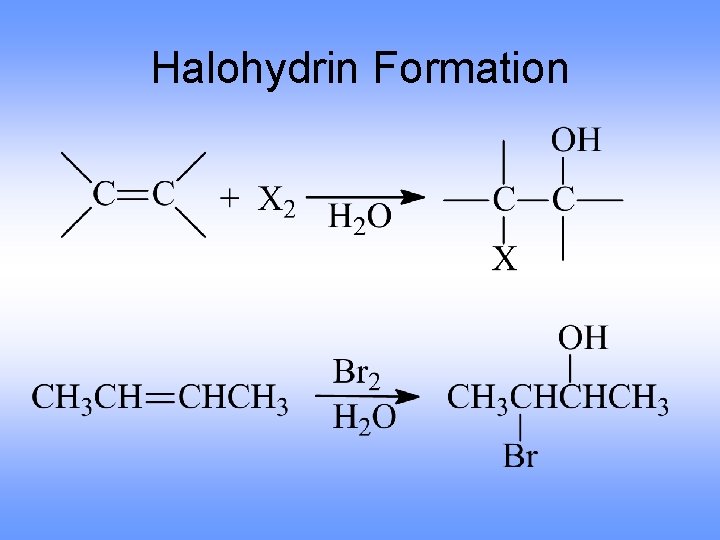
Halohydrin Formation

Mechanism


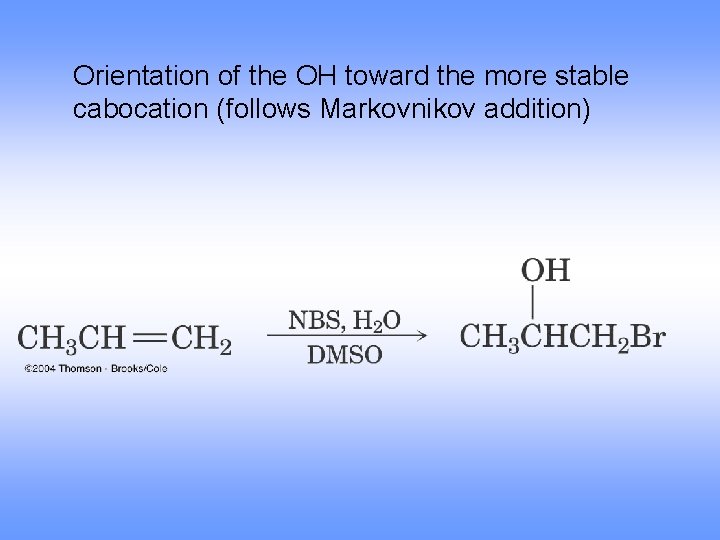
Orientation of the OH toward the more stable cabocation (follows Markovnikov addition)

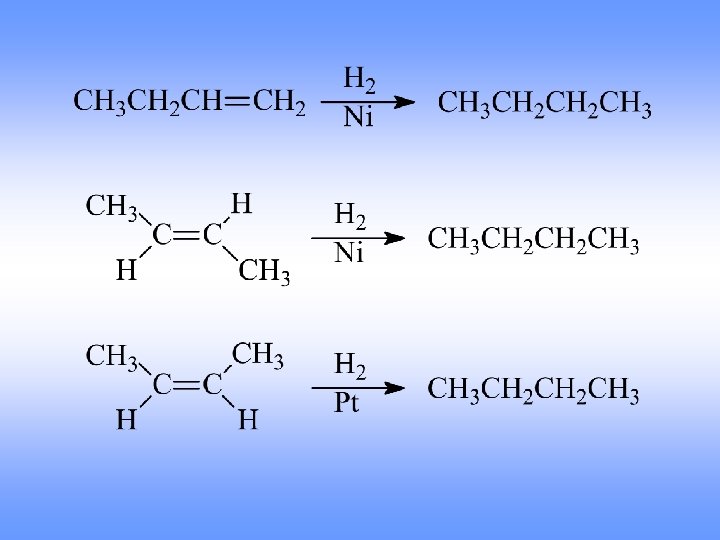
Hydrogenation Addition of Hydrogen

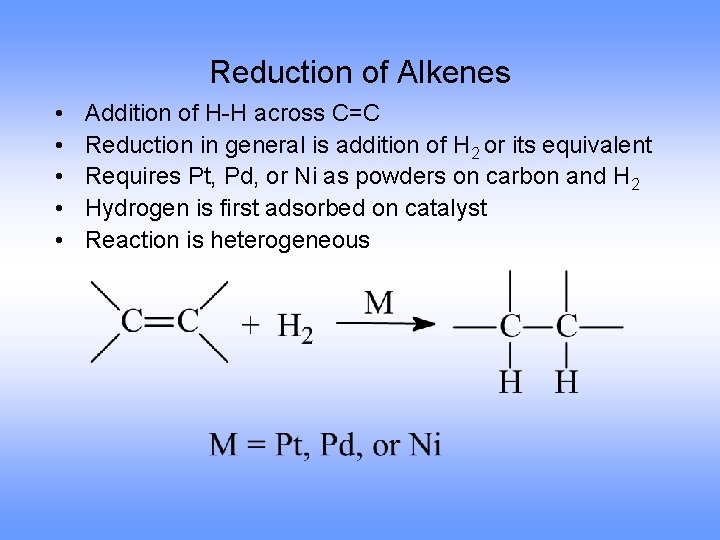
Reduction of Alkenes • • • Addition of H-H across C=C Reduction in general is addition of H 2 or its equivalent Requires Pt, Pd, or Ni as powders on carbon and H 2 Hydrogen is first adsorbed on catalyst Reaction is heterogeneous


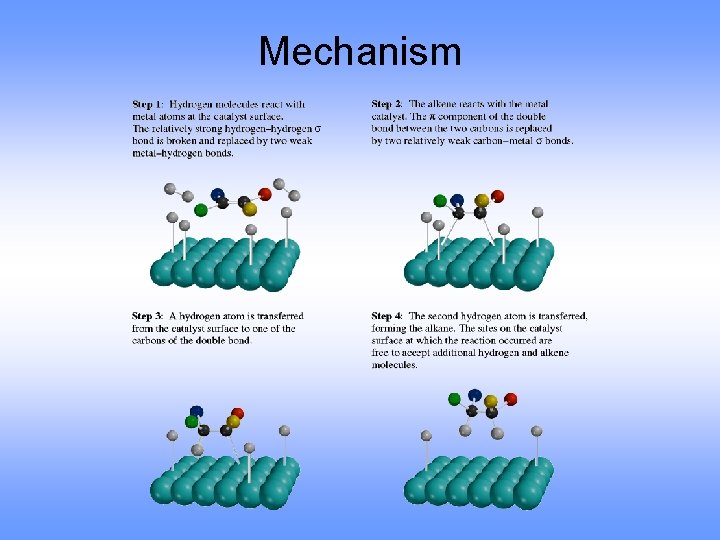
Mechanism

Addition of Water to Alkenes • Acid-Catalyzed Hydration • Oxymercuration-Demercuration • Hydroboration-Oxydation

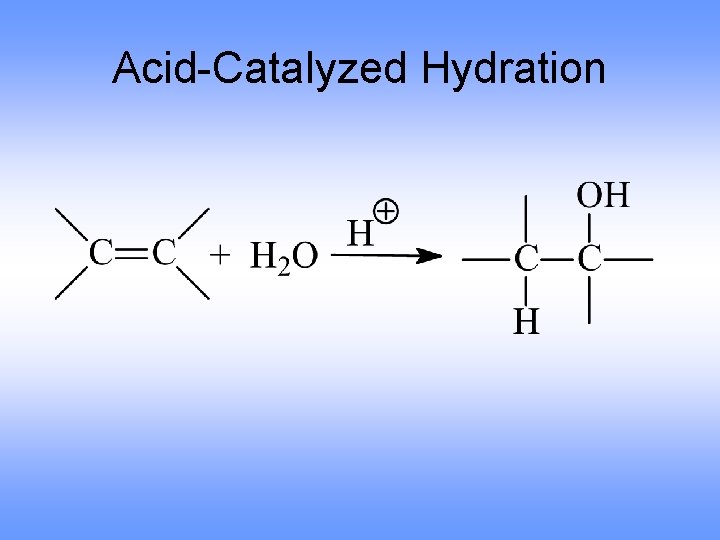
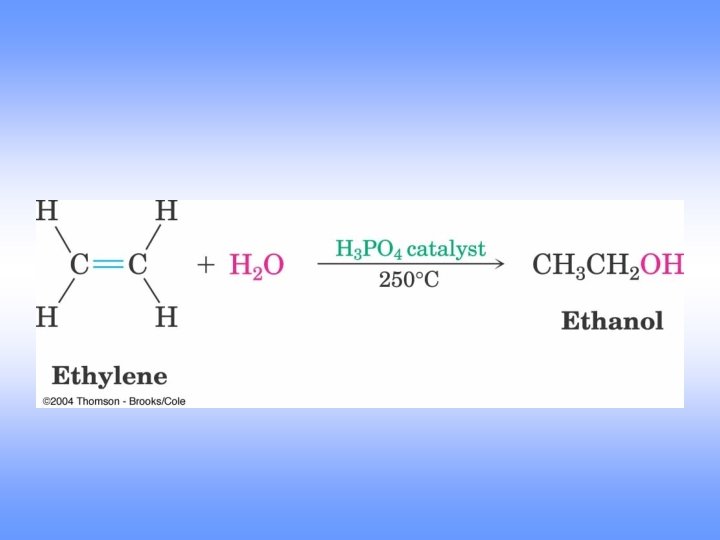
Acid-Catalyzed Hydration


Mechanism

Mechanism

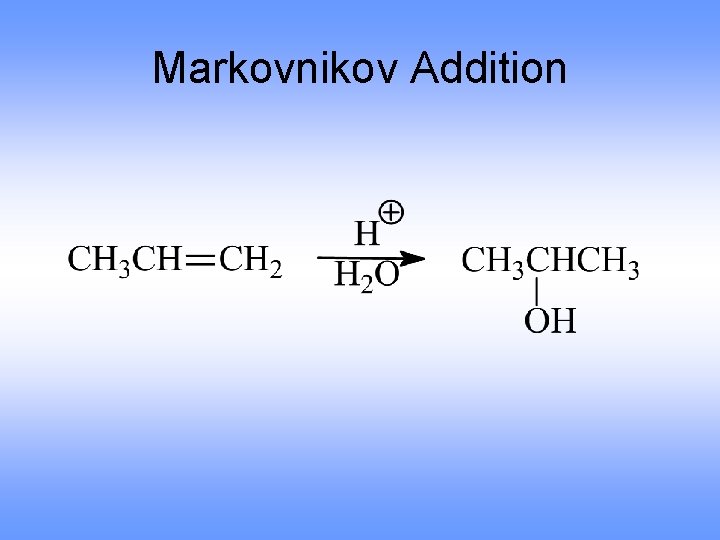
Markovnikov Addition


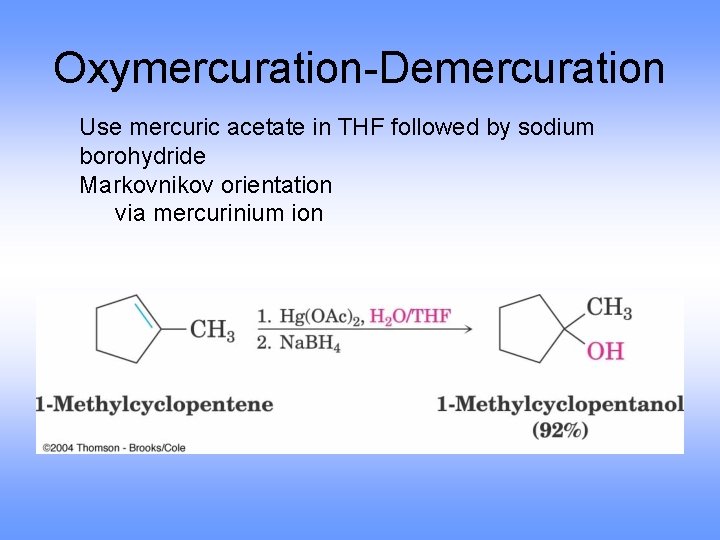
Oxymercuration-Demercuration Use mercuric acetate in THF followed by sodium borohydride Markovnikov orientation via mercurinium ion

Mechanism

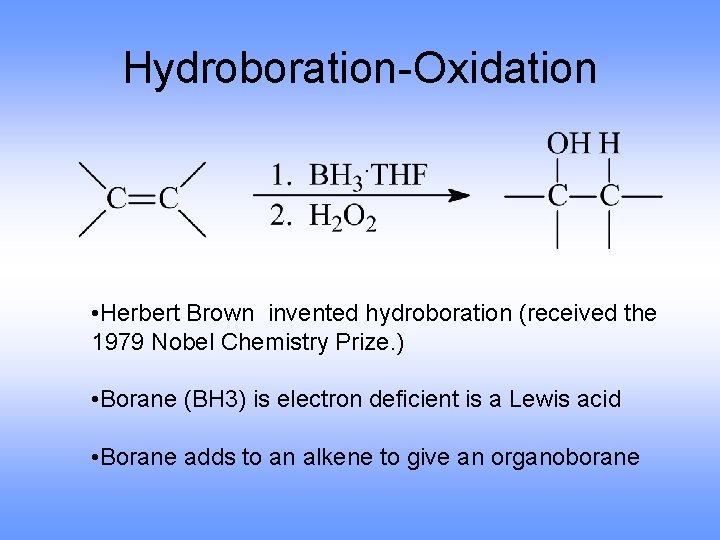
Hydroboration-Oxidation • Herbert Brown invented hydroboration (received the 1979 Nobel Chemistry Prize. ) • Borane (BH 3) is electron deficient is a Lewis acid • Borane adds to an alkene to give an organoborane

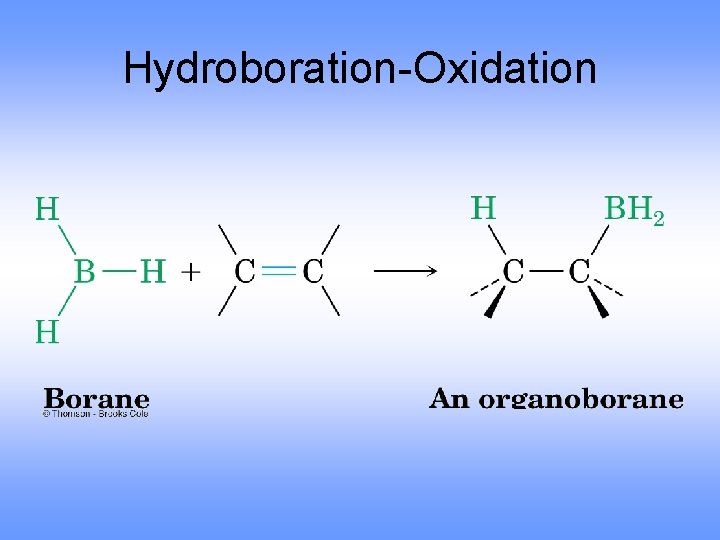
Hydroboration-Oxidation

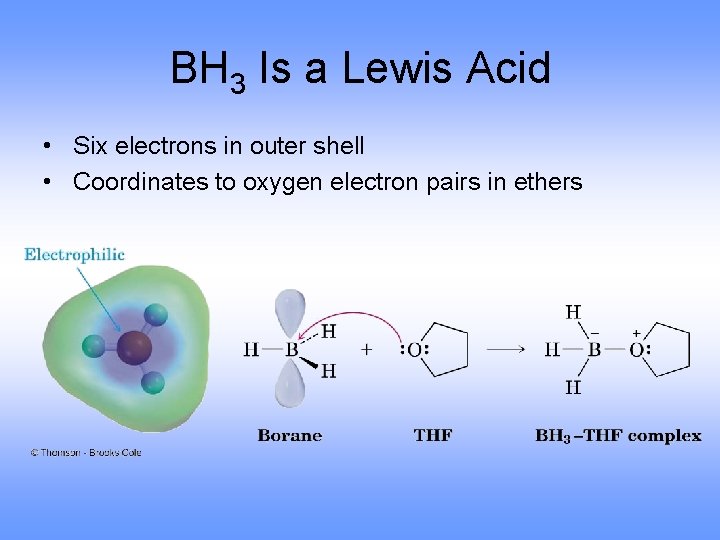
BH 3 Is a Lewis Acid • Six electrons in outer shell • Coordinates to oxygen electron pairs in ethers

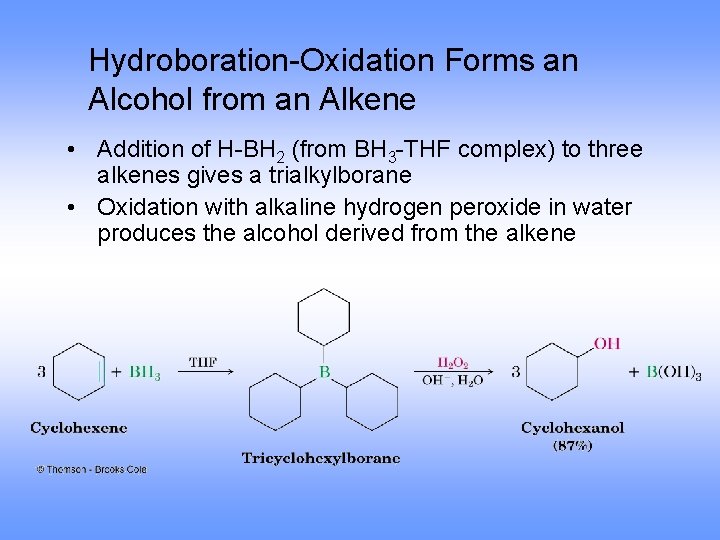
Hydroboration-Oxidation Forms an Alcohol from an Alkene • Addition of H-BH 2 (from BH 3 -THF complex) to three alkenes gives a trialkylborane • Oxidation with alkaline hydrogen peroxide in water produces the alcohol derived from the alkene

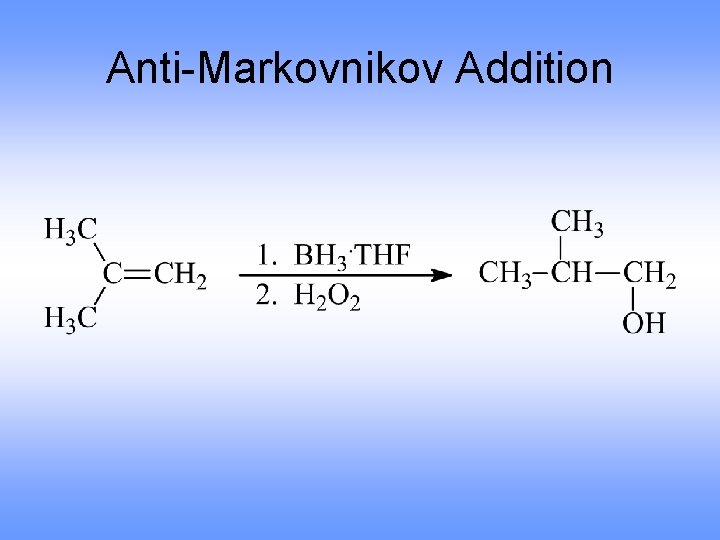
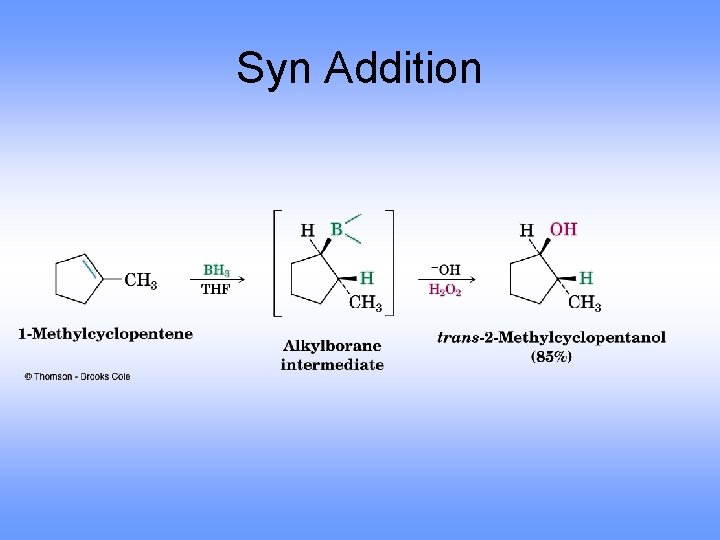
Orientation in Hydration via Hydroboration • Regiochemistry is opposite to Markovnikov orientation (Anti-Markovnikov) – OH is added to carbon with most H’s • H and OH add with syn stereochemistry, to the same face of the alkene (opposite of anti addition)

Anti-Markovnikov Addition

Syn Addition

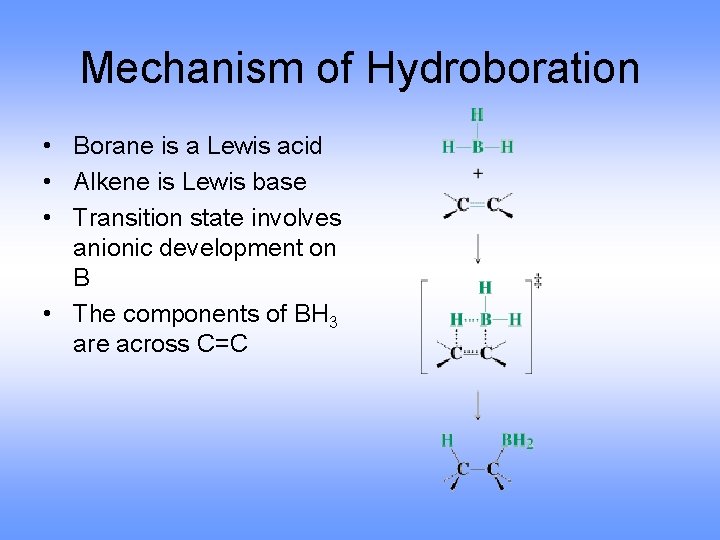
Mechanism of Hydroboration • Borane is a Lewis acid • Alkene is Lewis base • Transition state involves anionic development on B • The components of BH 3 are across C=C


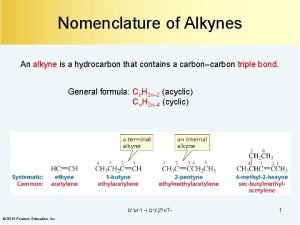
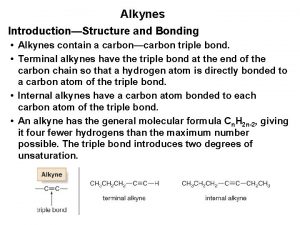
 Alkanes alkenes alkynes
Alkanes alkenes alkynes Diol formation from alkene
Diol formation from alkene Dissolving metal reduction
Dissolving metal reduction Chemsheets as 1128 answers
Chemsheets as 1128 answers Gauss jordan
Gauss jordan Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Redox reaction
Redox reaction Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Halogenation of alkynes
Halogenation of alkynes Lindlar pd
Lindlar pd Hydrogen halide example
Hydrogen halide example Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes General formula for alkenes
General formula for alkenes Hybridization of alkynes
Hybridization of alkynes Condensed structural formula of propyne
Condensed structural formula of propyne Preparation of alkynes
Preparation of alkynes Alkynes
Alkynes Alkynes
Alkynes First 10 members of alkynes
First 10 members of alkynes Alkynes to aldehydes
Alkynes to aldehydes Combustion reaction of alkanes
Combustion reaction of alkanes Properties of alkenes
Properties of alkenes Complete combustion of alkene
Complete combustion of alkene Alkane chemical formula
Alkane chemical formula Rco3h reaction
Rco3h reaction Properties of alkenes
Properties of alkenes Sp2 hybridization in alkenes
Sp2 hybridization in alkenes Name the following alkenes
Name the following alkenes Naming alkenes
Naming alkenes Test for alkenes
Test for alkenes Carbocyclic acid
Carbocyclic acid