Considerations for Optimizing Transplant in Multiple Myeloma Patient



![Incidence and Mortality by Race/Ethnicity and Gender Male Age, [1] years Female Incidence*[2] Mortality*[2] Incidence and Mortality by Race/Ethnicity and Gender Male Age, [1] years Female Incidence*[2] Mortality*[2]](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-6.jpg)

![Imaging of Residual Disease in MM PET/CT[1, 2] • PET may be useful for Imaging of Residual Disease in MM PET/CT[1, 2] • PET may be useful for](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-13.jpg)

![Risk-adapted Melphalan Dosing Suggested Melphalan Dose-adjustment for Patients with Renal Impairment[1] Cr. Cl >15 Risk-adapted Melphalan Dosing Suggested Melphalan Dose-adjustment for Patients with Renal Impairment[1] Cr. Cl >15](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-22.jpg)
![Intensification of MEL 220 mg/m 2 (French single arm study)[1] Escalating MEL to 300 Intensification of MEL 220 mg/m 2 (French single arm study)[1] Escalating MEL to 300](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-24.jpg)




![Prospective, Randomized Studies Evaluating HSCT for the Treatment of MM Study Barlogie 2006[1] Bladé Prospective, Randomized Studies Evaluating HSCT for the Treatment of MM Study Barlogie 2006[1] Bladé](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-50.jpg)
![Regimens Survival Bortezomib/lenalidomide/ dexamethasone (RVD)[1] 18 -mo PFS: 75% 18 -mo OS: 97% Carfilzomib/lenalidomide/ Regimens Survival Bortezomib/lenalidomide/ dexamethasone (RVD)[1] 18 -mo PFS: 75% 18 -mo OS: 97% Carfilzomib/lenalidomide/](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-55.jpg)








![Maintenance in Myeloma • PFS advantage[1 -3] • OS improvements? [2] • Toxicities of Maintenance in Myeloma • PFS advantage[1 -3] • OS improvements? [2] • Toxicities of](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-95.jpg)

![Methods for Assessing MRD to Predict Outcome 8 -color Flow[1] Next Gen Sequencing[2] TTP Methods for Assessing MRD to Predict Outcome 8 -color Flow[1] Next Gen Sequencing[2] TTP](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-101.jpg)











- Slides: 161

Considerations for Optimizing Transplant in Multiple Myeloma Patient Management The first 68 slides of this library should be considered a sample presentation only. You should customize your presentation using the slides in the library, keeping in mind that the learning objectives must be met.

Considerations for Optimizing Transplant in Multiple Myeloma Patient Management

Learning Objectives After completing this program, participants should be able to: 1. Identify factors that might determine eligibility for and timing of high-dose chemotherapy (HDT) followed by autologous stem cell transplant (ASCT) in patients with multiple myeloma (MM) 2. Differentiate treatment strategies for patients with newly diagnosed multiple myeloma who are eligible for HDT/ASCT 3. Identify barriers in access to HDT/ASCT for MM 4. Review the rationale for conditioning regimens in patients with multiple myeloma who are eligible for HDT/ASCT 5. Compare and contrast pertinent data regarding a new formulation of melphalan

MYELOMA OVERVIEW

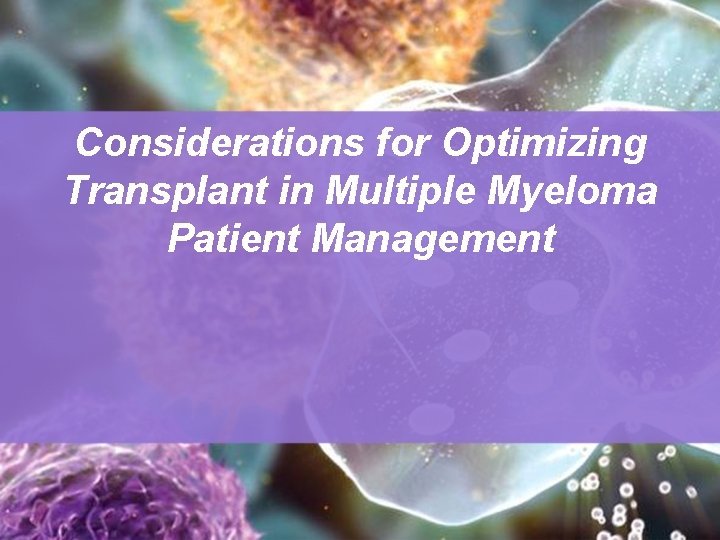
Hallmarks of MM Lytic lesions, Pathologic fractures, Hypercalcemia Anemia Plasma cell Bone destruction Marrow infiltration MULTIPLE MYELOMA Monoclonal globulins Urine: Renal failure Blood: Hyperviscosity, Cryoglobulins, Neuropathy Tissue: Amyloidosis Carr et al, 1999. Reduced globulins Infection
![Incidence and Mortality by RaceEthnicity and Gender Male Age 1 years Female Incidence2 Mortality2 Incidence and Mortality by Race/Ethnicity and Gender Male Age, [1] years Female Incidence*[2] Mortality*[2]](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-6.jpg)
Incidence and Mortality by Race/Ethnicity and Gender Male Age, [1] years Female Incidence*[2] Mortality*[2] 7. 9 4. 2 (range) All Races Age, [1] years (range) Incidence*[2] Mortality*[2] 5. 1 2. 7 White 70 (61 -78) 7. 5 4. 0 72 (62 -81) 4. 5 2. 4 Black 65 (57 -74) 15. 1 7. 6 67 (57 -76) 11. 2 5. 3 Asian/Pacific Islander 69 (60 -77) 4. 6 2. 2 70 (59 -78) 3. 0 1. 4 4. 6 3. 2 4. 2 2. 3 7. 3 3. 5 4. 8 2. 3 American Indian/Alaska Native Hispanic 65 (55 -74) 66 (56 -76) *Age-adjusted rates per 100, 000 [1] Costa et al. Biol Blood Marrow Transplant. 2015 Apr; 21: 701 -706. [2] SEER Stat Fact Sheets: Myeloma. Available at http: //seer. cancer. gov/statfacts/html/mulmy. html. Accessed August 2015.

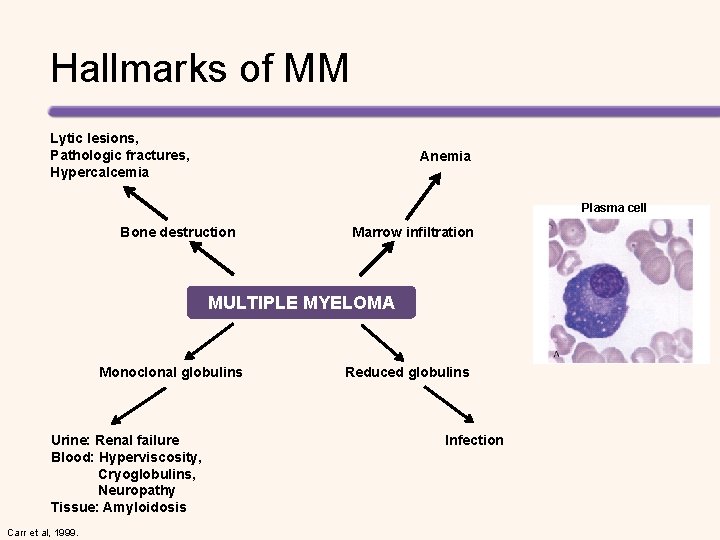
Revised International Staging System (R-ISS) for MM • R-ISS I (n = 871) – Including ISS stage I (serum β 2 -microglobulin level < 3. 5 mg/L and serum albumin level ≥ 3. 5 g/d. L) – No high-risk CA [del(17 p) and/or t(4; 14) and/or t(14; 16)] – Normal LDH level (less than the upper limit of normal range) • R-ISS III (n = 295) – Including ISS stage III (serum β 2 -microglobulin level > 5. 5 mg/L) – High-risk CA or high LDH level • R-ISS II (n = 1, 894) – Including all the other possible combinations 5 -Year OS* 5 -Year PFS* R-ISS I 82% 55% R-ISS II 62% 36% R-ISS III 40% 24% Palumbo, et al. JCO. 2015; 33(26): 2863 -2869. *At a median follow-up of 46 months

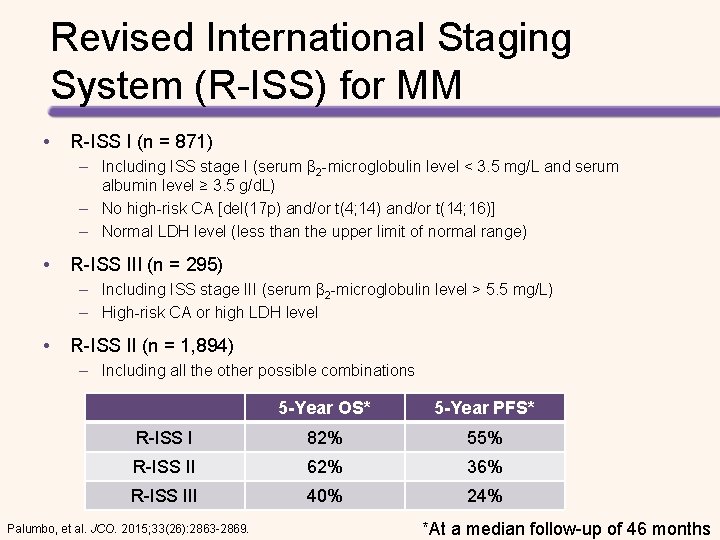
Indications for Considering Treatment (IMWG Consensus Guidelines) • At least one of the CRAB Criteria (evidence of end organ damage) CRAB Criteria • • Hypercalcemia Serum calcium >2. 75 mmol/L (>11 mg/d. L) Renal Failure Serum creatinine ≥ 2 mg/d. L or creatinine clearance <40 m. L per min Anemia Hemoglobin >20 g/L below the lower limit of normal, or a hemoglobin value <100 g/L Bone Lytic lesions, pathologic fractures, or severe osteopenia ≥ 60% clonal bone marrow plasma cells Serum involved/uninvolved free light chain ratio ≥ 100 >1 Focal bone lesion (≥ 5 mm) on MRI Clinical judgement Rajkumar, et al. Lancet Oncology. 2014; 15(12): e 538 -48.

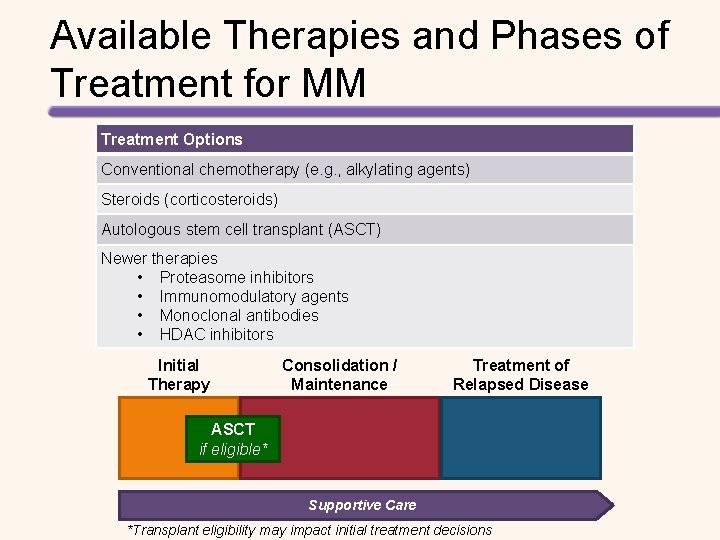
Available Therapies and Phases of Treatment for MM Treatment Options Conventional chemotherapy (e. g. , alkylating agents) Steroids (corticosteroids) Autologous stem cell transplant (ASCT) Newer therapies • Proteasome inhibitors • Immunomodulatory agents • Monoclonal antibodies • HDAC inhibitors Initial Therapy Consolidation / Maintenance Treatment of Relapsed Disease ASCT if eligible* Supportive Care *Transplant eligibility may impact initial treatment decisions

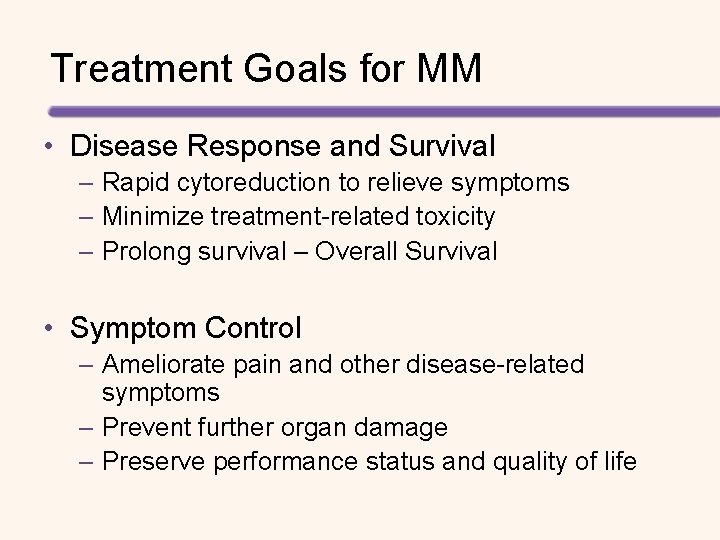
Treatment Goals for MM • Disease Response and Survival – Rapid cytoreduction to relieve symptoms – Minimize treatment-related toxicity – Prolong survival – Overall Survival • Symptom Control – Ameliorate pain and other disease-related symptoms – Prevent further organ damage – Preserve performance status and quality of life

ASSESSING AND MONITORING RESPONSE TO THERAPY

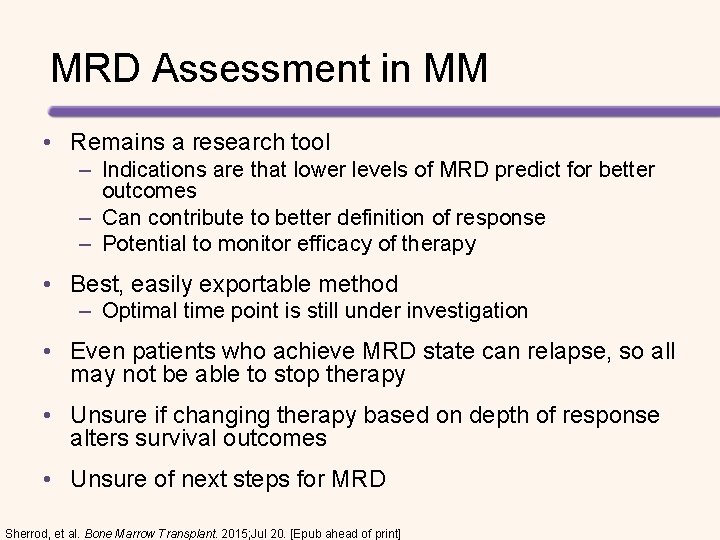
MRD Assessment in MM • Remains a research tool – Indications are that lower levels of MRD predict for better outcomes – Can contribute to better definition of response – Potential to monitor efficacy of therapy • Best, easily exportable method – Optimal time point is still under investigation • Even patients who achieve MRD state can relapse, so all may not be able to stop therapy • Unsure if changing therapy based on depth of response alters survival outcomes • Unsure of next steps for MRD Sherrod, et al. Bone Marrow Transplant. 2015; Jul 20. [Epub ahead of print]
![Imaging of Residual Disease in MM PETCT1 2 PET may be useful for Imaging of Residual Disease in MM PET/CT[1, 2] • PET may be useful for](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-13.jpg)
Imaging of Residual Disease in MM PET/CT[1, 2] • PET may be useful for some, but not all, patients 1. 0 PFS (Proportion) • Variability in PET approaches across different studies and institutions PFS (CR Pts After First-line Therapy) PET CR median: 90 mos 0. 8 0. 6 0. 4 NO PET CR median: 50 mos 0. 2 P =. 010 0 0 12 24 [1] Zamagni E, et al. Blood. 2011; 118: 5989 -95. [2] Zamagni E, et al. ASH 2013. Abstract 1936. 36 Mos 48 60 72

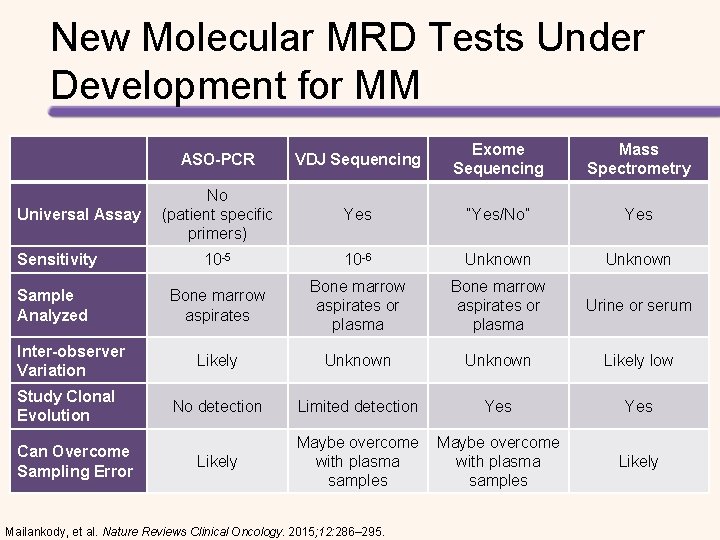
New Molecular MRD Tests Under Development for MM Universal Assay Sensitivity Sample Analyzed Inter-observer Variation Study Clonal Evolution Can Overcome Sampling Error ASO-PCR VDJ Sequencing Exome Sequencing Mass Spectrometry No (patient specific primers) Yes “Yes/No” Yes 10 -5 10 -6 Unknown Bone marrow aspirates or plasma Urine or serum Likely Unknown Likely low No detection Limited detection Yes Likely Maybe overcome with plasma samples Mailankody, et al. Nature Reviews Clinical Oncology. 2015; 12: 286– 295. Likely

AUTOLOGOUS TRANSPLANT When and How?

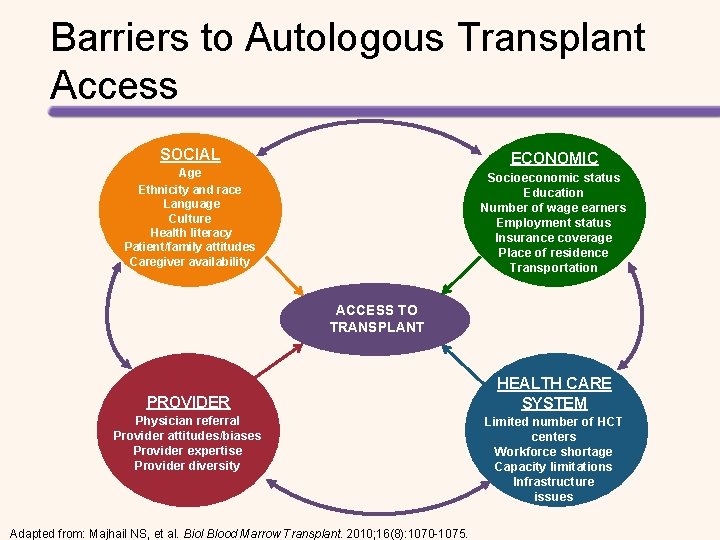
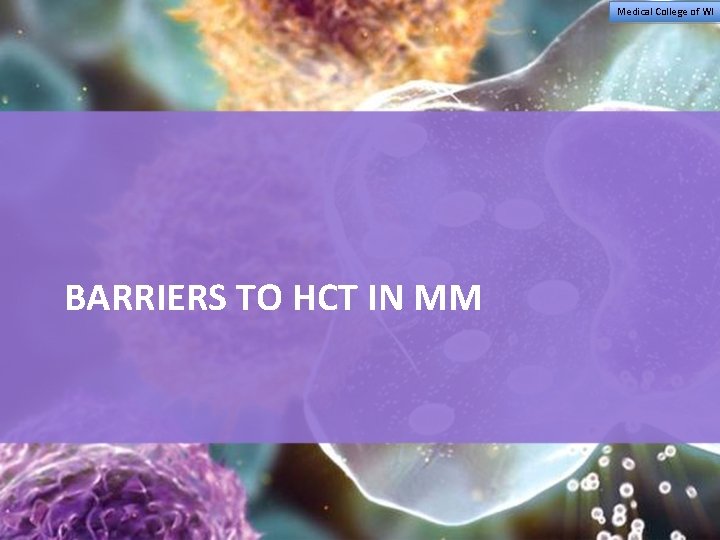
Barriers to Autologous Transplant Access SOCIAL ECONOMIC Age Ethnicity and race Language Culture Health literacy Patient/family attitudes Caregiver availability Socioeconomic status Education Number of wage earners Employment status Insurance coverage Place of residence Transportation ACCESS TO TRANSPLANT PROVIDER Physician referral Provider attitudes/biases Provider expertise Provider diversity Adapted from: Majhail NS, et al. Biol Blood Marrow Transplant. 2010; 16(8): 1070 -1075. HEALTH CARE SYSTEM Limited number of HCT centers Workforce shortage Capacity limitations Infrastructure issues

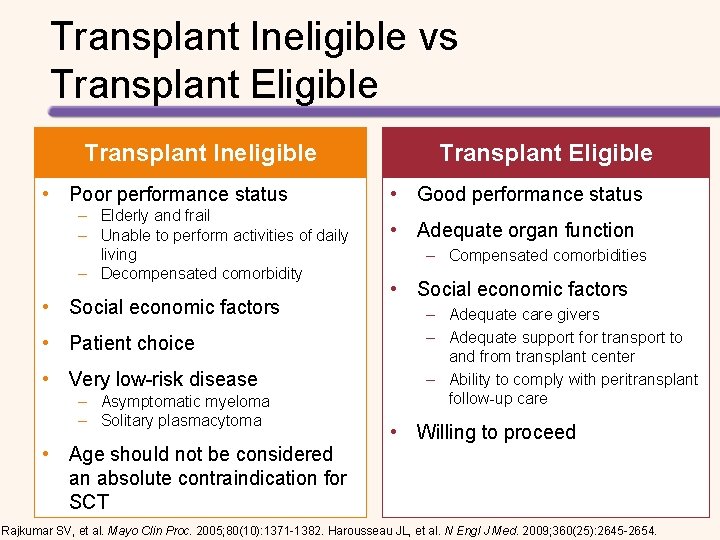
Transplant Ineligible vs Transplant Eligible Transplant Ineligible • Poor performance status – Elderly and frail – Unable to perform activities of daily living – Decompensated comorbidity • Social economic factors • Patient choice • Very low-risk disease – Asymptomatic myeloma – Solitary plasmacytoma • Age should not be considered an absolute contraindication for SCT Transplant Eligible • Good performance status • Adequate organ function – Compensated comorbidities • Social economic factors – Adequate care givers – Adequate support for transport to and from transplant center – Ability to comply with peritransplant follow-up care • Willing to proceed Rajkumar SV, et al. Mayo Clin Proc. 2005; 80(10): 1371 -1382. Harousseau JL, et al. N Engl J Med. 2009; 360(25): 2645 -2654.

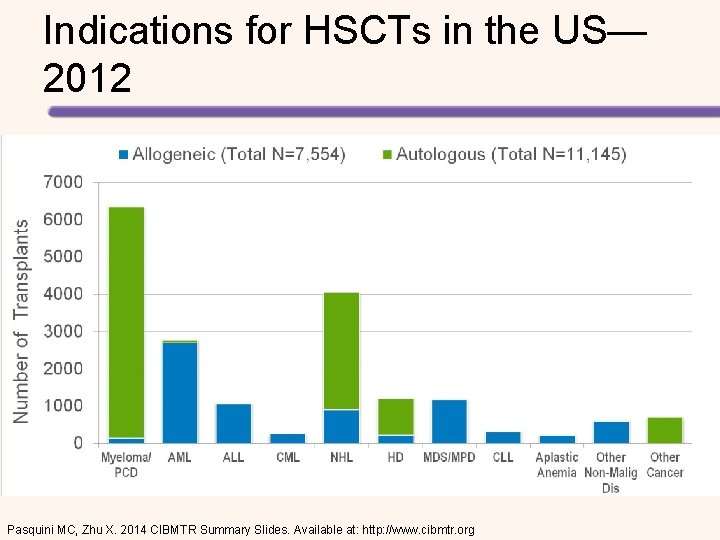
Indications for HSCTs in the US— 2012 Pasquini MC, Zhu X. 2014 CIBMTR Summary Slides. Available at: http: //www. cibmtr. org

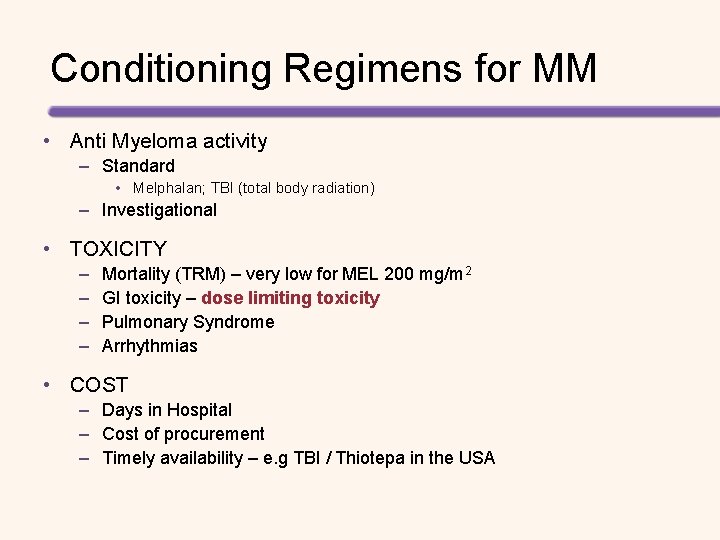
Conditioning Regimens for MM • Anti Myeloma activity – Standard • Melphalan; TBI (total body radiation) – Investigational • TOXICITY – – Mortality (TRM) – very low for MEL 200 mg/m 2 GI toxicity – dose limiting toxicity Pulmonary Syndrome Arrhythmias • COST – Days in Hospital – Cost of procurement – Timely availability – e. g TBI / Thiotepa in the USA

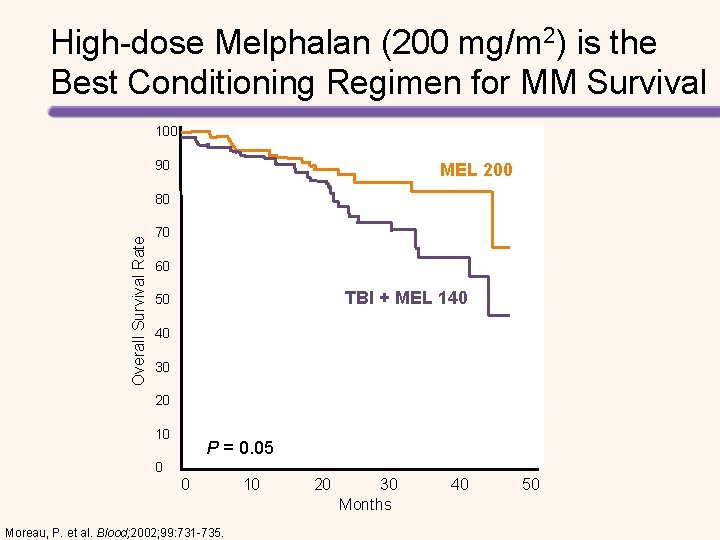
High-dose Melphalan (200 mg/m 2) is the Best Conditioning Regimen for MM Survival 100 90 MEL 200 Overall Survival Rate 80 70 60 TBI + MEL 140 50 40 30 20 10 P = 0. 05 0 0 10 20 30 40 50 Months Moreau, P. et al. Blood; 2002; 99: 731 -735.

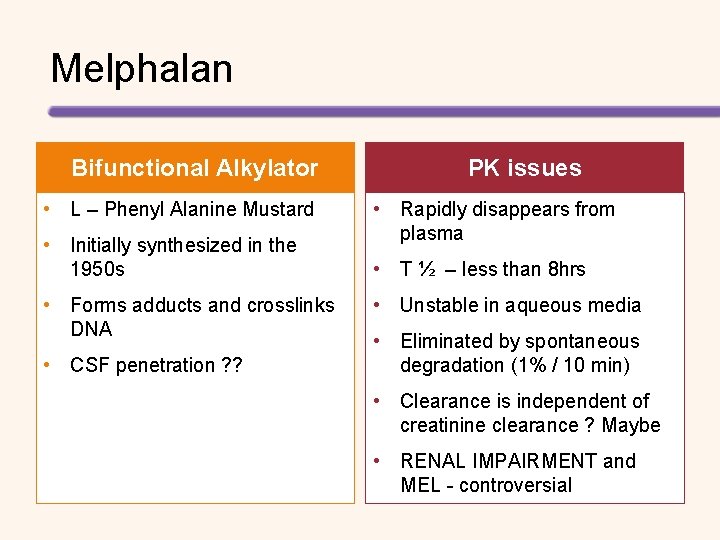
Melphalan Bifunctional Alkylator • L – Phenyl Alanine Mustard • Initially synthesized in the 1950 s • Forms adducts and crosslinks DNA • CSF penetration ? ? PK issues • Rapidly disappears from plasma • T ½ – less than 8 hrs • Unstable in aqueous media • Eliminated by spontaneous degradation (1% / 10 min) • Clearance is independent of creatinine clearance ? Maybe • RENAL IMPAIRMENT and MEL - controversial
![Riskadapted Melphalan Dosing Suggested Melphalan Doseadjustment for Patients with Renal Impairment1 Cr Cl 15 Risk-adapted Melphalan Dosing Suggested Melphalan Dose-adjustment for Patients with Renal Impairment[1] Cr. Cl >15](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-22.jpg)
Risk-adapted Melphalan Dosing Suggested Melphalan Dose-adjustment for Patients with Renal Impairment[1] Cr. Cl >15 < 60 m. L/min Cr. Cl < 15 m. L/min or the patient is on hemodialysis 140 mg/m 2 High-dose Melphalan Suggested Age-adjusted Dosing of Melphalan[2] Melphalan Age <65 years Dose level 0 Age 65– 75 years Dose level − 1 Age >75 years Dose level − 2 0· 25 mg/kg days 1– 4 every 4– 6 weeks 0· 18 mg/kg days 1– 4 every 4– 6 weeks 0· 13 mg/kg days 1– 4 every 4– 6 weeks • Melphalan dose may also be adjusted due to comorbidities [1] Dimopoulos, et al. JCO. 2010; 28: 4976 -4984. [2] Palumbo A, Anderson K. N Engl J Med. 2011; 364: 1046 -1060.

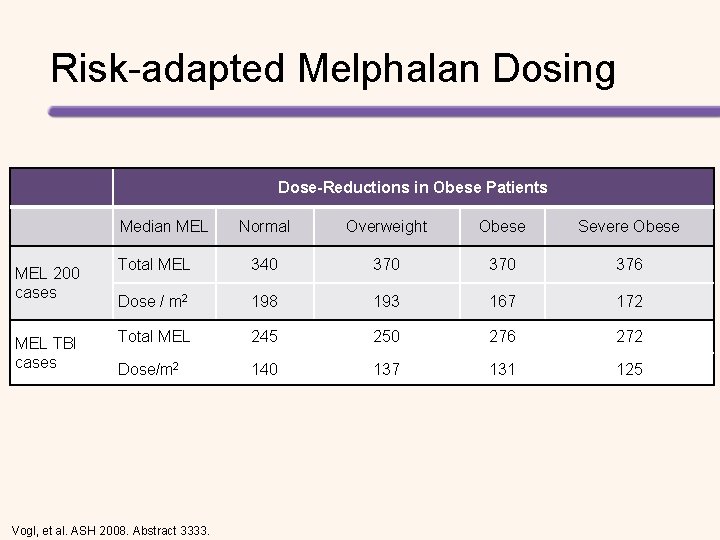
Risk-adapted Melphalan Dosing Dose-Reductions in Obese Patients Median MEL 200 cases MEL TBI cases Normal Overweight Obese Severe Obese Total MEL 340 370 376 Dose / m 2 198 193 167 172 Total MEL 245 250 276 272 Dose/m 2 140 137 131 125 Vogl, et al. ASH 2008. Abstract 3333.
![Intensification of MEL 220 mgm 2 French single arm study1 Escalating MEL to 300 Intensification of MEL 220 mg/m 2 (French single arm study)[1] Escalating MEL to 300](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-24.jpg)
Intensification of MEL 220 mg/m 2 (French single arm study)[1] Escalating MEL to 300 mg/m 2 [2] • Cardiac toxicity reported • Amifostine for protection • No obvious superiority over MEL 200 (historical) • MTD was MEL 280 mg/m 2 • At least 2 other MEL 280 studies in progress or completed • At higher MEL doses – – – Atrial Fibrillation Hepatic Necrosis Cardiac Death Severe Mucositis Increased deaths [1] Moreau, et al. Bone Marrow Transplant. 1999; 23: 1003 -1006. [2] Philips, et al. Biol Blood Marrow Transplant. 2004; 10: 473 -483.

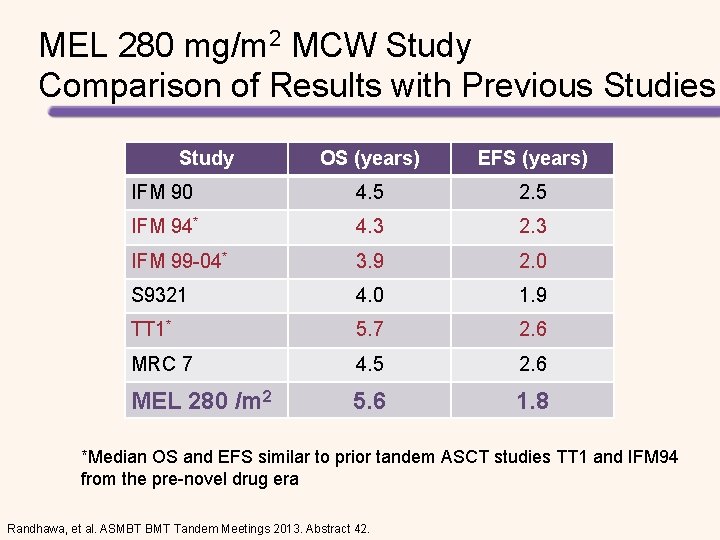
MEL 280 mg/m 2 MCW Study Comparison of Results with Previous Studies Study OS (years) EFS (years) IFM 90 4. 5 2. 5 IFM 94* 4. 3 2. 3 IFM 99 -04* 3. 9 2. 0 S 9321 4. 0 1. 9 TT 1* 5. 7 2. 6 MRC 7 4. 5 2. 6 MEL 280 /m 2 5. 6 1. 8 *Median OS and EFS similar to prior tandem ASCT studies TT 1 and IFM 94 from the pre-novel drug era Randhawa, et al. ASMBT BMT Tandem Meetings 2013. Abstract 42.

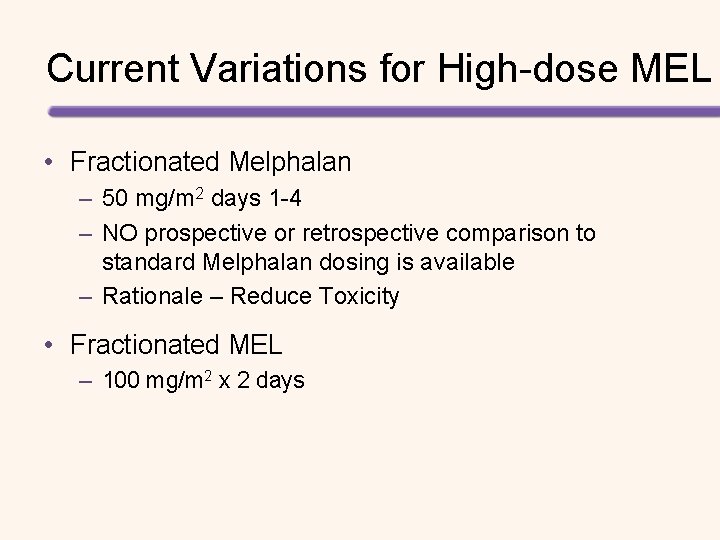
Current Variations for High-dose MEL • Fractionated Melphalan – 50 mg/m 2 days 1 -4 – NO prospective or retrospective comparison to standard Melphalan dosing is available – Rationale – Reduce Toxicity • Fractionated MEL – 100 mg/m 2 x 2 days

MEL-100 x 2 vs MEL-200 X 1 Response Category 2 -Day Dosing Melphalan 1 -Day Dosing Melphalan (n=185) (n=93) P-value s. CR 23 (12%) 8 (8%) . 3 CR 55 (30%) 21 (23%) VGPR 41 (22%) 21 (23%) PR 45 (24%) 35 (38%) s. CR+CR 78 (42%) 29 (31%) . 09 ORR 164 (89%) 85 (91%) . 5 Parmar, et al. Bone Marrow Transplantation. 2014; 49: 761– 766.

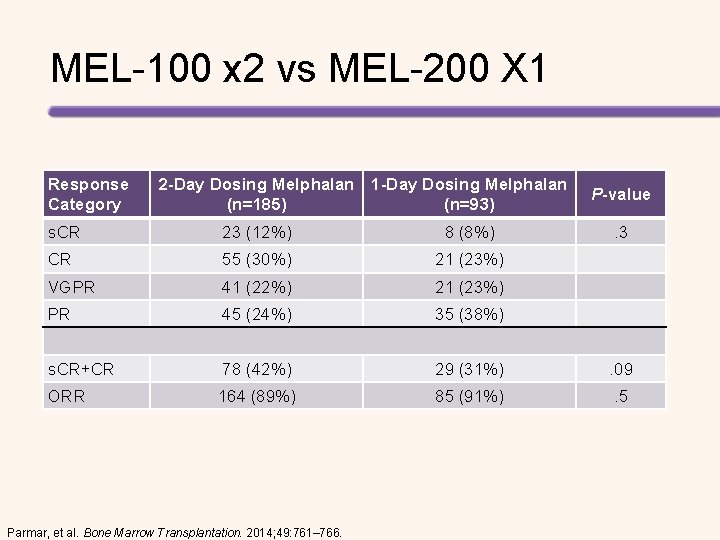
MEL 200 vs Bortezomib-MEL 200 vs. Bortezomib MEL – Matched pair BOR-MEL (n = 46) MEL 200 (n = 115) P CR 35% 11% . 001 VGPR 35% 43% PR 26% 43% CR+VGPR 70% 54% Response Mucositis Grade 3 -4 Median duration Mucositis . 078 47% 9 days (2 -13) GI – diarrhea G 1 -2 72% Skin Reactions G 1 -2 34% Neuropathy 8% Headache 28% Roussel, et al. Blood. 2010; 115: 32 -37.

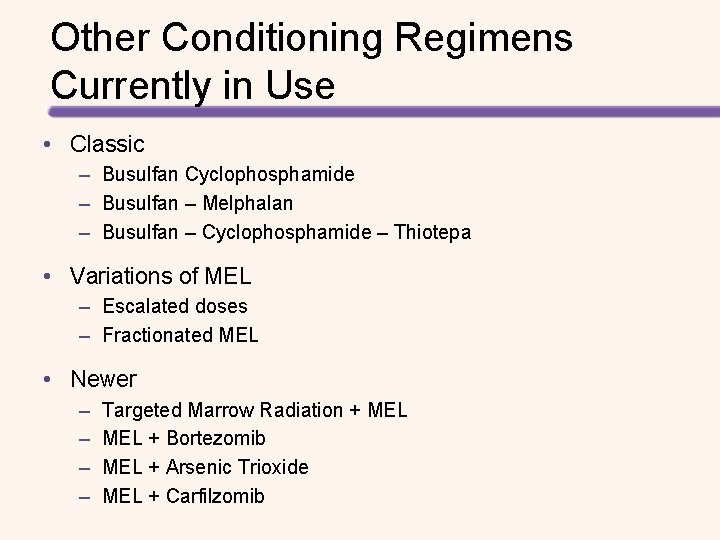
Other Conditioning Regimens Currently in Use • Classic – Busulfan Cyclophosphamide – Busulfan – Melphalan – Busulfan – Cyclophosphamide – Thiotepa • Variations of MEL – Escalated doses – Fractionated MEL • Newer – – Targeted Marrow Radiation + MEL + Bortezomib MEL + Arsenic Trioxide MEL + Carfilzomib

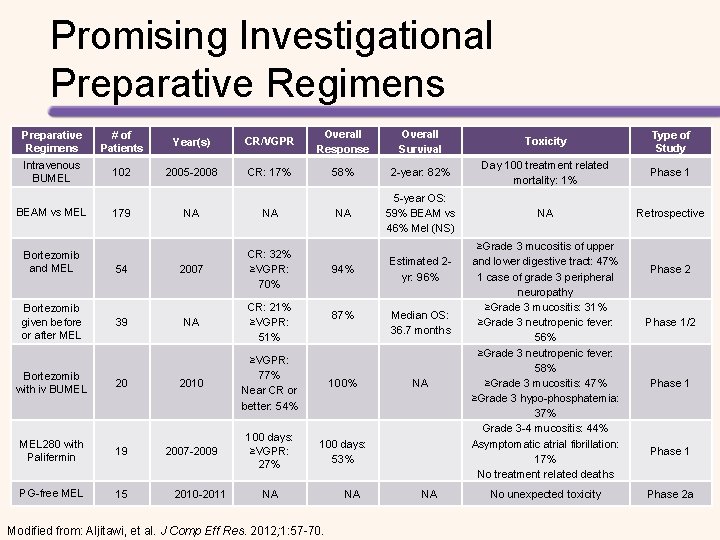
Promising Investigational Preparative Regimens # of Patients Year(s) CR/VGPR Overall Response Overall Survival Toxicity Type of Study Intravenous BUMEL 102 2005 -2008 CR: 17% 58% 2 -year: 82% Day 100 treatment related mortality: 1% Phase 1 NA 5 -year OS: 59% BEAM vs 46% Mel (NS) NA Retrospective BEAM vs MEL Bortezomib and MEL Bortezomib given before or after MEL 179 54 39 NA NA 2007 CR: 32% ≥VGPR: 70% 94% Estimated 2 yr: 96% NA CR: 21% ≥VGPR: 51% 87% Median OS: 36. 7 months 100% NA 100 days: 53% Bortezomib with iv BUMEL 20 2010 ≥VGPR: 77% Near CR or better: 54% MEL 280 with Palifermin 19 2007 -2009 100 days: ≥VGPR: 27% PG-free MEL 15 2010 -2011 NA Modified from: Aljitawi, et al. J Comp Eff Res. 2012; 1: 57 -70. NA NA ≥Grade 3 mucositis of upper and lower digestive tract: 47% 1 case of grade 3 peripheral neuropathy ≥Grade 3 mucositis: 31% ≥Grade 3 neutropenic fever: 56% ≥Grade 3 neutropenic fever: 58% ≥Grade 3 mucositis: 47% ≥Grade 3 hypo-phosphatemia: 37% Grade 3 -4 mucositis: 44% Asymptomatic atrial fibrillation: 17% No treatment related deaths No unexpected toxicity Phase 2 Phase 1/2 Phase 1 Phase 2 a

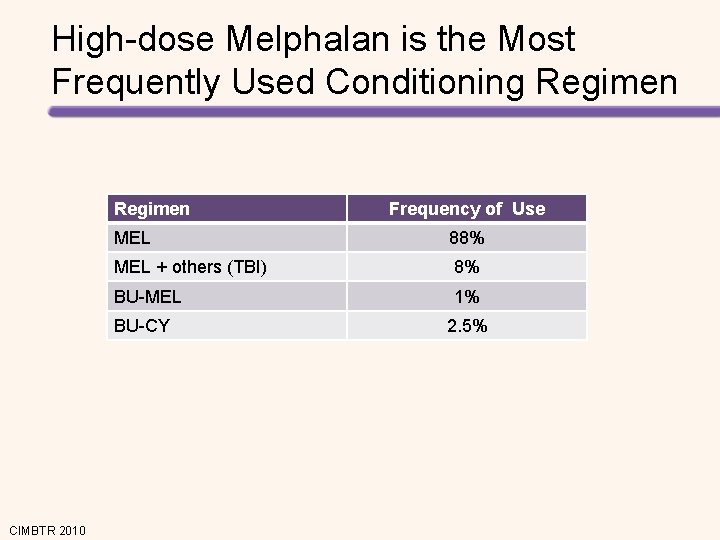
High-dose Melphalan is the Most Frequently Used Conditioning Regimen MEL 88% MEL + others (TBI) 8% BU-MEL 1% BU-CY CIMBTR 2010 Frequency of Use 2. 5%

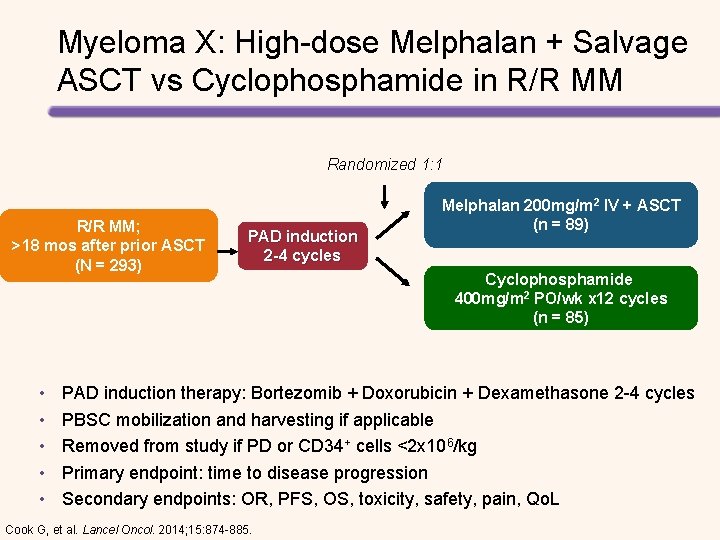
Myeloma X: High-dose Melphalan + Salvage ASCT vs Cyclophosphamide in R/R MM Randomized 1: 1 R/R MM; >18 mos after prior ASCT (N = 293) • • • PAD induction 2 -4 cycles Melphalan 200 mg/m 2 IV + ASCT (n = 89) Cyclophosphamide 400 mg/m 2 PO/wk x 12 cycles (n = 85) PAD induction therapy: Bortezomib + Doxorubicin + Dexamethasone 2 -4 cycles PBSC mobilization and harvesting if applicable Removed from study if PD or CD 34+ cells <2 x 106/kg Primary endpoint: time to disease progression Secondary endpoints: OR, PFS, OS, toxicity, safety, pain, Qo. L Cook G, et al. Lancel Oncol. 2014; 15: 874 -885.

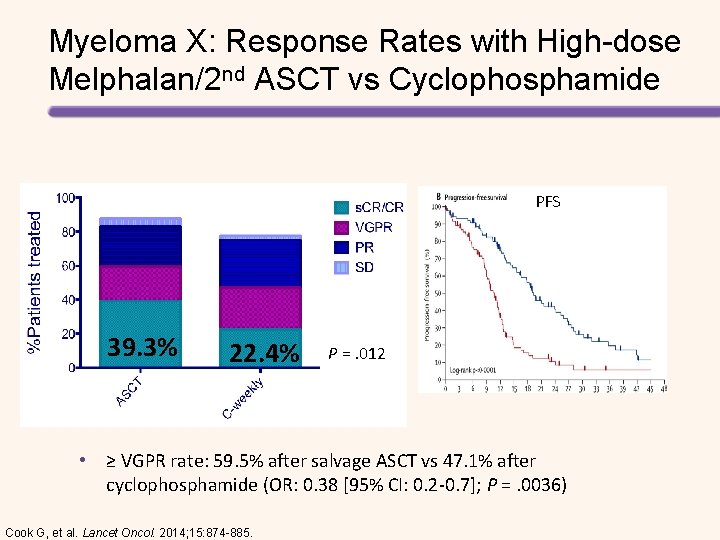
Myeloma X: Response Rates with High-dose Melphalan/2 nd ASCT vs Cyclophosphamide PFS 39. 3% 22. 4% P =. 012 • ≥ VGPR rate: 59. 5% after salvage ASCT vs 47. 1% after cyclophosphamide (OR: 0. 38 [95% CI: 0. 2 -0. 7]; P =. 0036) Cook G, et al. Lancet Oncol. 2014; 15: 874 -885.

MELPHALAN CHALLENGES PK Variability Propylene Glycol Special Issues

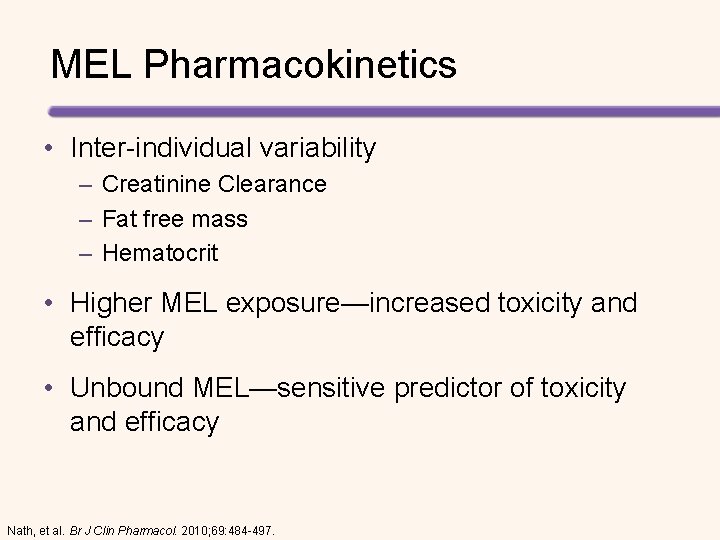
MEL Pharmacokinetics • Inter-individual variability – Creatinine Clearance – Fat free mass – Hematocrit • Higher MEL exposure—increased toxicity and efficacy • Unbound MEL—sensitive predictor of toxicity and efficacy Nath, et al. Br J Clin Pharmacol. 2010; 69: 484 -497.

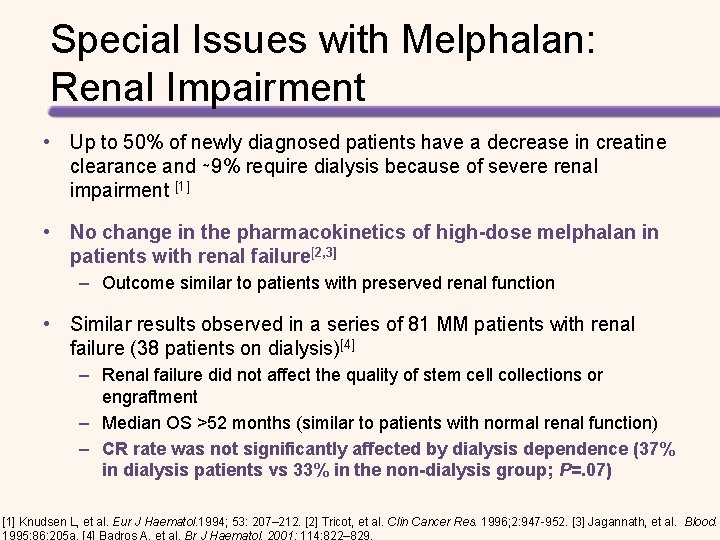
Special Issues with Melphalan: Renal Impairment • Up to 50% of newly diagnosed patients have a decrease in creatine clearance and ∼ 9% require dialysis because of severe renal impairment [1] • No change in the pharmacokinetics of high-dose melphalan in patients with renal failure[2, 3] – Outcome similar to patients with preserved renal function • Similar results observed in a series of 81 MM patients with renal failure (38 patients on dialysis)[4] – Renal failure did not affect the quality of stem cell collections or engraftment – Median OS >52 months (similar to patients with normal renal function) – CR rate was not significantly affected by dialysis dependence (37% in dialysis patients vs 33% in the non-dialysis group; P=. 07) [1] Knudsen L, et al. Eur J Haematol. 1994; 53: 207– 212. [2] Tricot, et al. Clin Cancer Res. 1996; 2: 947 -952. [3] Jagannath, et al. Blood.

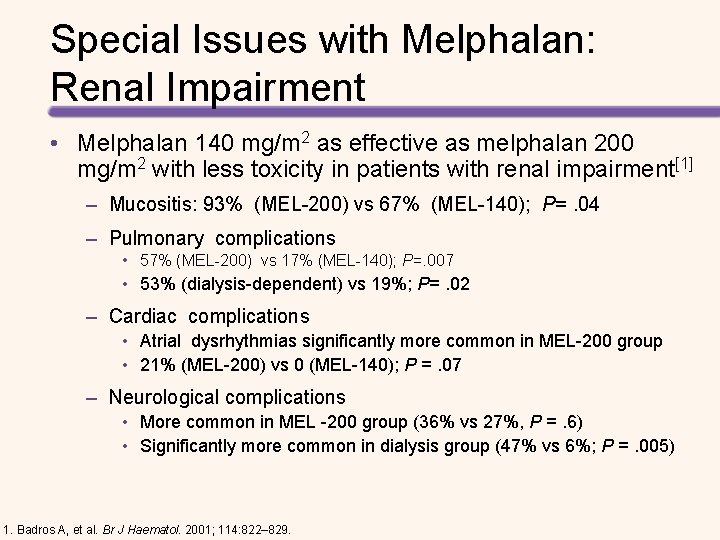
Special Issues with Melphalan: Renal Impairment • Melphalan 140 mg/m 2 as effective as melphalan 200 mg/m 2 with less toxicity in patients with renal impairment[1] – Mucositis: 93% (MEL-200) vs 67% (MEL-140); P=. 04 – Pulmonary complications • 57% (MEL-200) vs 17% (MEL-140); P=. 007 • 53% (dialysis-dependent) vs 19%; P=. 02 – Cardiac complications • Atrial dysrhythmias significantly more common in MEL-200 group • 21% (MEL-200) vs 0 (MEL-140); P =. 07 – Neurological complications • More common in MEL -200 group (36% vs 27%, P =. 6) • Significantly more common in dialysis group (47% vs 6%; P =. 005) 1. Badros A, et al. Br J Haematol. 2001; 114: 822– 829.

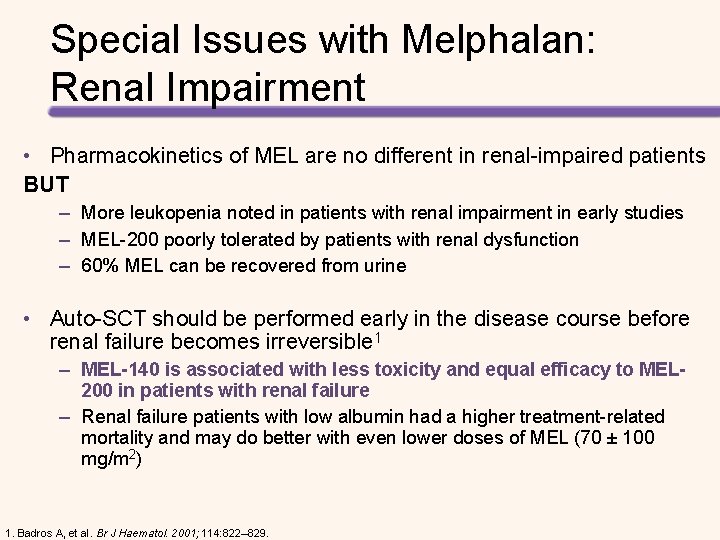
Special Issues with Melphalan: Renal Impairment • Pharmacokinetics of MEL are no different in renal-impaired patients BUT – More leukopenia noted in patients with renal impairment in early studies – MEL-200 poorly tolerated by patients with renal dysfunction – 60% MEL can be recovered from urine • Auto-SCT should be performed early in the disease course before renal failure becomes irreversible 1 – MEL-140 is associated with less toxicity and equal efficacy to MEL 200 in patients with renal failure – Renal failure patients with low albumin had a higher treatment-related mortality and may do better with even lower doses of MEL (70 ± 100 mg/m 2) 1. Badros A, et al. Br J Haematol. 2001; 114: 822– 829.

Special Issues with Melphalan: Mucositis • MEL-ASCT is associated with severe oral mucositis (OM) – OM develops in 45%-75% of patients[1, 2] – Predictors of severe OM[1] • High serum creatinine • High mg/kg melphalan – Severe OM risk and/or duration was significantly associated with: [2] • Higher chemotherapy dose/kg of body weight • Poor performance status • NOT related to age [1] Grazziutti ML, et al. Bone Marrow Transplant. 2006; 38(7): 501 -506. [2] Blijlevens N, et al. J Clin Oncol. 2008; 26(9): 1519 -1525.

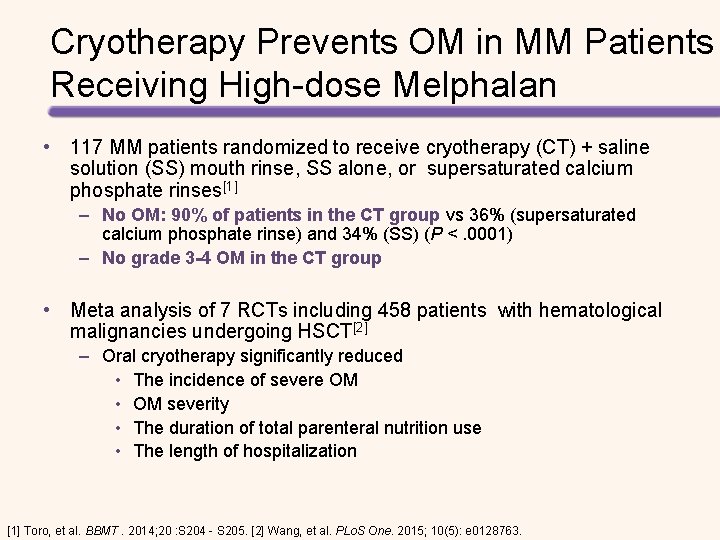
Cryotherapy Prevents OM in MM Patients Receiving High-dose Melphalan • 117 MM patients randomized to receive cryotherapy (CT) + saline solution (SS) mouth rinse, SS alone, or supersaturated calcium phosphate rinses[1] – No OM: 90% of patients in the CT group vs 36% (supersaturated calcium phosphate rinse) and 34% (SS) (P <. 0001) – No grade 3 -4 OM in the CT group • Meta analysis of 7 RCTs including 458 patients with hematological malignancies undergoing HSCT[2] – Oral cryotherapy significantly reduced • The incidence of severe OM • OM severity • The duration of total parenteral nutrition use • The length of hospitalization [1] Toro, et al. BBMT. 2014; 20 : S 204 - S 205. [2] Wang, et al. PLo. S One. 2015; 10(5): e 0128763.

Special Issues with Melphalan: Administration • When reconstituted, Melphalan rapidly hydrolyzes ~1% every 10 minutes • Manufacturer recommendations: – Dilute dose in NS to </= 0. 45 mg/m. L and infuse over at least 15 minutes – Complete the infusion within 60 minutes of reconstitution of the vial • BMT programs should verify that infusions have ended before the Melphalan expiration time/date

Special Issues with Melphalan: Administration • Example: Patient 2. 1 m 2 ordered 200 mg/m 2 • A dose of 420 mg diluted in 933 m. L NS (0. 45 mg/m. L) must infuse @ 1867 m. L/h administer the dose over 30 minutes • The dose is prepared as 2 bags (466. 5 m. L each) to infuse simultaneously with each pump @ 933 m. L/h – A typical infusion pump has a maximum infusion rate of 999 m. L/h

Special Issues with Melphalan: Stability • Highly unstable in solution • 10% per loss of activity/ hr • Propylene Glycol (PG) – Additive to MEL – Toxic in the ICU setting when given as continuous infusion – Rate of PG infusion exceeds FDA guidelines when MEL bolus given currently

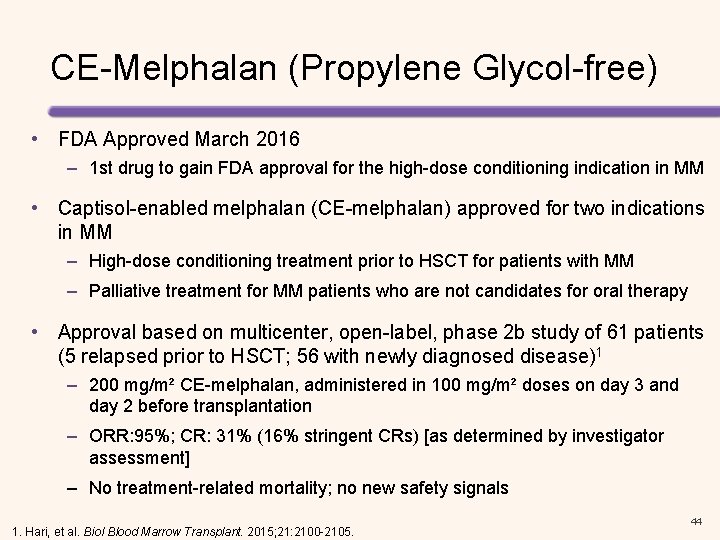
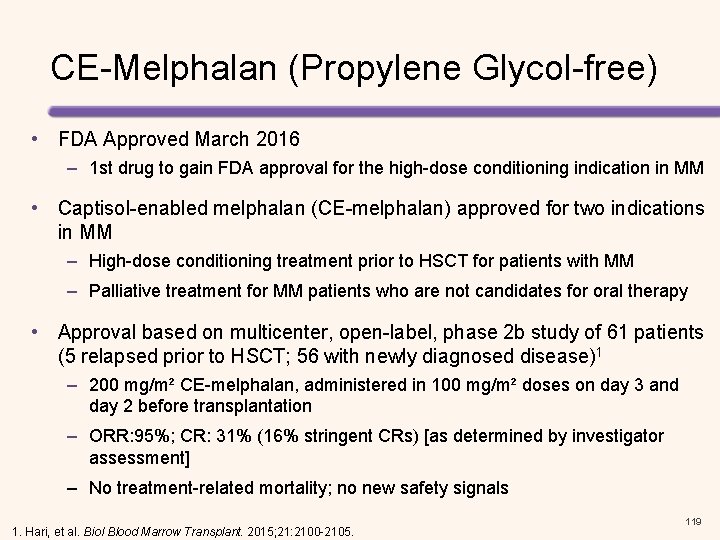
CE-Melphalan (Propylene Glycol-free) • FDA Approved March 2016 – 1 st drug to gain FDA approval for the high-dose conditioning indication in MM • Captisol-enabled melphalan (CE-melphalan) approved for two indications in MM – High-dose conditioning treatment prior to HSCT for patients with MM – Palliative treatment for MM patients who are not candidates for oral therapy • Approval based on multicenter, open-label, phase 2 b study of 61 patients (5 relapsed prior to HSCT; 56 with newly diagnosed disease)1 – 200 mg/m² CE-melphalan, administered in 100 mg/m² doses on day 3 and day 2 before transplantation – ORR: 95%; CR: 31% (16% stringent CRs) [as determined by investigator assessment] – No treatment-related mortality; no new safety signals 1. Hari, et al. Biol Blood Marrow Transplant. 2015; 21: 2100 -2105. 44

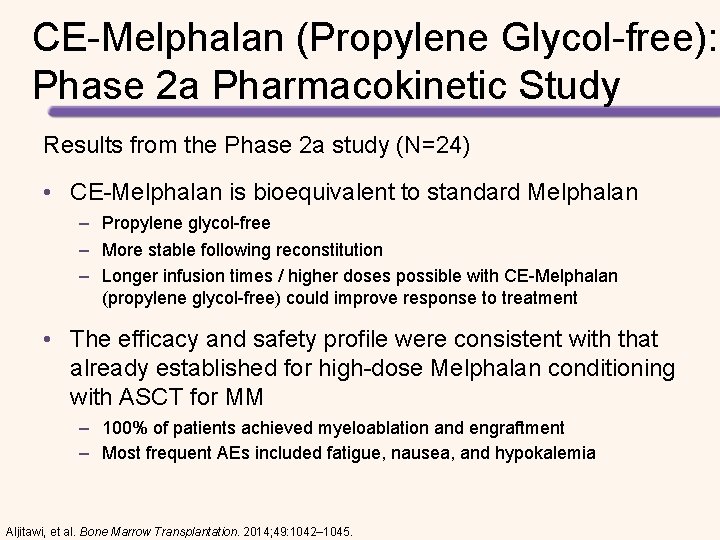
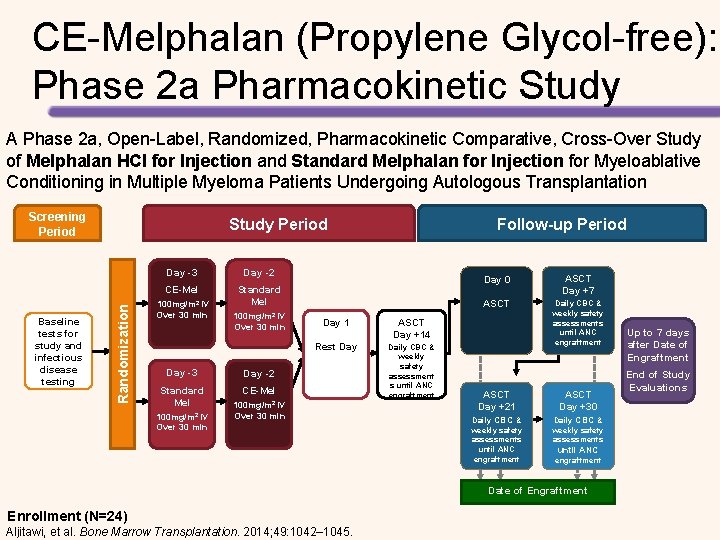
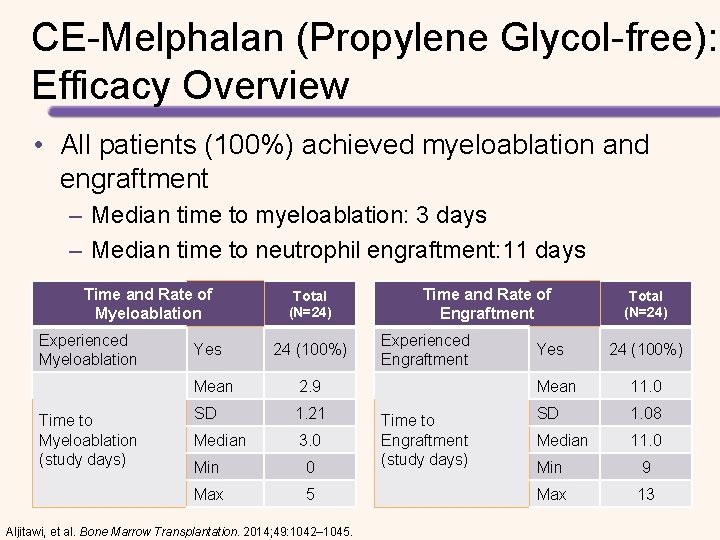
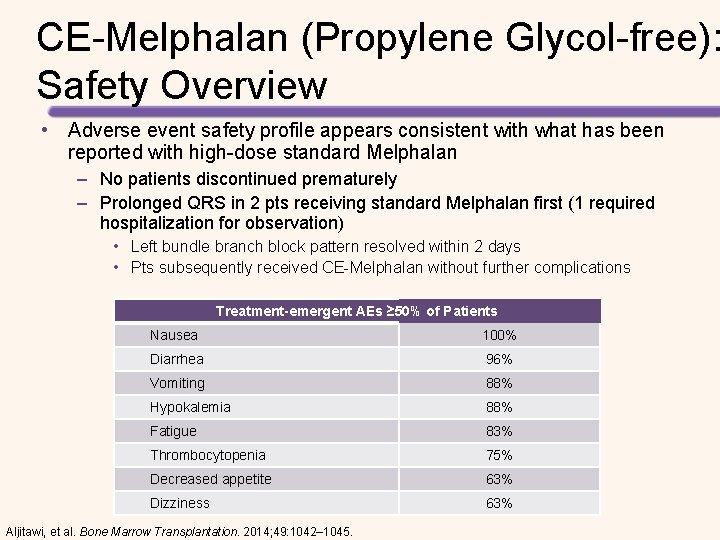
CE-Melphalan (Propylene Glycol-free): Phase 2 a Pharmacokinetic Study Results from the Phase 2 a study (N=24) • CE-Melphalan is bioequivalent to standard Melphalan – Propylene glycol-free – More stable following reconstitution – Longer infusion times / higher doses possible with CE-Melphalan (propylene glycol-free) could improve response to treatment • The efficacy and safety profile were consistent with that already established for high-dose Melphalan conditioning with ASCT for MM – 100% of patients achieved myeloablation and engraftment – Most frequent AEs included fatigue, nausea, and hypokalemia Aljitawi, et al. Bone Marrow Transplantation. 2014; 49: 1042– 1045.

CE-Melphalan (Propylene Glycol-free): Phase 2 a Pharmacokinetic Study Melphalan Plasma Concentration l Bioequivalence demonstrated Concentration (ng/m. L) • Cmax: 112% • AUC 0 -t: 110% • AUC-inf: 110% Successful myeloablation (Day +3) l Successful engraftment (Day +11) l CE-Melphalan HCl Melphalan No additional toxicities: l Treatment-emergent AEs (100%) – Common AEs: nausea, vomiting, hypokalemia, fatigue, decreased appetite, dizziness, and thrombocytopenia l Treatment-emergent SAEs (29%) – Febrile neutropenia, mucosal inflammation, sepsis and extreme fatigue Aljitawi, et al. Bone Marrow Transplantation. 2014; 49: 1042– 1045.

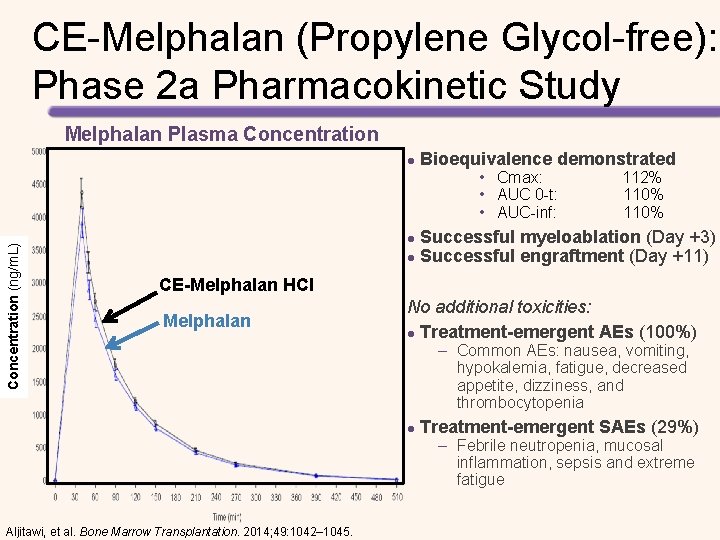
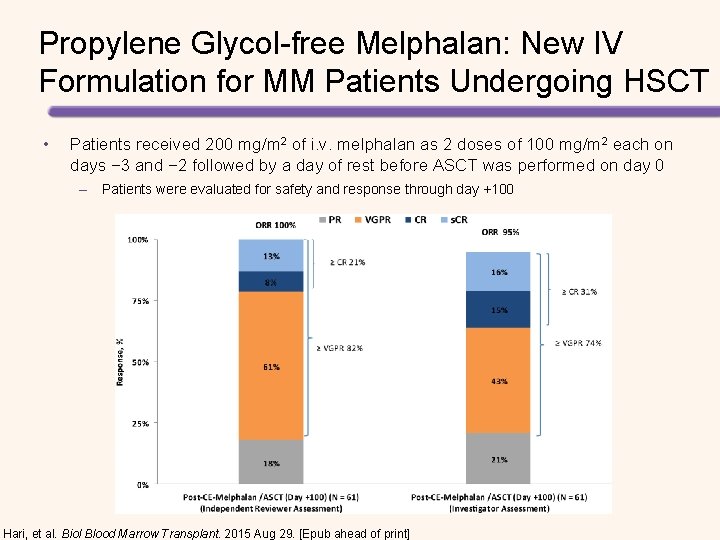
Propylene Glycol-free Melphalan: New IV Formulation for MM Patients Undergoing HSCT • Patients received 200 mg/m 2 of i. v. melphalan as 2 doses of 100 mg/m 2 each on days − 3 and − 2 followed by a day of rest before ASCT was performed on day 0 – Patients were evaluated for safety and response through day +100 Hari, et al. Biol Blood Marrow Transplant. 2015 Aug 29. [Epub ahead of print]

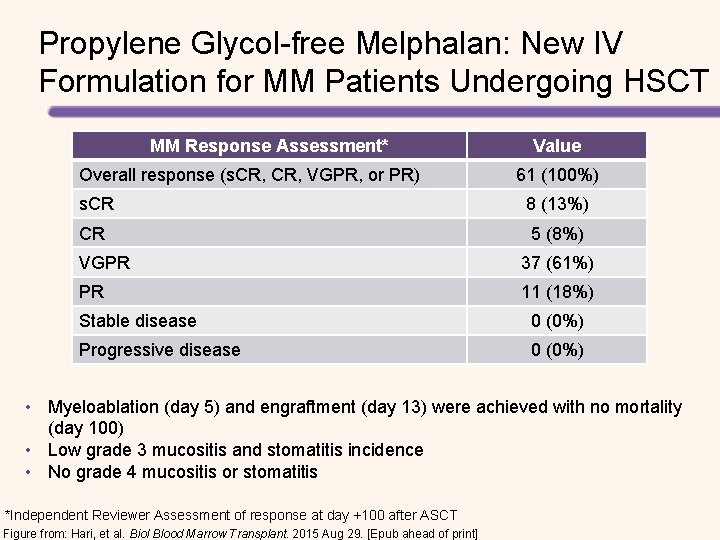
Propylene Glycol-free Melphalan: New IV Formulation for MM Patients Undergoing HSCT MM Response Assessment* Overall response (s. CR, VGPR, or PR) Value 61 (100%) s. CR 8 (13%) CR 5 (8%) VGPR 37 (61%) PR 11 (18%) Stable disease 0 (0%) Progressive disease 0 (0%) • Myeloablation (day 5) and engraftment (day 13) were achieved with no mortality (day 100) • Low grade 3 mucositis and stomatitis incidence • No grade 4 mucositis or stomatitis *Independent Reviewer Assessment of response at day +100 after ASCT Figure from: Hari, et al. Biol Blood Marrow Transplant. 2015 Aug 29. [Epub ahead of print]

AUTOLOGOUS TRANSPLANTATION Transplant Vs Conventional Therapy
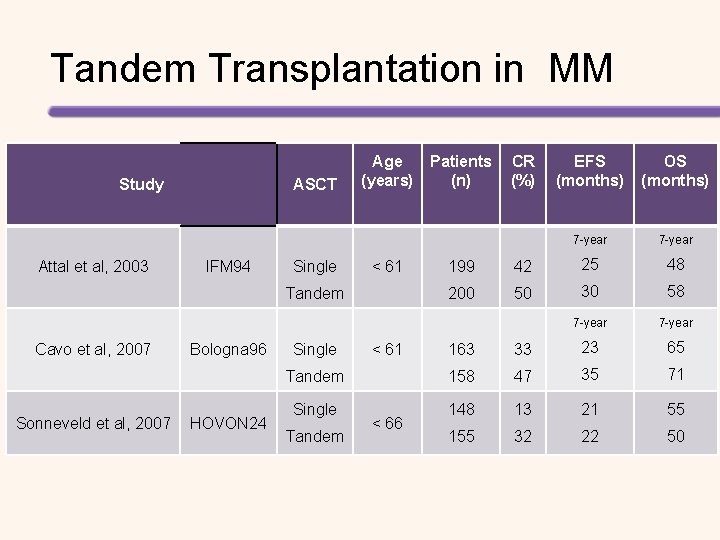
![Prospective Randomized Studies Evaluating HSCT for the Treatment of MM Study Barlogie 20061 Bladé Prospective, Randomized Studies Evaluating HSCT for the Treatment of MM Study Barlogie 2006[1] Bladé](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-50.jpg)
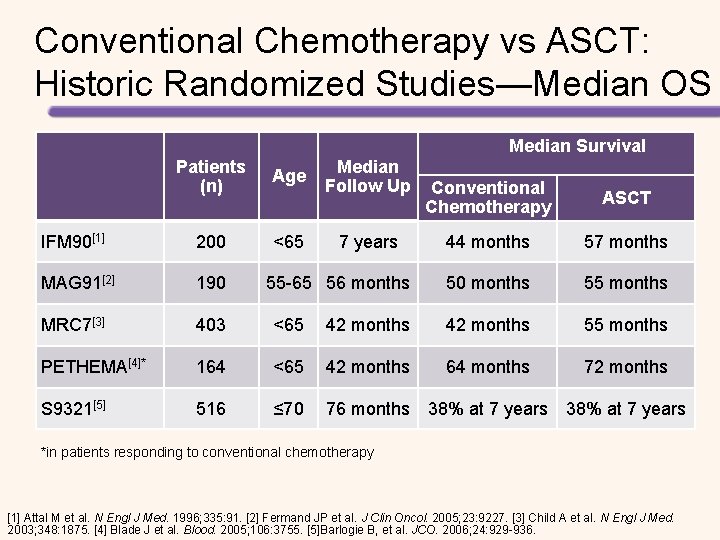
Prospective, Randomized Studies Evaluating HSCT for the Treatment of MM Study Barlogie 2006[1] Bladé 2005[2] Fermand 2005[3] Palumbo 2004[4] Child 2003[5] Segeren 2003[6] Attal 2003[7] Attal 1996[8] Treatment n Chemotherapy 255 HDT + HSCT 261 Chemotherapy 83 HDT + HSCT 81 Chemotherapy 96 HDT + HSCT 94 Chemotherapy 99 HDT + HSCT 95 Chemotherapy 196 HDT + HSCT 197 Chemotherapy 129 HDT + HSCT 132 Single HSCT 199 Double HSCT 200 Chemotherapy 100 HDT + HSCT 100 OS Benefit EFS Benefit NS NS* NS NS Yes Yes* NS NS Yes Yes *Progression-free survival. All studies included were published between 1996 and 2006. OS, overall survival; EFS, event-free survival HDT, high-dose chemotherapy; HSCT, haematopoietic stem cell transplant; NS, not significant. [1] Barlogie B, et al. J Clin Oncol 2006; 24: 929– 936. [2] Bladé J, et al. Blood 2005; 106: 3755– 3759. [3] Fermand JP, et al. J Clin Oncol 2005; 23: 9227– 9233. [4] Palumbo A, et al. Blood 2004; 104: 3052– 3057. [5] Child. JA, et al. N Engl J Med 2003; 348: 1875– 1883. [6] Segeren CM, et al. Blood 2003; 101: 2144– 2151. 7. Attal M, et al. N Engl J Med. 2003; 349: 2495– 2502. [8] Attal M, et al. N Engl J Med. 1996; 335: 91– 97.

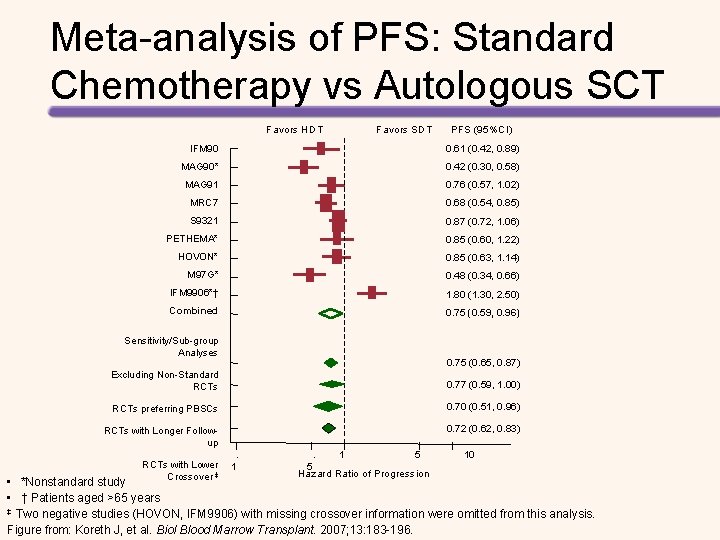
Meta-analysis of PFS: Standard Chemotherapy vs Autologous SCT Favors HDT Favors SDT PFS (95%CI) IFM 90 0. 61 (0. 42, 0. 89) MAG 90* 0. 42 (0. 30, 0. 58) MAG 91 0. 76 (0. 57, 1. 02) MRC 7 0. 68 (0. 54, 0. 85) S 9321 0. 87 (0. 72, 1. 06) PETHEMA* 0. 85 (0. 60, 1. 22) HOVON* 0. 85 (0. 63, 1. 14) M 97 G* 0. 48 (0. 34, 0. 66) IFM 9906*† 1. 80 (1. 30, 2. 50) Combined 0. 75 (0. 59, 0. 96) Sensitivity/Sub-group Analyses 0. 75 (0. 65, 0. 87) Excluding Non-Standard RCTs 0. 77 (0. 59, 1. 00) RCTs preferring PBSCs 0. 70 (0. 51, 0. 96) RCTs with Longer Followup 0. 72 (0. 62, 0. 83) . RCTs with Lower 1 Crossover ‡ . 5 1 5 Hazard Ratio of Progression 10 • *Nonstandard study • † Patients aged >65 years ‡ Two negative studies (HOVON, IFM 9906) with missing crossover information were omitted from this analysis. Figure from: Koreth J, et al. Biol Blood Marrow Transplant. 2007; 13: 183 -196.

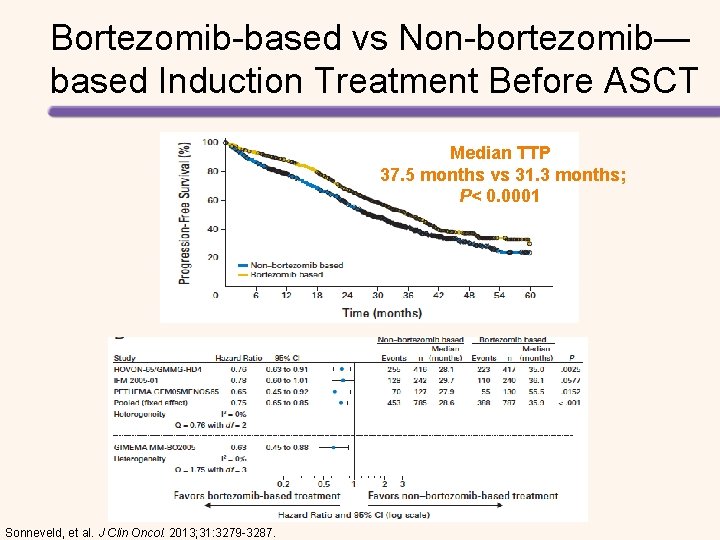
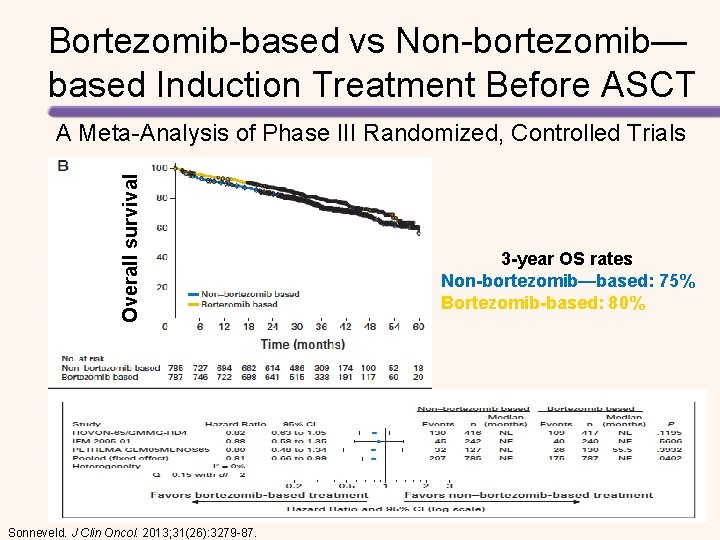
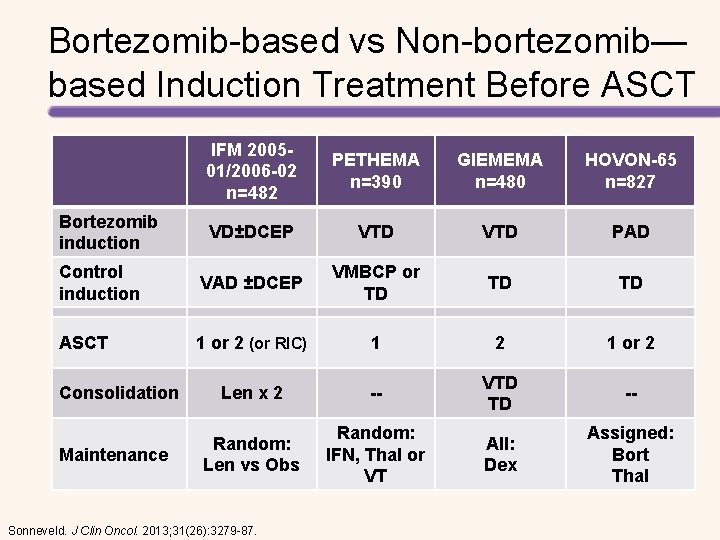
Bortezomib-based vs Non-bortezomib— based Induction Treatment Before ASCT Median TTP 37. 5 months vs 31. 3 months; P< 0. 0001 Sonneveld, et al. J Clin Oncol. 2013; 31: 3279 -3287.

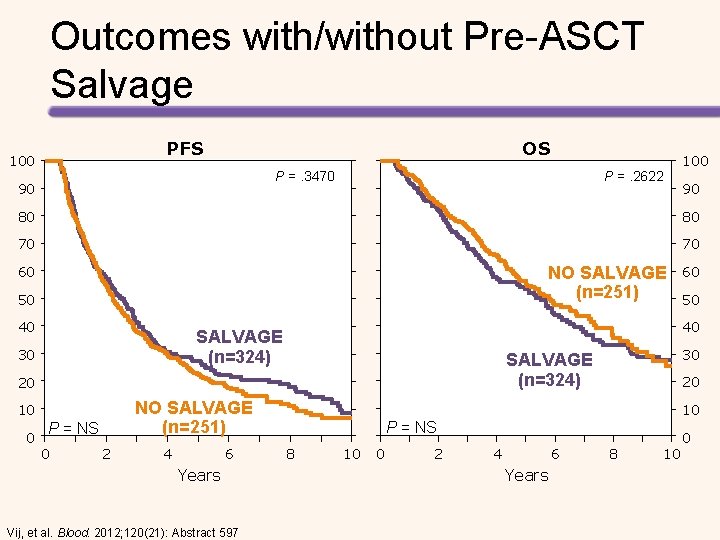
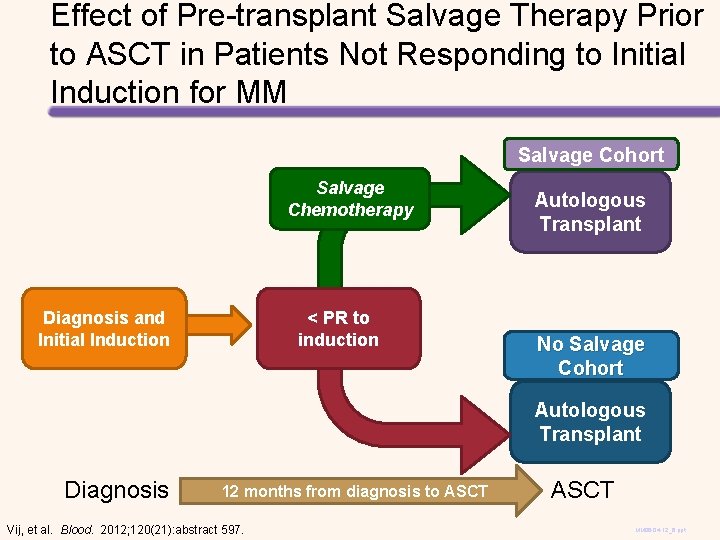
Outcomes with/without Pre-ASCT Salvage PFS 100 OS P =. 3470 90 P =. 2622 100 90 80 80 70 70 NO SALVAGE (n=251) 60 50 40 0 NO SALVAGE (n=251) P = NS 0 2 4 6 Years Vij, et al. Blood. 2012; 120(21): Abstract 597 30 SALVAGE (n=324) 20 10 50 40 SALVAGE (n=324) 30 60 20 10 P = NS 8 10 0 2 4 6 Years 8 10 0

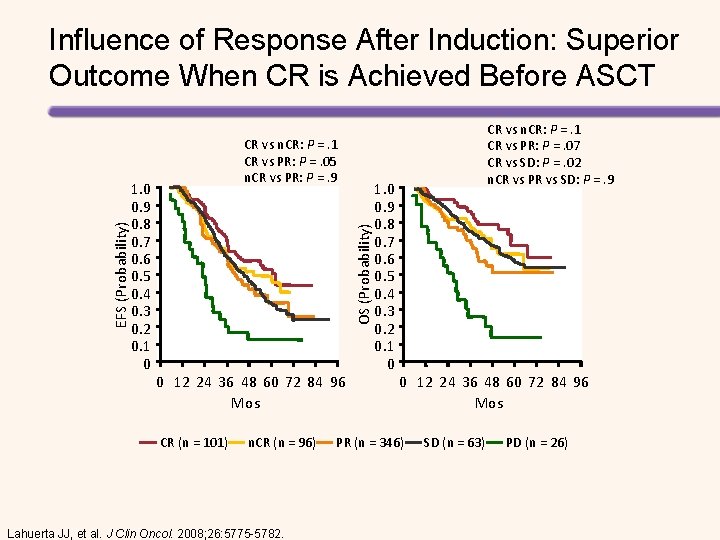
1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 CR vs n. CR: P =. 1 CR vs PR: P =. 05 n. CR vs PR: P =. 9 OS (Probability) EFS (Probability) Influence of Response After Induction: Superior Outcome When CR is Achieved Before ASCT 0 12 24 36 48 60 72 84 96 Mos CR (n = 101) n. CR (n = 96) Lahuerta JJ, et al. J Clin Oncol. 2008; 26: 5775 -5782. 1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 CR vs n. CR: P =. 1 CR vs PR: P =. 07 CR vs SD: P =. 02 n. CR vs PR vs SD: P =. 9 0 12 24 36 48 60 72 84 96 Mos PR (n = 346) SD (n = 63) PD (n = 26)
![Regimens Survival Bortezomiblenalidomide dexamethasone RVD1 18 mo PFS 75 18 mo OS 97 Carfilzomiblenalidomide Regimens Survival Bortezomib/lenalidomide/ dexamethasone (RVD)[1] 18 -mo PFS: 75% 18 -mo OS: 97% Carfilzomib/lenalidomide/](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-55.jpg)
Regimens Survival Bortezomib/lenalidomide/ dexamethasone (RVD)[1] 18 -mo PFS: 75% 18 -mo OS: 97% Carfilzomib/lenalidomide/ dexamethasone (KRd)[2, 3] 12 -mo PFS: 97%[2] 24 -mo PFS: 92%[2] 3 -yr PFS: 79%[3] 3 -yr OS: 96%[3] Carfilzomib/thalidomide/ dexamethasone (KTd)[4] 3 -yr PFS: 72% Bortezomib/ cyclophosphamide/ dexamethasone (Cy. Bor. D)[5] 5 -yr PFS: 42%[6] 5 -yr OS: 70%[6] Ixazomib/lenalidomide/ dexamethasone[7] 12 -mo PFS: 88% 12 -mo OS: 94% Pts Achieving ≥ VGPR (%) Earlier Phase Studies: Induction Regimens for Transplantation-Eligible Pts 100 80 81 67 68 60 60 58 40 20 0 [1 D V R ] ] [2 d R K ]] [4 d T K [5 ] r. D RD / o B ib y m C zo a Ix [1] Richardson, PG et al. Blood. 2010; 116: 679 -686. [2] Jakubowiak A, et al. Blood. 2012; 120: 1801 -1809. [3] Jasielec J, et al. ASH 2013. Abstract 3220. [4] Sonneveld P, et al. Blood. 2015; 125: 449 -456. [5] Reeder CB, et al. Blood. 2010; 115: 3416 -3417. [6] Reeder CB, et al. ASH 2013. Abstract 3192. [7] Kumar SK, et al. Lancet Oncol. 2014; 15: 1503 -1512. ] [7

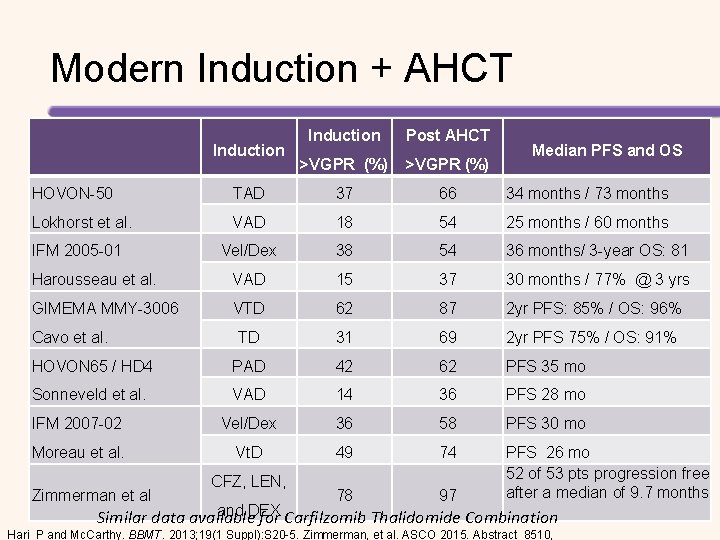
Modern Induction + AHCT Induction Post AHCT >VGPR (%) Median PFS and OS HOVON-50 TAD 37 66 34 months / 73 months Lokhorst et al. VAD 18 54 25 months / 60 months IFM 2005 -01 Vel/Dex 38 54 36 months/ 3 -year OS: 81 Harousseau et al. VAD 15 37 30 months / 77% @ 3 yrs GIMEMA MMY-3006 VTD 62 87 2 yr PFS: 85% / OS: 96% TD 31 69 2 yr PFS 75% / OS: 91% HOVON 65 / HD 4 PAD 42 62 PFS 35 mo Sonneveld et al. VAD 14 36 PFS 28 mo IFM 2007 -02 Vel/Dex 36 58 PFS 30 mo Moreau et al. Vt. D 49 74 PFS 26 mo 52 of 53 pts progression free after a median of 9. 7 months Cavo et al. Zimmerman et al CFZ, LEN, 78 97 and DEX Similar data available for Carfilzomib Thalidomide Combination Hari P and Mc. Carthy. BBMT. 2013; 19(1 Suppl): S 20 -5. Zimmerman, et al. ASCO 2015. Abstract 8510,

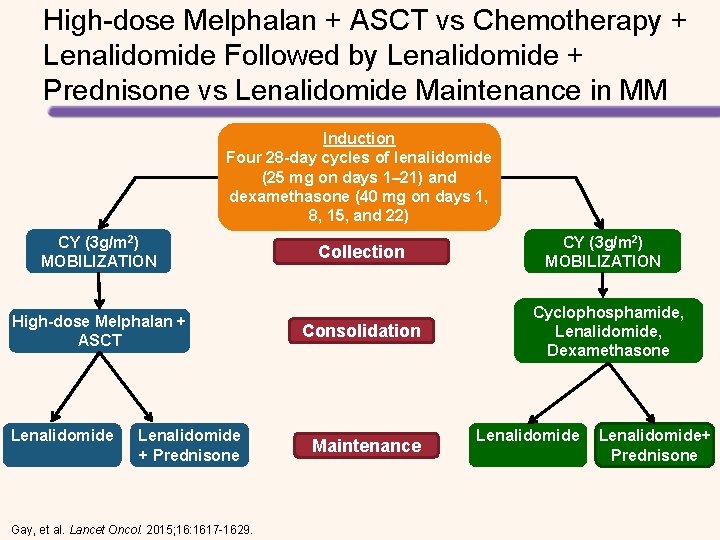
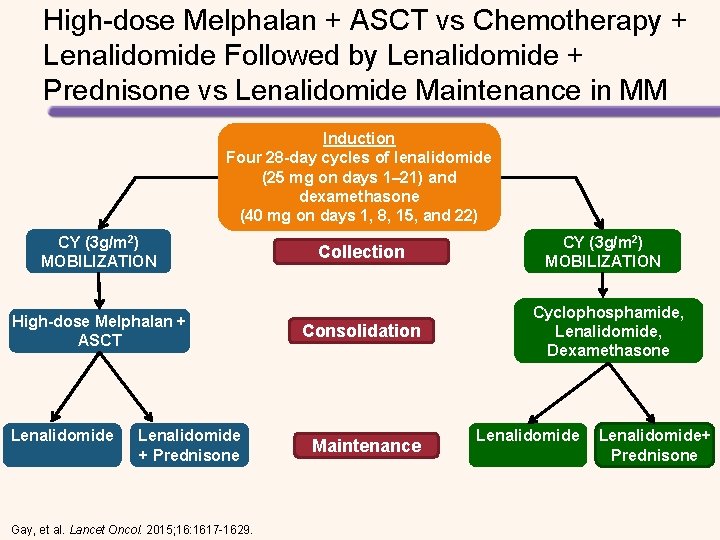
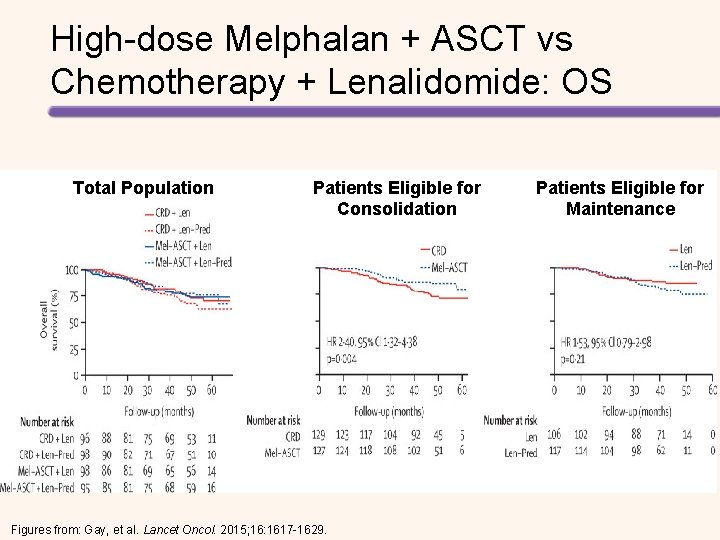
High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide Followed by Lenalidomide + Prednisone vs Lenalidomide Maintenance in MM Induction Four 28 -day cycles of lenalidomide (25 mg on days 1– 21) and dexamethasone (40 mg on days 1, 8, Induction 15, and 22) CY (3 g/m 2) MOBILIZATION High-dose Melphalan + ASCT Lenalidomide + Prednisone Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629. Collection Consolidation Maintenance CY (3 g/m 2) MOBILIZATION Cyclophosphamide, Lenalidomide, Dexamethasone Lenalidomide+ Prednisone

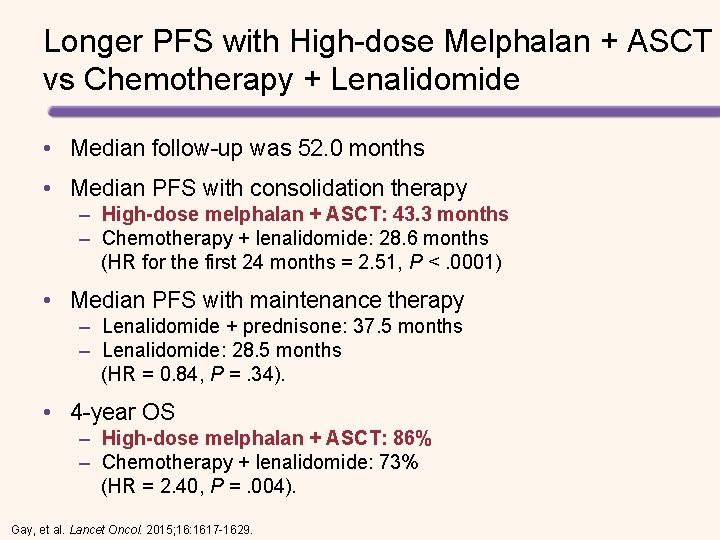
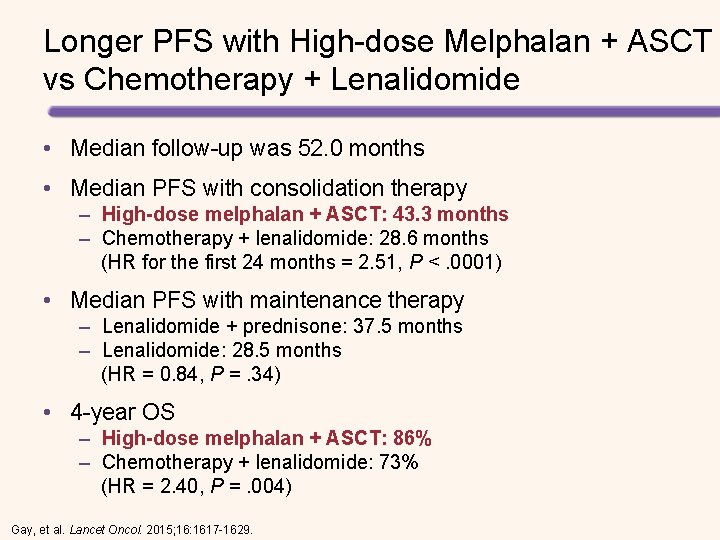
Longer PFS with High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide • Median follow-up was 52. 0 months • Median PFS with consolidation therapy – High-dose melphalan + ASCT: 43. 3 months – Chemotherapy + lenalidomide: 28. 6 months (HR for the first 24 months = 2. 51, P <. 0001) • Median PFS with maintenance therapy – Lenalidomide + prednisone: 37. 5 months – Lenalidomide: 28. 5 months (HR = 0. 84, P =. 34). • 4 -year OS – High-dose melphalan + ASCT: 86% – Chemotherapy + lenalidomide: 73% (HR = 2. 40, P =. 004). Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629.

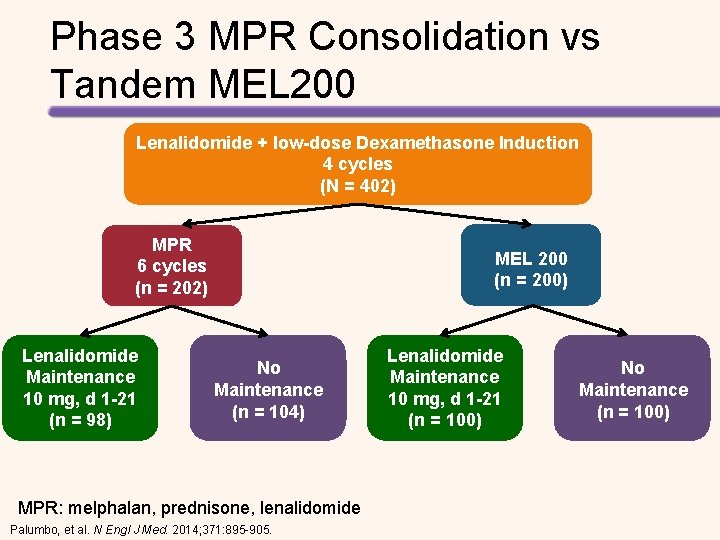
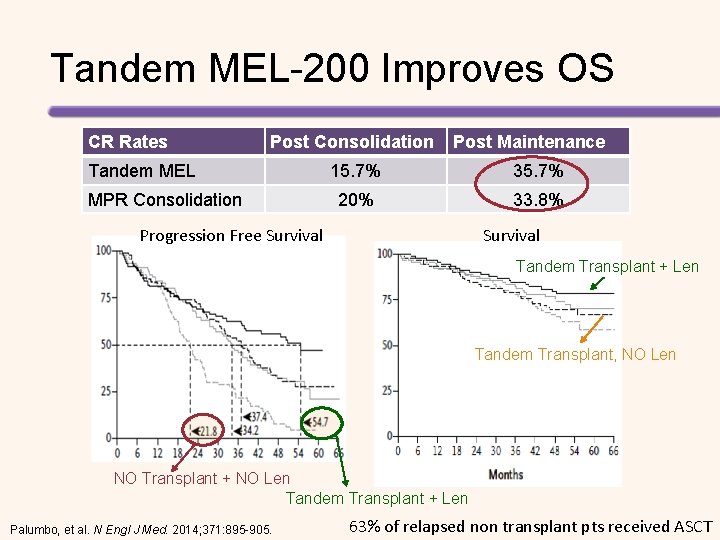
Phase 3 MPR Consolidation vs Tandem MEL 200 Lenalidomide + low-dose Dexamethasone Induction 4 cycles (N = 402) MPR 6 cycles (n = 202) Lenalidomide Maintenance 10 mg, d 1 -21 (n = 98) MEL 200 (n = 200) No Maintenance (n = 104) MPR: melphalan, prednisone, lenalidomide Palumbo, et al. N Engl J Med. 2014; 371: 895 -905. Lenalidomide Maintenance 10 mg, d 1 -21 (n = 100) No Maintenance (n = 100)

Tandem MEL-200 Improves OS CR Rates Post Consolidation Tandem MEL MPR Consolidation Post Maintenance 15. 7% 35. 7% 20% 33. 8% Progression Free Survival Tandem Transplant + Len Tandem Transplant, NO Len NO Transplant + NO Len Tandem Transplant + Len Palumbo, et al. N Engl J Med. 2014; 371: 895 -905. 63% of relapsed non transplant pts received ASCT

AUTOLOGOUS TRANSPLANTATION Early vs Late HCT

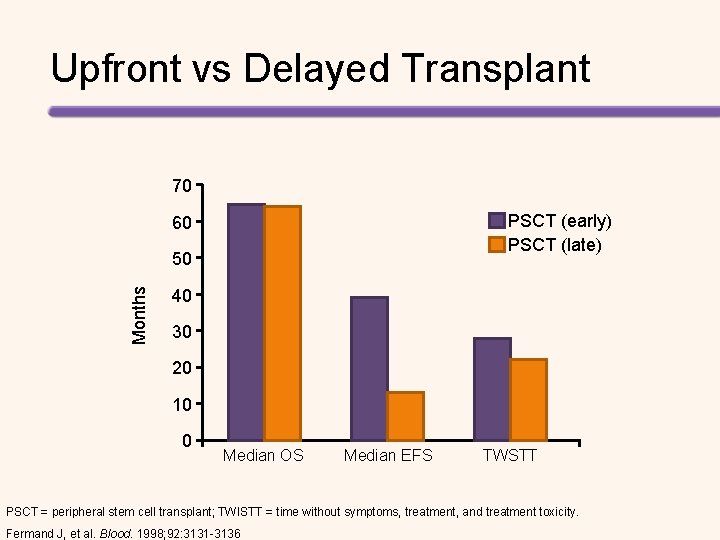
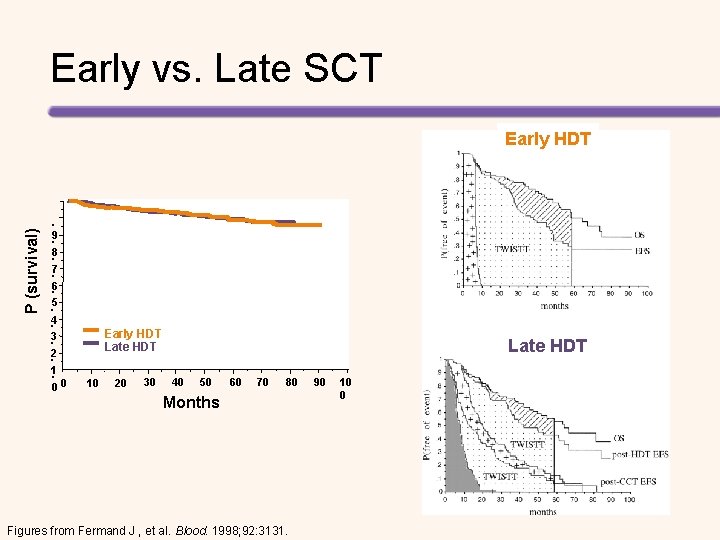
Upfront vs Delayed Transplant 70 PSCT (early) PSCT (late) 60 Months 50 40 30 20 10 0 Median OS Median EFS TWSTT PSCT = peripheral stem cell transplant; TWISTT = time without symptoms, treatment, and treatment toxicity. Fermand J, et al. Blood. 1998; 92: 3131 -3136

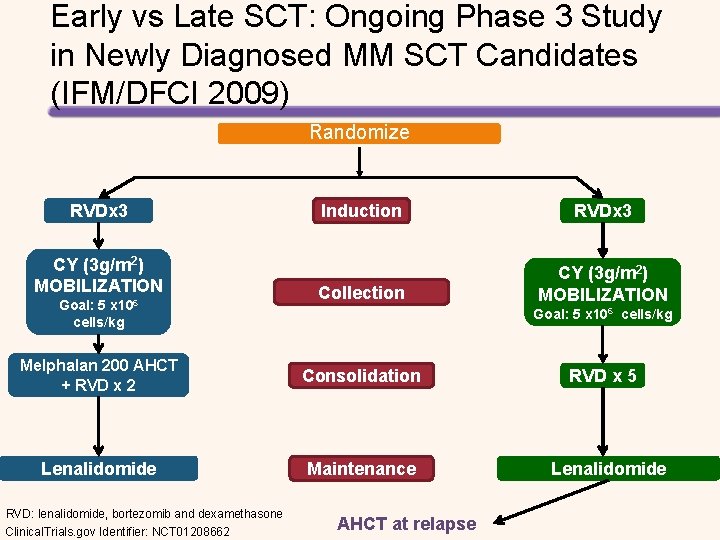
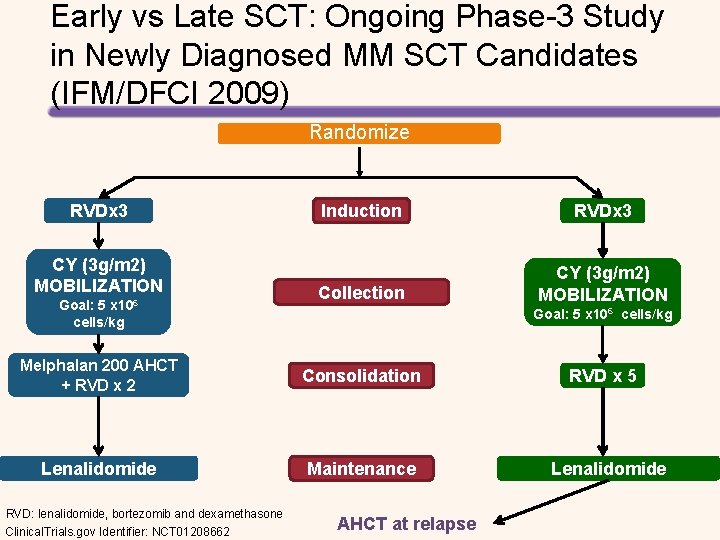
Early vs Late SCT: Ongoing Phase 3 Study in Newly Diagnosed MM SCT Candidates (IFM/DFCI 2009) Randomize RVDx 3 CY (3 g/m 2) MOBILIZATION Goal: 5 x 106 cells/kg Induction RVDx 3 Collection CY (3 g/m 2) MOBILIZATION Goal: 5 x 106 cells/kg Melphalan 200 AHCT + RVD x 2 Consolidation Lenalidomide Maintenance RVD: lenalidomide, bortezomib and dexamethasone Clinical. Trials. gov Identifier: NCT 01208662 AHCT at relapse RVD x 5 Lenalidomide

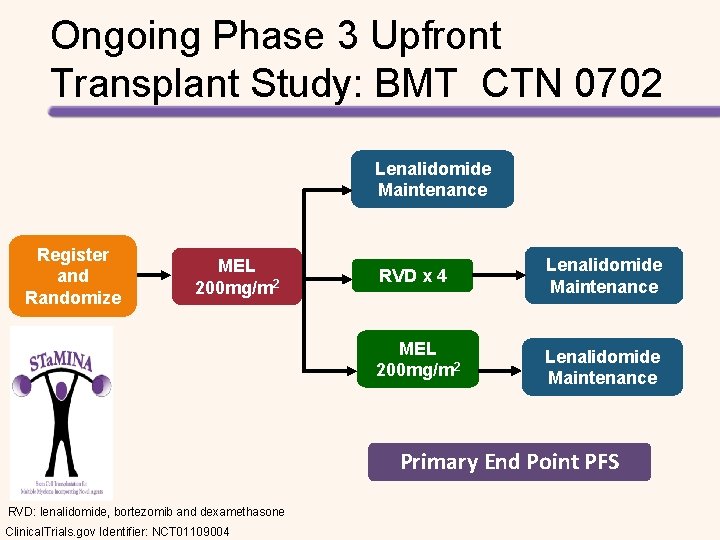
Ongoing Phase 3 Upfront Transplant Study: BMT CTN 0702 Lenalidomide Maintenance Register and Randomize MEL 200 mg/m 2 RVD x 4 Lenalidomide Maintenance MEL 200 mg/m 2 Lenalidomide Maintenance Primary End Point PFS RVD: lenalidomide, bortezomib and dexamethasone Clinical. Trials. gov Identifier: NCT 01109004

ALLOGENEIC TRANSPLANTATION

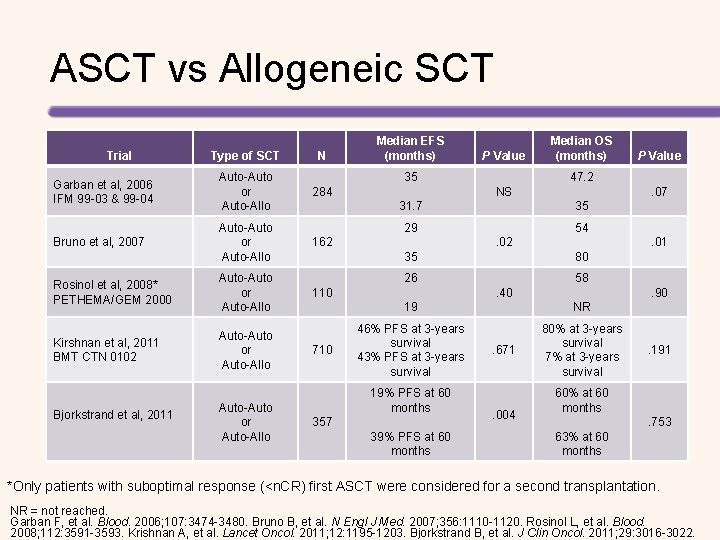
ASCT vs Allogeneic SCT Trial Type of SCT N Garban et al, 2006 IFM 99 -03 & 99 -04 Auto-Auto or Auto-Allo 284 Bruno et al, 2007 Auto-Auto or Auto-Allo Rosinol et al, 2008* PETHEMA/GEM 2000 Auto-Auto or Auto-Allo Kirshnan et al, 2011 BMT CTN 0102 Auto-Auto or Auto-Allo Bjorkstrand et al, 2011 Auto-Auto or Auto-Allo Median EFS (months) P Value 35 . 07 31. 7 35 29 54. 02 . 01 35 80 26 58 110 . 40 . 90 19 NR 46% PFS at 3 -years survival 43% PFS at 3 -years survival 80% at 3 -years survival 7% at 3 -years survival 19% PFS at 60 months 357 39% PFS at 60 months P Value 47. 2 NS 162 710 Median OS (months) . 671 . 004 . 191 60% at 60 months. 753 63% at 60 months *Only patients with suboptimal response (<n. CR) first ASCT were considered for a second transplantation. NR = not reached. Garban F, et al. Blood. 2006; 107: 3474 -3480. Bruno B, et al. N Engl J Med. 2007; 356: 1110 -1120. Rosinol L, et al. Blood. 2008; 112: 3591 -3593. Krishnan A, et al. Lancet Oncol. 2011; 12: 1195 -1203. Bjorkstrand B, et al. J Clin Oncol. 2011; 29: 3016 -3022.

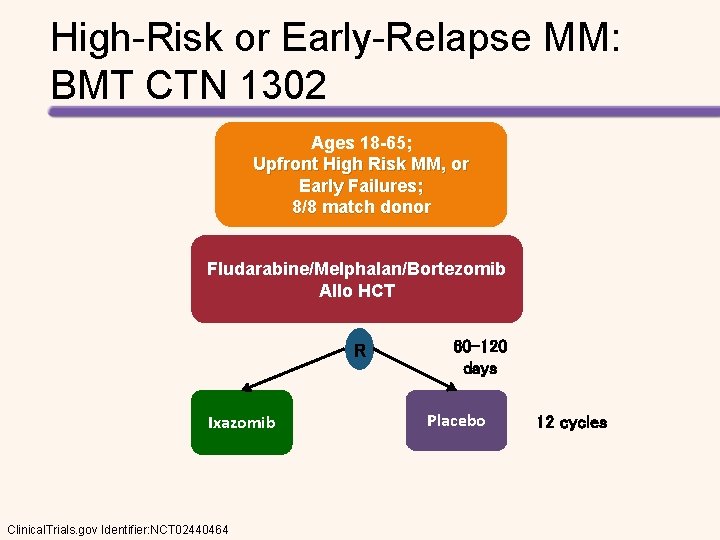
High-Risk or Early-Relapse MM: BMT CTN 1302 Ages 18 -65; Upfront High Risk MM, or Early Failures; 8/8 match donor Fludarabine/Melphalan/Bortezomib Allo HCT R Ixazomib Clinical. Trials. gov Identifier: NCT 02440464 60 -120 days Placebo 12 cycles

CONCLUSIONS

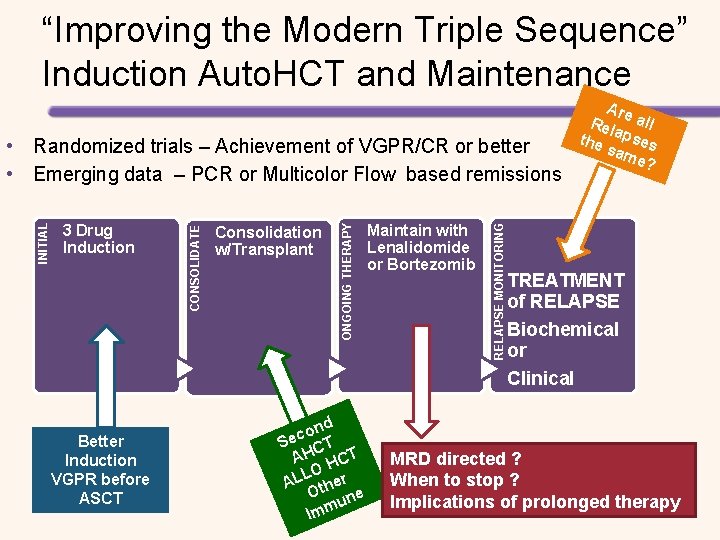
“Improving the Modern Triple Sequence” Induction Auto. HCT and Maintenance Better Induction VGPR before ASCT nd o c Se CT AH HCT LO AL ther O ne u Imm Maintain with Lenalidomide or Bortezomib RELAPSE MONITORING Consolidation w/Transplant ONGOING THERAPY 3 Drug Induction CONSOLIDATE INITIAL • Randomized trials – Achievement of VGPR/CR or better • Emerging data – PCR or Multicolor Flow based remissions Are Rel all the apses sam e? TREATMENT of RELAPSE Biochemical or Clinical MRD directed ? When to stop ? Implications of prolonged therapy

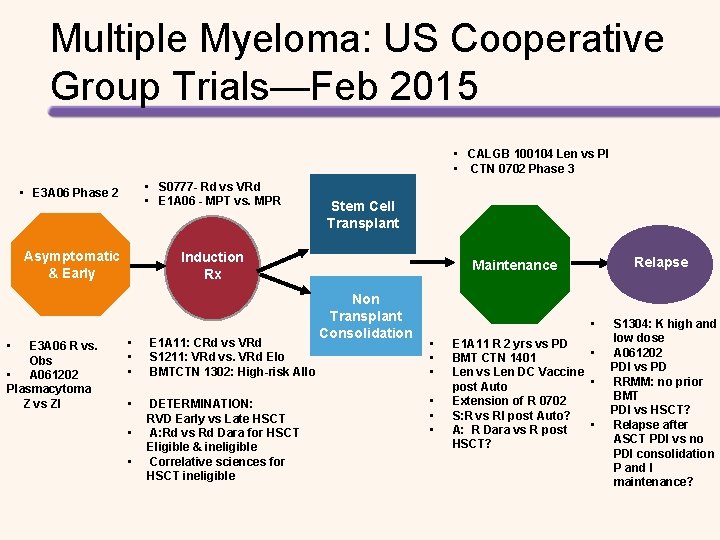
Multiple Myeloma: US Cooperative Group Trials—Feb 2015 • CALGB 100104 Len vs Pl • CTN 0702 Phase 3 • • E 3 A 06 Phase 2 • S 0777 - Rd vs VRd • E 1 A 06 - MPT vs. MPR Asymptomatic & Early Induction Rx E 3 A 06 R vs. Obs • A 061202 Plasmacytoma Z vs ZI • • • E 1 A 11: CRd vs VRd S 1211: VRd vs. VRd Elo BMTCTN 1302: High-risk Allo • DETERMINATION: RVD Early vs Late HSCT A: Rd vs Rd Dara for HSCT Eligible & ineligible Correlative sciences for HSCT ineligible • • Stem Cell Transplant Relapse Maintenance Non Transplant Consolidation • • E 1 A 11 R 2 yrs vs PD • BMT CTN 1401 Len vs Len DC Vaccine • post Auto Extension of R 0702 S: R vs RI post Auto? • A: R Dara vs R post HSCT? S 1304: K high and low dose A 061202 PDI vs PD RRMM: no prior BMT PDI vs HSCT? Relapse after ASCT PDI vs no PDI consolidation P and I maintenance?


APPENDIX OF ADDITIONAL SLIDES FOR CUSTOMIZING PRESENTATIONS

MYELOMA OVERVIEW

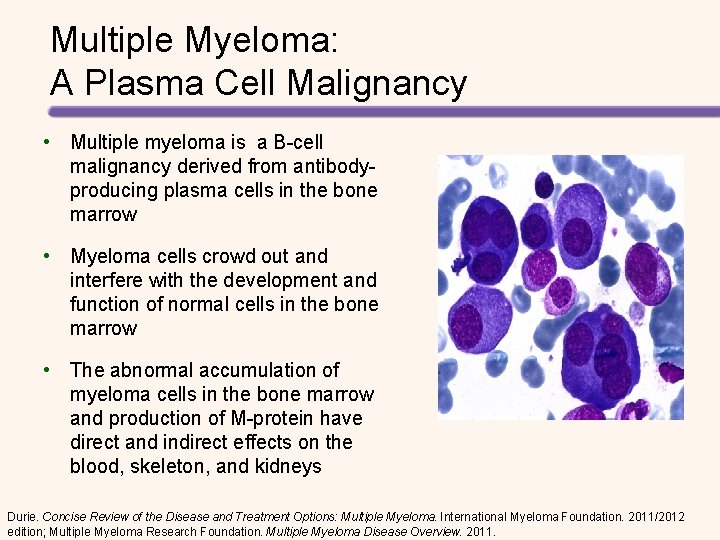
Multiple Myeloma: A Plasma Cell Malignancy • Multiple myeloma is a B-cell malignancy derived from antibodyproducing plasma cells in the bone marrow • Myeloma cells crowd out and interfere with the development and function of normal cells in the bone marrow • The abnormal accumulation of myeloma cells in the bone marrow and production of M-protein have direct and indirect effects on the blood, skeleton, and kidneys Durie. Concise Review of the Disease and Treatment Options: Multiple Myeloma. International Myeloma Foundation. 2011/2012 edition; Multiple Myeloma Research Foundation. Multiple Myeloma Disease Overview. 2011.

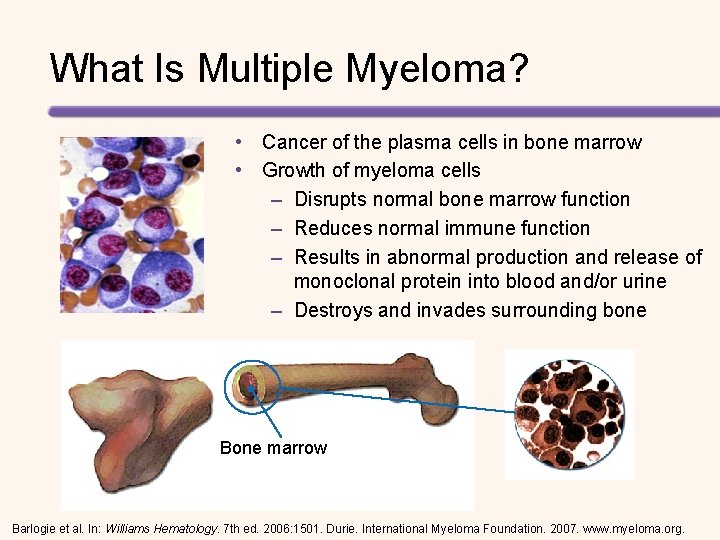
What Is Multiple Myeloma? • Cancer of the plasma cells in bone marrow • Growth of myeloma cells – Disrupts normal bone marrow function – Reduces normal immune function – Results in abnormal production and release of monoclonal protein into blood and/or urine – Destroys and invades surrounding bone Bone marrow Barlogie et al. In: Williams Hematology. 7 th ed. 2006: 1501. Durie. International Myeloma Foundation. 2007. www. myeloma. org.

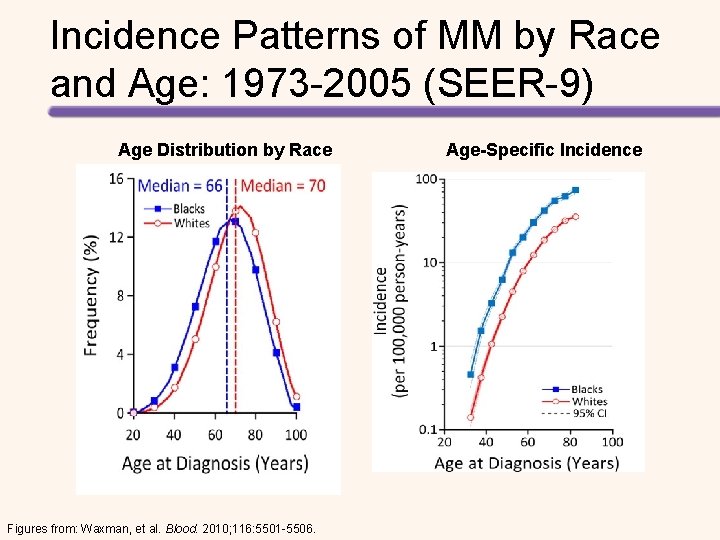
Incidence Patterns of MM by Race and Age: 1973 -2005 (SEER-9) Age Distribution by Race Figures from: Waxman, et al. Blood. 2010; 116: 5501 -5506. Age-Specific Incidence

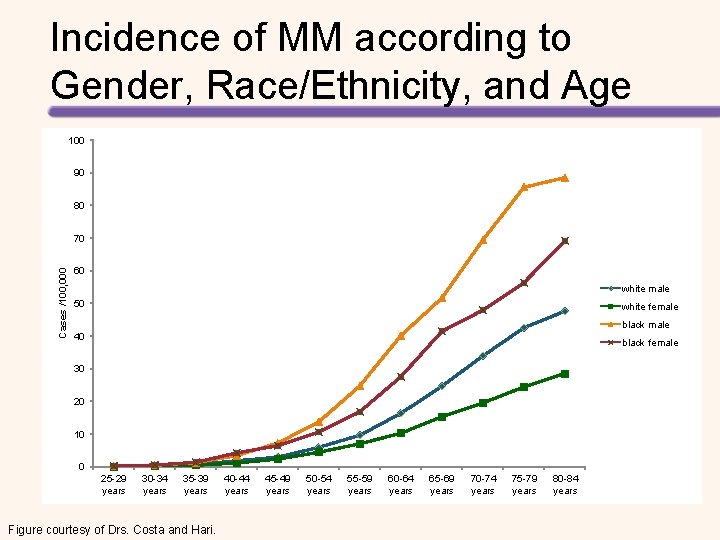
Incidence of MM according to Gender, Race/Ethnicity, and Age 100 90 80 Cases /100, 000 70 60 white male 50 white female black male 40 black female 30 20 10 0 25 -29 years 30 -34 years 35 -39 years Figure courtesy of Drs. Costa and Hari. 40 -44 years 45 -49 years 50 -54 years 55 -59 years 60 -64 years 65 -69 years 70 -74 years 75 -79 years 80 -84 years

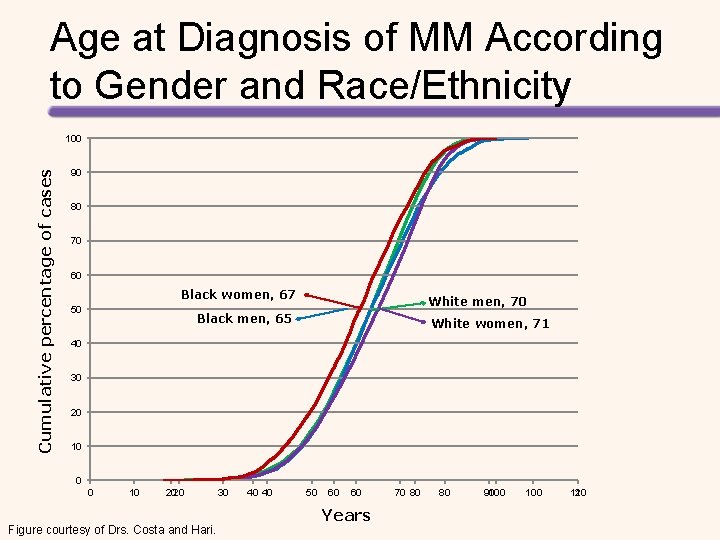
Age at Diagnosis of MM According to Gender and Race/Ethnicity Cumulative percentage of cases 100 90 80 70 60 Black women, 67 50 White men, 70 Black men, 65 White women, 71 40 30 20 10 0 0 10 2020 Figure courtesy of Drs. Costa and Hari. 30 40 40 50 60 60 Years 70 80 80 90 100 110 120

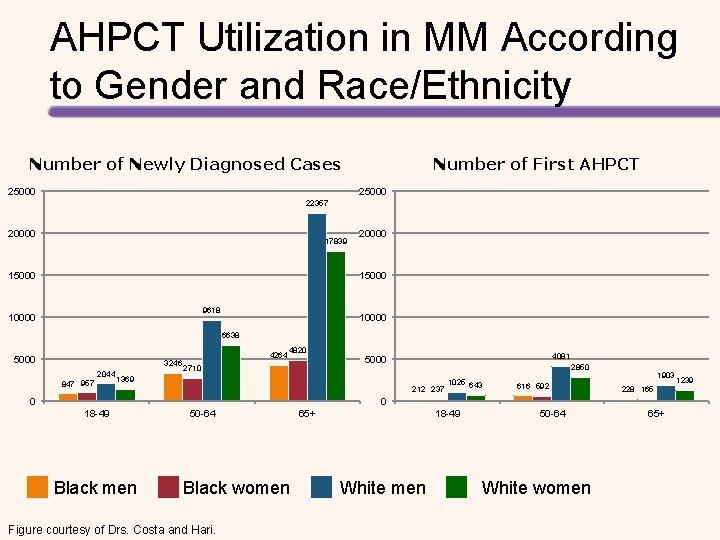
AHPCT Utilization in MM According to Gender and Race/Ethnicity Number of Newly Diagnosed Cases 25000 Number of First AHPCT 25000 22357 20000 17839 15000 20000 15000 9618 10000 6638 5000 3246 2044 847 957 4264 4820 2710 4081 5000 2850 1369 212 237 0 1025 643 616 592 1903 228 165 0 18 -49 Black men 50 -64 Black women Figure courtesy of Drs. Costa and Hari. 65+ 18 -49 White men 50 -64 White women 65+ 1239

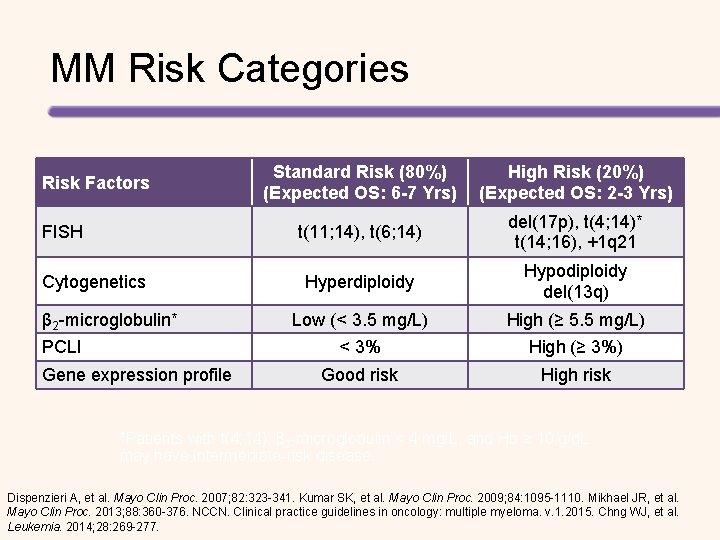
MM Risk Categories Risk Factors FISH Cytogenetics β 2 -microglobulin* PCLI Gene expression profile Standard Risk (80%) (Expected OS: 6 -7 Yrs) High Risk (20%) (Expected OS: 2 -3 Yrs) t(11; 14), t(6; 14) del(17 p), t(4; 14)* t(14; 16), +1 q 21 Hyperdiploidy Hypodiploidy del(13 q) Low (< 3. 5 mg/L) High (≥ 5. 5 mg/L) < 3% High (≥ 3%) Good risk High risk *Patients with t(4; 14), β 2 -microglobulin < 4 mg/L, and Hb ≥ 10 g/d. L may have intermediate-risk disease. Dispenzieri A, et al. Mayo Clin Proc. 2007; 82: 323 -341. Kumar SK, et al. Mayo Clin Proc. 2009; 84: 1095 -1110. Mikhael JR, et al. Mayo Clin Proc. 2013; 88: 360 -376. NCCN. Clinical practice guidelines in oncology: multiple myeloma. v. 1. 2015. Chng WJ, et al. Leukemia. 2014; 28: 269 -277.

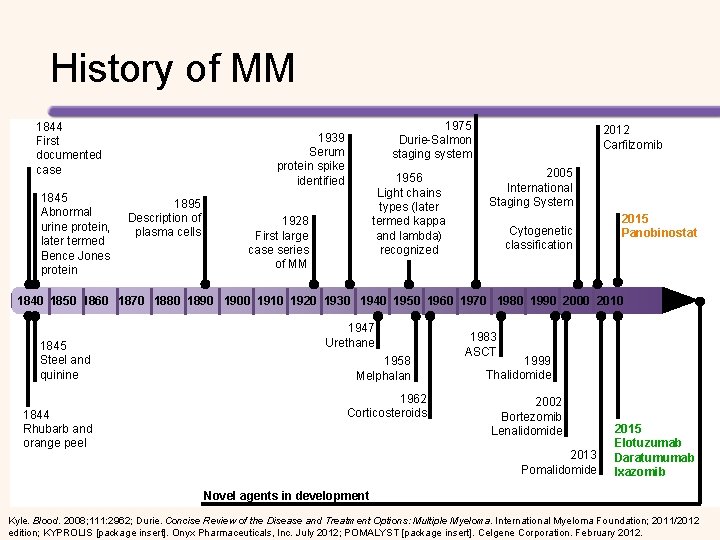
History of MM 1844 First documented case 1845 Abnormal urine protein, later termed Bence Jones protein 1975 Durie-Salmon staging system 1939 Serum protein spike identified 1895 Description of plasma cells 1956 Light chains types (later termed kappa and lambda) recognized 1928 First large case series of MM 2012 Carfilzomib 2005 International Staging System Cytogenetic classification 2015 Panobinostat 1840 1850 1860 1870 1880 1890 1900 1910 1920 1930 1940 1950 1960 1970 1980 1990 2000 2010 1845 Steel and quinine 1844 Rhubarb and orange peel 1947 Urethane 1958 Melphalan 1962 Corticosteroids 1983 ASCT 1999 Thalidomide 2002 Bortezomib Lenalidomide 2013 Pomalidomide 2015 Elotuzumab Daratumumab Ixazomib Novel agents in development Kyle. Blood. 2008; 111: 2962; Durie. Concise Review of the Disease and Treatment Options: Multiple Myeloma. International Myeloma Foundation; 2011/2012 edition; KYPROLIS [package insert]. Onyx Pharmaceuticals, Inc. July 2012; POMALYST [package insert]. Celgene Corporation. February 2012.

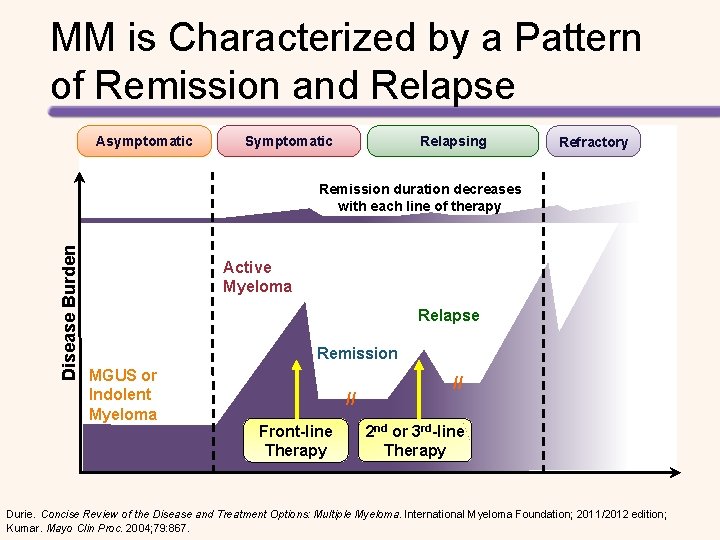
MM is Characterized by a Pattern of Remission and Relapse Asymptomatic Symptomatic Relapsing Refractory Disease Burden Remission duration decreases with each line of therapy Active Myeloma Relapse Remission MGUS or Indolent Myeloma // Front-line Therapy // 2 nd or 3 rd-line Therapy Durie. Concise Review of the Disease and Treatment Options: Multiple Myeloma. International Myeloma Foundation; 2011/2012 edition; Kumar. Mayo Clin Proc. 2004; 79: 867.

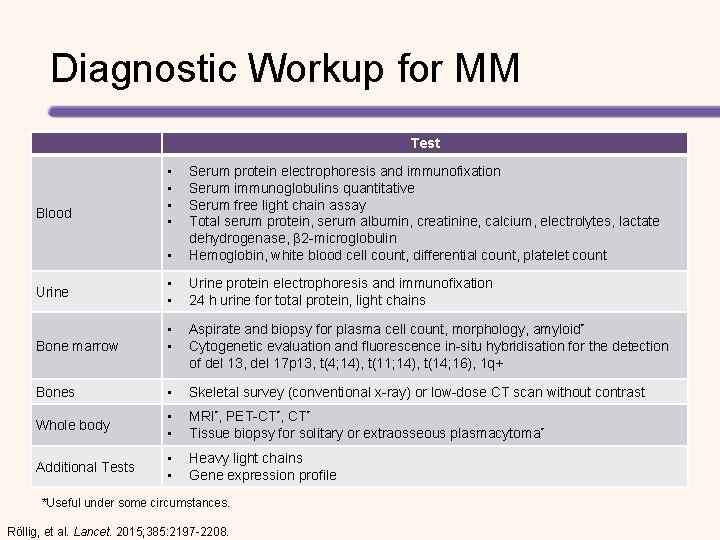
Diagnostic Workup for MM Test • • • Serum protein electrophoresis and immunofixation Serum immunoglobulins quantitative Serum free light chain assay Total serum protein, serum albumin, creatinine, calcium, electrolytes, lactate dehydrogenase, β 2 -microglobulin Hemoglobin, white blood cell count, differential count, platelet count • • Urine protein electrophoresis and immunofixation 24 h urine for total protein, light chains Bone marrow • • Aspirate and biopsy for plasma cell count, morphology, amyloid* Cytogenetic evaluation and fluorescence in-situ hybridisation for the detection of del 13, del 17 p 13, t(4; 14), t(11; 14), t(14; 16), 1 q+ Bones • Skeletal survey (conventional x-ray) or low-dose CT scan without contrast Whole body • • MRI*, PET-CT*, CT* Tissue biopsy for solitary or extraosseous plasmacytoma* Additional Tests • • Heavy light chains Gene expression profile Blood Urine *Useful under some circumstances. Röllig, et al. Lancet. 2015; 385: 2197 -2208.

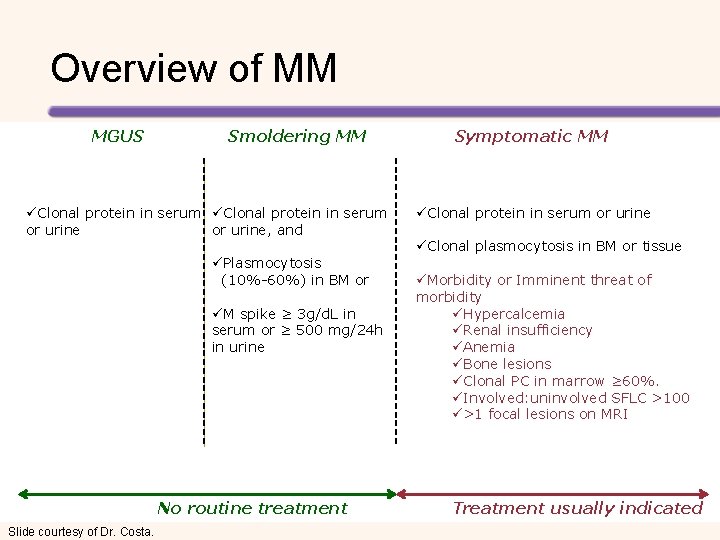
Overview of MM MGUS Smoldering MM üClonal protein in serum or urine, and or urine üPlasmocytosis (10%-60%) in BM or üM spike ≥ 3 g/d. L in serum or ≥ 500 mg/24 h in urine No routine treatment Slide courtesy of Dr. Costa. Symptomatic MM üClonal protein in serum or urine üClonal plasmocytosis in BM or tissue üMorbidity or Imminent threat of morbidity üHypercalcemia üRenal insufficiency üAnemia üBone lesions üClonal PC in marrow ≥ 60%. üInvolved: uninvolved SFLC >100 ü>1 focal lesions on MRI Treatment usually indicated

TREATMENT PARADIGM AND GOALS

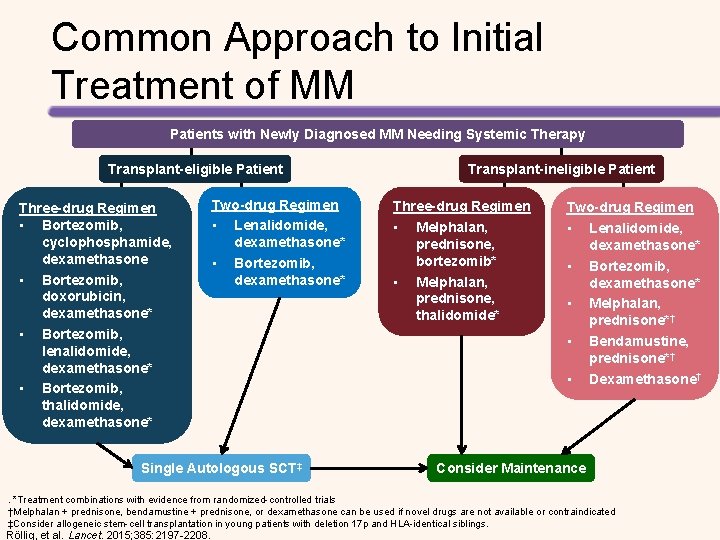
Common Approach to Initial Treatment of MM Patients with Newly Diagnosed MM Needing Systemic Therapy Transplant-eligible Patient Three-drug Regimen • Bortezomib, cyclophosphamide, dexamethasone • Bortezomib, doxorubicin, dexamethasone* • Bortezomib, lenalidomide, dexamethasone* • Bortezomib, thalidomide, dexamethasone* Two-drug Regimen • Lenalidomide, dexamethasone* • Bortezomib, dexamethasone* Single Autologous SCT‡ Transplant-ineligible Patient Three-drug Regimen • Melphalan, prednisone, bortezomib* • Melphalan, prednisone, thalidomide* Two-drug Regimen • Lenalidomide, dexamethasone* • Bortezomib, dexamethasone* • Melphalan, prednisone*† • Bendamustine, prednisone*† • Dexamethasone† Consider Maintenance . *Treatment combinations with evidence from randomized-controlled trials †Melphalan + prednisone, bendamustine + prednisone, or dexamethasone can be used if novel drugs are not available or contraindicated ‡Consider allogeneic stem-cell transplantation in young patients with deletion 17 p and HLA-identical siblings. Röllig, et al. Lancet. 2015; 385: 2197 -2208.

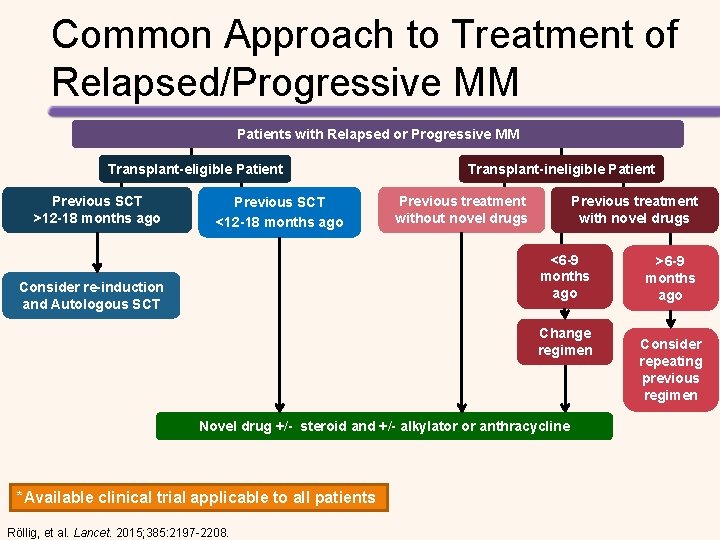
Common Approach to Treatment of Relapsed/Progressive MM Patients with Relapsed or Progressive MM Transplant-eligible Patient Previous SCT >12 -18 months ago Previous SCT <12 -18 months ago Transplant-ineligible Patient Previous treatment without novel drugs Previous treatment with novel drugs <6 -9 months ago Consider re-induction and Autologous SCT Change regimen Novel drug +/- steroid and +/- alkylator or anthracycline *Available clinical trial applicable to all patients Röllig, et al. Lancet. 2015; 385: 2197 -2208. >6 -9 months ago Consider repeating previous regimen

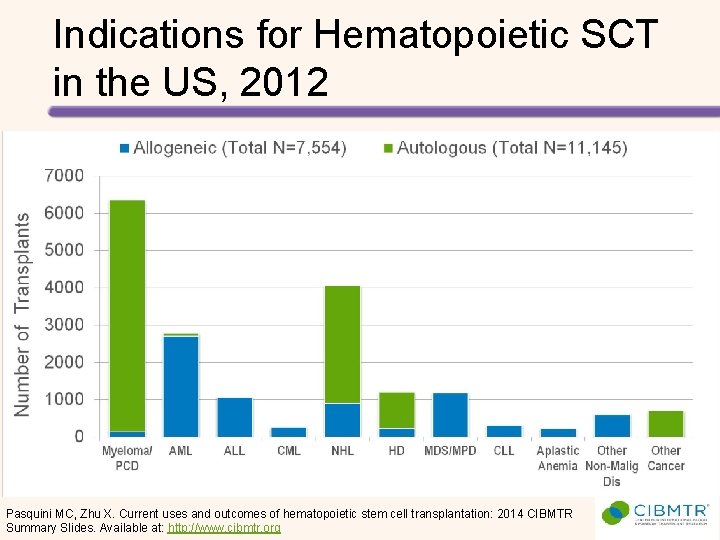
Indications for Hematopoietic SCT in the US, 2012 Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. Available at: http: //www. cibmtr. org

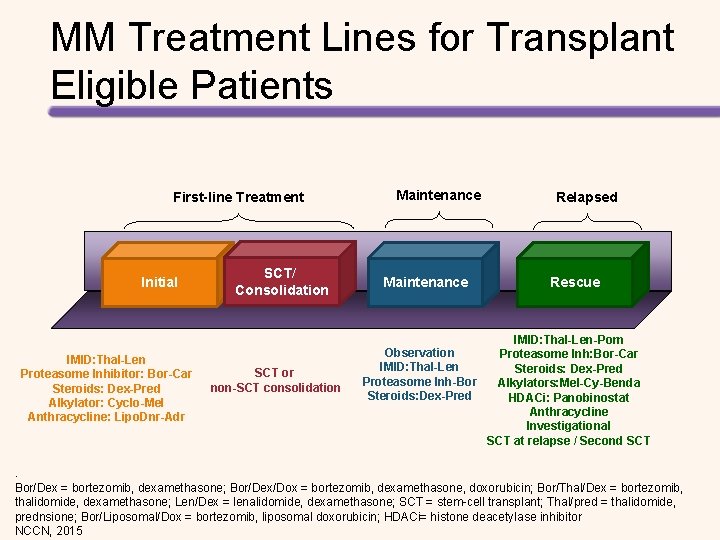
MM Treatment Lines for Transplant Eligible Patients First-line Treatment Initial IMID: Thal-Len Proteasome Inhibitor: Bor-Car Steroids: Dex-Pred Alkylator: Cyclo-Mel Anthracycline: Lipo. Dnr-Adr SCT/ Consolidation SCT or non-SCT consolidation Maintenance Relapsed Rescue IMID: Thal-Len-Pom Observation Proteasome Inh: Bor-Car IMID: Thal-Len Steroids: Dex-Pred Proteasome Inh-Bor Alkylators: Mel-Cy-Benda Steroids: Dex-Pred HDACi: Panobinostat Anthracycline Investigational SCT at relapse / Second SCT . Bor/Dex = bortezomib, dexamethasone; Bor/Dex/Dox = bortezomib, dexamethasone, doxorubicin; Bor/Thal/Dex = bortezomib, thalidomide, dexamethasone; Len/Dex = lenalidomide, dexamethasone; SCT = stem-cell transplant; Thal/pred = thalidomide, prednsione; Bor/Liposomal/Dox = bortezomib, liposomal doxorubicin; HDACi= histone deacetylase inhibitor NCCN, 2015

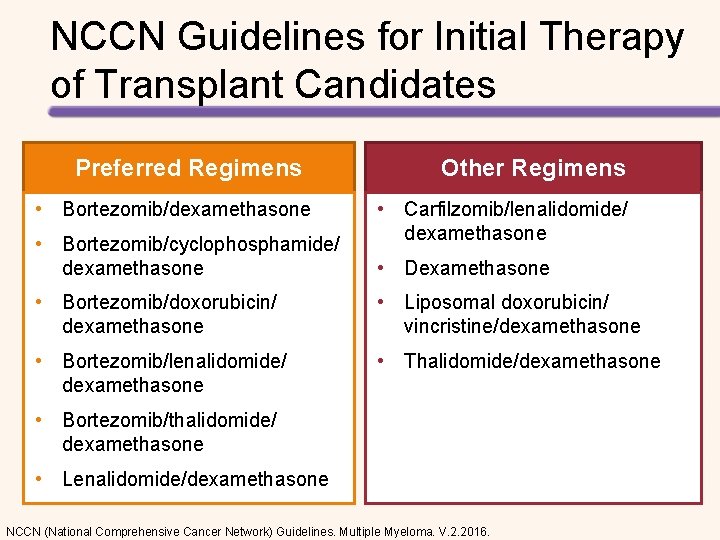
NCCN Guidelines for Initial Therapy of Transplant Candidates Preferred Regimens Other Regimens • Bortezomib/dexamethasone • Carfilzomib/lenalidomide/ dexamethasone • Bortezomib/cyclophosphamide/ dexamethasone • Dexamethasone • Bortezomib/doxorubicin/ dexamethasone • Liposomal doxorubicin/ vincristine/dexamethasone • Bortezomib/lenalidomide/ dexamethasone • Thalidomide/dexamethasone • Bortezomib/thalidomide/ dexamethasone • Lenalidomide/dexamethasone NCCN (National Comprehensive Cancer Network) Guidelines. Multiple Myeloma. V. 2. 2016.

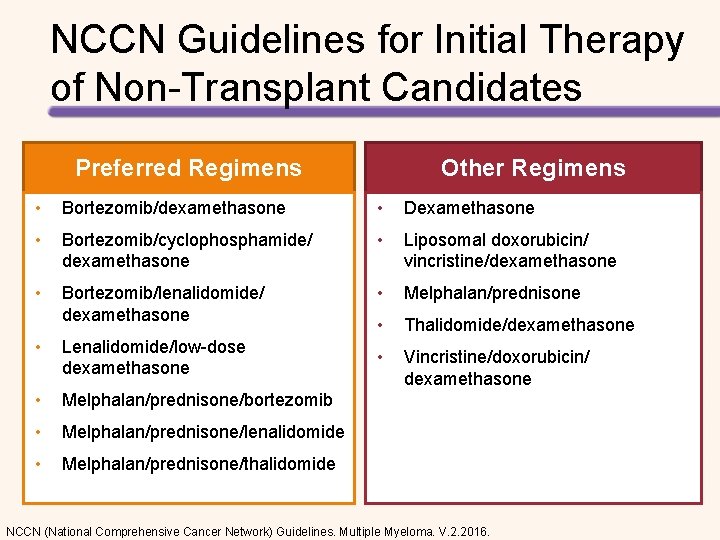
NCCN Guidelines for Initial Therapy of Non-Transplant Candidates Preferred Regimens Other Regimens • Bortezomib/dexamethasone • Dexamethasone • Bortezomib/cyclophosphamide/ dexamethasone • Liposomal doxorubicin/ vincristine/dexamethasone • Bortezomib/lenalidomide/ dexamethasone • Melphalan/prednisone • Thalidomide/dexamethasone • Vincristine/doxorubicin/ dexamethasone • Lenalidomide/low-dose dexamethasone • Melphalan/prednisone/bortezomib • Melphalan/prednisone/lenalidomide • Melphalan/prednisone/thalidomide NCCN (National Comprehensive Cancer Network) Guidelines. Multiple Myeloma. V. 2. 2016.

NCCN Guidelines for Previously Treated MM Preferred Regimens • • • • • Repeat induction regimen if relapse at > 6 months Bortezomib/dexamethasone Bortezomib/cyclophosphamide/ dexamethasone Bortezomib/lenalidomide/ dexamethasone Bortezomib/liposomal doxorubicin Carfilzomib/dexamethasone Carfilzomib/lenalidomide/ dexamethasone Cyclophosphamide/lenalidomide/dexamethasone Dexamethasone/cyclophosphamide/etoposide/cisplatin Dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide± bortezomib High-dose cyclophosphamide Lenalidomide/dexamethasone Panobinostat/bortezomib/dexamethasone Pomalidomide/dexamethasone Thalidomide/dexamethasone NCCN (National Comprehensive Cancer Network) Guidelines. Multiple Myeloma. V. 2. 2016.

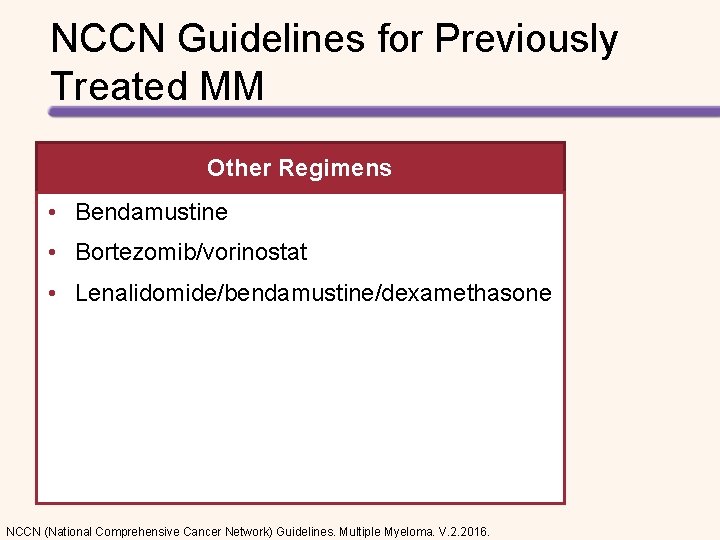
NCCN Guidelines for Previously Treated MM Other Regimens • Bendamustine • Bortezomib/vorinostat • Lenalidomide/bendamustine/dexamethasone NCCN (National Comprehensive Cancer Network) Guidelines. Multiple Myeloma. V. 2. 2016.

ROLE OF MAINTENANCE THERAPY
![Maintenance in Myeloma PFS advantage1 3 OS improvements 2 Toxicities of Maintenance in Myeloma • PFS advantage[1 -3] • OS improvements? [2] • Toxicities of](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-95.jpg)
Maintenance in Myeloma • PFS advantage[1 -3] • OS improvements? [2] • Toxicities of treatment – Myelosuppression[3] – Second primary malignancies[3, 4] – Quality of life • Unclear whether all patients benefit from maintenance • Unclear which agent and duration of therapy [1] Attal M, et al. ASH 2013. Abstract 406. [2] Mc. Carthy PL, et al. N Engl J Med. 2012; 366: 1770 -1781. [3] Attal M, et al. N Engl J Med. 2012; 366: 1782 -1791. [4] Palumbo A, et al. Lancet Oncol. 2014; 15: 333 -342.

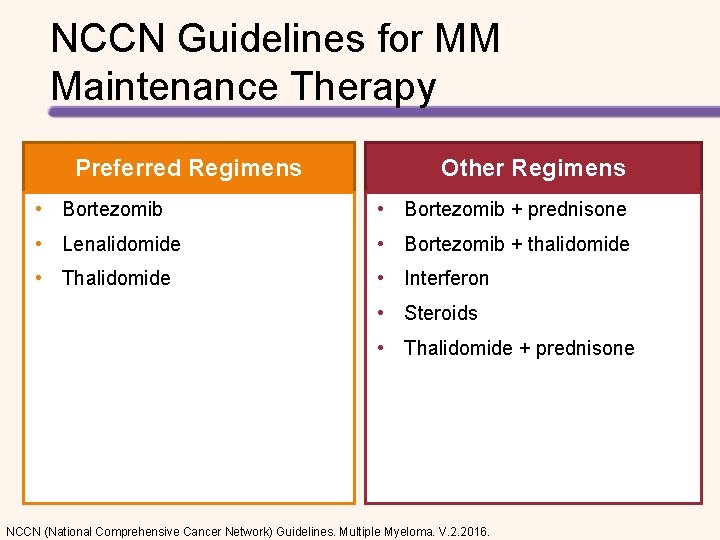
NCCN Guidelines for MM Maintenance Therapy Preferred Regimens Other Regimens • Bortezomib + prednisone • Lenalidomide • Bortezomib + thalidomide • Thalidomide • Interferon • Steroids • Thalidomide + prednisone NCCN (National Comprehensive Cancer Network) Guidelines. Multiple Myeloma. V. 2. 2016.

Maintenance Therapy in MM: Summary • Median PFS after ASCT has improved without maintenance using better induction • Maintenance with novel agents further improves PFS – Toxicity issues are critical • OS results are improved in some studies of thalidomide, lenalidomide, and bortezomib maintenance • Decisions regarding maintenance will be influenced by – Incidence of toxicity such as secondary cancers – Outcome after myeloma progression – Identification of subgroups most likely to benefit

ASSESSING AND MONITORING RESPONSE TO THERAPY

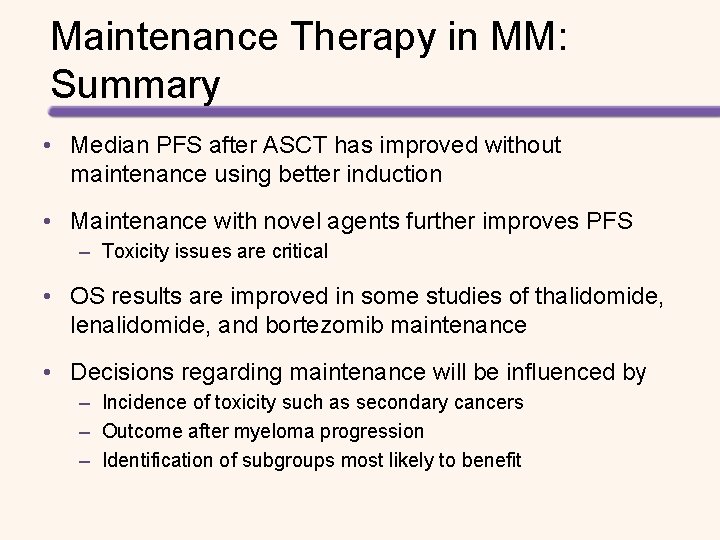
The Deeper the Response, the Longer PFS Figure from: Bruno Paiva et al. Blood. 2015; 125: 3059 -3068

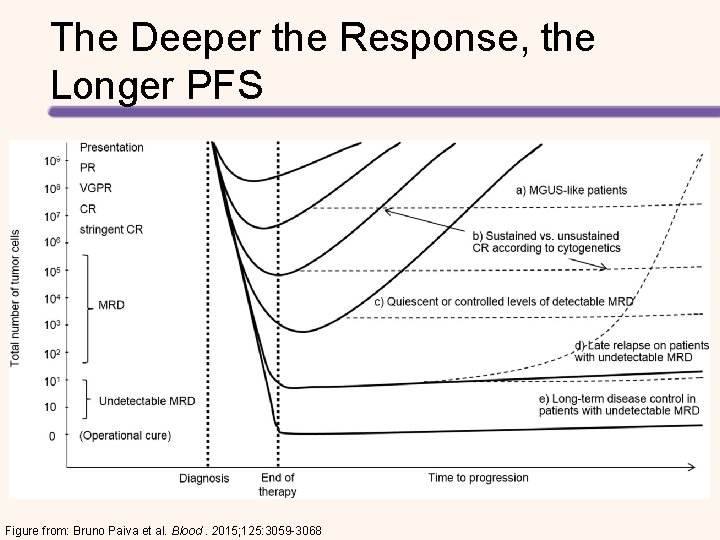
Features of Currently Available Techniques to Monitor MRD in MM MFC (≥ 8 -color) ASO-PCR NGS PET/CT ∼ 100% 60% to 70% ∼ 90% ∼ 100%* Reproducibility among centers High Not reported Moderate at MRD Availability in individual laboratories around the world High Intermediate Limited Intermediate Important but not mandatory Mandatory Important but not mandatory 2 -3 h ≥ 5 d (follow-up), 3 -4 wk (target identification) ≥ 7 d 2 h Cost per sample ∼ 350 USD ∼ 500 USD (follow-up), ∼ 1500 USD at diagnosis (target identification) ∼ 700 USD ∼ 2000 USD Sensitivity 10− 5 to 10− 6 High (4 mm) Yes (directly; high accuracy) Yes Yes Fresh sample Needed (<36 h) Not needed NA Patchy sample Impacts No impact Yes No No No Ongoing (Euro. Flow/IMF) Yes, since 15 y (Euro. MRD) Not reported No Applicability Diagnostic sample Time Quantitative Global cell characterization Standardization Table from: Bruno Paiva et al. Blood. 2015; 125: 3059 -3068
![Methods for Assessing MRD to Predict Outcome 8 color Flow1 Next Gen Sequencing2 TTP Methods for Assessing MRD to Predict Outcome 8 -color Flow[1] Next Gen Sequencing[2] TTP](https://slidetodoc.com/presentation_image_h/816f0c34ed9fe8d07cdd351739d4b52f/image-101.jpg)
Methods for Assessing MRD to Predict Outcome 8 -color Flow[1] Next Gen Sequencing[2] TTP (CR Patients) 1. 0 100 0. 8 80 0. 6 60 0. 4 MRD – MRD + Overall 0. 2 0 0 6 12 18 24 30 36 42 Mos TTP (%) PFS (proportion) PFS P =. 001 n = 26 40 n = 36 20 0 MRD – MRD + 0 50 100 150 Mos [1] Roussel M, et al. J Clin Oncol. 2014; 32: 2712 -2717. [2] Martinez-Lopez J, et al. Blood. 2014; 123: 3073 -3079.

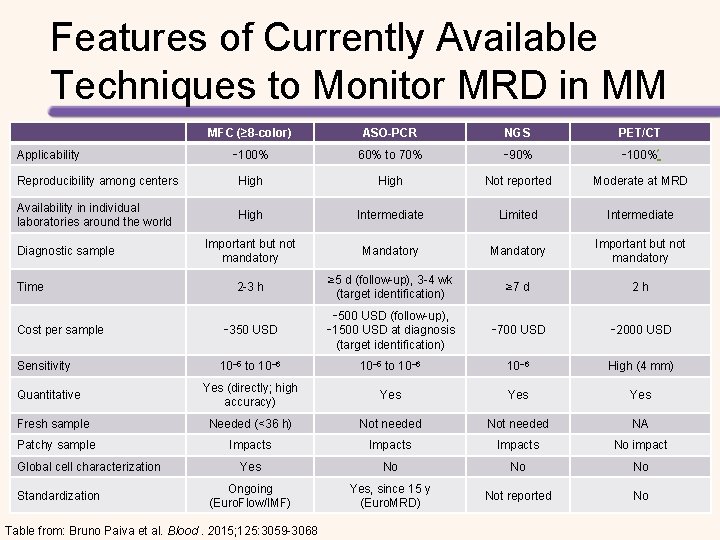
Response to Therapy in MM Patients 1 × 1012 Number of Myeloma Cells At diagnosis Partial response – 50% reduction in M protein Near complete remission – immunofixation positive only Complete remission – immunofixation negative 1 × 108 1 × 106 1 × 104 Nonquantitative ASO-PCR MRD Rajkumar, et al (IMWG consensus criteria). Blood. 2011; 117: 4691– 4695. Quantitative ASO-PCR High quality flow cytometry Deep sequencing

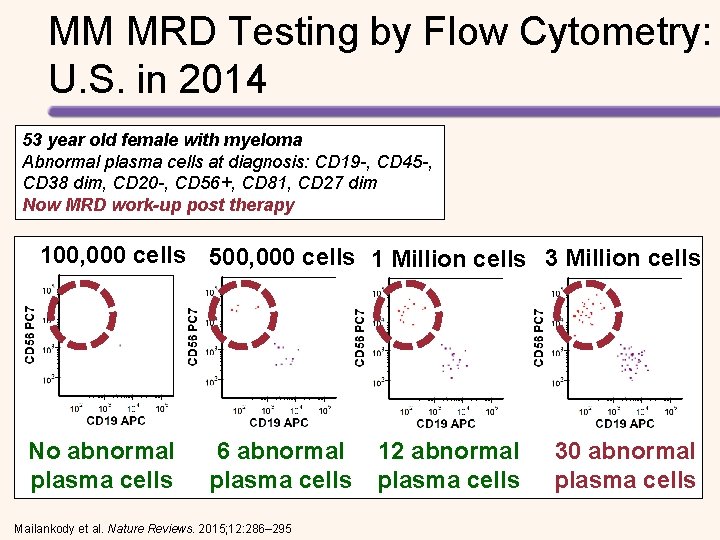
MM MRD Testing by Flow Cytometry: U. S. in 2014 53 year old female with myeloma Abnormal plasma cells at diagnosis: CD 19 -, CD 45 -, CD 38 dim, CD 20 -, CD 56+, CD 81, CD 27 dim Now MRD work-up post therapy 100, 000 cells 500, 000 cells 1 Million cells 3 Million cells No abnormal plasma cells 6 abnormal plasma cells Mailankody et al. Nature Reviews. 2015; 12: 286– 295 12 abnormal plasma cells 30 abnormal plasma cells

STEM CELL MOBILIZATION

International Myeloma Working Group (IMWG) Consensus Recommendations for Cell Doses • Is there an optimum CD 34+ cell dose to be infused? – Cell doses > 3 × 106 CD 34+ cells/kg associated with better outcomes[1 -3] – Studies primarily retrospective – Recommendation: the issue of optimal CD 34+ cell dosing in a. HSCT for MM requires a prospective clinical trial • Is there an optimal dose of CD 34+ cells to be collected? – Recommendations[1] • Minimum target of 4 × 106 CD 34+ cells/kg should be collected • If feasible an average of 8 -10 × 106 CD 34+ cells/kg should be collected – These targets allow most patients to undergo at least 2 a. HSCT, each with an optimal cell dose a. HSCT, autologous hematopoietic stem cell transplantation; MM, multiple myeloma. [1] Desikan JR, et al. Br J Haematol. 2001; 112: 242 -247. [2]Bensinger W, et al. J Clin Oncol. 1995; 13: 2547 -2555. [3] Weaver CH, et al. Blood. 1995; 86: 3961 -3969. [4] Giralt S, et al. Leukemia. 2009; 23: 1904 -1912

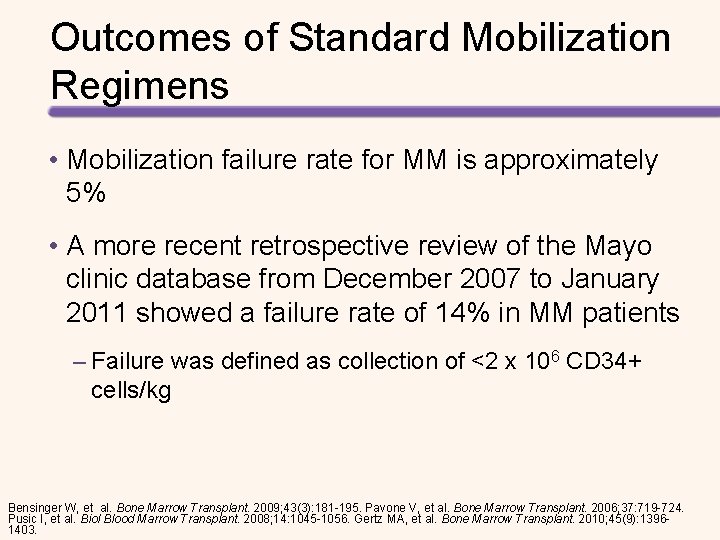
Outcomes of Standard Mobilization Regimens • Mobilization failure rate for MM is approximately 5% • A more recent retrospective review of the Mayo clinic database from December 2007 to January 2011 showed a failure rate of 14% in MM patients – Failure was defined as collection of <2 x 106 CD 34+ cells/kg Bensinger W, et al. Bone Marrow Transplant. 2009; 43(3): 181 -195. Pavone V, et al. Bone Marrow Transplant. 2006; 37: 719 -724. Pusic I, et al. Biol Blood Marrow Transplant. 2008; 14: 1045 -1056. Gertz MA, et al. Bone Marrow Transplant. 2010; 45(9): 13961403.

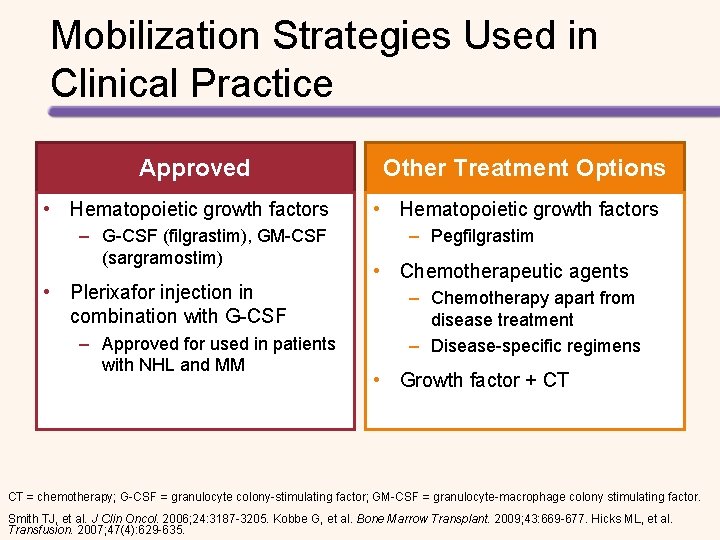
Mobilization Strategies Used in Clinical Practice Approved • Hematopoietic growth factors – G-CSF (filgrastim), GM-CSF (sargramostim) • Plerixafor injection in combination with G-CSF – Approved for used in patients with NHL and MM Other Treatment Options • Hematopoietic growth factors – Pegfilgrastim • Chemotherapeutic agents – Chemotherapy apart from disease treatment – Disease-specific regimens • Growth factor + CT CT = chemotherapy; G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony stimulating factor. Smith TJ, et al. J Clin Oncol. 2006; 24: 3187 -3205. Kobbe G, et al. Bone Marrow Transplant. 2009; 43: 669 -677. Hicks ML, et al. Transfusion. 2007; 47(4): 629 -635.

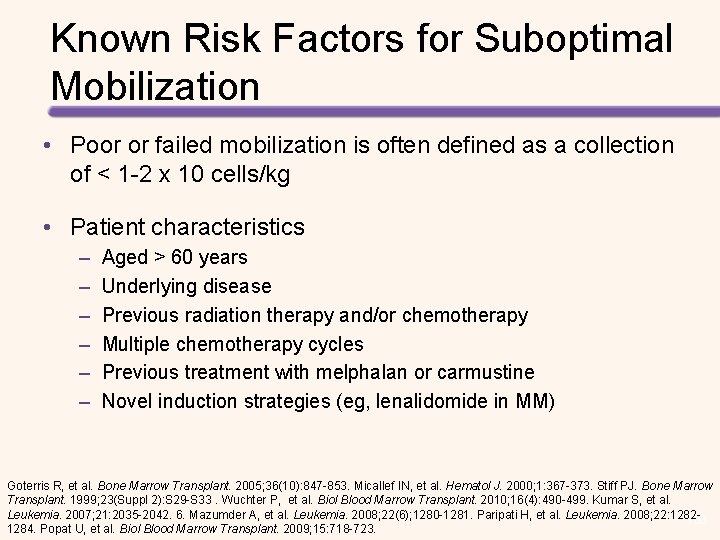
Known Risk Factors for Suboptimal Mobilization • Poor or failed mobilization is often defined as a collection of < 1 -2 x 10 cells/kg • Patient characteristics – – – Aged > 60 years Underlying disease Previous radiation therapy and/or chemotherapy Multiple chemotherapy cycles Previous treatment with melphalan or carmustine Novel induction strategies (eg, lenalidomide in MM) Goterris R, et al. Bone Marrow Transplant. 2005; 36(10): 847 -853. Micallef IN, et al. Hematol J. 2000; 1: 367 -373. Stiff PJ. Bone Marrow Transplant. 1999; 23(Suppl 2): S 29 -S 33. Wuchter P, et al. Biol Blood Marrow Transplant. 2010; 16(4): 490 -499. Kumar S, et al. Leukemia. 2007; 21: 2035 -2042. 6. Mazumder A, et al. Leukemia. 2008; 22(6); 1280 -1281. Paripati H, et al. Leukemia. 2008; 22: 1282108 1284. Popat U, et al. Biol Blood Marrow Transplant. 2009; 15: 718 -723.

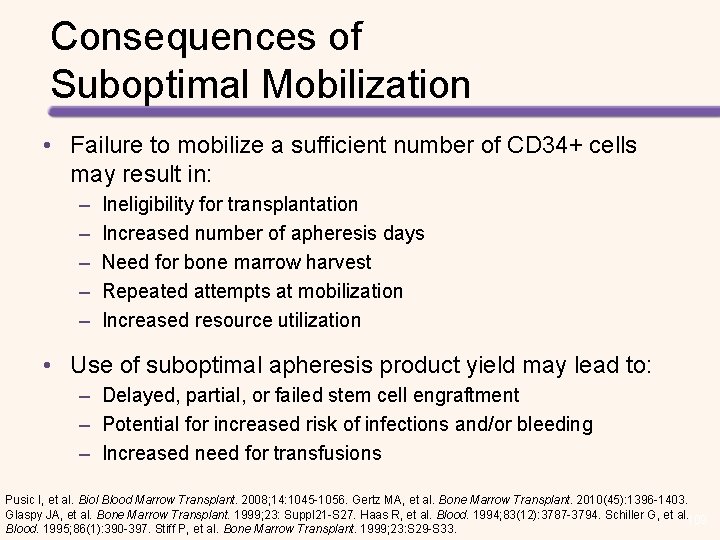
Consequences of Suboptimal Mobilization • Failure to mobilize a sufficient number of CD 34+ cells may result in: – – – Ineligibility for transplantation Increased number of apheresis days Need for bone marrow harvest Repeated attempts at mobilization Increased resource utilization • Use of suboptimal apheresis product yield may lead to: – Delayed, partial, or failed stem cell engraftment – Potential for increased risk of infections and/or bleeding – Increased need for transfusions Pusic I, et al. Biol Blood Marrow Transplant. 2008; 14: 1045 -1056. Gertz MA, et al. Bone Marrow Transplant. 2010(45): 1396 -1403. Glaspy JA, et al. Bone Marrow Transplant. 1999; 23: Suppl 21 -S 27. Haas R, et al. Blood. 1994; 83(12): 3787 -3794. Schiller G, et al. 109 Blood. 1995; 86(1): 390 -397. Stiff P, et al. Bone Marrow Transplant. 1999; 23: S 29 -S 33.

Conventional Mobilization Strategies Growth Factor • Advantages – Predictable collection schedule – Earlier engraftment • Disadvantages – Lower stem cells yields – More apheresis days CT • Advantages – High stem cell yields • Disadvantages – Risk for infection – Neutropenic fever and hospitalization – Less predictable collection schedule Pusic I, et al. Curr Pharm Des. 2008; 14(20): 1950 -1961. Gertz MA. Br J Haematol. 2010; 150(6): 647 -662.

AUTOLOGOUS TRANSPLANT When and How?

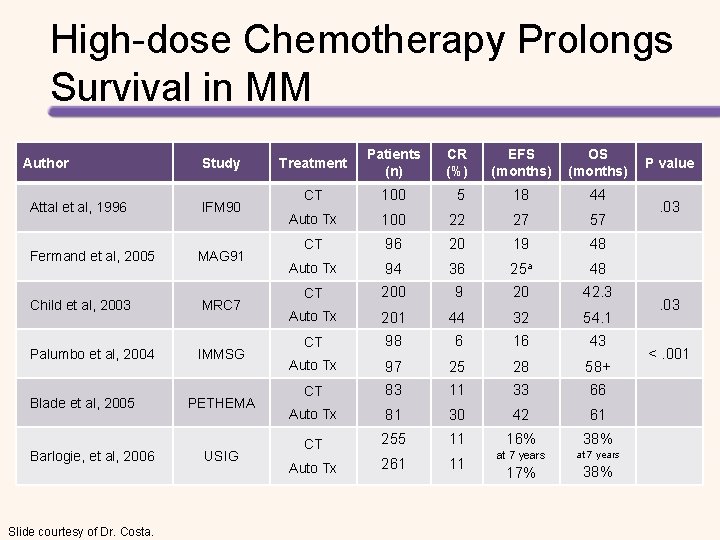
High-dose Chemotherapy Prolongs Survival in MM Author Study Attal et al, 1996 IFM 90 Fermand et al, 2005 MAG 91 Child et al, 2003 MRC 7 Palumbo et al, 2004 IMMSG Blade et al, 2005 Barlogie, et al, 2006 Slide courtesy of Dr. Costa. PETHEMA USIG Treatment Patients (n) CR (%) EFS (months) OS (months) CT 100 5 18 44 Auto Tx 100 22 27 57 CT 96 20 19 48 Auto Tx 94 36 25 a 48 CT 200 9 20 42. 3 Auto Tx 201 44 32 54. 1 CT 98 6 16 43 Auto Tx 97 25 28 58+ CT 83 11 33 66 Auto Tx 81 30 42 61 CT 255 11 Auto Tx 261 11 16% 38% at 7 years 17% 38% P value . 03 <. 001

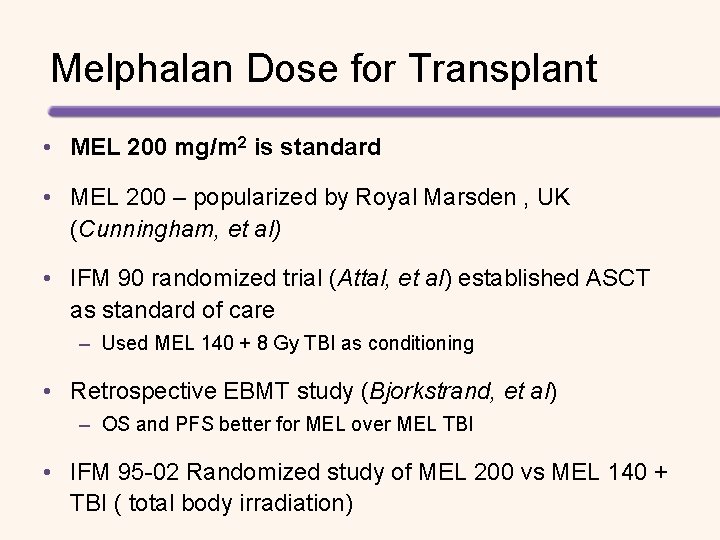
Melphalan Dose for Transplant • MEL 200 mg/m 2 is standard • MEL 200 – popularized by Royal Marsden , UK (Cunningham, et al) • IFM 90 randomized trial (Attal, et al) established ASCT as standard of care – Used MEL 140 + 8 Gy TBI as conditioning • Retrospective EBMT study (Bjorkstrand, et al) – OS and PFS better for MEL over MEL TBI • IFM 95 -02 Randomized study of MEL 200 vs MEL 140 + TBI ( total body irradiation)

MELPHALAN CHALLENGES PK Variability Propylene Glycol Special Issues

CE-Melphalan (Propylene Glycol-free): Phase 2 a Pharmacokinetic Study A Phase 2 a, Open-Label, Randomized, Pharmacokinetic Comparative, Cross-Over Study of Melphalan HCl for Injection and Standard Melphalan for Injection for Myeloablative Conditioning in Multiple Myeloma Patients Undergoing Autologous Transplantation Screening Period Randomization Baseline tests for study and infectious disease testing Study Period Day -3 Day -2 CE-Mel Standard Mel 100 mg/m 2 IV Over 30 min Day -3 Standard Mel 100 mg/m 2 IV Over 30 min Follow-up Period Day 1 ASCT Day +14 Rest Day Daily CBC & weekly safety assessment s until ANC engraftment Day -2 CE-Mel 100 mg/m 2 IV Over 30 min Day 0 ASCT Day +7 ASCT Daily CBC & weekly safety assessments until ANC engraftment ASCT Day +21 ASCT Day +30 Daily CBC & weekly safety assessments until ANC engraftment Date of Engraftment Enrollment (N=24) Aljitawi, et al. Bone Marrow Transplantation. 2014; 49: 1042– 1045. Up to 7 days after Date of Engraftment End of Study Evaluations

CE-Melphalan (Propylene Glycol-free): Efficacy Overview • All patients (100%) achieved myeloablation and engraftment – Median time to myeloablation: 3 days – Median time to neutrophil engraftment: 11 days Time and Rate of Myeloablation Experienced Myeloablation Time to Myeloablation (study days) Yes Total (N=24) 24 (100%) Mean 2. 9 SD 1. 21 Median 3. 0 Min 0 Max 5 Aljitawi, et al. Bone Marrow Transplantation. 2014; 49: 1042– 1045. Time and Rate of Engraftment Experienced Engraftment Time to Engraftment (study days) Yes Total (N=24) 24 (100%) Mean 11. 0 SD 1. 08 Median 11. 0 Min 9 Max 13

CE-Melphalan (Propylene Glycol-free): Safety Overview • Adverse event safety profile appears consistent with what has been reported with high-dose standard Melphalan – No patients discontinued prematurely – Prolonged QRS in 2 pts receiving standard Melphalan first (1 required hospitalization for observation) • Left bundle branch block pattern resolved within 2 days • Pts subsequently received CE-Melphalan without further complications Treatment-emergent AEs ≥ 50% of Patients Nausea 100% Diarrhea 96% Vomiting 88% Hypokalemia 88% Fatigue 83% Thrombocytopenia 75% Decreased appetite 63% Dizziness 63% Aljitawi, et al. Bone Marrow Transplantation. 2014; 49: 1042– 1045.

CE-Melphalan (Propylene Glycol-free): Results Overview Results from the Phase 2 a study (N=24): • CE-Melphalan is propylene glycol-free – More stable following reconstitution – Longer infusion times / higher doses possible with CE-Melphalan (propylene glycol-free) could improve response to treatment • CE-Melphalan (propylene glycol-free) is bioequivalent to standard Melphalan – CE-Melphalan (propylene glycol-free) peak and systemic exposure were slightly greater than with standard Melphalan • The efficacy and safety profile were consistent with that already established for high-dose Melphalan conditioning with ASCT for MM – 100% of patients achieved myeloablation and engraftment – Most frequent AEs included fatigue, nausea, and hypokalemia Aljitawi, et al. Bone Marrow Transplantation. 2014; 49: 1042– 1045.

CE-Melphalan (Propylene Glycol-free) • FDA Approved March 2016 – 1 st drug to gain FDA approval for the high-dose conditioning indication in MM • Captisol-enabled melphalan (CE-melphalan) approved for two indications in MM – High-dose conditioning treatment prior to HSCT for patients with MM – Palliative treatment for MM patients who are not candidates for oral therapy • Approval based on multicenter, open-label, phase 2 b study of 61 patients (5 relapsed prior to HSCT; 56 with newly diagnosed disease)1 – 200 mg/m² CE-melphalan, administered in 100 mg/m² doses on day 3 and day 2 before transplantation – ORR: 95%; CR: 31% (16% stringent CRs) [as determined by investigator assessment] – No treatment-related mortality; no new safety signals 1. Hari, et al. Biol Blood Marrow Transplant. 2015; 21: 2100 -2105. 119

AUTOLOGOUS TRANSPLANTATION Transplant vs Conventional Therapy Initial Therapy Tandem Early vs Late

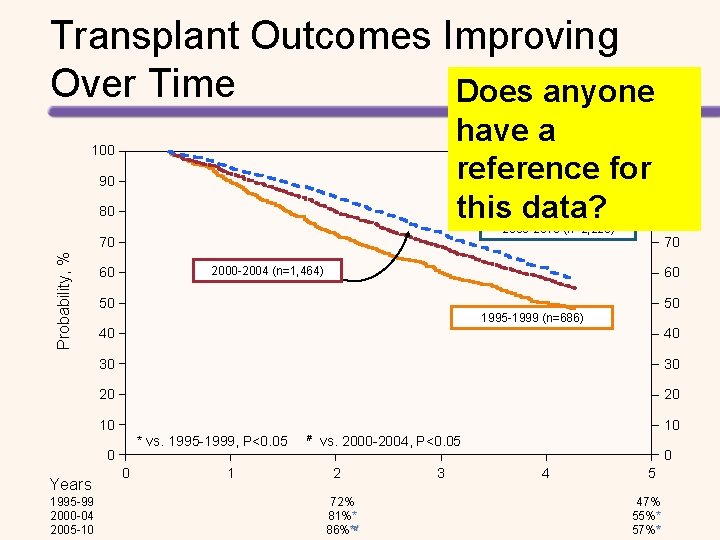
Transplant Outcomes Improving Over Time Does anyone have a reference for this data? 100 90 80 2005 -2010 (n=2, 223) Probability, % 70 50 50 1995 -1999 (n=686) 40 40 30 30 20 20 10 * vs. 1995 -1999, P<0. 05 # vs. 2000 -2004, P<0. 05 0 1995 -99 2000 -04 2005 -10 80 60 10 Years 90 70 2000 -2004 (n=1, 464) 60 100 0 1 2 72% 81%** 86%**# 3 0 4 5 47% 55%** 57%**

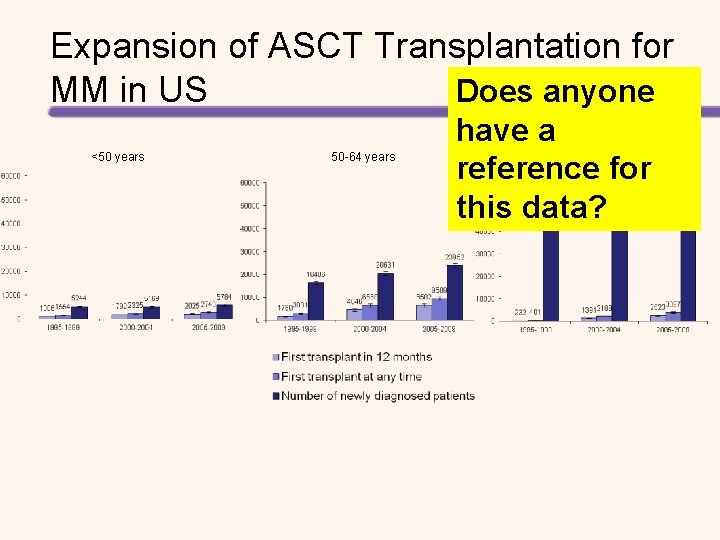
Expansion of ASCT Transplantation for MM in US Does anyone <50 years 50 -64 years have a reference for this data? ≥ 65 years

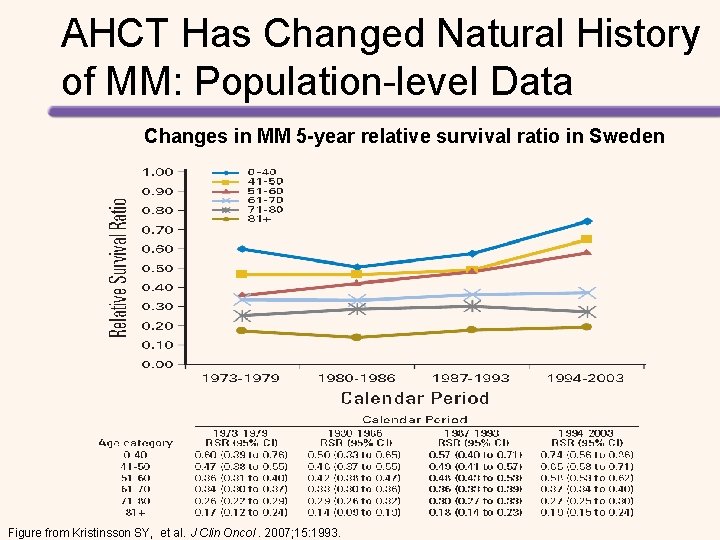
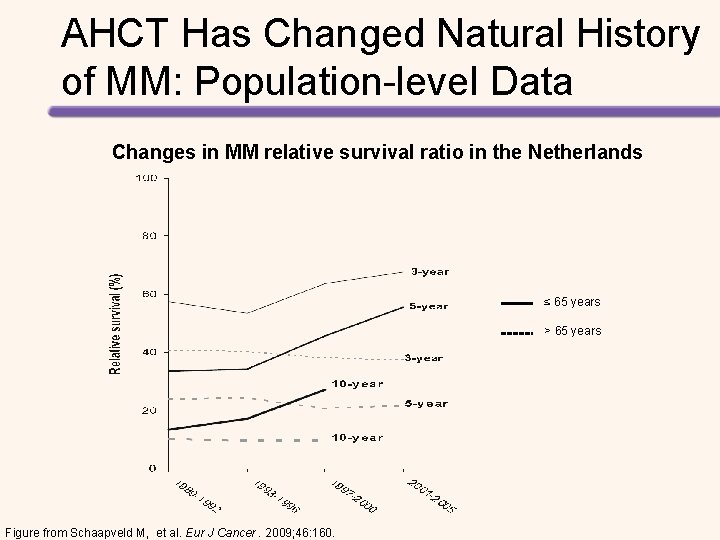
AHCT Has Changed Natural History of MM: Population-level Data Changes in MM 5 -year relative survival ratio in Sweden Figure from Kristinsson SY, et al. J Clin Oncol. 2007; 15: 1993.

AHCT Has Changed Natural History of MM: Population-level Data Changes in MM relative survival ratio in the Netherlands ≤ 65 years > 65 years Figure from Schaapveld M, et al. Eur J Cancer. 2009; 46: 160.

AUTOLOGOUS TRANSPLANTATION Transplant vs Conventional Therapy

Conventional Chemotherapy vs ASCT: Historic Randomized Studies—Median OS Median Survival Patients (n) Age IFM 90[1] 200 <65 MAG 91[2] 190 MRC 7[3] 403 <65 PETHEMA[4]* 164 S 9321[5] 516 Median Follow Up Conventional Chemotherapy ASCT 44 months 57 months 50 months 55 months 42 months 55 months <65 42 months 64 months 72 months ≤ 70 76 months 38% at 7 years 55 -65 56 months 38% at 7 years *in patients responding to conventional chemotherapy [1] Attal M et al. N Engl J Med. 1996; 335: 91. [2] Fermand JP et al. J Clin Oncol. 2005; 23: 9227. [3] Child A et al. N Engl J Med. 2003; 348: 1875. [4] Blade J et al. Blood. 2005; 106: 3755. [5]Barlogie B, et al. JCO. 2006; 24: 929 -936.

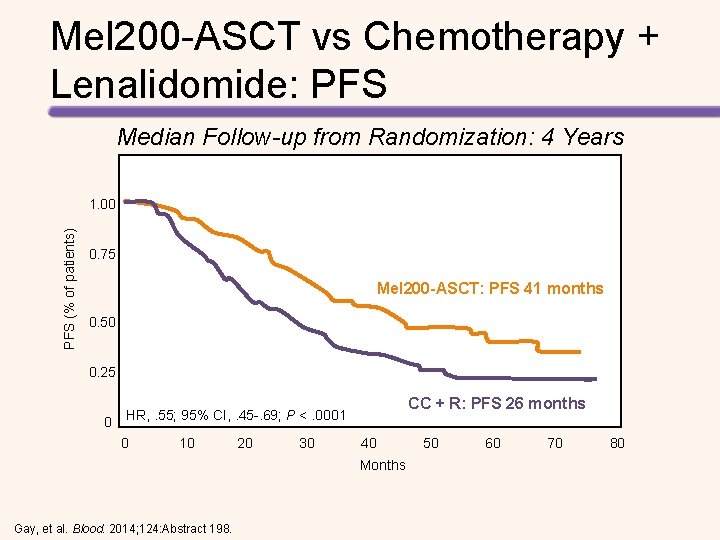
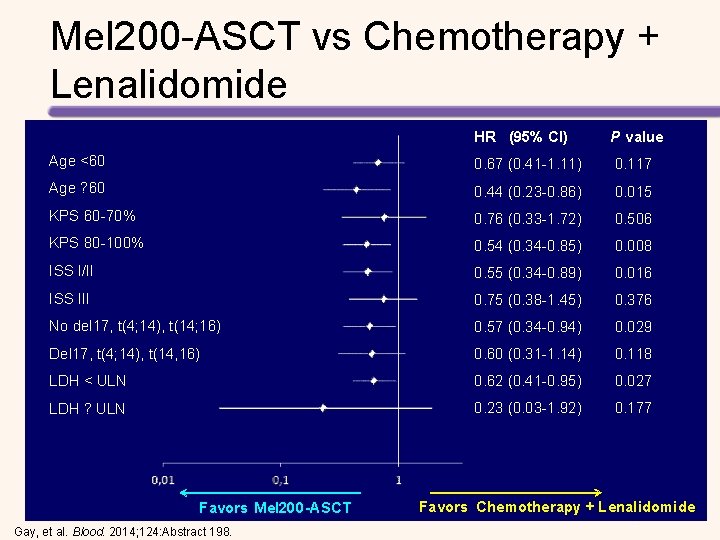
Mel 200 -ASCT vs Chemotherapy + Lenalidomide: PFS Median Follow-up from Randomization: 4 Years PFS (% of patients) 1. 00 0. 75 Mel 200 -ASCT: PFS 41 months 0. 50 0. 25 0 CC + R: PFS 26 months HR, . 55; 95% CI, . 45 -. 69; P <. 0001 0 10 20 30 40 50 60 70 80 Months Gay, et al. Blood. 2014; 124: Abstract 198.

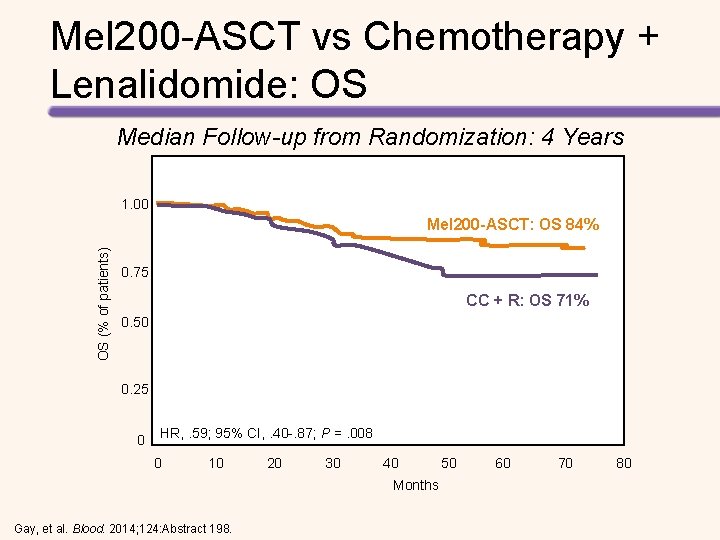
Mel 200 -ASCT vs Chemotherapy + Lenalidomide: OS Median Follow-up from Randomization: 4 Years 1. 00 OS (% of patients) Mel 200 -ASCT: OS 84% 0. 75 CC + R: OS 71% 0. 50 0. 25 0 HR, . 59; 95% CI, . 40 -. 87; P =. 008 0 10 20 30 40 50 60 70 80 Months Gay, et al. Blood. 2014; 124: Abstract 198.

Mel 200 -ASCT vs Chemotherapy + Lenalidomide HR (95% CI) P value Age <60 0. 67 (0. 41 -1. 11) 0. 117 Age ? 60 0. 44 (0. 23 -0. 86) 0. 015 KPS 60 -70% 0. 76 (0. 33 -1. 72) 0. 506 KPS 80 -100% 0. 54 (0. 34 -0. 85) 0. 008 ISS I/II 0. 55 (0. 34 -0. 89) 0. 016 ISS III 0. 75 (0. 38 -1. 45) 0. 376 No del 17, t(4; 14), t(14; 16) 0. 57 (0. 34 -0. 94) 0. 029 Del 17, t(4; 14), t(14, 16) 0. 60 (0. 31 -1. 14) 0. 118 LDH < ULN 0. 62 (0. 41 -0. 95) 0. 027 LDH ? ULN 0. 23 (0. 03 -1. 92) 0. 177 Favors Mel 200 -ASCT Gay, et al. Blood. 2014; 124: Abstract 198. Favors Chemotherapy + Lenalidomide -

High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide Followed by Lenalidomide + Prednisone vs Lenalidomide Maintenance in MM Induction Four 28 -day cycles of lenalidomide (25 mg on days 1– 21) and dexamethasone Induction (40 mg on days 1, 8, 15, and 22) CY (3 g/m 2) MOBILIZATION High-dose Melphalan + ASCT Lenalidomide + Prednisone Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629. Collection Consolidation Maintenance CY (3 g/m 2) MOBILIZATION Cyclophosphamide, Lenalidomide, Dexamethasone Lenalidomide+ Prednisone

Longer PFS with High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide • Median follow-up was 52. 0 months • Median PFS with consolidation therapy – High-dose melphalan + ASCT: 43. 3 months – Chemotherapy + lenalidomide: 28. 6 months (HR for the first 24 months = 2. 51, P <. 0001) • Median PFS with maintenance therapy – Lenalidomide + prednisone: 37. 5 months – Lenalidomide: 28. 5 months (HR = 0. 84, P =. 34) • 4 -year OS – High-dose melphalan + ASCT: 86% – Chemotherapy + lenalidomide: 73% (HR = 2. 40, P =. 004) Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629.

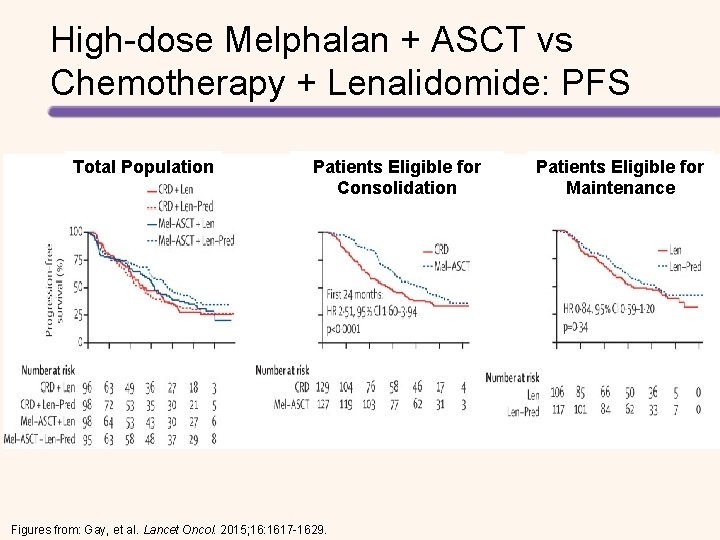
High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide: PFS Total Population Patients Eligible for Consolidation Figures from: Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629. Patients Eligible for Maintenance

High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide: OS Total Population Patients Eligible for Consolidation Figures from: Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629. Patients Eligible for Maintenance

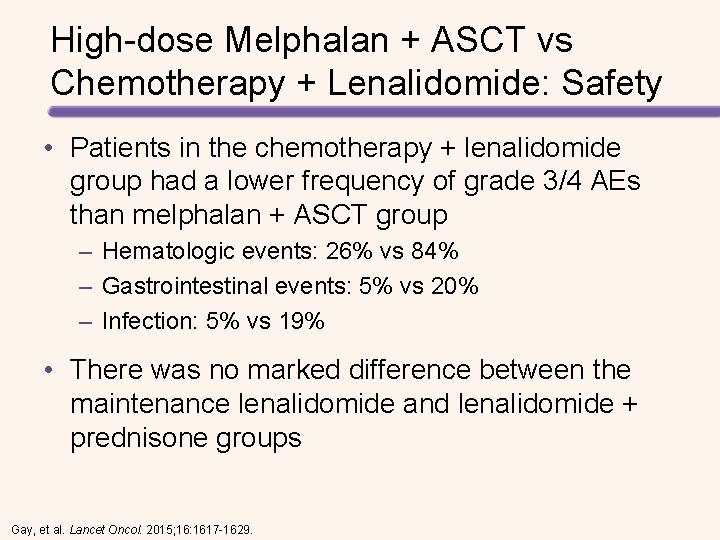
High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide: Safety • Patients in the chemotherapy + lenalidomide group had a lower frequency of grade 3/4 AEs than melphalan + ASCT group – Hematologic events: 26% vs 84% – Gastrointestinal events: 5% vs 20% – Infection: 5% vs 19% • There was no marked difference between the maintenance lenalidomide and lenalidomide + prednisone groups Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629.

AUTOLOGOUS TRANSPLANTATION Initial Treatment

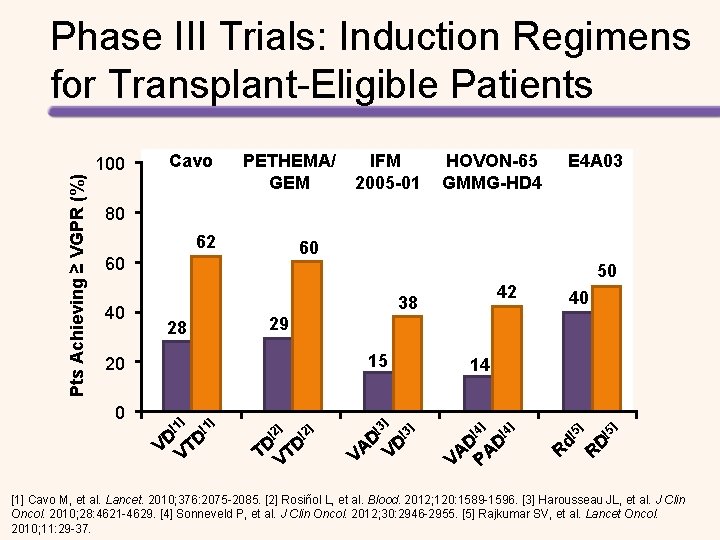
Pts Achieving ≥ VGPR (%) Phase III Trials: Induction Regimens for Transplant-Eligible Patients 100 Cavo PETHEMA/ GEM IFM 2005 -01 HOVON-65 GMMG-HD 4 E 4 A 03 80 62 60 60 40 50 15 ] [1 40 29 28 20 0 42 38 ] [1 VD VTD ] [2 TD VTD [3 ] 14 [3 D D A V V ] [4 ] ] [4 D AD A V P [5 ] ] [5 Rd RD [1] Cavo M, et al. Lancet. 2010; 376: 2075 -2085. [2] Rosiñol L, et al. Blood. 2012; 120: 1589 -1596. [3] Harousseau JL, et al. J Clin Oncol. 2010; 28: 4621 -4629. [4] Sonneveld P, et al. J Clin Oncol. 2012; 30: 2946 -2955. [5] Rajkumar SV, et al. Lancet Oncol. 2010; 11: 29 -37.

Bortezomib-based vs Non-bortezomib— based Induction Treatment Before ASCT Overall survival A Meta-Analysis of Phase III Randomized, Controlled Trials Sonneveld. J Clin Oncol. 2013; 31(26): 3279 -87. 3 -year OS rates Non-bortezomib—based: 75% Bortezomib-based: 80%

Bortezomib-based vs Non-bortezomib— based Induction Treatment Before ASCT IFM 200501/2006 -02 n=482 PETHEMA n=390 GIEMEMA n=480 HOVON-65 n=827 VD±DCEP VTD PAD Control induction VAD ±DCEP VMBCP or TD TD TD ASCT 1 or 2 (or RIC) 1 2 1 or 2 Len x 2 -- VTD TD -- Random: Len vs Obs Random: IFN, Thal or VT All: Dex Assigned: Bort Thal Bortezomib induction Consolidation Maintenance Sonneveld. J Clin Oncol. 2013; 31(26): 3279 -87.

Effect of Pre-transplant Salvage Therapy Prior to ASCT in Patients Not Responding to Initial Induction for MM Salvage Cohort Salvage Chemotherapy Diagnosis and Initial Induction < PR to induction Autologous Transplant No Salvage Cohort Autologous Transplant Diagnosis 12 months from diagnosis to ASCT Vij, et al. Blood. 2012; 120(21): abstract 597. ASCT MM 06 -04 -12_6. ppt

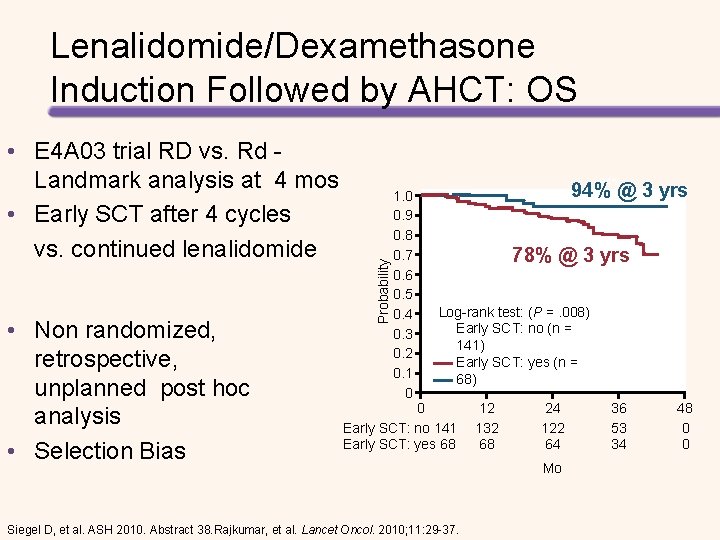
• E 4 A 03 trial RD vs. Rd - Landmark analysis at 4 mos • Early SCT after 4 cycles vs. continued lenalidomide • Non randomized, retrospective, unplanned post hoc analysis • Selection Bias Probability Lenalidomide/Dexamethasone Induction Followed by AHCT: OS 1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 94% @ 3 yrs 78% @ 3 yrs Log-rank test: (P =. 008) Early SCT: no (n = 141) Early SCT: yes (n = 68) 0 Early SCT: no 141 Early SCT: yes 68 Siegel D, et al. ASH 2010. Abstract 38. Rajkumar, et al. Lancet Oncol. 2010; 11: 29 -37. 12 132 68 24 122 64 Mo 36 53 34 48 0 0

AUTOLOGOUS TRANSPLANTATION Tandem

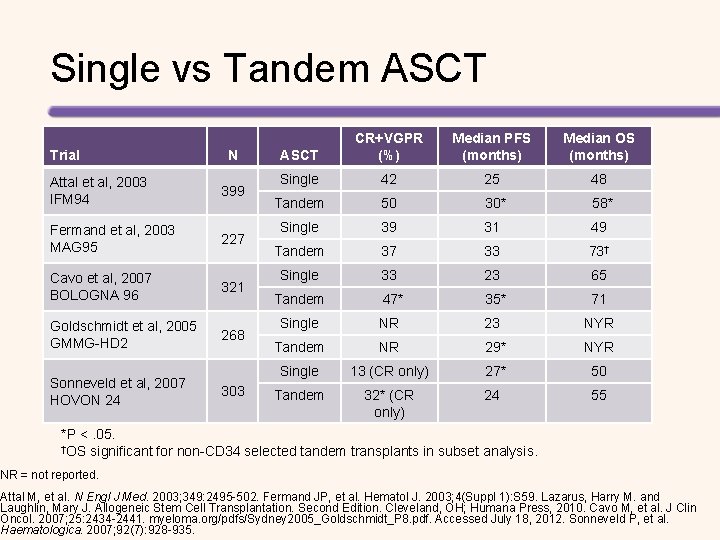
Single vs Tandem ASCT Trial N Attal et al, 2003 IFM 94 399 Fermand et al, 2003 MAG 95 227 Cavo et al, 2007 BOLOGNA 96 321 Goldschmidt et al, 2005 GMMG-HD 2 268 Sonneveld et al, 2007 HOVON 24 303 ASCT CR+VGPR (%) Median PFS (months) Median OS (months) Single 42 25 48 Tandem 50 30* 58* Single 39 31 49 Tandem 37 33 73† Single 33 23 65 Tandem 47* 35* 71 Single NR 23 NYR Tandem NR 29* NYR Single 13 (CR only) 27* 50 Tandem 32* (CR only) 24 55 *P <. 05. †OS significant for non-CD 34 selected tandem transplants in subset analysis. NR = not reported. Attal M, et al. N Engl J Med. 2003; 349: 2495 -502. Fermand JP, et al. Hematol J. 2003; 4(Suppl 1): S 59. Lazarus, Harry M. and Laughlin, Mary J. Allogeneic Stem Cell Transplantation. Second Edition. Cleveland, OH; Humana Press, 2010. Cavo M, et al. J Clin Oncol. 2007; 25: 2434 -2441. myeloma. org/pdfs/Sydney 2005_Goldschmidt_P 8. pdf. Accessed July 18, 2012. Sonneveld P, et al. Haematologica. 2007; 92(7): 928 -935.

Tandem Transplantation in MM Study Attal et al, 2003 ASCT IFM 94 Single Age (years) < 61 Tandem Cavo et al, 2007 Sonneveld et al, 2007 Bologna 96 HOVON 24 Patients (n) CR (%) EFS (months) OS (months) 7 -year 199 42 25 48 200 50 30 58 7 -year 163 33 23 65 Tandem 158 47 35 71 Single 148 13 21 55 155 32 22 50 Single Tandem < 61 < 66

AUTOLOGOUS TRANSPLANTATION Early vs Late HCT

Early vs. Late SCT P (survival) Early HDT . 9. . 8 7. . 6. 5 4. 3. . 2 1. 00 Early HDT Late HDT 10 20 30 Late HDT 40 50 60 70 80 Months Figures from Fermand J , et al. Blood. 1998; 92: 3131. 90 10 0

Early vs Late SCT: Ongoing Phase-3 Study in Newly Diagnosed MM SCT Candidates (IFM/DFCI 2009) Randomize RVDx 3 CY (3 g/m 2) MOBILIZATION Goal: 5 x 106 cells/kg Induction RVDx 3 Collection CY (3 g/m 2) MOBILIZATION Goal: 5 x 106 cells/kg Melphalan 200 AHCT + RVD x 2 Consolidation Lenalidomide Maintenance RVD: lenalidomide, bortezomib and dexamethasone Clinical. Trials. gov Identifier: NCT 01208662 AHCT at relapse RVD x 5 Lenalidomide

Medical College of WI ALLOGENEIC TRANSPLANTATION

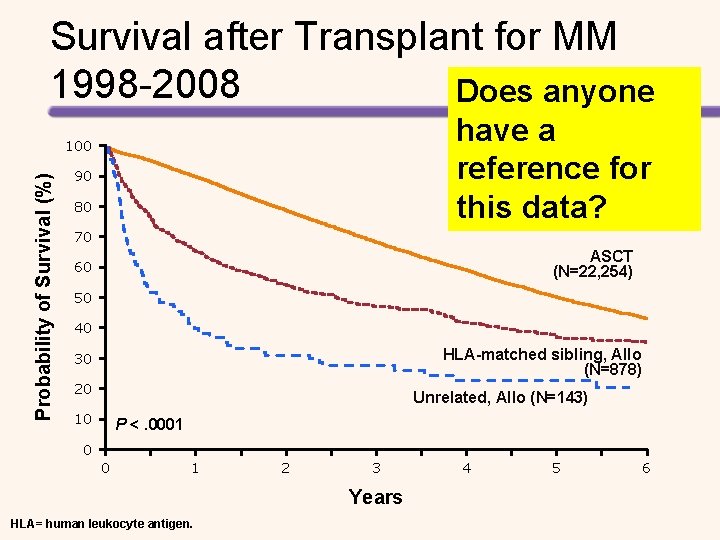
Survival after Transplant for MM 1998 -2008 Does anyone have a reference for this data? Probability of Survival (%) 100 90 80 70 ASCT (N=22, 254) 60 50 40 HLA-matched sibling, Allo (N=878) 30 20 Unrelated, Allo (N=143) 10 P <. 0001 0 0 1 2 3 Years HLA= human leukocyte antigen. 4 5 6

Medical College of WI BARRIERS TO HCT IN MM

Barriers to Autologous Transplant Access SOCIAL ECONOMIC Age Ethnicity and race Language Culture Health literacy Patient/family attitudes Caregiver availability Socioeconomic status Education Number of wage earners Employment status Insurance coverage Place of residence Transportation ACCESS TO TRANSPLANT PROVIDER Physician referral Provider attitudes/biases Provider expertise Provider diversity Adapted from: Majhail NS, et al. Biol Blood Marrow Transplant. 2010; 16(8): 1070 -1075. HEALTH CARE SYSTEM Limited number of HCT centers Workforce shortage Capacity limitations Infrastructure issues

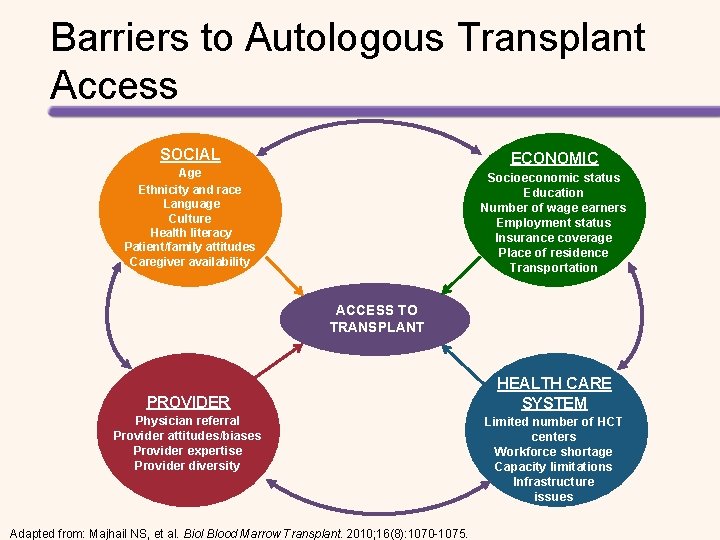
Elderly Patients and Black Patients Are Less Likely to Obtain HCT Referral • Survey of hematologists/oncologists in the United States about HCT referral practices • The odds of not receiving HCT are listed in the table OR (95% CI) P Age, 60 years vs 30 years 8. 29 (5. 89, 11. 69) <. 001 Race, black vs white patients 2. 35 (1. 93, 2. 87) <. 001 Characteristic CI, confidence interval; OR, odds ratio. Pidala J, et al. Bone Marrow Transplant. 2013; 48: 63– 67.

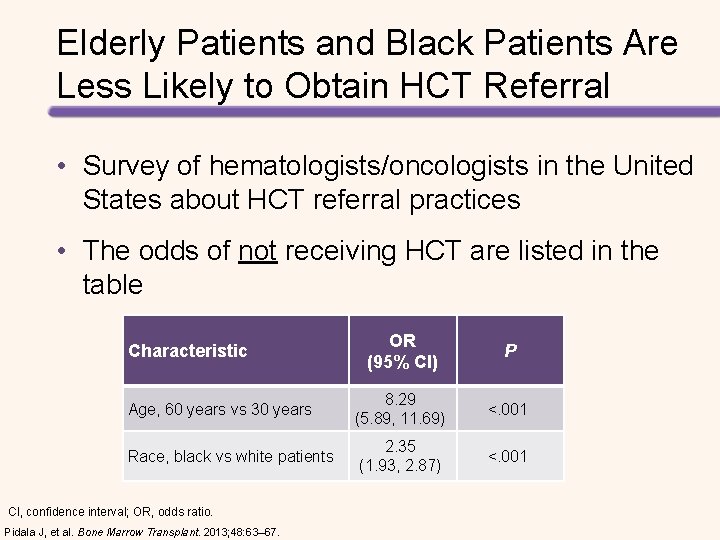
Factors to be Considered in the Treatment of Elderly Patients • Lower functional capacity (performance status, activities of daily living [ADL score], cognitive function) • Comorbidities (renal, pulmonary, hepatic, cardiac, bone marrow) • Disability • Frailty (weakness, poor endurance, weight loss, low physical activity, slow gait speed) • A higher prevalence of unfavorable prognostic factors (β 2 -microglobulin ≥ 3. 5 μg/m. L, albumin <3. 5 g/d. L, hemoblobin <10 g/d. L, International Staging System [ISS] stage III) • Polypharmacy • Lower capacity to tolerate toxicity • Therapy should be adjusted according to risk groups defined by age, comorbidity, organ function, disability, and frailty Ludwig, et al. Oncologist. 2012; 17: 592– 606

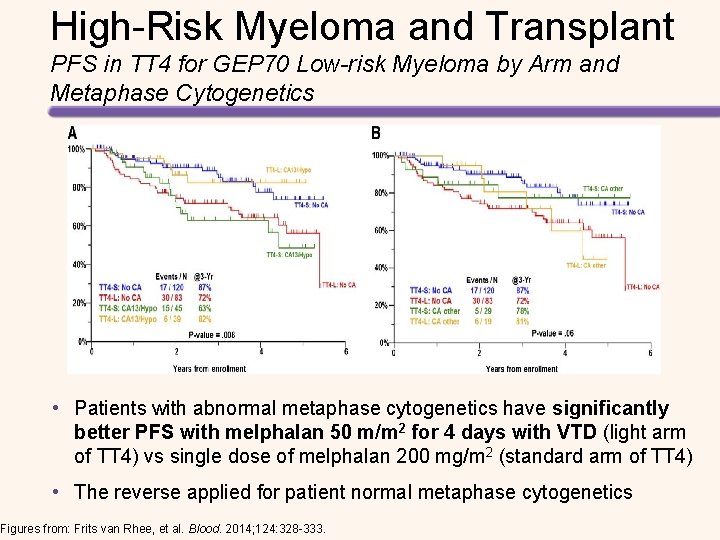
High-Risk Myeloma and Transplant PFS in TT 4 for GEP 70 Low-risk Myeloma by Arm and Metaphase Cytogenetics • Patients with abnormal metaphase cytogenetics have significantly better PFS with melphalan 50 m/m 2 for 4 days with VTD (light arm of TT 4) vs single dose of melphalan 200 mg/m 2 (standard arm of TT 4) • The reverse applied for patient normal metaphase cytogenetics Figures from: Frits van Rhee, et al. Blood. 2014; 124: 328 -333.

NEWER AGENTS/REGIMENS

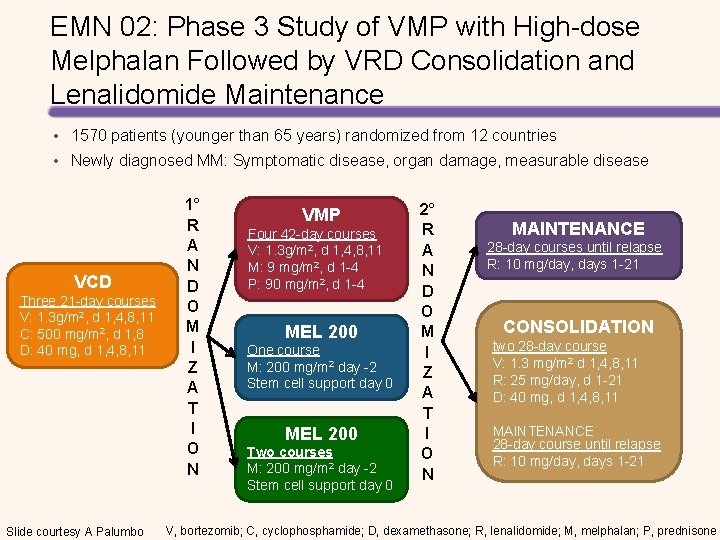
EMN 02: Phase 3 Study of VMP with High-dose Melphalan Followed by VRD Consolidation and Lenalidomide Maintenance • 1570 patients (younger than 65 years) randomized from 12 countries • Newly diagnosed MM: Symptomatic disease, organ damage, measurable disease VCD Three 21 -day courses V: 1. 3 g/m 2, d 1, 4, 8, 11 C: 500 mg/m 2, d 1, 8 D: 40 mg, d 1, 4, 8, 11 Slide courtesy A Palumbo 1° R A N D O M I Z A T I O N VMP Four 42 -day courses V: 1. 3 g/m 2, d 1, 4, 8, 11 M: 9 mg/m 2, d 1 -4 P: 90 mg/m 2, d 1 -4 MEL 200 One course M: 200 mg/m 2 day -2 Stem cell support day 0 MEL 200 Two courses M: 200 mg/m 2 day -2 Stem cell support day 0 2° R A N D O M I Z A T I O N MAINTENANCE 28 -day courses until relapse R: 10 mg/day, days 1 -21 CONSOLIDATION two 28 -day course V: 1. 3 mg/m 2 d 1, 4, 8, 11 R: 25 mg/day, d 1 -21 D: 40 mg, d 1, 4, 8, 11 MAINTENANCE 28 -day course until relapse R: 10 mg/day, days 1 -21 V, bortezomib; C, cyclophosphamide; D, dexamethasone; R, lenalidomide; M, melphalan; P, prednisone

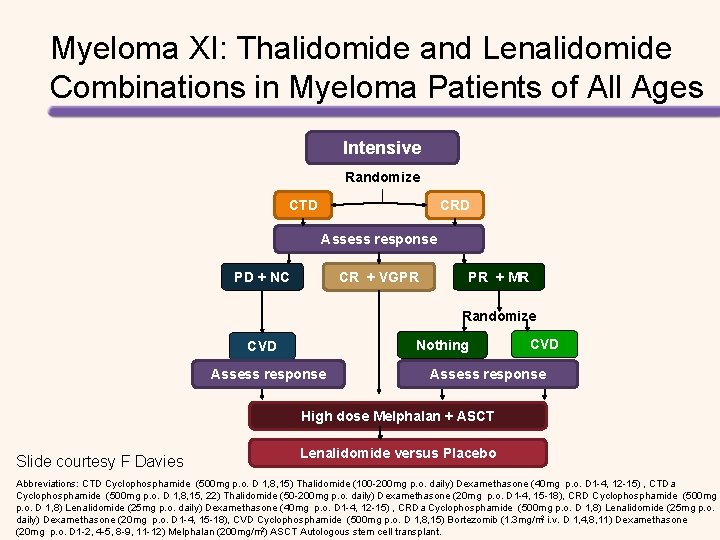
Myeloma XI: Thalidomide and Lenalidomide Combinations in Myeloma Patients of All Ages Intensive Randomize CTD CRD Assess response PD + NC CR + VGPR PR + MR Randomize Nothing CVD Assess response High dose Melphalan + ASCT Slide courtesy F Davies Lenalidomide versus Placebo Abbreviations: CTD Cyclophosphamide (500 mg p. o. D 1, 8, 15) Thalidomide (100 -200 mg p. o. daily) Dexamethasone (40 mg p. o. D 1 -4, 12 -15) , CTDa Cyclophosphamide (500 mg p. o. D 1, 8, 15, 22) Thalidomide (50 -200 mg p. o. daily) Dexamethasone (20 mg p. o. D 1 -4, 15 -18), CRD Cyclophosphamide (500 mg p. o. D 1, 8) Lenalidomide (25 mg p. o. daily) Dexamethasone (40 mg p. o. D 1 -4, 12 -15) , CRDa Cyclophosphamide (500 mg p. o. D 1, 8) Lenalidomide (25 mg p. o. daily) Dexamethasone (20 mg p. o. D 1 -4, 15 -18), CVD Cyclophosphamide (500 mg p. o. D 1, 8, 15) Bortezomib (1. 3 mg/m 2 i. v. D 1, 4, 8, 11) Dexamethasone (20 mg p. o. D 1 -2, 4 -5, 8 -9, 11 -12) Melphalan (200 mg/m 2) ASCT Autologous stem cell transplant.

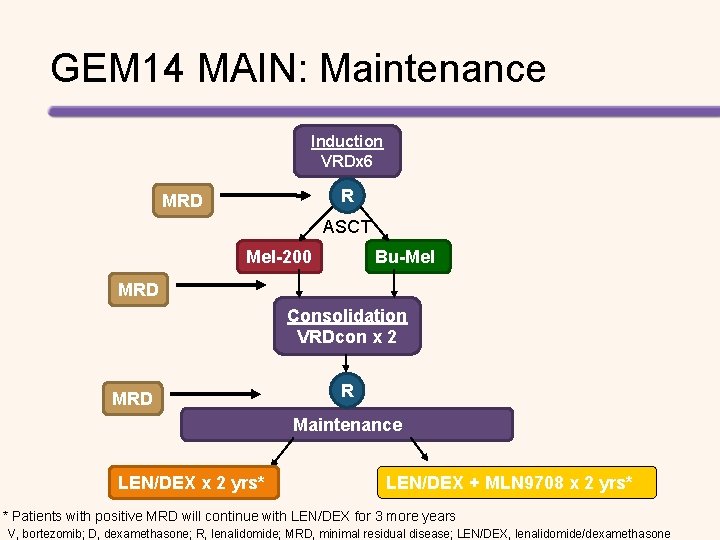
GEM 14 MAIN: Maintenance Induction VRDx 6 R MRD ASCT Mel-200 Bu-Mel MRD Consolidation VRDcon x 2 MRD R Maintenance LEN/DEX x 2 yrs* LEN/DEX + MLN 9708 x 2 yrs* * Patients with positive MRD will continue with LEN/DEX for 3 more years V, bortezomib; D, dexamethasone; R, lenalidomide; MRD, minimal residual disease; LEN/DEX, lenalidomide/dexamethasone

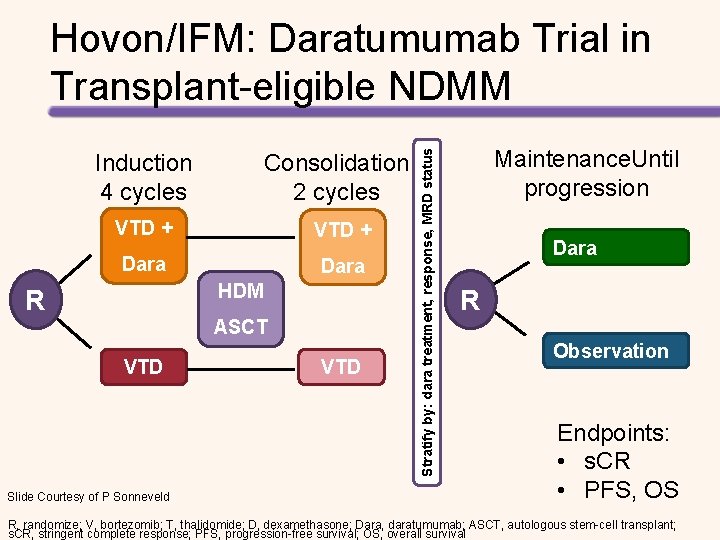
Induction 4 cycles Consolidation 2 cycles VTD + Dara HDM R ASCT VTD Slide Courtesy of P Sonneveld VTD Stratify by: dara treatment, response, MRD status Hovon/IFM: Daratumumab Trial in Transplant-eligible NDMM Maintenance. Until progression Dara R Observation Endpoints: • s. CR • PFS, OS R, randomize; V, bortezomib; T, thalidomide; D, dexamethasone; Dara, daratumumab; ASCT, autologous stem-cell transplant; s. CR, stringent complete response; PFS, progression-free survival; OS, overall survival

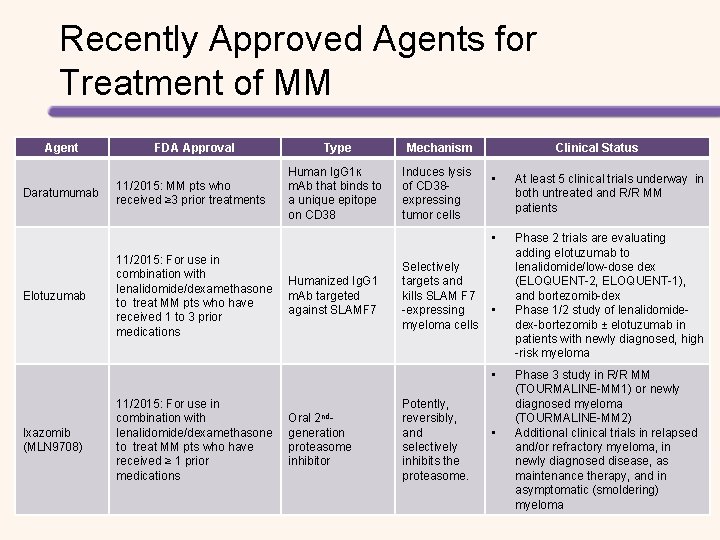
Recently Approved Agents for Treatment of MM Agent Daratumumab Elotuzumab FDA Approval 11/2015: MM pts who received ≥ 3 prior treatments 11/2015: For use in combination with lenalidomide/dexamethasone to treat MM pts who have received 1 to 3 prior medications Type Mechanism Human Ig. G 1ĸ m. Ab that binds to a unique epitope on CD 38 Induces lysis of CD 38 expressing tumor cells Humanized Ig. G 1 m. Ab targeted against SLAMF 7 Clinical Status • At least 5 clinical trials underway in both untreated and R/R MM patients • Phase 2 trials are evaluating adding elotuzumab to lenalidomide/low-dose dex (ELOQUENT-2, ELOQUENT-1), and bortezomib-dex Phase 1/2 study of lenalidomidedex-bortezomib ± elotuzumab in patients with newly diagnosed, high -risk myeloma Selectively targets and kills SLAM F 7 -expressing • myeloma cells • Ixazomib (MLN 9708) 11/2015: For use in combination with lenalidomide/dexamethasone to treat MM pts who have received ≥ 1 prior medications Oral 2 ndgeneration proteasome inhibitor Potently, reversibly, and selectively inhibits the proteasome. • Phase 3 study in R/R MM (TOURMALINE-MM 1) or newly diagnosed myeloma (TOURMALINE-MM 2) Additional clinical trials in relapsed and/or refractory myeloma, in newly diagnosed disease, as maintenance therapy, and in asymptomatic (smoldering) myeloma

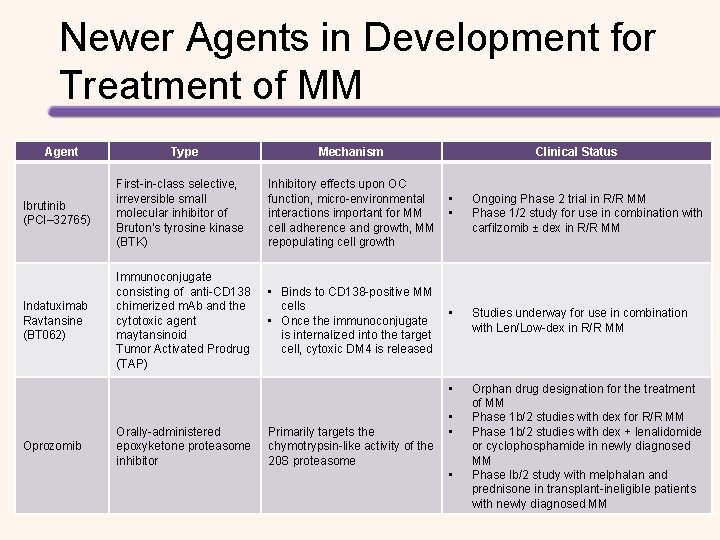
Newer Agents in Development for Treatment of MM Agent Type Mechanism Clinical Status Ibrutinib (PCI– 32765) First-in-class selective, irreversible small molecular inhibitor of Bruton’s tyrosine kinase (BTK) Inhibitory effects upon OC function, micro-environmental • interactions important for MM • cell adherence and growth, MM repopulating cell growth Ongoing Phase 2 trial in R/R MM Phase 1/2 study for use in combination with carfilzomib ± dex in R/R MM Indatuximab Ravtansine (BT 062) Immunoconjugate consisting of anti-CD 138 chimerized m. Ab and the cytotoxic agent maytansinoid Tumor Activated Prodrug (TAP) • Binds to CD 138 -positive MM cells • • Once the immunoconjugate is internalized into the target cell, cytoxic DM 4 is released Studies underway for use in combination with Len/Low-dex in R/R MM • Oprozomib Orally-administered epoxyketone proteasome inhibitor Primarily targets the chymotrypsin-like activity of the 20 S proteasome • • • Orphan drug designation for the treatment of MM Phase 1 b/2 studies with dex for R/R MM Phase 1 b/2 studies with dex + lenalidomide or cyclophosphamide in newly diagnosed MM Phase Ib/2 study with melphalan and prednisone in transplant-ineligible patients with newly diagnosed MM

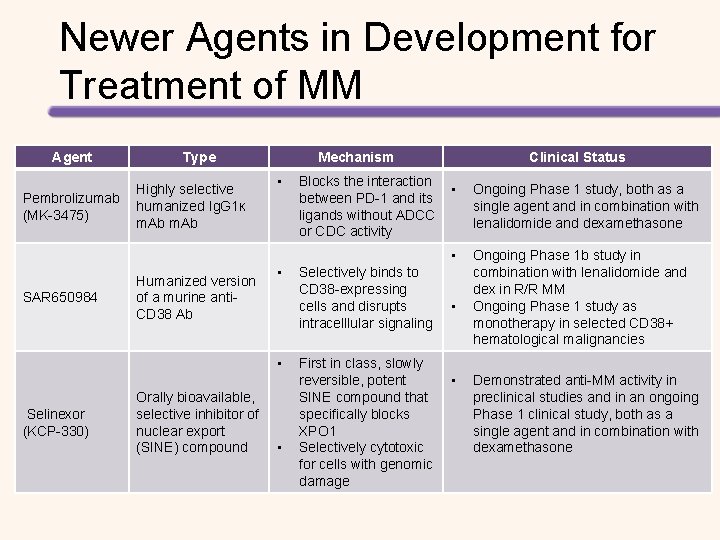
Newer Agents in Development for Treatment of MM Agent Type Highly selective Pembrolizumab humanized Ig. G 1ĸ (MK-3475) m. Ab Mechanism • Clinical Status Blocks the interaction • between PD-1 and its ligands without ADCC or CDC activity • SAR 650984 Humanized version of a murine anti. CD 38 Ab • • Selinexor (KCP-330) Orally bioavailable, selective inhibitor of nuclear export (SINE) compound • Selectively binds to CD 38 -expressing cells and disrupts intracelllular signaling • First in class, slowly reversible, potent • SINE compound that specifically blocks XPO 1 Selectively cytotoxic for cells with genomic damage Ongoing Phase 1 study, both as a single agent and in combination with lenalidomide and dexamethasone Ongoing Phase 1 b study in combination with lenalidomide and dex in R/R MM Ongoing Phase 1 study as monotherapy in selected CD 38+ hematological malignancies Demonstrated anti-MM activity in preclinical studies and in an ongoing Phase 1 clinical study, both as a single agent and in combination with dexamethasone