Chapter Eight Alkenes and Alkynes I Addition Reactions















- Slides: 69

Chapter Eight Alkenes and Alkynes I. Addition Reactions WWU -- Chemistry

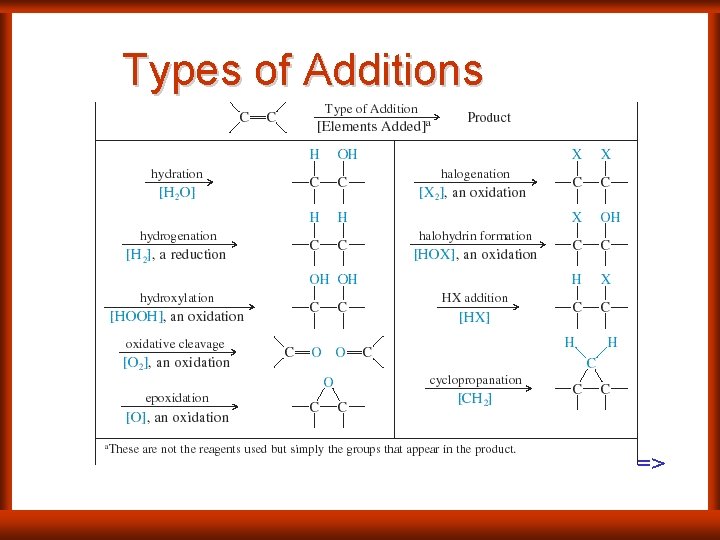
Types of Additions =>

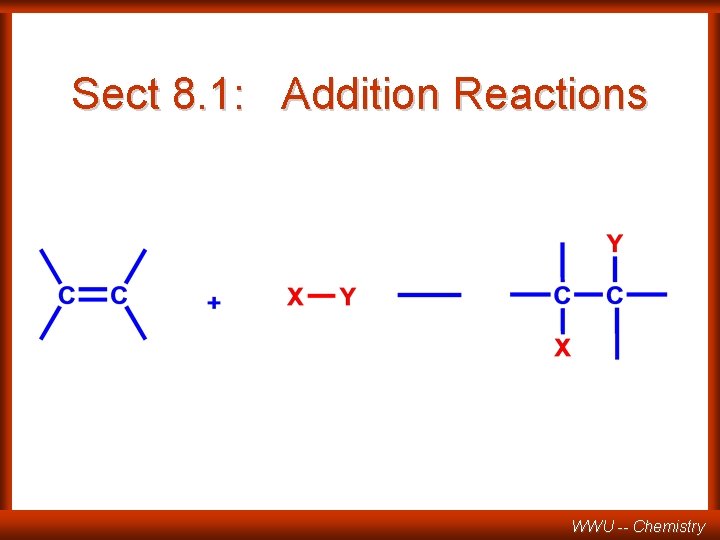
Sect 8. 1: Addition Reactions WWU -- Chemistry

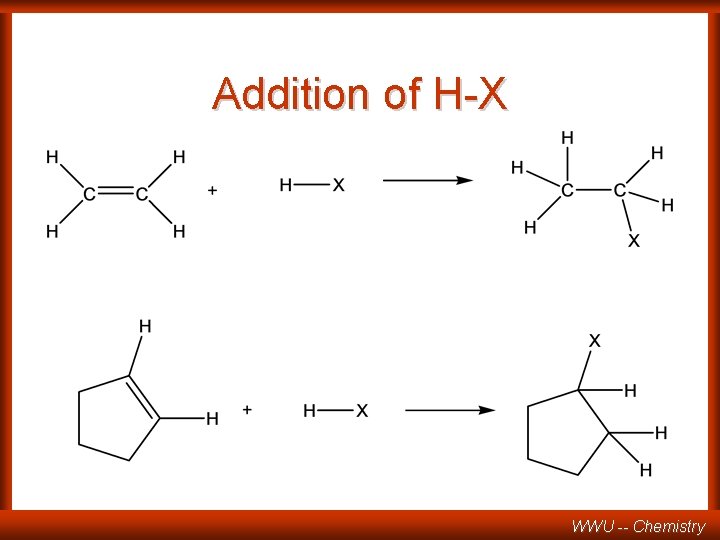
Addition of H-X WWU -- Chemistry

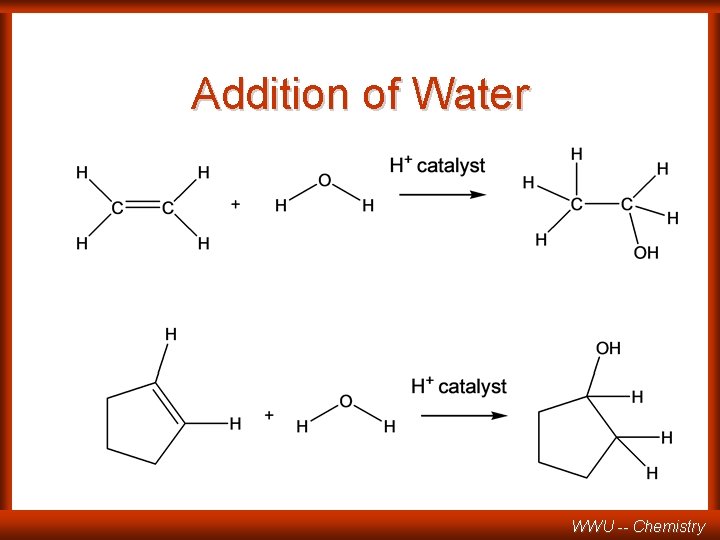
Addition of Water WWU -- Chemistry

Sect. 8. 2: Introduction to Mechanisms • • • mechanism two step rate determining step (i. e. slow step) energy diagram transition states intermediates WWU -- Chemistry

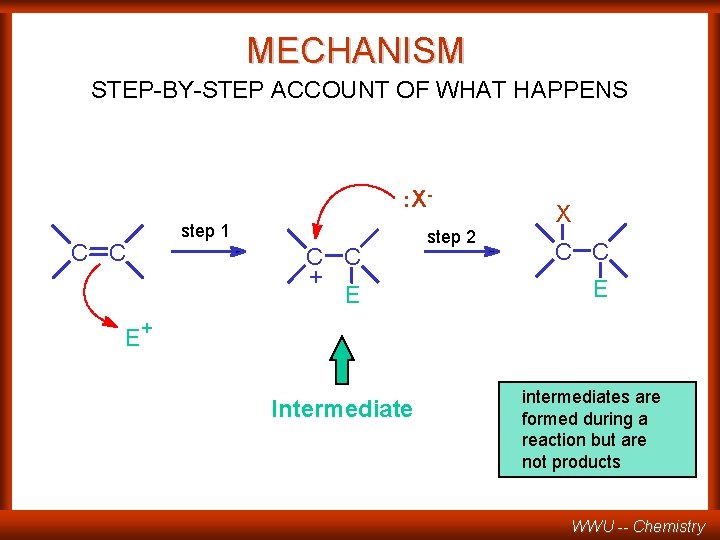
MECHANISM STEP-BY-STEP ACCOUNT OF WHAT HAPPENS : Xstep 1 C C + E E step 2 X C C E + Intermediate intermediates are formed during a reaction but are not products WWU -- Chemistry

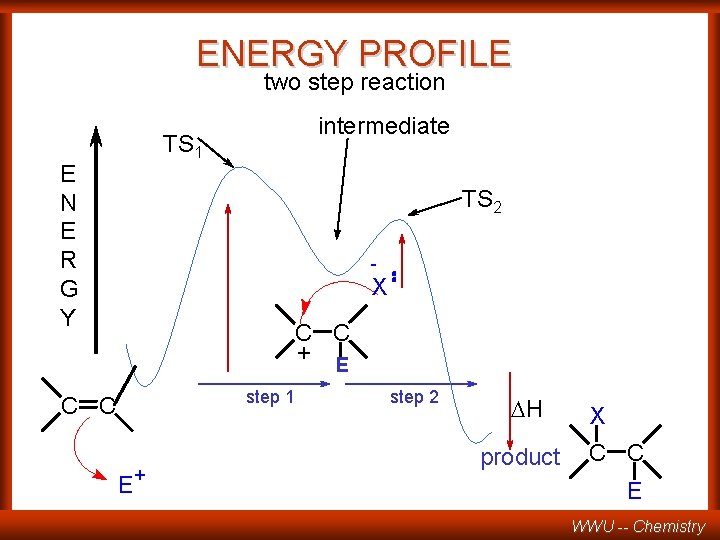
ENERGY PROFILE two step reaction intermediate TS 1 E N E R G Y TS 2 - X C C + E step 1 C C E + step 2 DH product X C C E WWU -- Chemistry

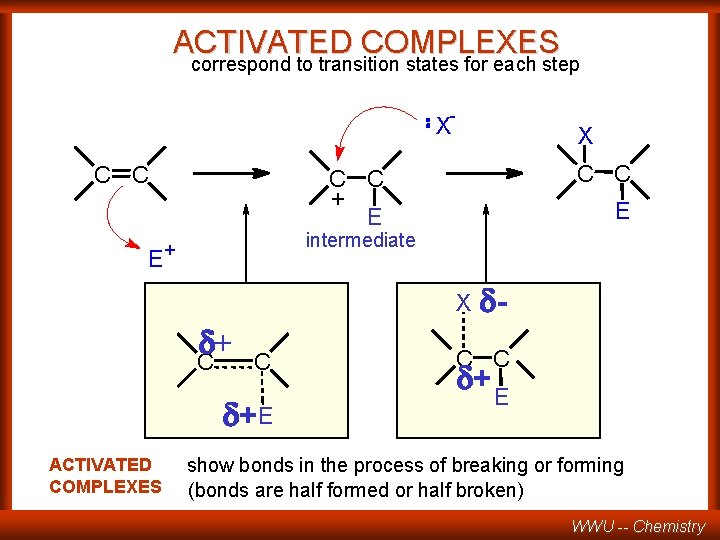
ACTIVATED COMPLEXES correspond to transition states for each step - X C C E X C C + E E intermediate + X + C C +E ACTIVATED COMPLEXES - C C + E show bonds in the process of breaking or forming (bonds are half formed or half broken) WWU -- Chemistry

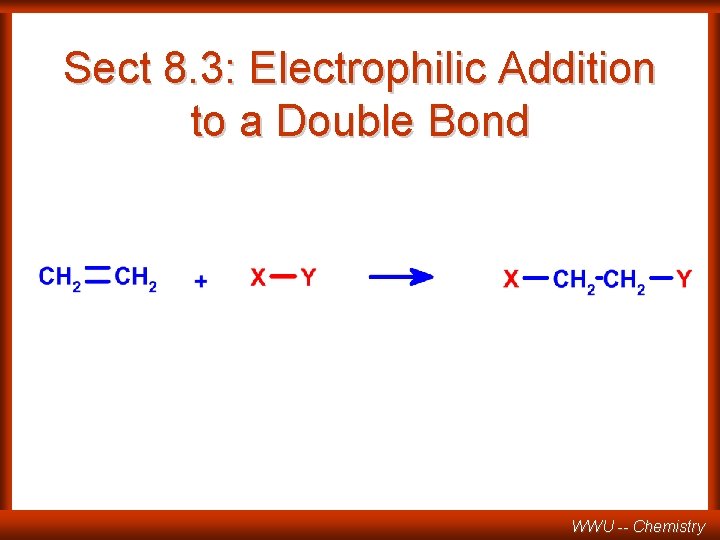
Sect 8. 3: Electrophilic Addition to a Double Bond WWU -- Chemistry

Hyperconjugation WWU -- Chemistry

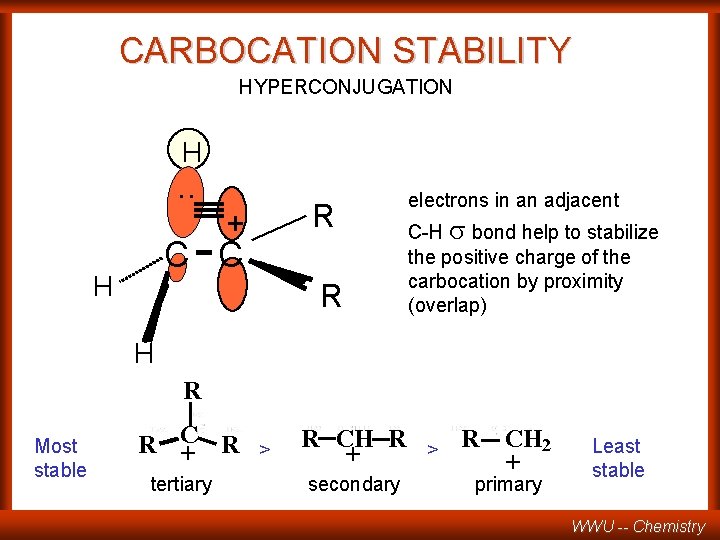
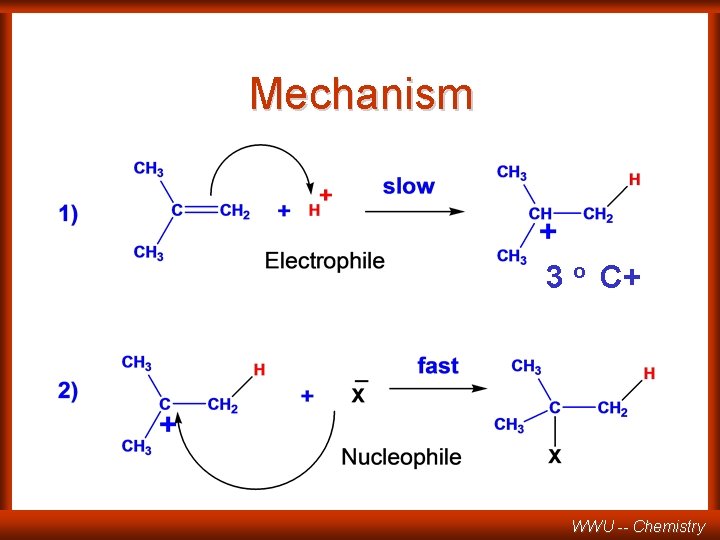
CARBOCATION STABILITY HYPERCONJUGATION H . . R + C C H R electrons in an adjacent C-H s bond help to stabilize the positive charge of the carbocation by proximity (overlap) H R Most stable C R R + tertiary > R CH R + secondary > R CH 2 + primary Least stable WWU -- Chemistry

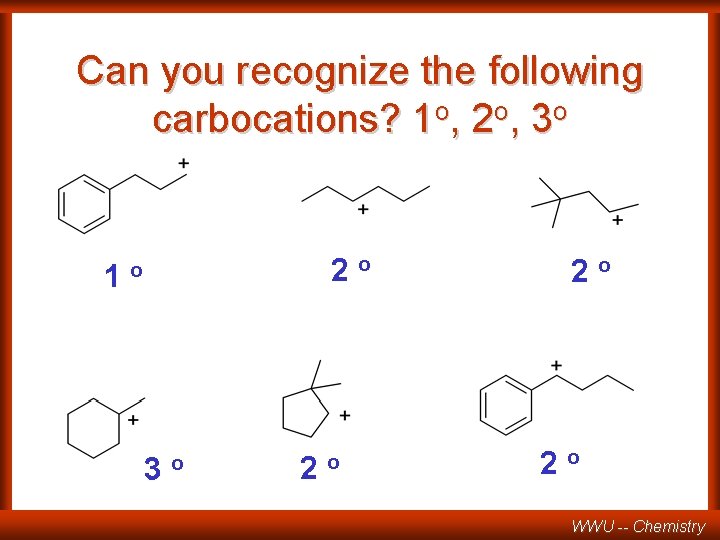
Can you recognize the following carbocations? 1 o, 2 o, 3 o 1 2 o o 3 o 2 o 2 o 2 o WWU -- Chemistry

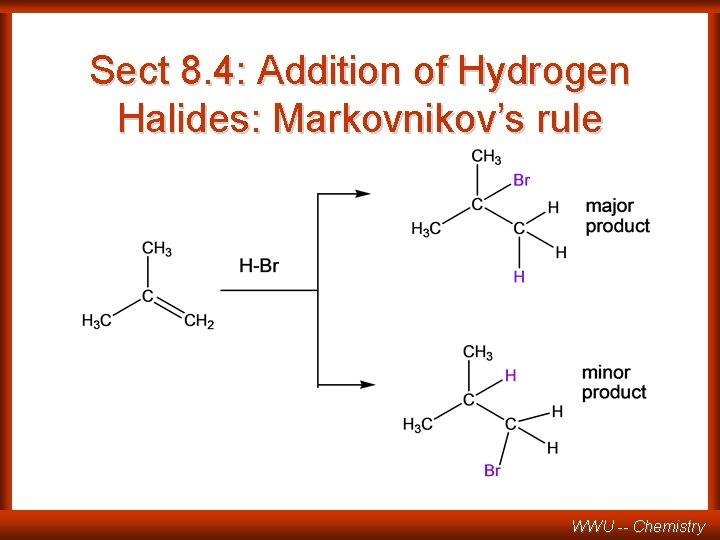
Sect 8. 4: Addition of Hydrogen Halides: Markovnikov’s rule WWU -- Chemistry

Markovnikov’s Rule In the ionic addition of an acid to the carbon-carbon double bond of an alkene, the hydrogen of the acid attaches itself to the carbon atom which already holds the greater number of hydrogens. –“Them that has, gets!” –“The richer get richer!” (V. W. Markovnikov -- 1838 - 1904) WWU -- Chemistry

Markovnikov WWU -- Chemistry

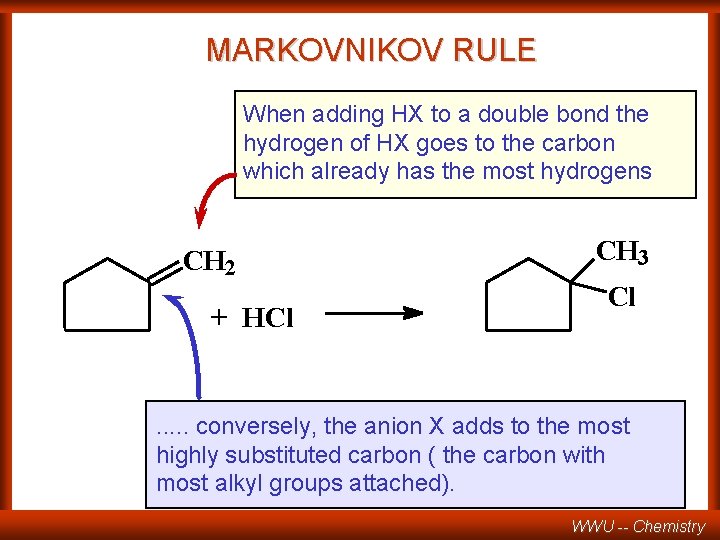
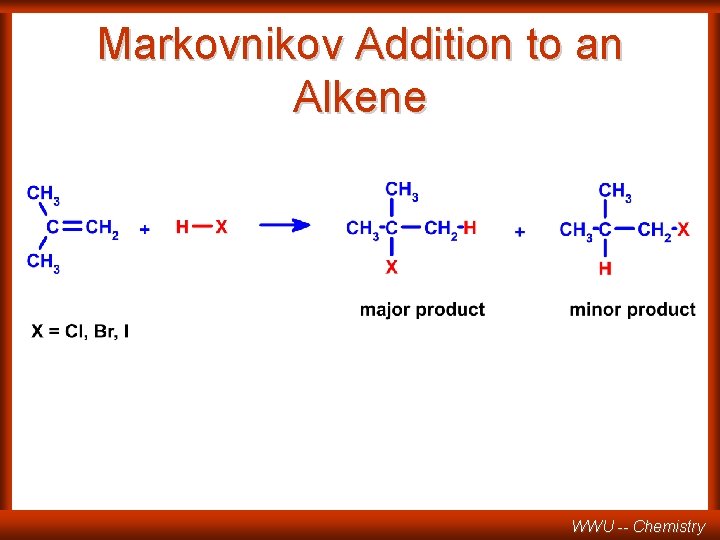
MARKOVNIKOV RULE When adding HX to a double bond the hydrogen of HX goes to the carbon which already has the most hydrogens CH 2 + HCl CH 3 Cl . . . conversely, the anion X adds to the most highly substituted carbon ( the carbon with most alkyl groups attached). WWU -- Chemistry

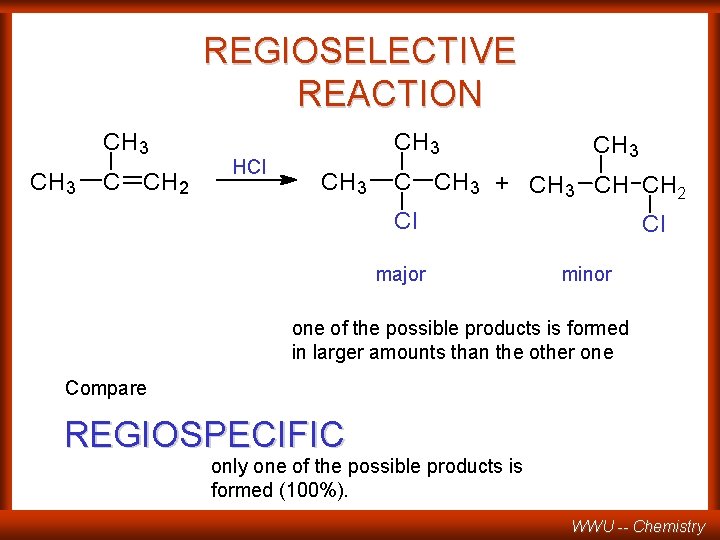
REGIOSELECTIVE REACTION CH 3 C CH 2 HCl CH 3 C CH 3 + CH 3 CH CH 2 Cl major Cl minor one of the possible products is formed in larger amounts than the other one Compare REGIOSPECIFIC only one of the possible products is formed (100%). WWU -- Chemistry

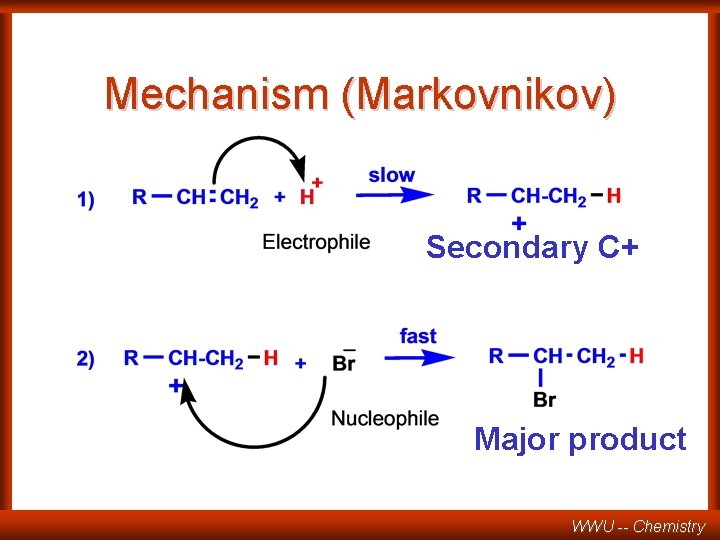
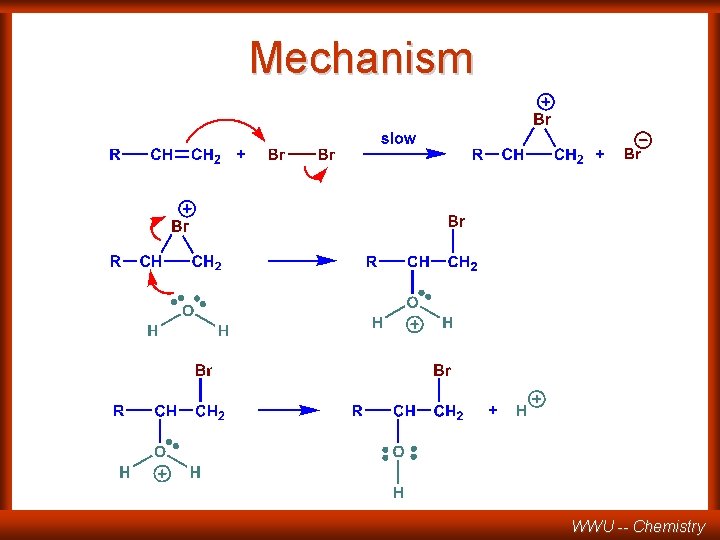
Mechanism (Markovnikov) Secondary C+ Major product WWU -- Chemistry

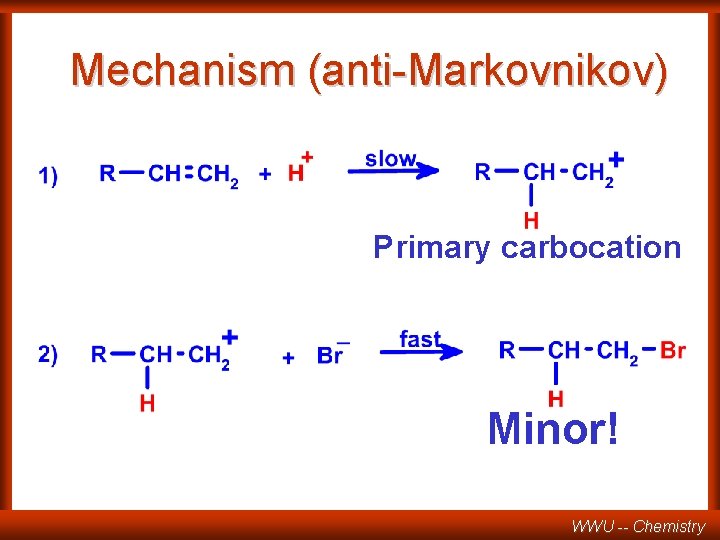
Mechanism (anti-Markovnikov) Primary carbocation Minor! WWU -- Chemistry

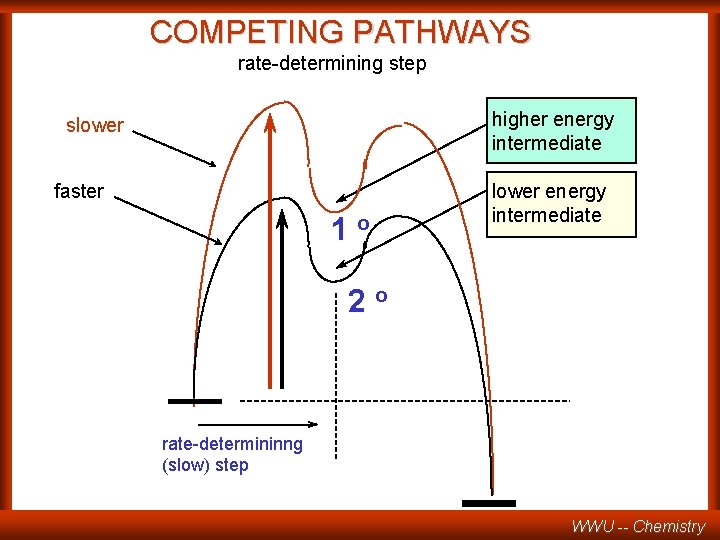
COMPETING PATHWAYS rate-determining step higher energy intermediate slower faster 1 o lower energy intermediate 2 o rate-determininng (slow) step WWU -- Chemistry

Markovnikov Addition to an Alkene WWU -- Chemistry

Mechanism 3 o C+ WWU -- Chemistry

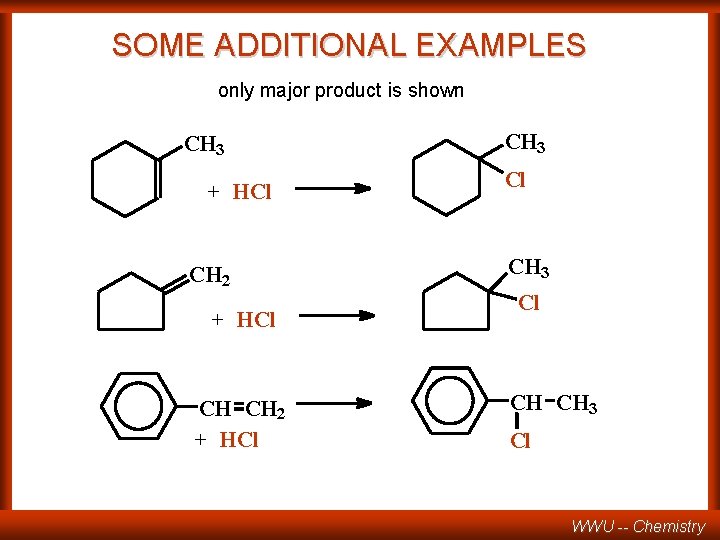
SOME ADDITIONAL EXAMPLES only major product is shown CH 3 + HCl CH 2 + HCl CH 3 Cl CH CH 3 Cl WWU -- Chemistry

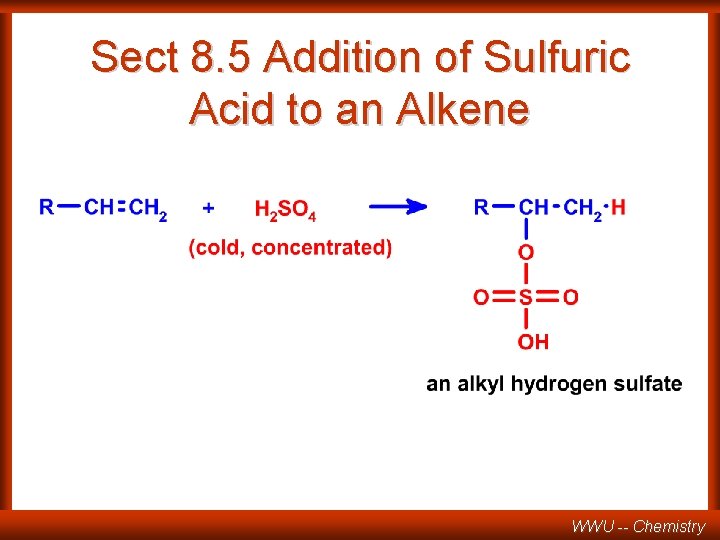
Sect 8. 5 Addition of Sulfuric Acid to an Alkene WWU -- Chemistry

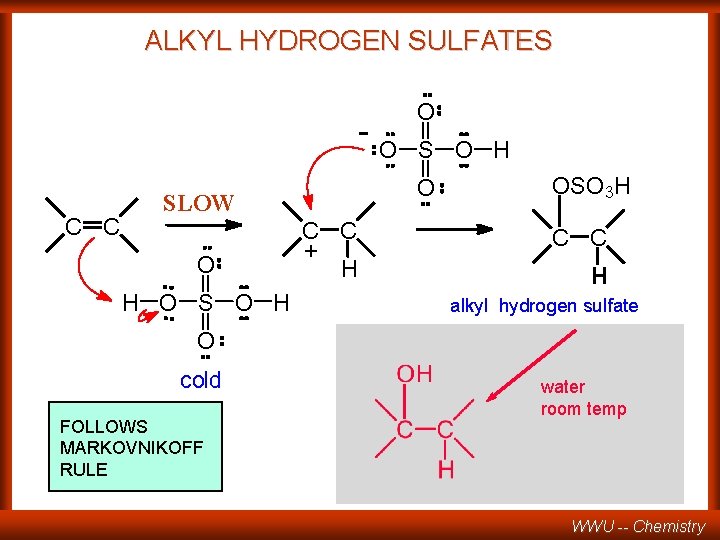
ALKYL HYDROGEN SULFATES -O C C H O S O H O SLOW O O C C + H OSO 3 H C C H alkyl hydrogen sulfate O cold FOLLOWS MARKOVNIKOFF RULE water room temp WWU -- Chemistry

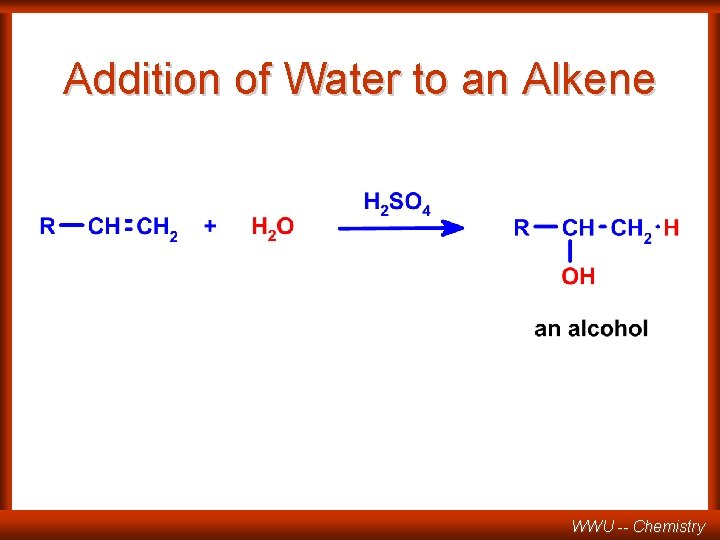
Addition of Water to an Alkene WWU -- Chemistry

Mechanism of Hydration WWU -- Chemistry

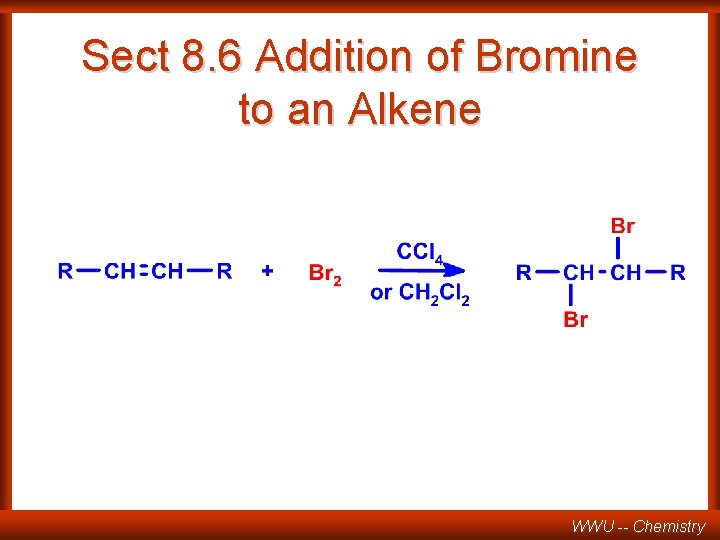
Sect 8. 6 Addition of Bromine to an Alkene WWU -- Chemistry

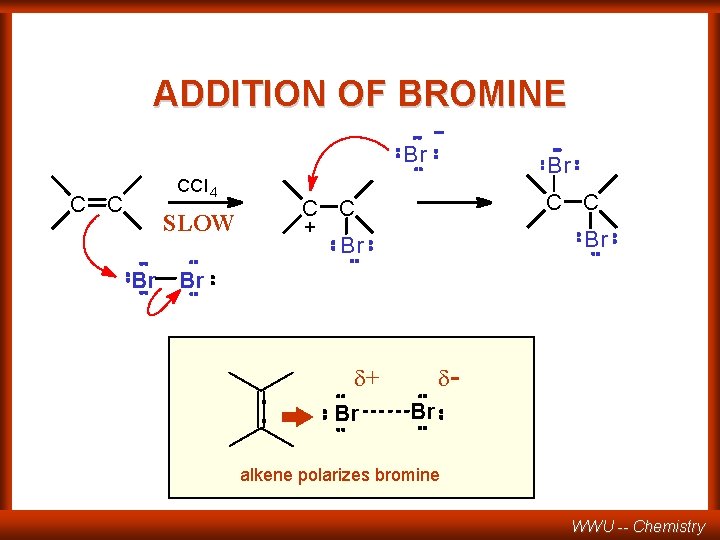
ADDITION OF BROMINE Br CCl 4 C C C + Br SLOW Br Br : d- d+ Br Br alkene polarizes bromine WWU -- Chemistry

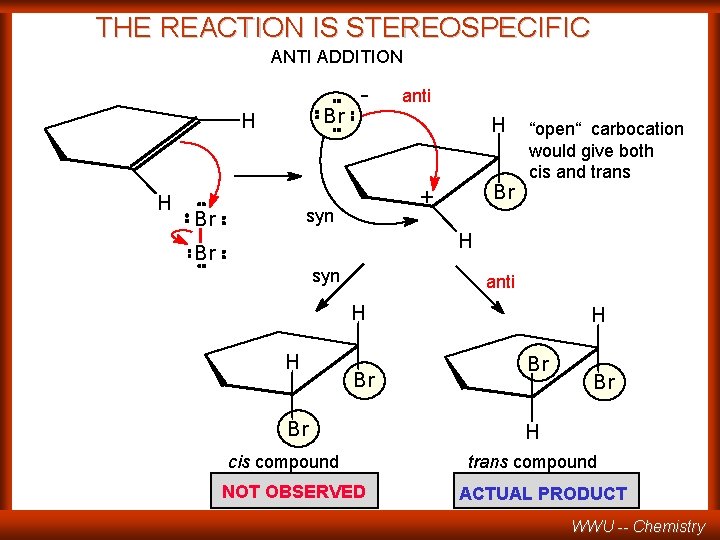
THE REACTION IS STEREOSPECIFIC ANTI ADDITION Br H H - H Br + syn Br anti “open“ carbocation would give both cis and trans H Br syn anti H H Br Br cis compound NOT OBSERVED H Br Br H trans compound ACTUAL PRODUCT WWU -- Chemistry

WHAT WOULD EXPLAIN FORMATION OF ONLY THE trans PRODUCT ? . . . A BRIDGED OR CYCLIC INTERMEDIATE WWU -- Chemistry

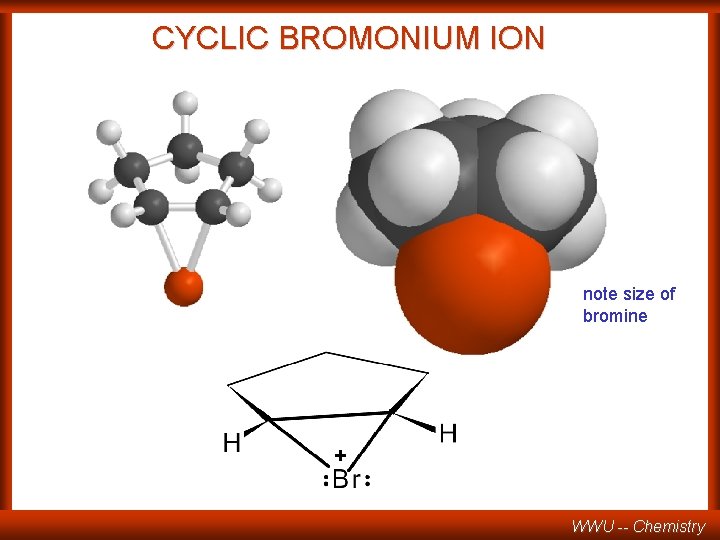
CYCLIC BROMONIUM ION note size of bromine + WWU -- Chemistry

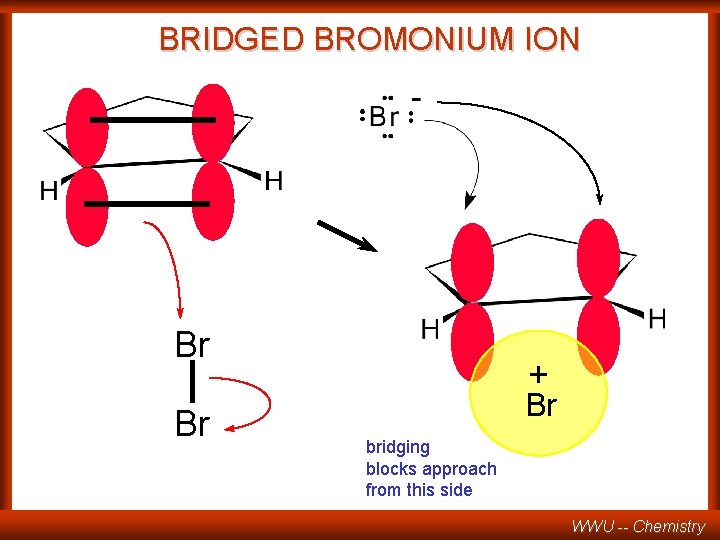
BRIDGED BROMONIUM ION Br Br + Br bridging blocks approach from this side WWU -- Chemistry

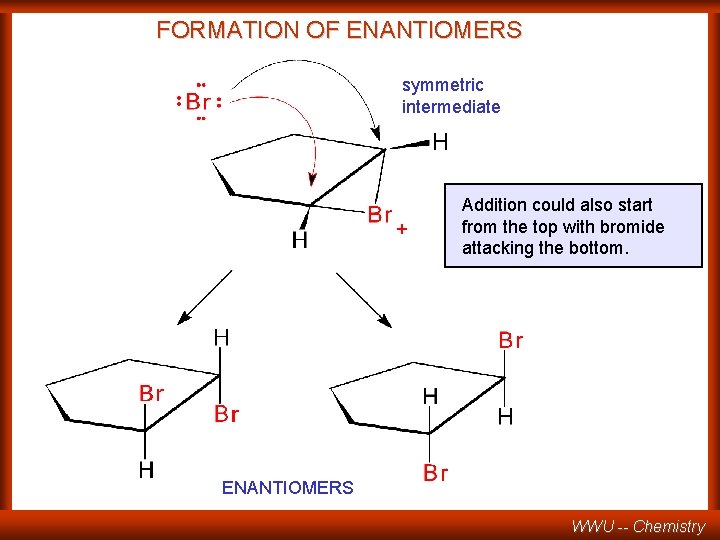
FORMATION OF ENANTIOMERS symmetric intermediate + Addition could also start from the top with bromide attacking the bottom. ENANTIOMERS WWU -- Chemistry

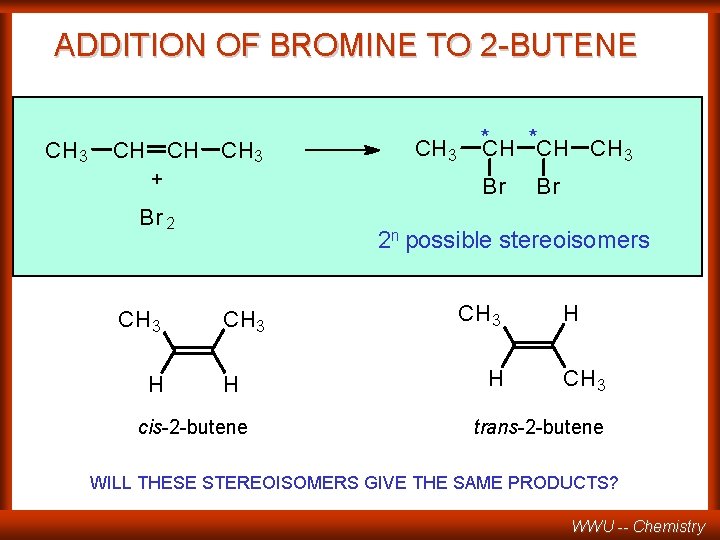
ADDITION OF BROMINE TO 2 -BUTENE CH 3 CH CH CH 3 + Br 2 CH 3 H CH 3 *CH CH 3 Br Br 2 n possible stereoisomers CH 3 H cis-2 -butene CH 3 H H CH 3 trans-2 -butene WILL THESE STEREOISOMERS GIVE THE SAME PRODUCTS? WWU -- Chemistry

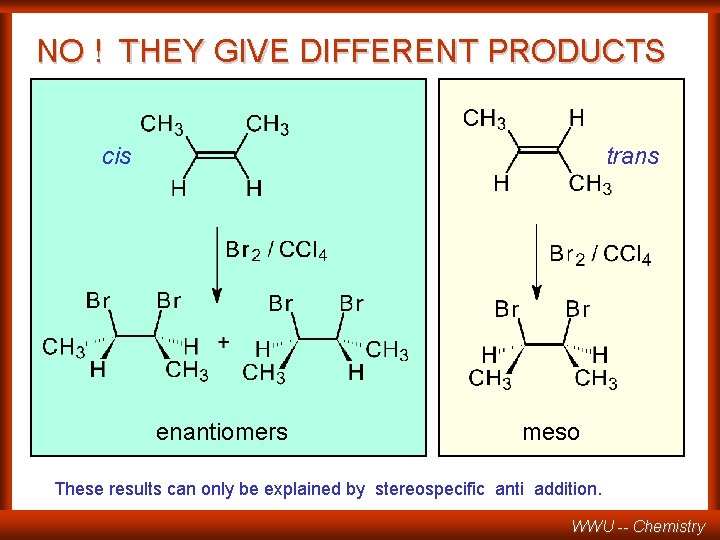
NO ! THEY GIVE DIFFERENT PRODUCTS cis trans enantiomers meso These results can only be explained by stereospecific anti addition. WWU -- Chemistry

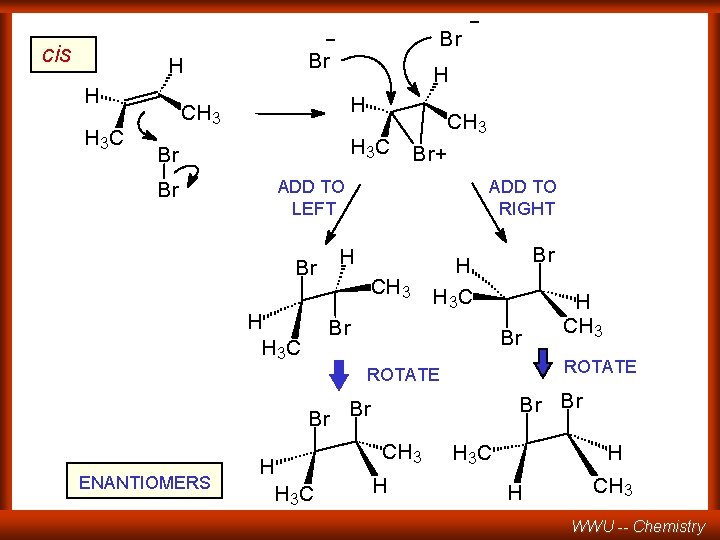
- cis Br H H H 3 C Br H H CH 3 H 3 C Br Br - CH 3 Br+ ADD TO LEFT Br H H 3 C ADD TO RIGHT H CH 3 Br H H 3 C Br Br ROTATE Br Br ENANTIOMERS H H 3 C H CH 3 H H 3 C H H CH 3 WWU -- Chemistry

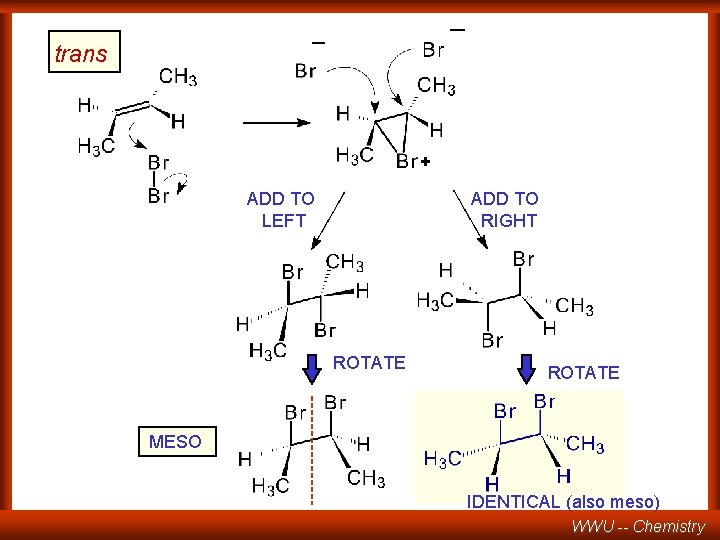
_ _ trans + ADD TO LEFT ADD TO RIGHT ROTATE MESO IDENTICAL (also meso) WWU -- Chemistry

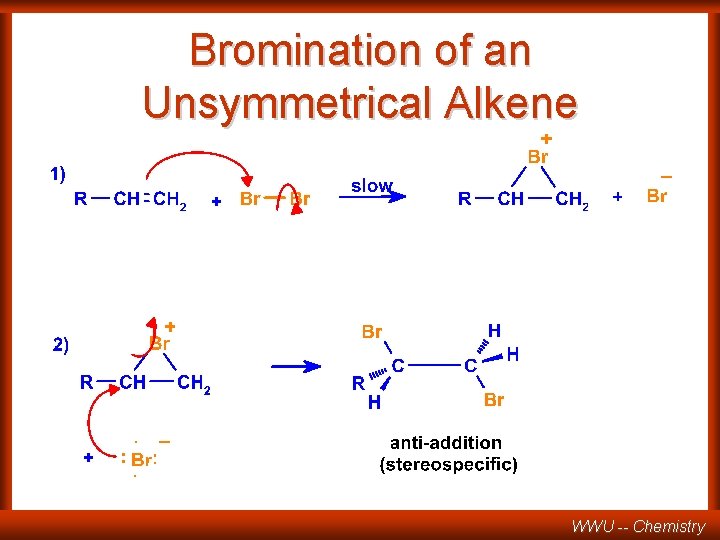
Bromination of an Unsymmetrical Alkene WWU -- Chemistry

Stereochemistry of Bromination of Alkenes • Simple alkenes: Addition of bromine or chlorine goes exclusively anti, with the formation of a bridged ion • If a resonance-stabilized open-chain carbocation is possible, there may be a mixture of mechanisms, with some molecules reacting via a bridged ion and some molecules reacting via an openchain carbocation WWU -- Chemistry

Stereochemistry of Bromination of Alkenes --Part Two • In cases where a resonance-stabilized carbocation is possible, if the solvent is made more polar (acetic acid or nitromethane), the proportion of molecules reacting via an open-chain carbocation increases. • For simple alkenes, changing solvents has little or no effect on stereochemistry. WWU -- Chemistry

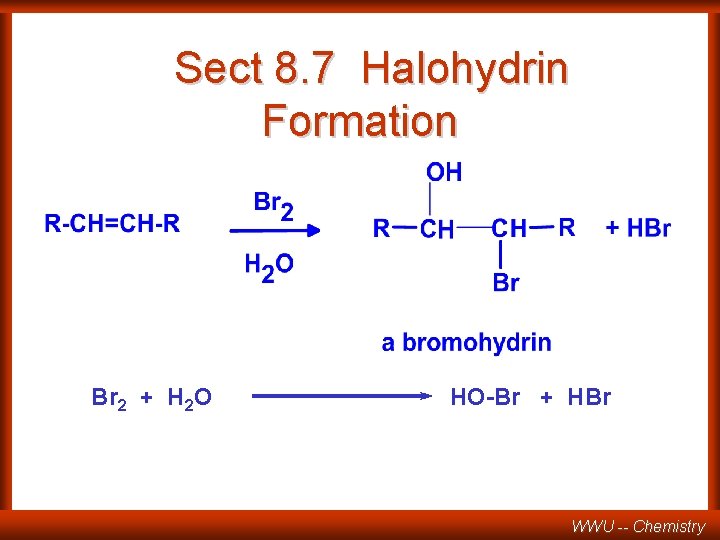
Sect 8. 7 Halohydrin Formation Br 2 + H 2 O HO-Br + HBr WWU -- Chemistry

Mechanism WWU -- Chemistry

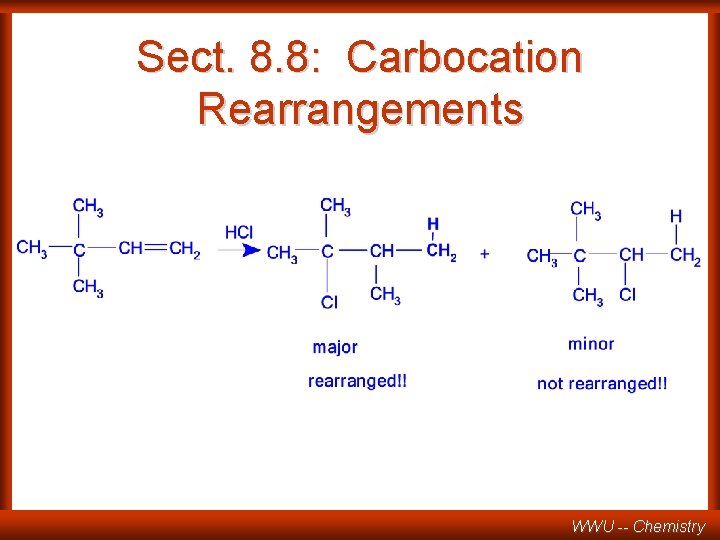
Sect. 8. 8: Carbocation Rearrangements WWU -- Chemistry

Sect 8. 10 Free Radical Addition of HBr to Alkenes (anti-Markovnikov!) WWU -- Chemistry

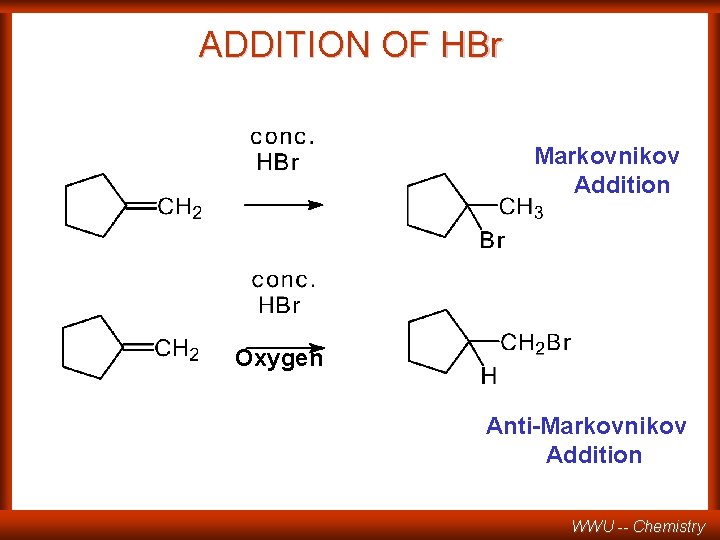
ADDITION OF HBr Markovnikov Addition Oxygen Anti-Markovnikov Addition WWU -- Chemistry

WWU -- Chemistry

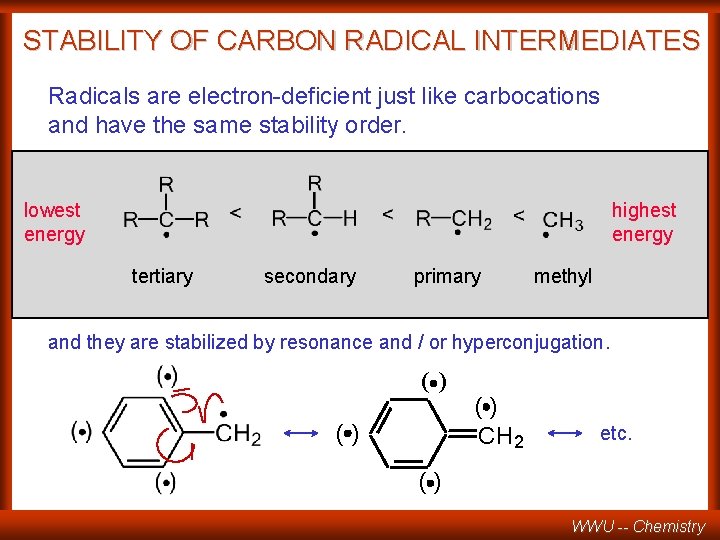
STABILITY OF CARBON RADICAL INTERMEDIATES Radicals are electron-deficient just like carbocations and have the same stability order. lowest energy highest energy tertiary secondary primary methyl and they are stabilized by resonance and / or hyperconjugation. ( ) () () CH 2 etc. () WWU -- Chemistry

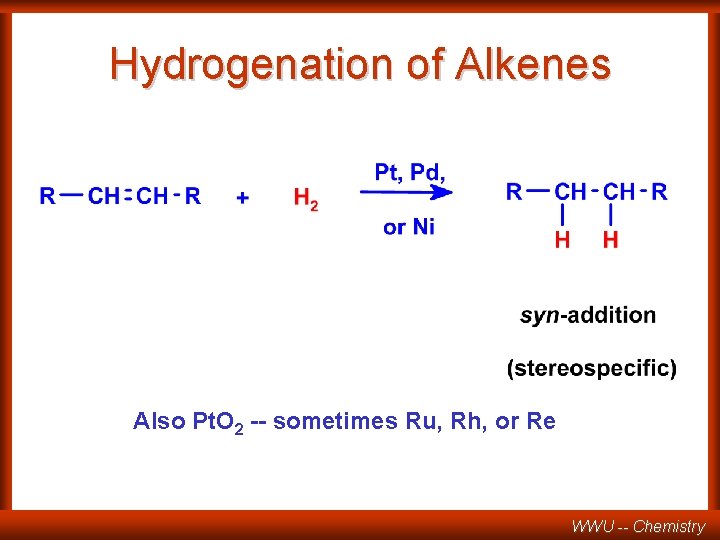
Sect. 8. 11 and 8. 12: Hydrogenation of Alkenes and alkynes WWU -- Chemistry

Hydrogenation of Alkenes Also Pt. O 2 -- sometimes Ru, Rh, or Re WWU -- Chemistry

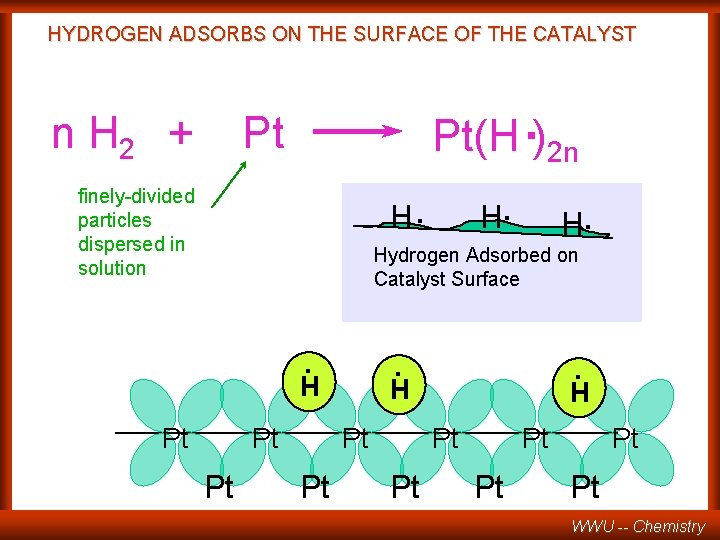
HYDROGEN ADSORBS ON THE SURFACE OF THE CATALYST n H 2 + Pt(H. )2 n Pt H. finely-divided particles dispersed in solution H. Hydrogen Adsorbed on Catalyst Surface . H Pt Pt Pt WWU -- Chemistry

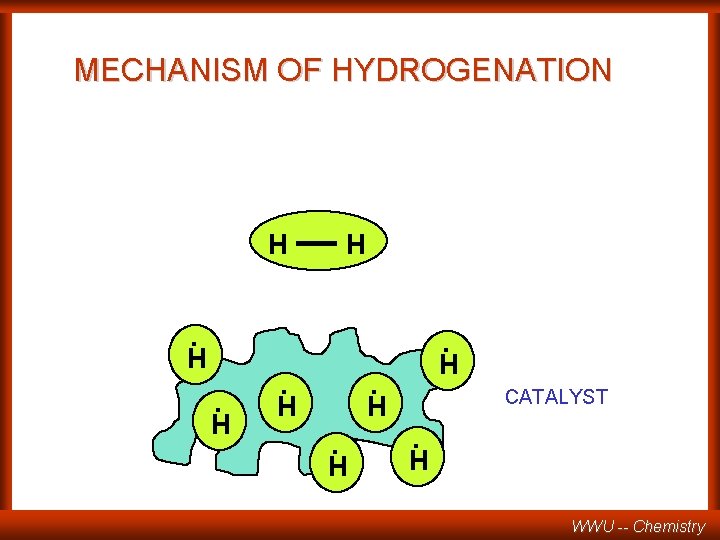
MECHANISM OF HYDROGENATION H . H . H . H. H CATALYST WWU -- Chemistry

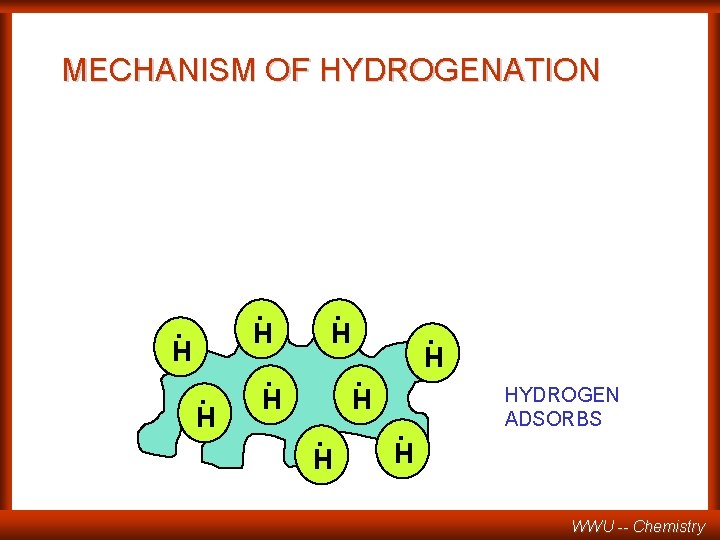
MECHANISM OF HYDROGENATION . . H H . H. H HYDROGEN ADSORBS WWU -- Chemistry

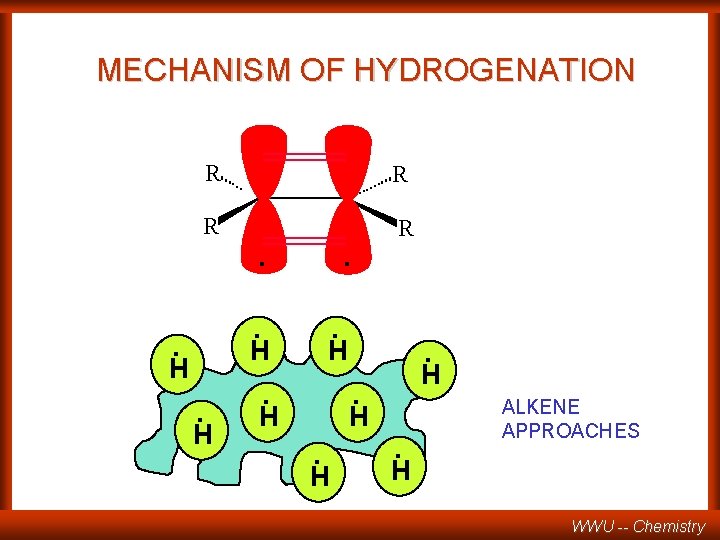
MECHANISM OF HYDROGENATION R R . . . H H . . H. H ALKENE APPROACHES WWU -- Chemistry

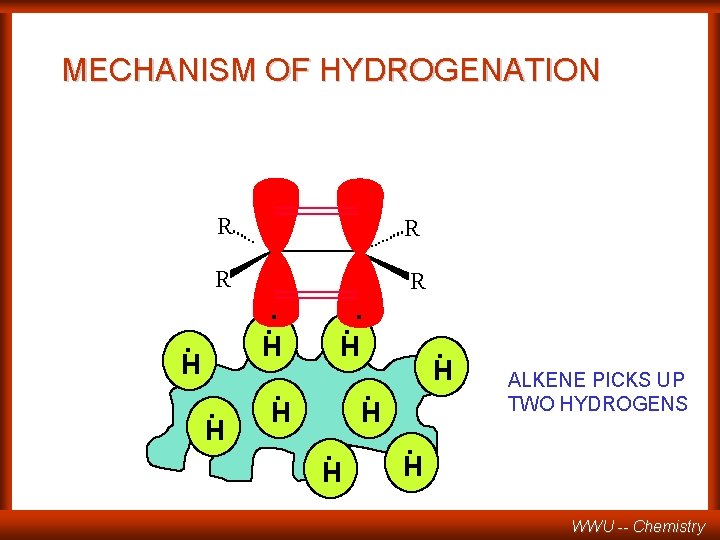
MECHANISM OF HYDROGENATION R R . . . H H . . H. H ALKENE PICKS UP TWO HYDROGENS WWU -- Chemistry

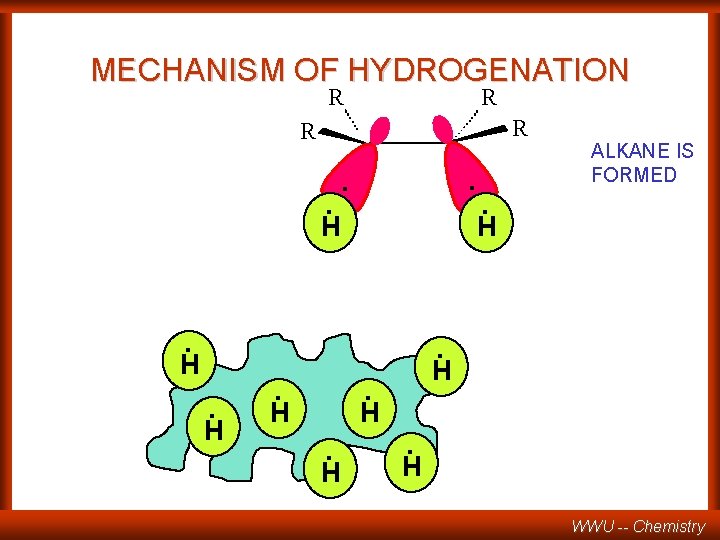
MECHANISM OF HYDROGENATION R R . . H . H ALKANE IS FORMED . H. H WWU -- Chemistry

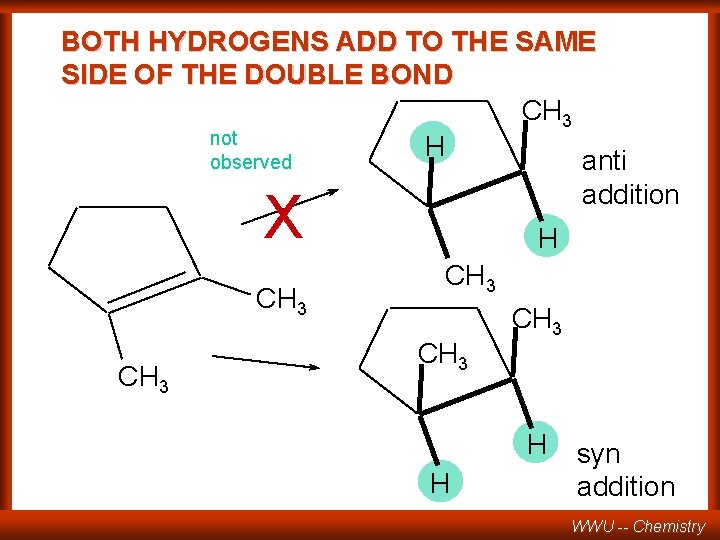
BOTH HYDROGENS ADD TO THE SAME SIDE OF THE DOUBLE BOND CH 3 not H observed anti addition X CH 3 H CH 3 H H syn addition WWU -- Chemistry

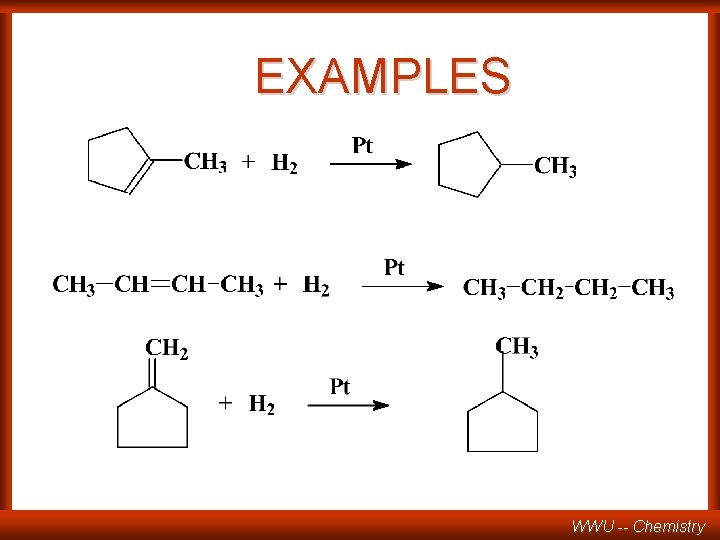
EXAMPLES WWU -- Chemistry

Sect. 8. 14: Addition Polymers WWU -- Chemistry

Polymerization of propene WWU -- Chemistry

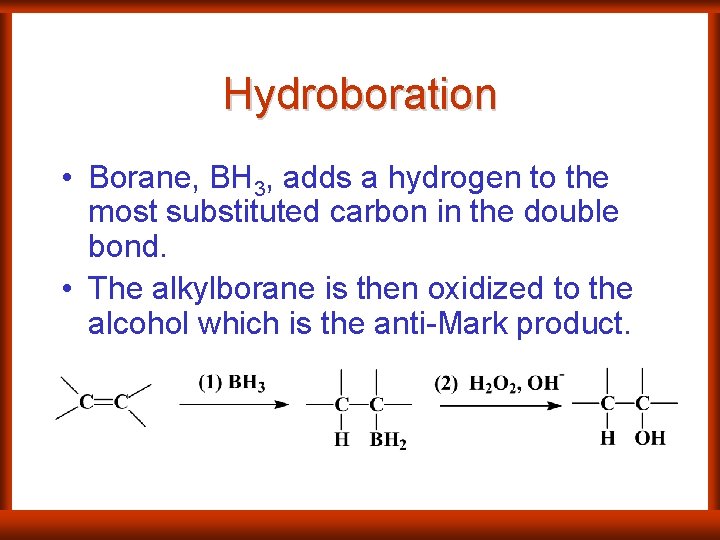
Indirect Hydration • Oxymercuration-Demercuration – Markovnikov product formed – Anti addition of H-OH – No rearrangements • Hydroboration – Anti-Markovnikov product formed – Syn addition of H-OH

Hydroboration • Borane, BH 3, adds a hydrogen to the most substituted carbon in the double bond. • The alkylborane is then oxidized to the alcohol which is the anti-Mark product.

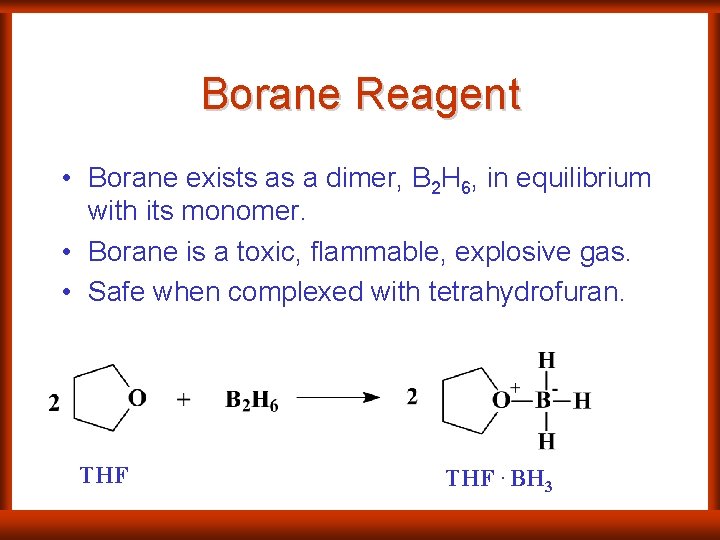
Borane Reagent • Borane exists as a dimer, B 2 H 6, in equilibrium with its monomer. • Borane is a toxic, flammable, explosive gas. • Safe when complexed with tetrahydrofuran. THF. BH 3

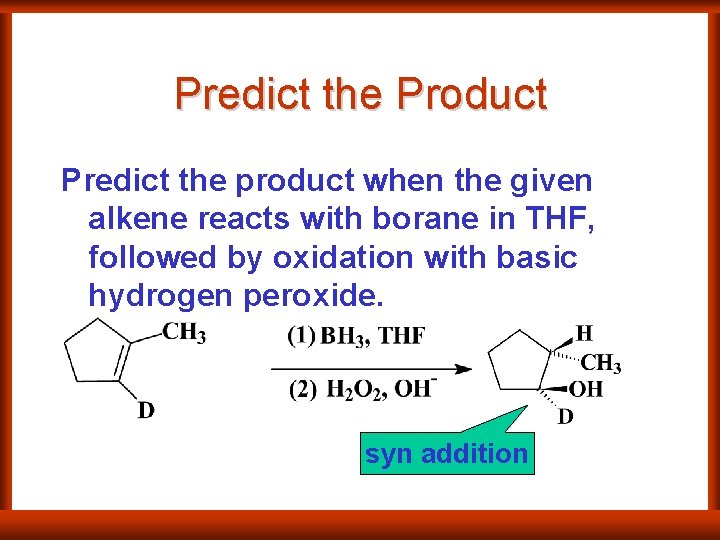
Predict the Product Predict the product when the given alkene reacts with borane in THF, followed by oxidation with basic hydrogen peroxide. syn addition

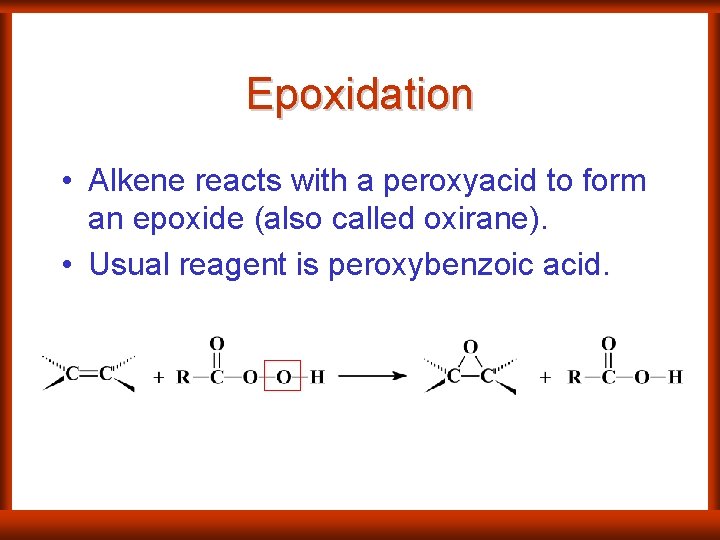
Epoxidation • Alkene reacts with a peroxyacid to form an epoxide (also called oxirane). • Usual reagent is peroxybenzoic acid.

One-Step Reaction • To synthesize the glycol without isolating the epoxide, use aqueous peroxyacetic acid or peroxyformic acid. • The reaction is stereospecific.

Syn Hydroxylation of Alkenes • Alkene is converted to a cis-1, 2 -diol, • Two reagents: – Osmium tetroxide (expensive!), followed by hydrogen peroxide or – Cold, dilute aqueous potassium permanganate, followed by hydrolysis with base Chapter 8 => 68

WWU -- Chemistry