Organic Chemistry Chemical reactions part 1 Alkanes chemical





- Slides: 32

Organic Chemistry Chemical reactions part 1

Alkanes – chemical reactions Combustion Free radical substitution

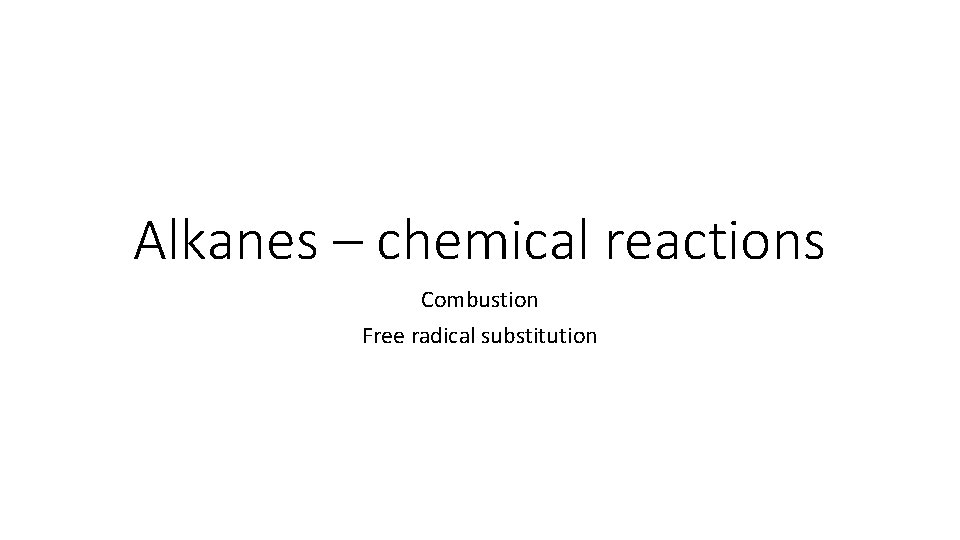
Complete combustion In excess oxygen, short chain alkanes can undergo complete combustion: alkane + oxygen → carbon dioxide + water For example: propane + oxygen → carbon dioxide + water C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) The combustion of alkanes is a highly exothermic process. This makes them good fuels because they release a relatively large amount of energy per gram of fuel.

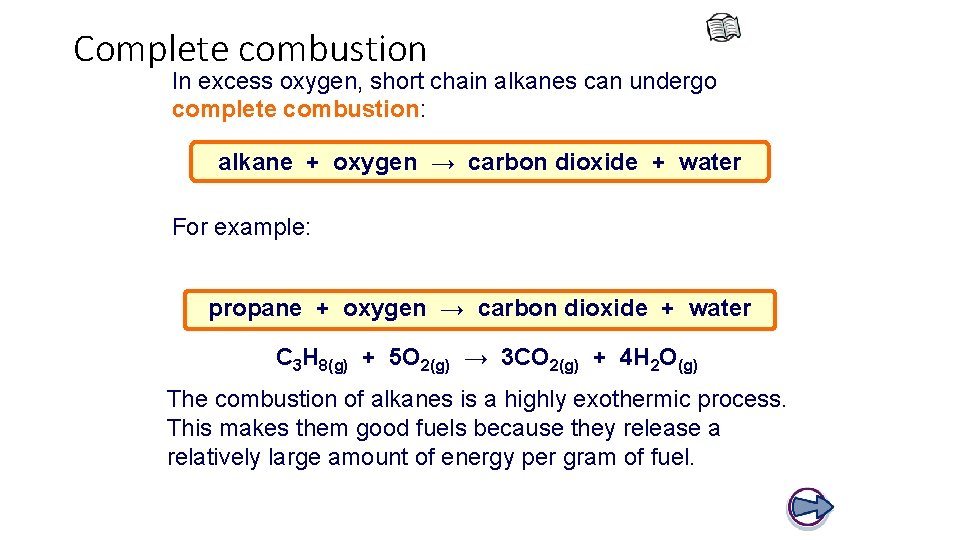
Incomplete combustion If oxygen is limited then incomplete combustion will occur: alkane + oxygen → carbon monoxide + water alkane + oxygen → carbon + water For example: propane + oxygen → carbon monoxide + water C 3 H 8(g) + 3½O 2(g) → 3 CO(g) + 4 H 2 O(g) propane + oxygen → carbon + water C 3 H 8(g) + 2 O 2(g) → 3 C(s) + 4 H 2 O(g)

Haloalkanes Making haloalkanes and reactions they can take part in

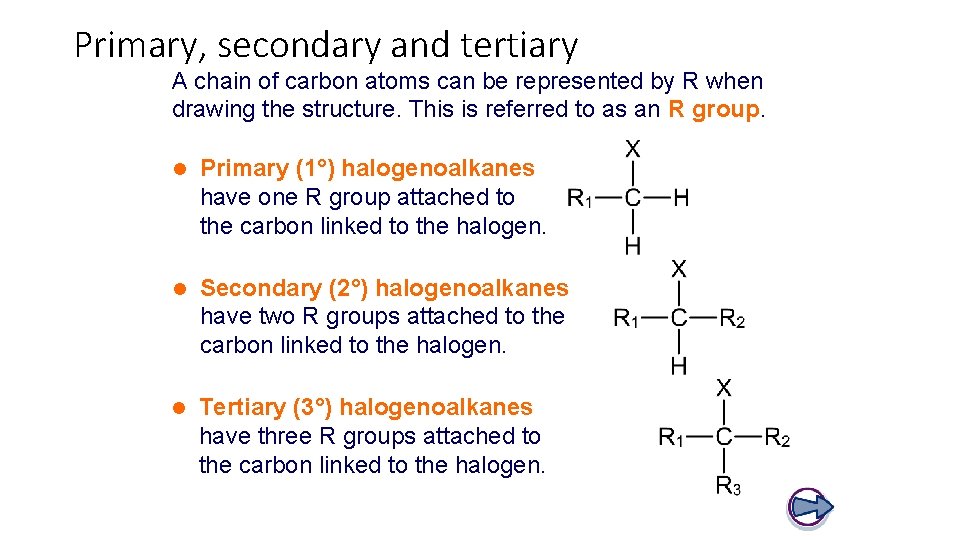
Primary, secondary and tertiary A chain of carbon atoms can be represented by R when drawing the structure. This is referred to as an R group. l Primary (1°) halogenoalkanes have one R group attached to the carbon linked to the halogen. l Secondary (2°) halogenoalkanes have two R groups attached to the carbon linked to the halogen. l Tertiary (3°) halogenoalkanes have three R groups attached to the carbon linked to the halogen.

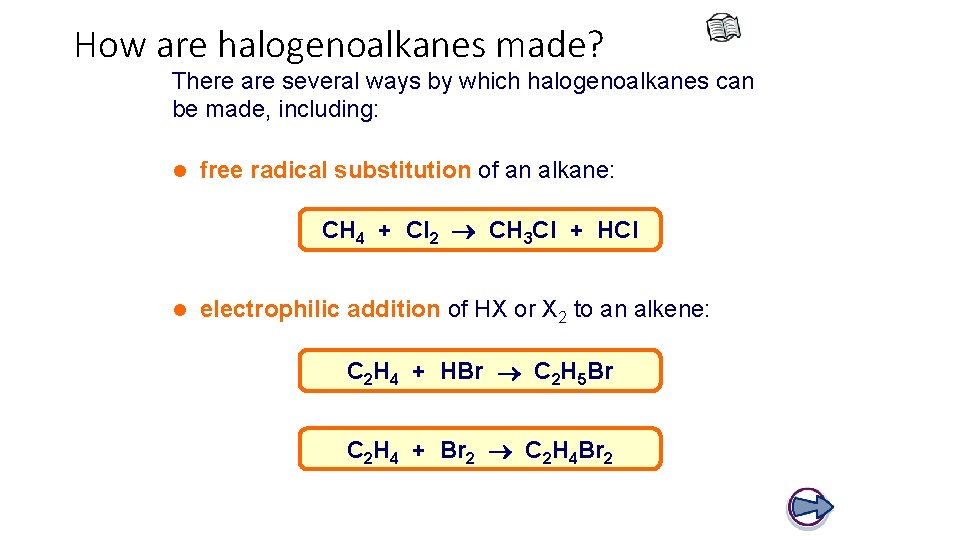
How are halogenoalkanes made? There are several ways by which halogenoalkanes can be made, including: l free radical substitution of an alkane: CH 4 + Cl 2 CH 3 Cl + HCl l electrophilic addition of HX or X 2 to an alkene: C 2 H 4 + HBr C 2 H 5 Br C 2 H 4 + Br 2 C 2 H 4 Br 2

Free radical substitution: Cl 2 + CH 4

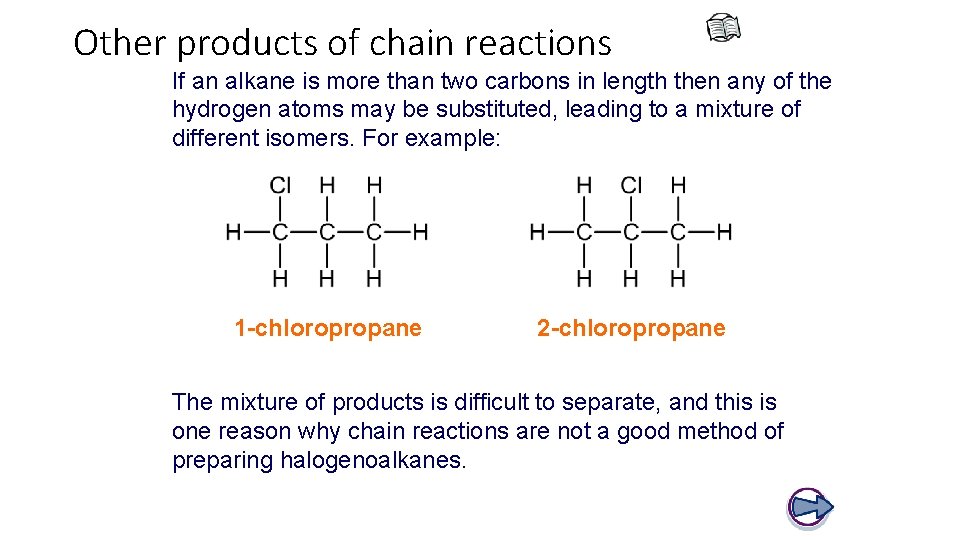
Other products of chain reactions If an alkane is more than two carbons in length then any of the hydrogen atoms may be substituted, leading to a mixture of different isomers. For example: 1 -chloropropane 2 -chloropropane The mixture of products is difficult to separate, and this is one reason why chain reactions are not a good method of preparing halogenoalkanes.

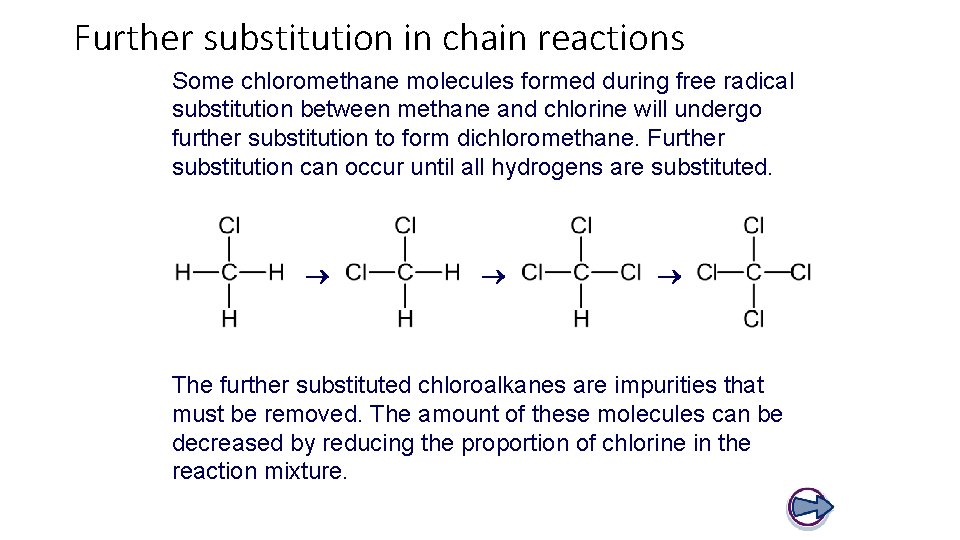
Further substitution in chain reactions Some chloromethane molecules formed during free radical substitution between methane and chlorine will undergo further substitution to form dichloromethane. Further substitution can occur until all hydrogens are substituted. The further substituted chloroalkanes are impurities that must be removed. The amount of these molecules can be decreased by reducing the proportion of chlorine in the reaction mixture.

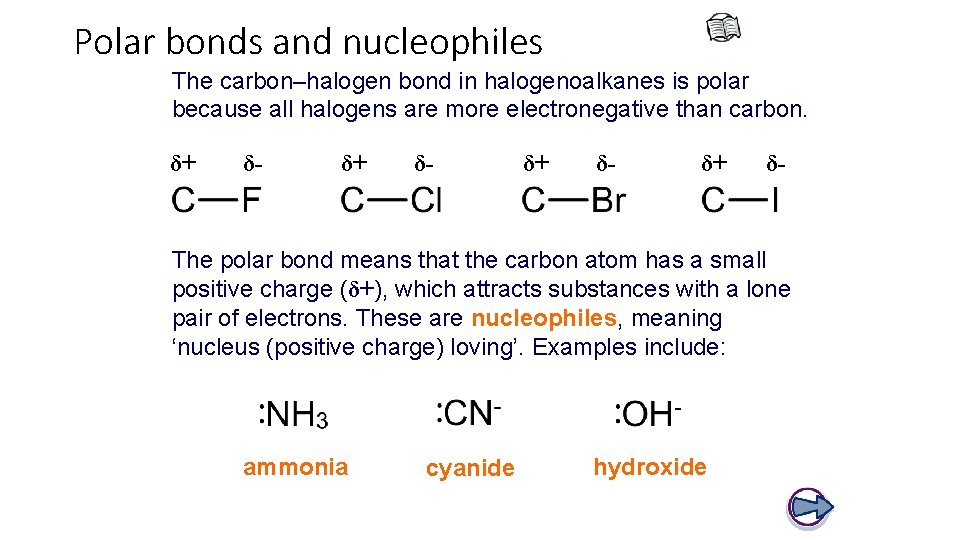
Polar bonds and nucleophiles The carbon–halogen bond in halogenoalkanes is polar because all halogens are more electronegative than carbon. δ+ δ- The polar bond means that the carbon atom has a small positive charge (δ+), which attracts substances with a lone pair of electrons. These are nucleophiles, meaning ‘nucleus (positive charge) loving’. Examples include: ammonia cyanide hydroxide

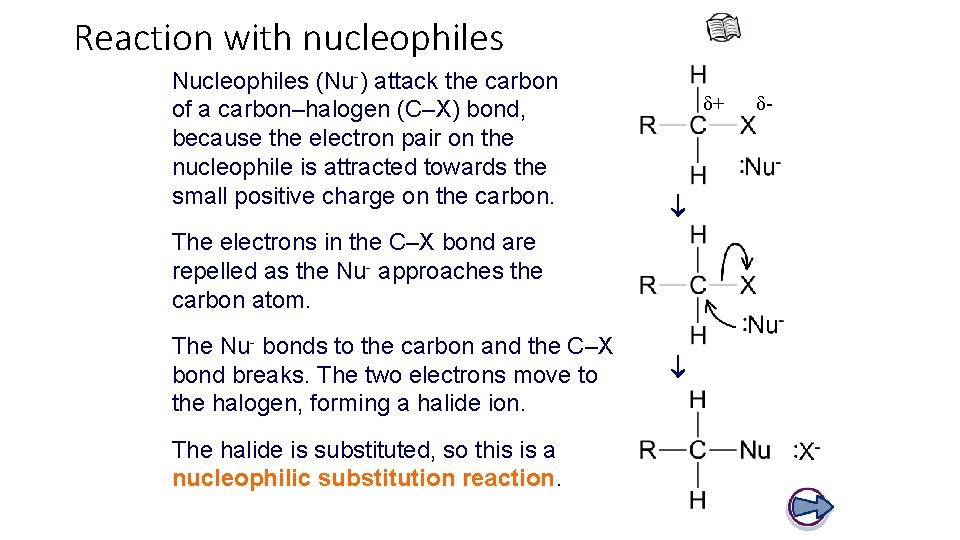
Reaction with nucleophiles δ+ Nucleophiles (Nu-) attack the carbon of a carbon–halogen (C–X) bond, because the electron pair on the nucleophile is attracted towards the small positive charge on the carbon. The electrons in the C–X bond are repelled as the Nu- approaches the carbon atom. The halide is substituted, so this is a nucleophilic substitution reaction. The Nu- bonds to the carbon and the C–X bond breaks. The two electrons move to the halogen, forming a halide ion. δ-

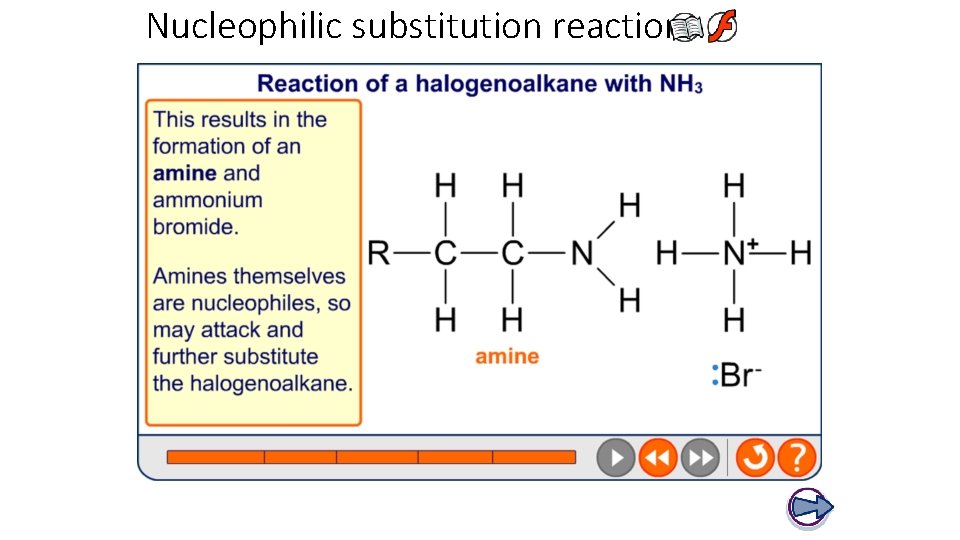
Nucleophilic substitution reactions

Alkenes Combustion reactions Addition reactions Polymerisation reactions

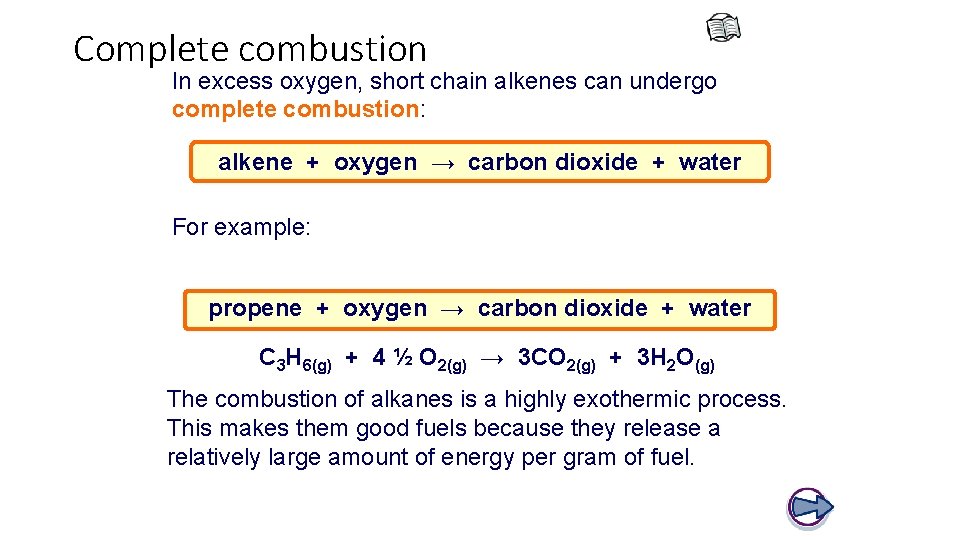
Complete combustion In excess oxygen, short chain alkenes can undergo complete combustion: alkene + oxygen → carbon dioxide + water For example: propene + oxygen → carbon dioxide + water C 3 H 6(g) + 4 ½ O 2(g) → 3 CO 2(g) + 3 H 2 O(g) The combustion of alkanes is a highly exothermic process. This makes them good fuels because they release a relatively large amount of energy per gram of fuel.

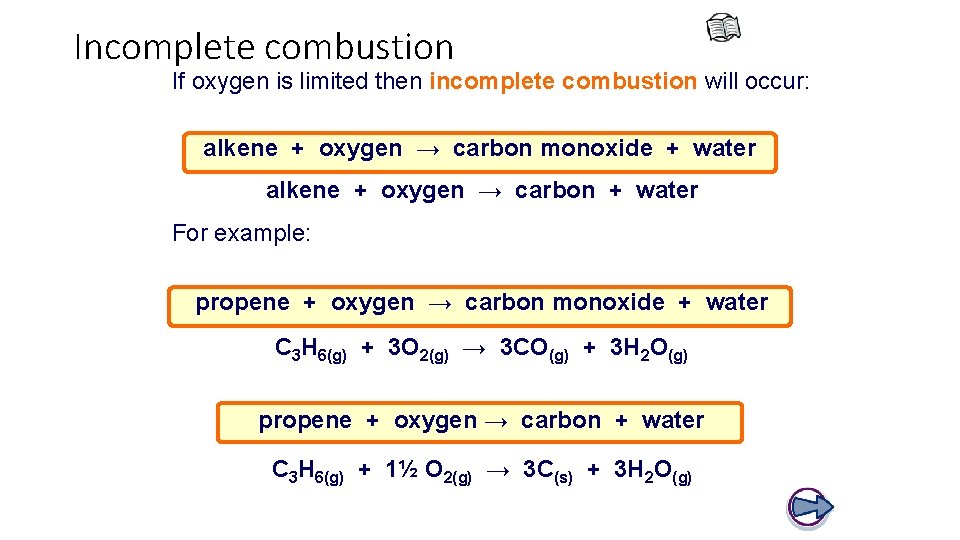
Incomplete combustion If oxygen is limited then incomplete combustion will occur: alkene + oxygen → carbon monoxide + water alkene + oxygen → carbon + water For example: propene + oxygen → carbon monoxide + water C 3 H 6(g) + 3 O 2(g) → 3 CO(g) + 3 H 2 O(g) propene + oxygen → carbon + water C 3 H 6(g) + 1½ O 2(g) → 3 C(s) + 3 H 2 O(g)

Double bonds and electrophiles The double bond of an alkene is an area of high electron density, and therefore an area of high negative charge. The negative charge of the double bond may be attacked by electron-deficient species, which will accept a lone pair of electrons. These species have either a full positive charge or slight positive charge on one or more of their atoms. They are called electrophiles, meaning ‘electron loving’. Alkenes undergo addition reactions when attacked by electrophiles. This is called electrophilic addition.

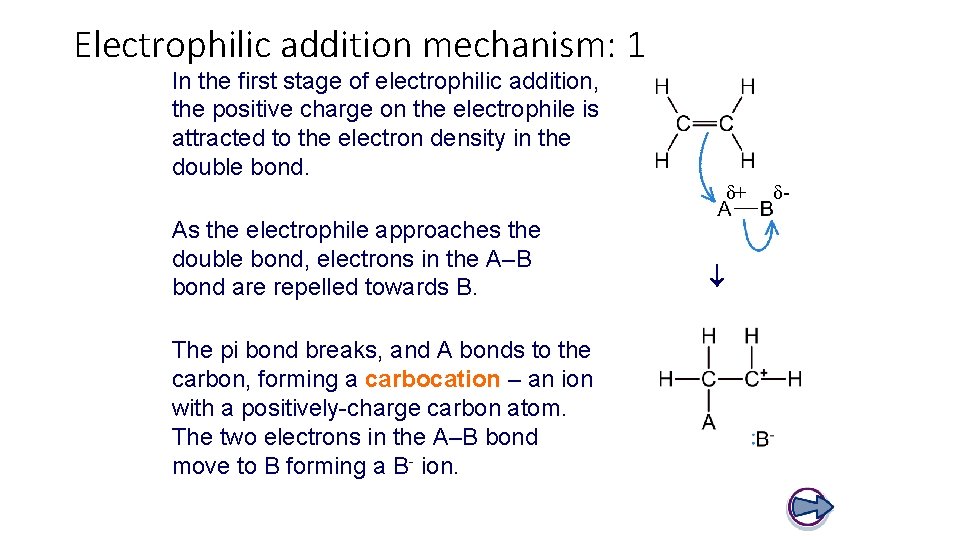
Electrophilic addition mechanism: 1 In the first stage of electrophilic addition, the positive charge on the electrophile is attracted to the electron density in the double bond. δ+ The pi bond breaks, and A bonds to the carbon, forming a carbocation – an ion with a positively-charge carbon atom. The two electrons in the A–B bond move to B forming a B- ion. As the electrophile approaches the double bond, electrons in the A–B bond are repelled towards B. δ-

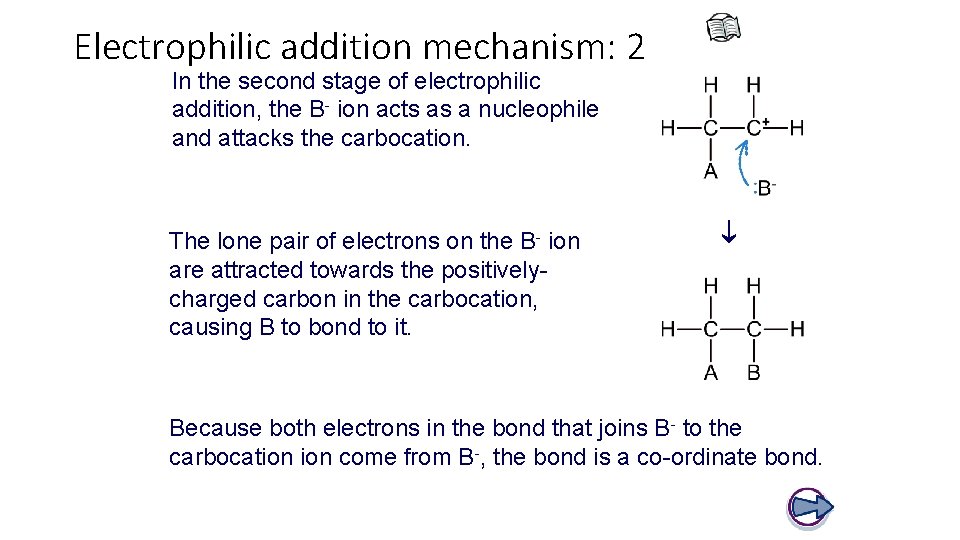
Electrophilic addition mechanism: 2 In the second stage of electrophilic addition, the B- ion acts as a nucleophile and attacks the carbocation. The lone pair of electrons on the B- ion are attracted towards the positivelycharged carbon in the carbocation, causing B to bond to it. Because both electrons in the bond that joins B- to the carbocation come from B-, the bond is a co-ordinate bond.

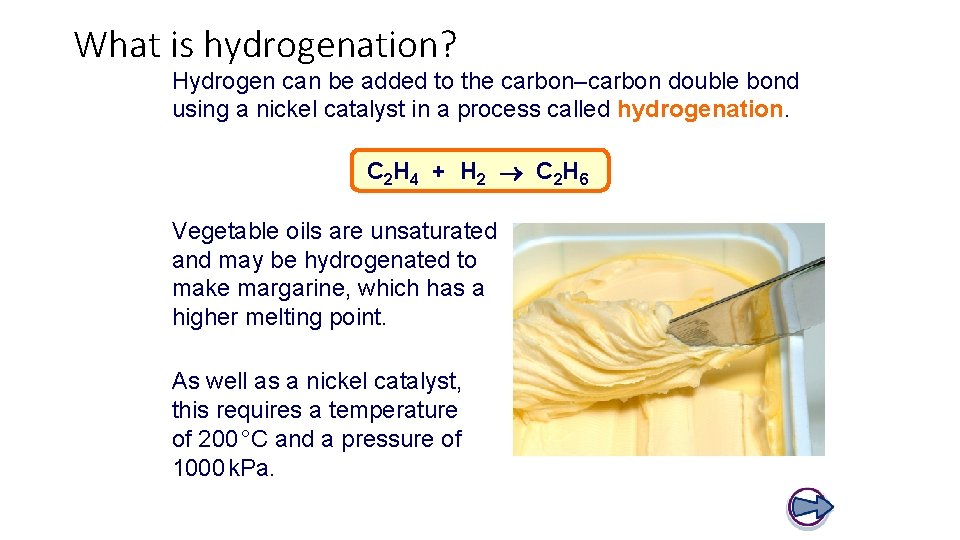
What is hydrogenation? Hydrogen can be added to the carbon–carbon double bond using a nickel catalyst in a process called hydrogenation. C 2 H 4 + H 2 C 2 H 6 Vegetable oils are unsaturated and may be hydrogenated to make margarine, which has a higher melting point. As well as a nickel catalyst, this requires a temperature of 200 °C and a pressure of 1000 k. Pa.

Testing for alkenes The presence of unsaturation (a carbon– carbon double bond) can be detected using bromine water, a red/orange coloured solution of bromine. A few drops of bromine water are added to the test liquid and shaken. If a carbon–carbon double bond is present, the bromine adds across it and the solution turns colourless.

More on the bromine water test A simple equation for the bromine water test with ethene is: CH 2=CH 2 + Br 2 + H 2 O CH 2 Br + H 2 O However, because water is present in such a large amount, a water molecule (which has a lone pair) adds to one of the carbon atoms, followed by the loss of a H+ ion. CH 2=CH 2 + Br 2 + H 2 O CH 2 Br. CH 2 OH + HBr The major product of the test is not 1, 2 -dibromoethane (CH 2 Br) but 2 -bromoethan-1 -ol (CH 2 Br. CH 2 OH).

Electrophilic addition reactions

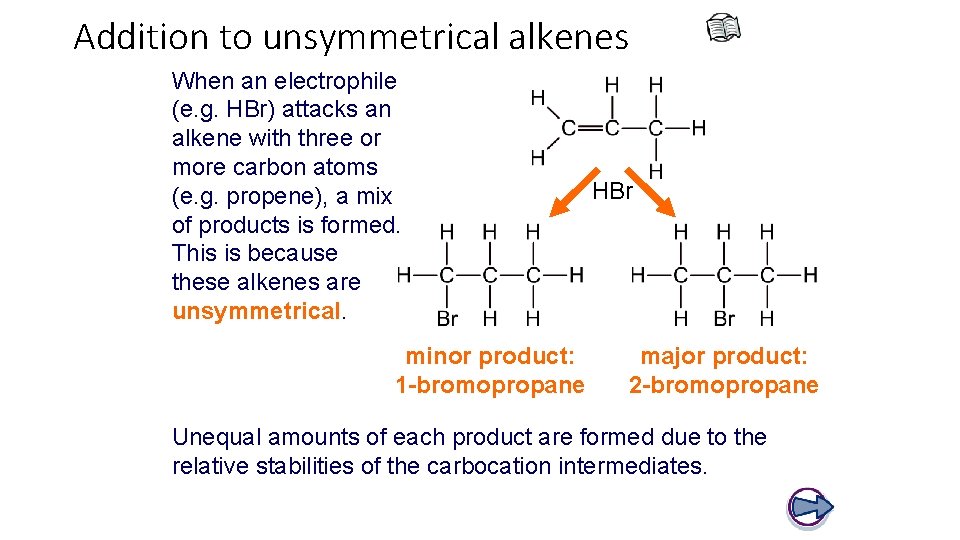
Addition to unsymmetrical alkenes When an electrophile (e. g. HBr) attacks an alkene with three or more carbon atoms (e. g. propene), a mix of products is formed. This is because these alkenes are unsymmetrical. minor product: 1 -bromopropane HBr major product: 2 -bromopropane Unequal amounts of each product are formed due to the relative stabilities of the carbocation intermediates.

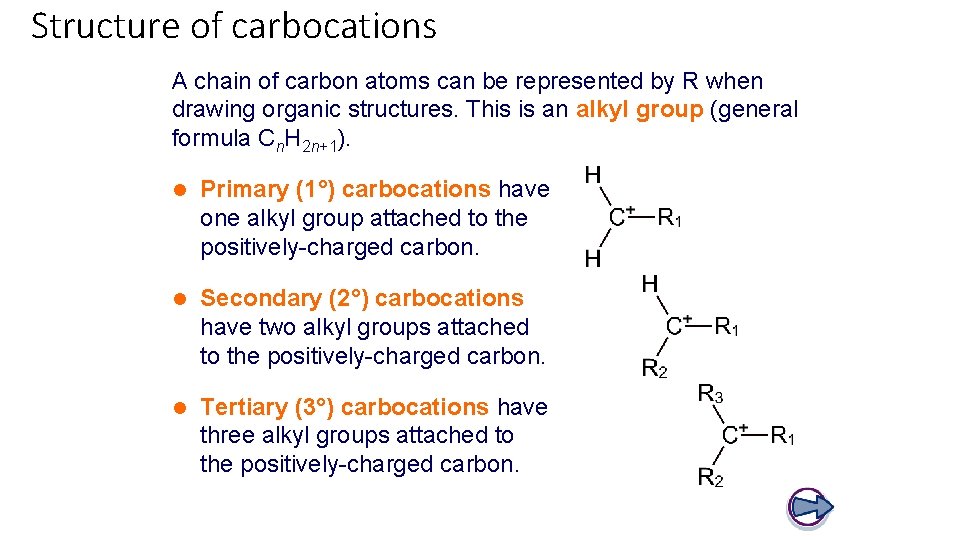
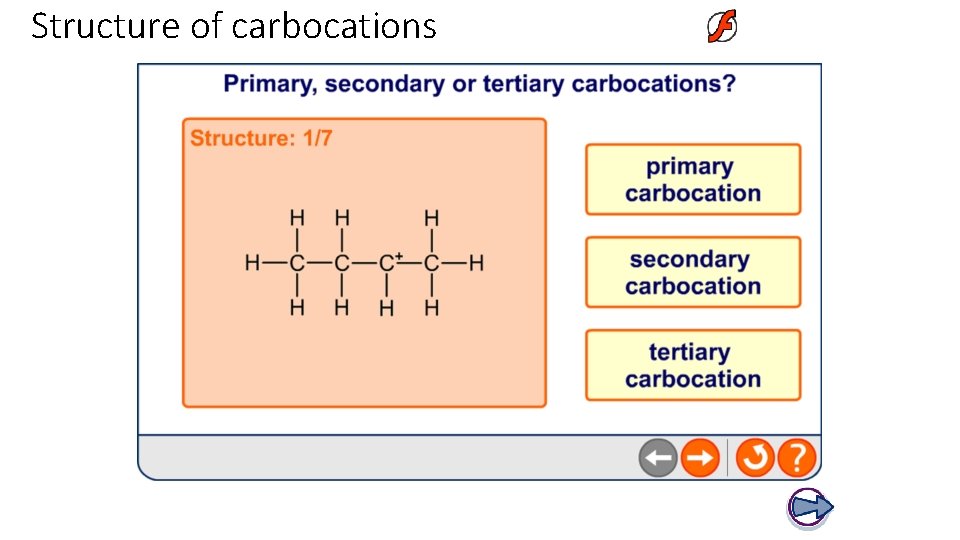
Structure of carbocations A chain of carbon atoms can be represented by R when drawing organic structures. This is an alkyl group (general formula Cn. H 2 n+1). l Primary (1°) carbocations have one alkyl group attached to the positively-charged carbon. l Secondary (2°) carbocations have two alkyl groups attached to the positively-charged carbon. l Tertiary (3°) carbocations have three alkyl groups attached to the positively-charged carbon.

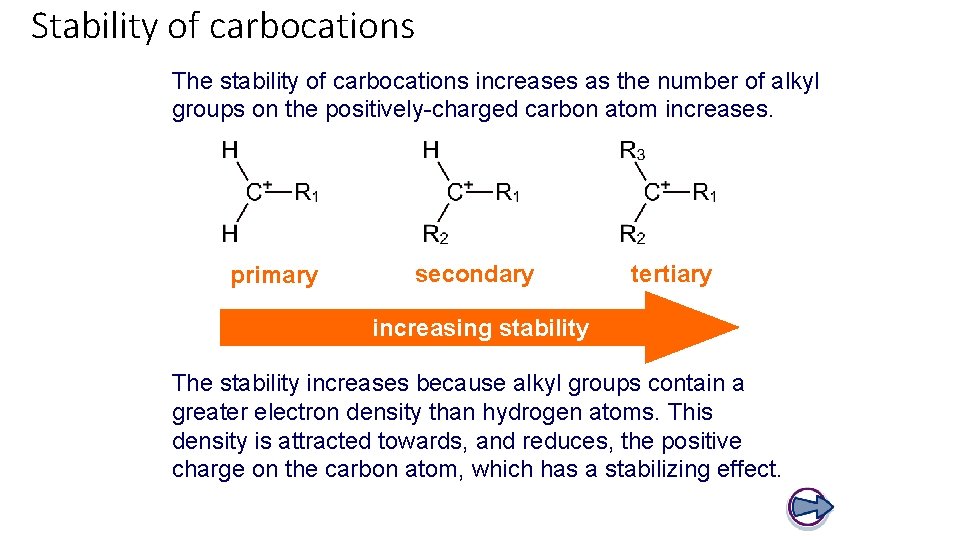
Stability of carbocations The stability of carbocations increases as the number of alkyl groups on the positively-charged carbon atom increases. primary secondary tertiary increasing stability The stability increases because alkyl groups contain a greater electron density than hydrogen atoms. This density is attracted towards, and reduces, the positive charge on the carbon atom, which has a stabilizing effect.

Structure of carbocations

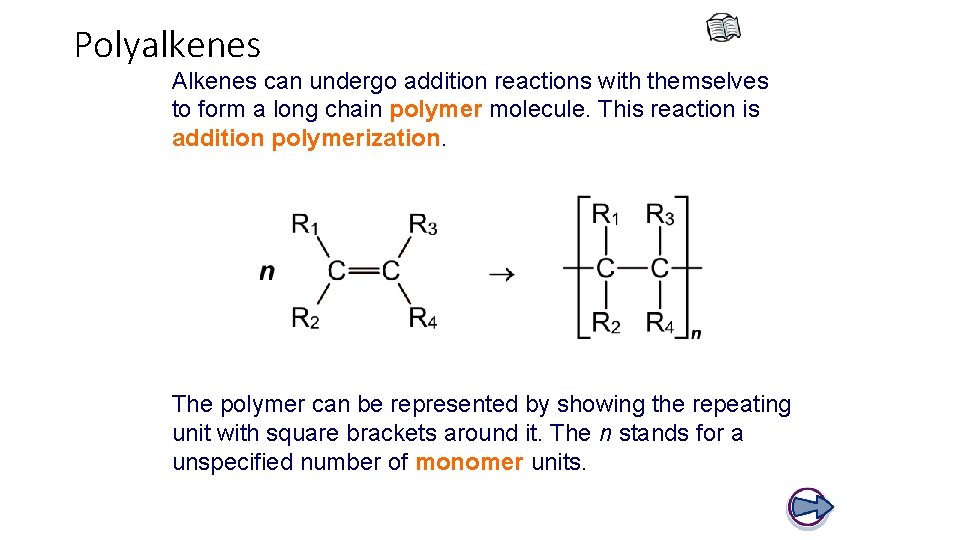
Polyalkenes Alkenes can undergo addition reactions with themselves to form a long chain polymer molecule. This reaction is addition polymerization. The polymer can be represented by showing the repeating unit with square brackets around it. The n stands for a unspecified number of monomer units.

Polymerization of ethene

LDPE and HDPE The reaction conditions under which ethene polymerizes affect the structure and properties of the poly(e)thene. l Low-density polythene (LDPE) is formed under a high pressure (1400 atm) and a temperature of about 170 °C. These conditions cause a high level of branching, meaning that the polymer chains cannot pack tightly together. l High-density polythene (HDPE) is formed with a catalyst, a pressure of 2 atm and a temperature of about 70 °C. Little branching occurs under these conditions, resulting in chains that can pack tightly together to create a denser material.

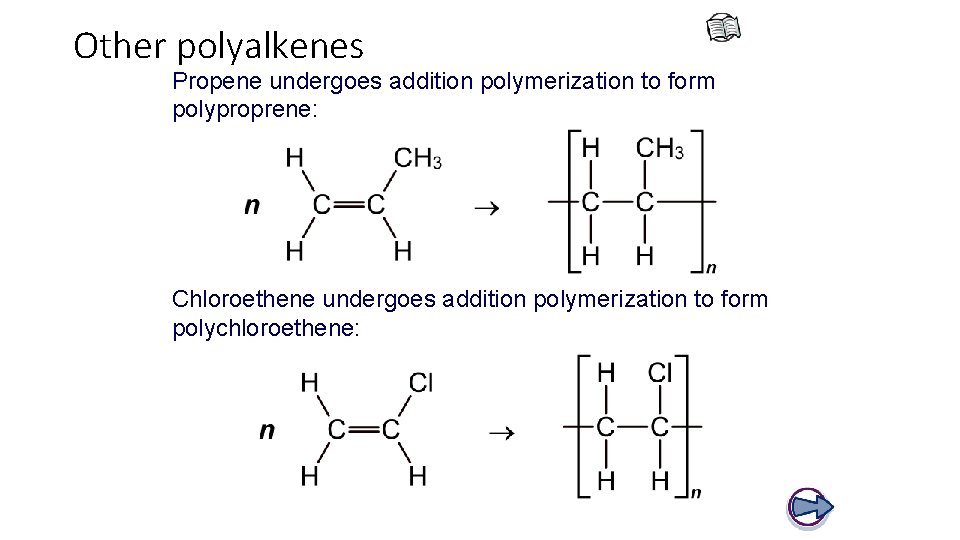
Other polyalkenes Propene undergoes addition polymerization to form polyproprene: Chloroethene undergoes addition polymerization to form polychloroethene:

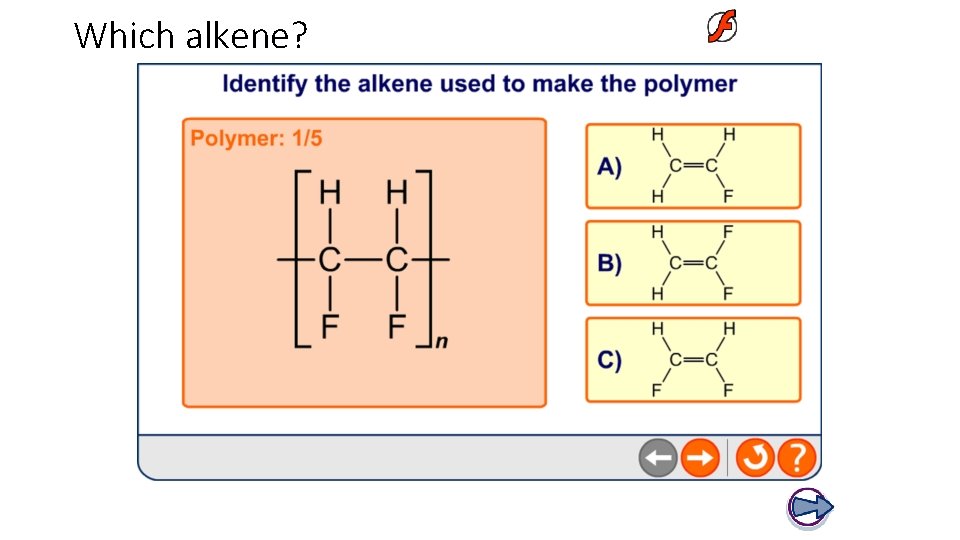
Which alkene?