Considerations for Optimizing Transplant in Multiple Myeloma Patient






![Regimens Survival Bortezomib/lenalidomide/ dexamethasone (RVD)[1] 18 -mo PFS: 75% 18 -mo OS: 97% Carfilzomib/lenalidomide/ Regimens Survival Bortezomib/lenalidomide/ dexamethasone (RVD)[1] 18 -mo PFS: 75% 18 -mo OS: 97% Carfilzomib/lenalidomide/](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-23.jpg)




![Maintenance in Myeloma • PFS advantage[1 -3] • OS improvements? [2] • Toxicities of Maintenance in Myeloma • PFS advantage[1 -3] • OS improvements? [2] • Toxicities of](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-40.jpg)
![Imaging of Residual Disease in MM PET/CT[1, 2] • PET may be useful for Imaging of Residual Disease in MM PET/CT[1, 2] • PET may be useful for](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-44.jpg)

![Intensification of MEL 220 mg/m 2 (French single arm study)[1] Escalating MEL to 300 Intensification of MEL 220 mg/m 2 (French single arm study)[1] Escalating MEL to 300](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-52.jpg)
![Risk-adapted Melphalan Dosing Suggested Melphalan Dose-adjustment for Patients with Renal Impairment[1] Cr. Cl >15 Risk-adapted Melphalan Dosing Suggested Melphalan Dose-adjustment for Patients with Renal Impairment[1] Cr. Cl >15](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-53.jpg)






- Slides: 77

Considerations for Optimizing Transplant in Multiple Myeloma Patient Management PARAMESWARAN HARI MD MEDICAL COLLEGE OF WISCONSIN

Support Acknowledgement This activity has been made possible through an unrestricted educational grant from Spectrum Pharmaceuticals 2

Accreditation Information • Physician Accreditation Statement: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint-sponsorship of Medical Education Resources (MER) and Ple. Xus Communications. MER is accredited by the ACCME to provide continuing medical education for physicians. Credit Designation: Medical Education Resources designates this live activity for a maximum of 1. 0 AMA PRA Category 1 Credit(s). Physicians should only claim credit commensurate with the extent of their participation in the activity. • Nursing Accreditation: Medical Education Resources is an approved provider of continuing education by the Colorado Nurses Association, an accredited approver by the American Nurses Credentialing Center's Commission on Accreditation. This CE activity provides 1. 0 contact hours of continuing nursing education. Medical Education Resources is a provider of continuing nursing education by the California Board of Registered Nursing, Provider #CEP 12299, for 1. 0 contact hour. • Pharmacists: Educational Review Systems is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education. This program is approved for 1 hour (0. 1 CEUs) of continuing pharmacy education credit. Proof of participation will be posted to your NABP CPE profile within 4 to 6 weeks to participants who have successfully completed the post-test. Participants must participate in the entire presentation and complete the course evaluation to receive continuing pharmacy education credit. UAN # 0761 -9999 -16 -015 -L 01 -P Certificates will be mailed to participants in 4 -6 weeks 3

Faculty Disclosure Faculty: Disclosures :

Learning Objectives After completing this program, participants should be able to: 1. Identify factors that might determine eligibility for and timing of high-dose chemotherapy (HDT) followed by autologous stem cell transplant (ASCT) in patients with multiple myeloma (MM) 2. Differentiate treatment strategies for patients with newly diagnosed multiple myeloma who are eligible for HDT/ASCT 3. Identify barriers in access to HDT/ASCT for MM 4. Review the rationale for conditioning regimens in patients with multiple myeloma who are eligible for HDT/ASCT 5. Compare and contrast pertinent data regarding a new formulation of melphalan

MYELOMA OVERVIEW

Revised International Staging System (R-ISS) for MM • R-ISS I (n = 871) – Including ISS stage I (serum β 2 -microglobulin level < 3. 5 mg/L and serum albumin level ≥ 3. 5 g/d. L) – No high-risk CA [del(17 p) and/or t(4; 14) and/or t(14; 16)] – Normal LDH level (less than the upper limit of normal range) • R-ISS III (n = 295) – Including ISS stage III (serum β 2 -microglobulin level > 5. 5 mg/L) – High-risk CA or high LDH level • R-ISS II (n = 1, 894) – Including all the other possible combinations 5 -Year OS* 5 -Year PFS* R-ISS I 82% 55% R-ISS II 62% 36% R-ISS III 40% 24% Palumbo, et al. JCO. 2015; 33(26): 2863 -2869. *At a median follow-up of 46 months

Indications for Considering Treatment (IMWG Consensus Guidelines) • At least one of the CRAB Criteria (evidence of end organ damage) CRAB Criteria • • Hypercalcemia Serum calcium >2. 75 mmol/L (>11 mg/d. L) Renal Failure Serum creatinine ≥ 2 mg/d. L or creatinine clearance <40 m. L per min Anemia Hemoglobin >20 g/L below the lower limit of normal, or a hemoglobin value <100 g/L Bone Lytic lesions, pathologic fractures, or severe osteopenia ≥ 60% clonal bone marrow plasma cells Serum involved/uninvolved free light chain ratio ≥ 100 >1 Focal bone lesion (≥ 5 mm) on MRI “Clinical judgement” Rajkumar, et al. Lancet Oncology. 2014; 15(12): e 538 -48.

Available Therapies and Phases of Treatment for MM Treatment Options Conventional chemotherapy (e. g. , alkylating agents) Steroids (corticosteroids) Autologous stem cell transplant (ASCT) Newer therapies • Proteasome inhibitors • Immunomodulatory agents • Monoclonal antibodies • HDAC inhibitors Initial Therapy Consolidation / Maintenance Treatment of Relapsed Disease ASCT if eligible* Supportive Care *Transplant eligibility may impact initial treatment decisions

Treatment Goals for MM • Disease Response and Survival – Rapid cytoreduction to relieve symptoms – Minimize treatment-related toxicity – Prolong survival – Overall Survival • Symptom Control – Ameliorate pain and other disease-related symptoms – Prevent further organ damage – Preserve performance status and quality of life

AUTOLOGOUS TRANSPLANTATION Transplant Vs Conventional Therapy

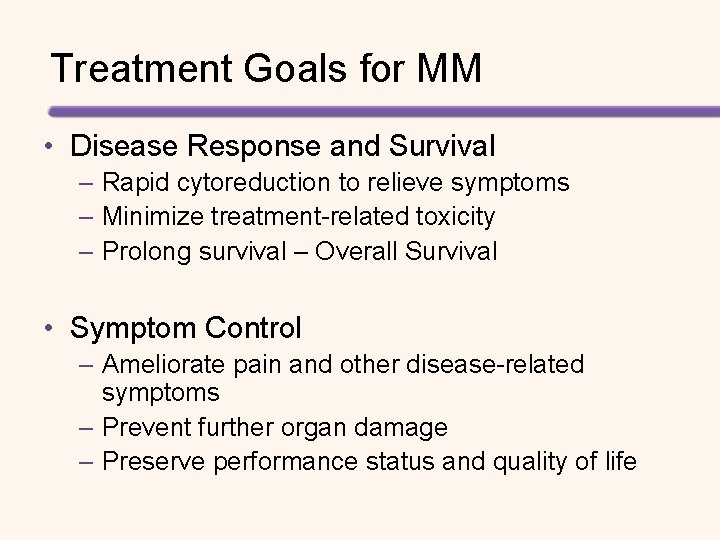
Meta-analysis of PFS: Standard Chemotherapy vs Autologous SCT Favors HDT Favors SDT PFS (95%CI) IFM 90 0. 61 (0. 42, 0. 89) MAG 90* 0. 42 (0. 30, 0. 58) MAG 91 0. 76 (0. 57, 1. 02) MRC 7 0. 68 (0. 54, 0. 85) S 9321 0. 87 (0. 72, 1. 06) PETHEMA* 0. 85 (0. 60, 1. 22) HOVON* 0. 85 (0. 63, 1. 14) M 97 G* 0. 48 (0. 34, 0. 66) IFM 9906*† 1. 80 (1. 30, 2. 50) Combined 0. 75 (0. 59, 0. 96) Sensitivity/Sub-group Analyses 0. 75 (0. 65, 0. 87) Excluding Non-Standard RCTs 0. 77 (0. 59, 1. 00) RCTs preferring PBSCs 0. 70 (0. 51, 0. 96) RCTs with Longer Followup 0. 72 (0. 62, 0. 83) . RCTs with Lower 1 Crossover ‡ . 5 1 5 Hazard Ratio of Progression 10 • *Nonstandard study • † Patients aged >65 years ‡ Two negative studies (HOVON, IFM 9906) with missing crossover information were omitted from this analysis. Figure from: Koreth J, et al. Biol Blood Marrow Transplant. 2007; 13: 183 -196.

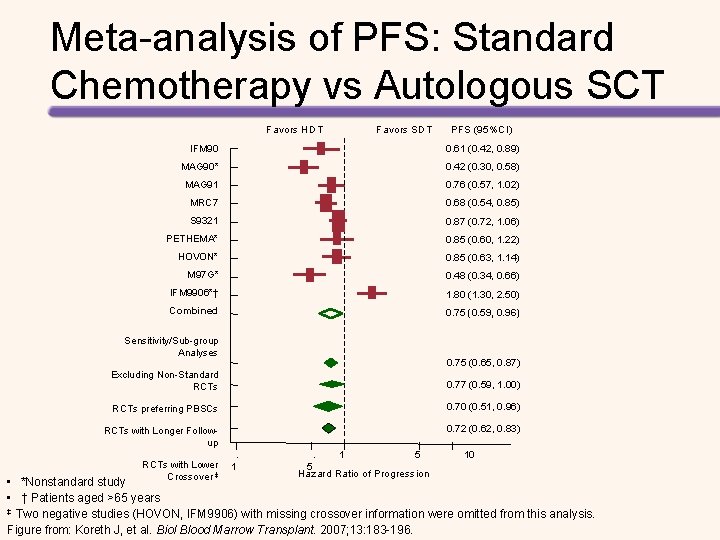
AHCT Has Changed Natural History of MM: Population-level Data Changes in MM relative survival ratio in the Netherlands ≤ 65 years > 65 years Figure from Schaapveld M, et al. Eur J Cancer. 2009; 46: 160.

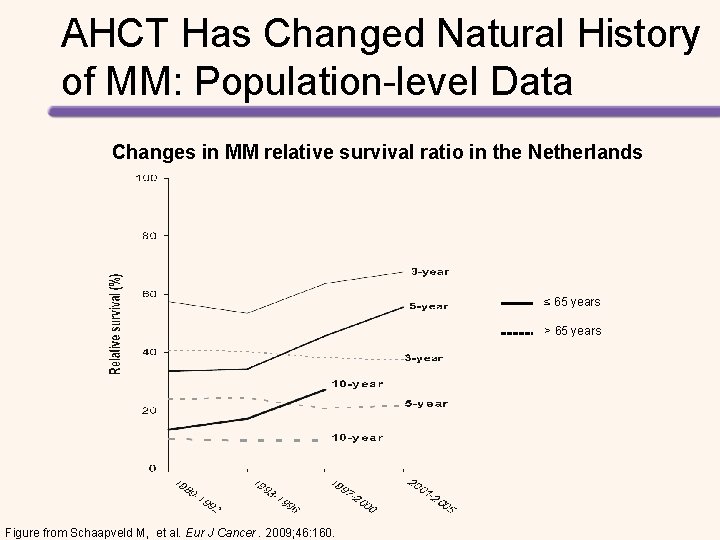
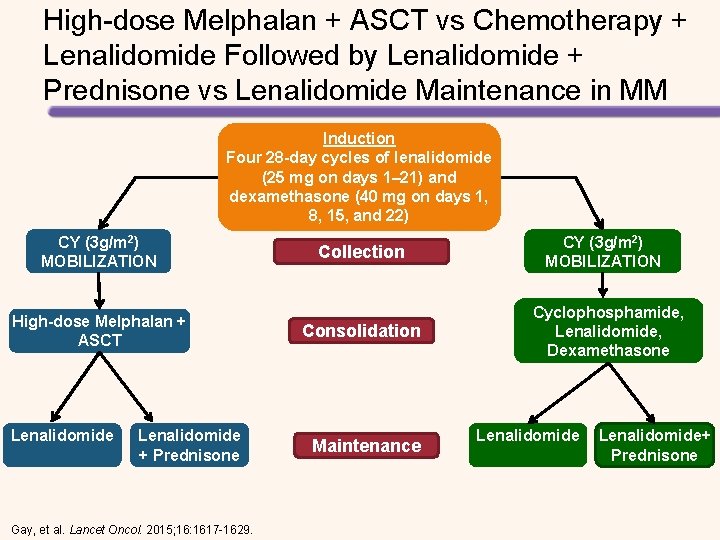
High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide Followed by Lenalidomide + Prednisone vs Lenalidomide Maintenance in MM Induction Four 28 -day cycles of lenalidomide (25 mg on days 1– 21) and dexamethasone (40 mg on days 1, Induction 8, 15, and 22) CY (3 g/m 2) MOBILIZATION High-dose Melphalan + ASCT Lenalidomide + Prednisone Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629. Collection Consolidation Maintenance CY (3 g/m 2) MOBILIZATION Cyclophosphamide, Lenalidomide, Dexamethasone Lenalidomide+ Prednisone

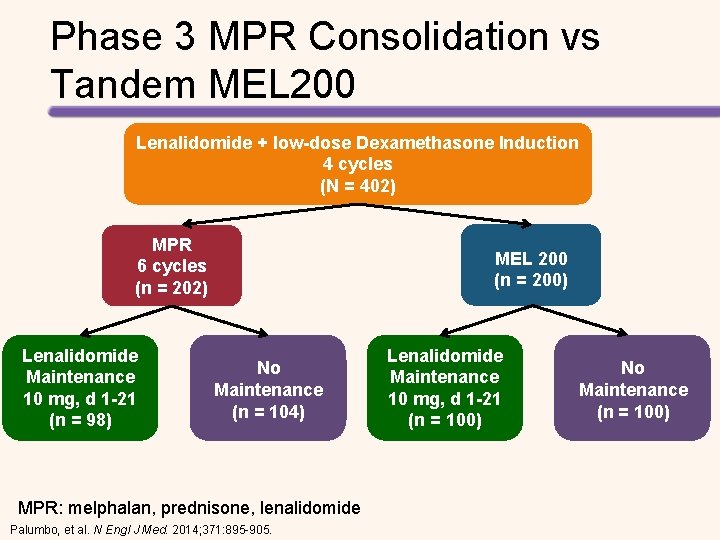
Longer PFS with High-dose Melphalan + ASCT vs Chemotherapy + Lenalidomide • Median follow-up was 52. 0 months • Median PFS with consolidation therapy – High-dose melphalan + ASCT: 43. 3 months – Chemotherapy + lenalidomide: 28. 6 months (HR for the first 24 months = 2. 51, P <. 0001) • Median PFS with maintenance therapy – Lenalidomide + prednisone: 37. 5 months – Lenalidomide: 28. 5 months (HR = 0. 84, P =. 34). • 4 -year OS – High-dose melphalan + ASCT: 86% – Chemotherapy + lenalidomide: 73% (HR = 2. 40, P =. 004). Gay, et al. Lancet Oncol. 2015; 16: 1617 -1629.

Phase 3 MPR Consolidation vs Tandem MEL 200 Lenalidomide + low-dose Dexamethasone Induction 4 cycles (N = 402) MPR 6 cycles (n = 202) Lenalidomide Maintenance 10 mg, d 1 -21 (n = 98) MEL 200 (n = 200) No Maintenance (n = 104) MPR: melphalan, prednisone, lenalidomide Palumbo, et al. N Engl J Med. 2014; 371: 895 -905. Lenalidomide Maintenance 10 mg, d 1 -21 (n = 100) No Maintenance (n = 100)

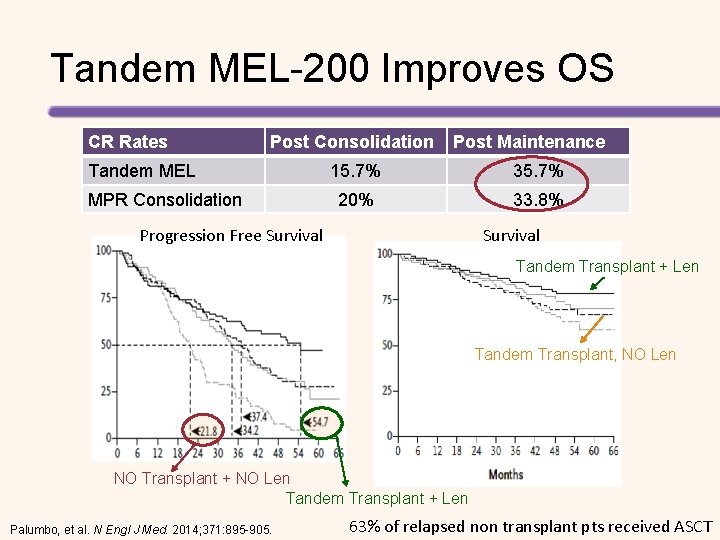
Tandem MEL-200 Improves OS CR Rates Post Consolidation Tandem MEL MPR Consolidation Post Maintenance 15. 7% 35. 7% 20% 33. 8% Progression Free Survival Tandem Transplant + Len Tandem Transplant, NO Len NO Transplant + NO Len Tandem Transplant + Len Palumbo, et al. N Engl J Med. 2014; 371: 895 -905. 63% of relapsed non transplant pts received ASCT

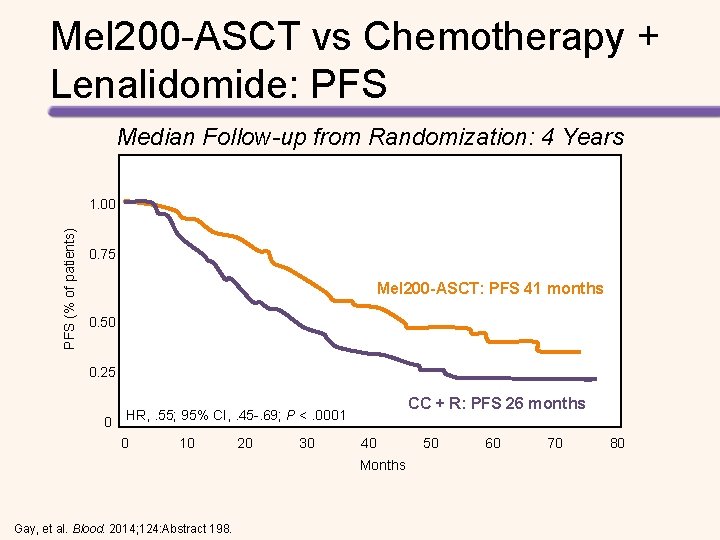
Mel 200 -ASCT vs Chemotherapy + Lenalidomide: PFS Median Follow-up from Randomization: 4 Years PFS (% of patients) 1. 00 0. 75 Mel 200 -ASCT: PFS 41 months 0. 50 0. 25 0 CC + R: PFS 26 months HR, . 55; 95% CI, . 45 -. 69; P <. 0001 0 10 20 30 40 50 60 70 80 Months Gay, et al. Blood. 2014; 124: Abstract 198.

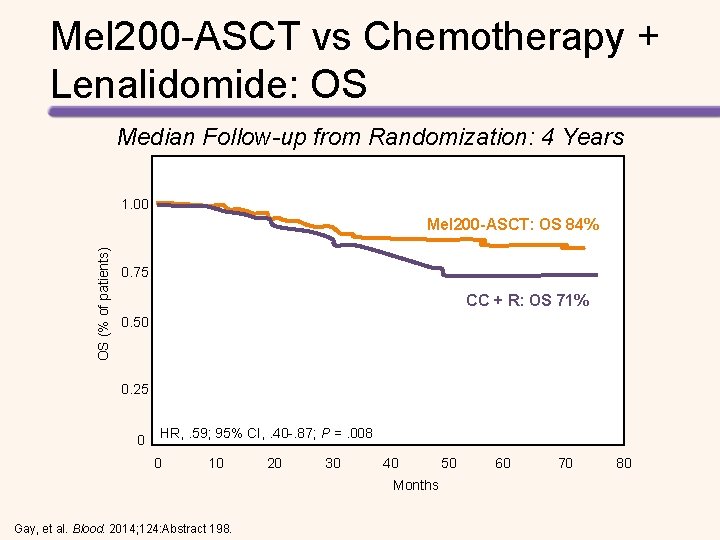
Mel 200 -ASCT vs Chemotherapy + Lenalidomide: OS Median Follow-up from Randomization: 4 Years 1. 00 OS (% of patients) Mel 200 -ASCT: OS 84% 0. 75 CC + R: OS 71% 0. 50 0. 25 0 HR, . 59; 95% CI, . 40 -. 87; P =. 008 0 10 20 30 40 50 60 70 80 Months Gay, et al. Blood. 2014; 124: Abstract 198.

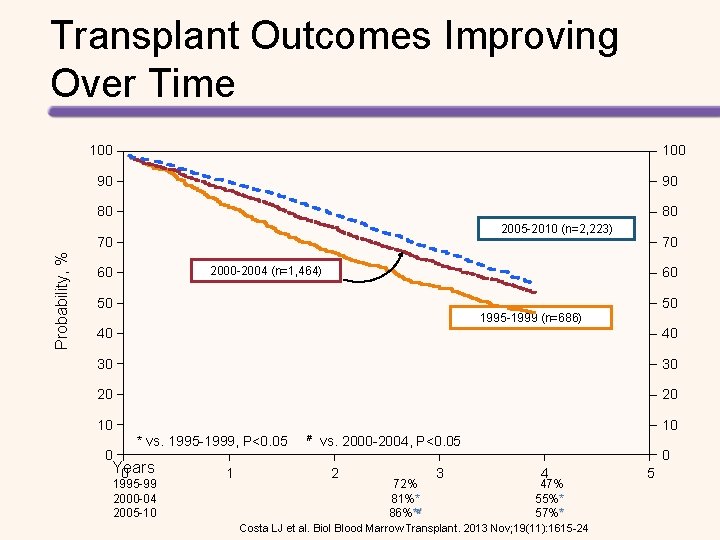
Transplant Outcomes Improving Over Time 100 90 90 80 80 2005 -2010 (n=2, 223) Probability, % 70 70 2000 -2004 (n=1, 464) 60 60 50 50 1995 -1999 (n=686) 40 40 30 30 20 20 10 * vs. 1995 -1999, P<0. 05 # vs. 2000 -2004, P<0. 05 Years 0 1995 -99 2000 -04 2005 -10 1 2 72% 81%** 86%**# 3 0 4 47% 55%** 57%** Costa LJ et al. Biol Blood Marrow Transplant. 2013 Nov; 19(11): 1615 -24 5

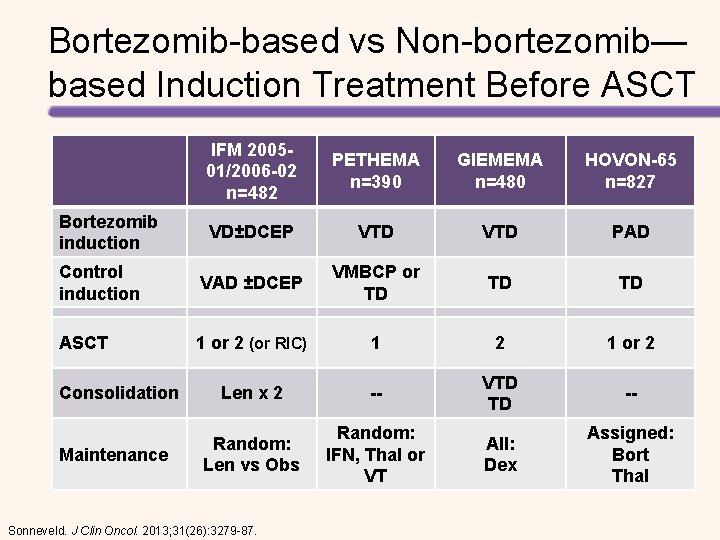
Bortezomib-based vs Non-bortezomib— based Induction Treatment Before ASCT IFM 200501/2006 -02 n=482 PETHEMA n=390 GIEMEMA n=480 HOVON-65 n=827 VD±DCEP VTD PAD Control induction VAD ±DCEP VMBCP or TD TD TD ASCT 1 or 2 (or RIC) 1 2 1 or 2 Len x 2 -- VTD TD -- Random: Len vs Obs Random: IFN, Thal or VT All: Dex Assigned: Bort Thal Bortezomib induction Consolidation Maintenance Sonneveld. J Clin Oncol. 2013; 31(26): 3279 -87.

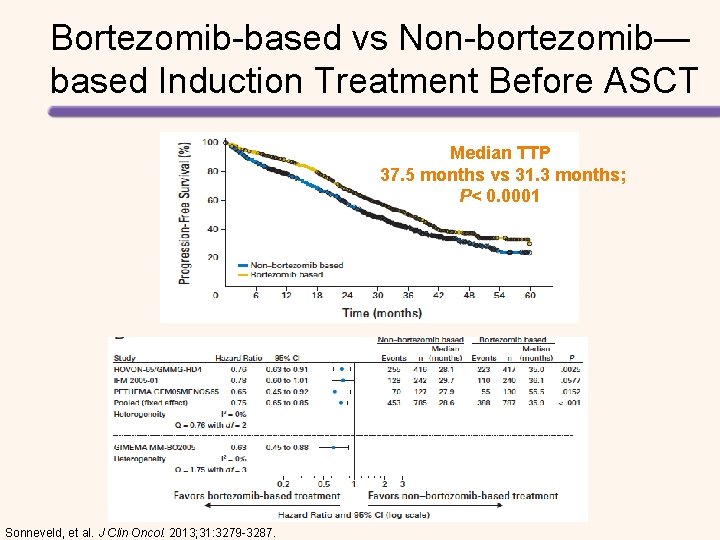
Bortezomib-based vs Non-bortezomib— based Induction Treatment Before ASCT Median TTP 37. 5 months vs 31. 3 months; P< 0. 0001 Sonneveld, et al. J Clin Oncol. 2013; 31: 3279 -3287.
![Regimens Survival Bortezomiblenalidomide dexamethasone RVD1 18 mo PFS 75 18 mo OS 97 Carfilzomiblenalidomide Regimens Survival Bortezomib/lenalidomide/ dexamethasone (RVD)[1] 18 -mo PFS: 75% 18 -mo OS: 97% Carfilzomib/lenalidomide/](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-23.jpg)
Regimens Survival Bortezomib/lenalidomide/ dexamethasone (RVD)[1] 18 -mo PFS: 75% 18 -mo OS: 97% Carfilzomib/lenalidomide/ dexamethasone (KRd)[2, 3] 12 -mo PFS: 97%[2] 24 -mo PFS: 92%[2] 3 -yr PFS: 79%[3] 3 -yr OS: 96%[3] Carfilzomib/thalidomide/ dexamethasone (KTd)[4] 3 -yr PFS: 72% Bortezomib/ cyclophosphamide/ dexamethasone (Cy. Bor. D)[5] 5 -yr PFS: 42%[6] 5 -yr OS: 70%[6] Ixazomib/lenalidomide/ dexamethasone[7] 12 -mo PFS: 88% 12 -mo OS: 94% Pts Achieving ≥ VGPR (%) Earlier Phase Studies: Induction Regimens for Transplantation-Eligible Pts 100 80 81 67 68 60 60 58 40 20 0 [1 D V R ] ] [2 d R K ]] [4 d T K [5 ] r. D RD / o B ib y m C zo a Ix [1] Richardson, PG et al. Blood. 2010; 116: 679 -686. [2] Jakubowiak A, et al. Blood. 2012; 120: 1801 -1809. [3] Jasielec J, et al. ASH 2013. Abstract 3220. [4] Sonneveld P, et al. Blood. 2015; 125: 449 -456. [5] Reeder CB, et al. Blood. 2010; 115: 3416 -3417. [6] Reeder CB, et al. ASH 2013. Abstract 3192. [7] Kumar SK, et al. Lancet Oncol. 2014; 15: 1503 -1512. ] [7

AUTOLOGOUS TRANSPLANTATION Early vs Late HCT

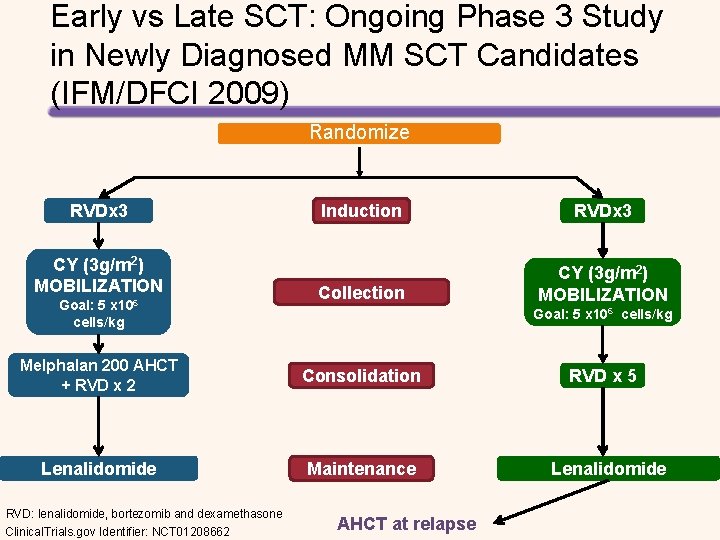
Early vs Late SCT: Ongoing Phase 3 Study in Newly Diagnosed MM SCT Candidates (IFM/DFCI 2009) Randomize RVDx 3 CY (3 g/m 2) MOBILIZATION Goal: 5 x 106 cells/kg Induction RVDx 3 Collection CY (3 g/m 2) MOBILIZATION Goal: 5 x 106 cells/kg Melphalan 200 AHCT + RVD x 2 Consolidation Lenalidomide Maintenance RVD: lenalidomide, bortezomib and dexamethasone Clinical. Trials. gov Identifier: NCT 01208662 AHCT at relapse RVD x 5 Lenalidomide

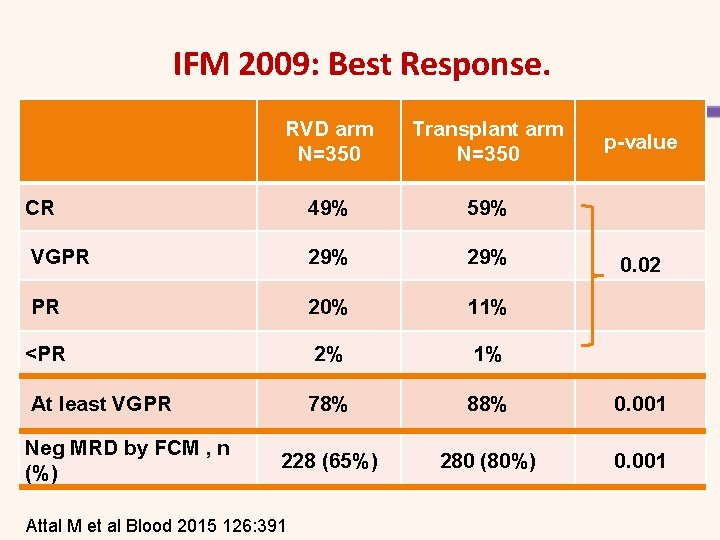
IFM 2009: Best Response. RVD arm N=350 Transplant arm N=350 p-value CR 49% 59% VGPR 29% 0. 02 PR 20% 11% <PR 2% 1% At least VGPR 78% 88% 0. 001 228 (65%) 280 (80%) 0. 001 Neg MRD by FCM , n (%) Attal M et al Blood 2015 126: 391

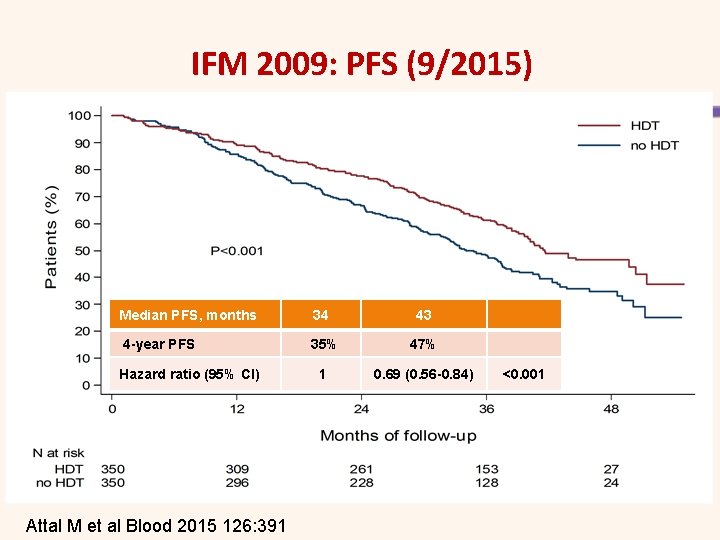
IFM 2009: PFS (9/2015) Median PFS, months 4 -year PFS Hazard ratio (95% CI) Attal M et al Blood 2015 126: 391 34 43 35% 47% 1 0. 69 (0. 56 -0. 84) <0. 001

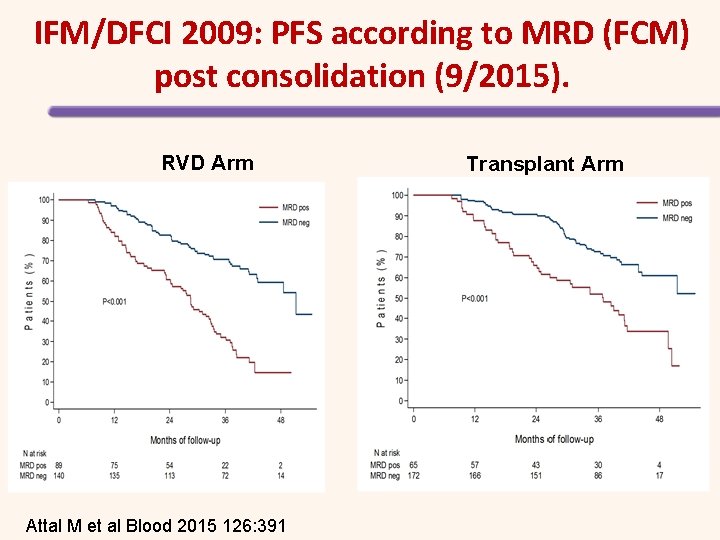
IFM/DFCI 2009: PFS according to MRD (FCM) post consolidation (9/2015). RVD Arm Attal M et al Blood 2015 126: 391 Transplant Arm

AUTOLOGOUS TRANSPLANT Who, When and How?

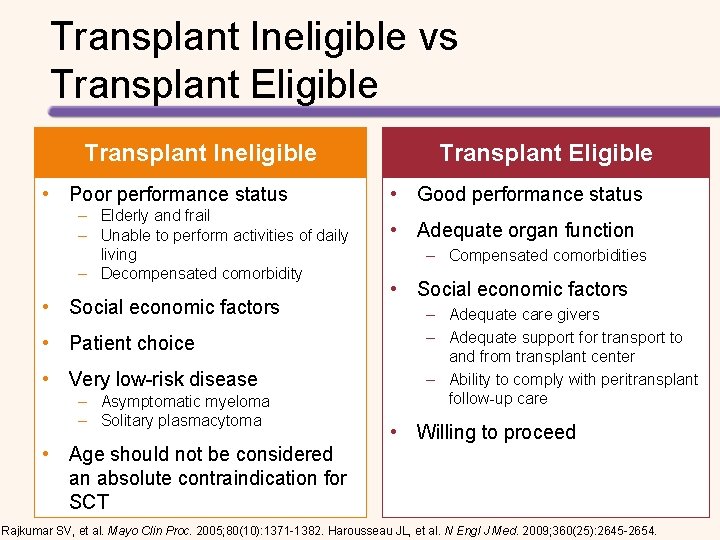
Transplant Ineligible vs Transplant Eligible Transplant Ineligible • Poor performance status – Elderly and frail – Unable to perform activities of daily living – Decompensated comorbidity • Social economic factors • Patient choice • Very low-risk disease – Asymptomatic myeloma – Solitary plasmacytoma • Age should not be considered an absolute contraindication for SCT Transplant Eligible • Good performance status • Adequate organ function – Compensated comorbidities • Social economic factors – Adequate care givers – Adequate support for transport to and from transplant center – Ability to comply with peritransplant follow-up care • Willing to proceed Rajkumar SV, et al. Mayo Clin Proc. 2005; 80(10): 1371 -1382. Harousseau JL, et al. N Engl J Med. 2009; 360(25): 2645 -2654.

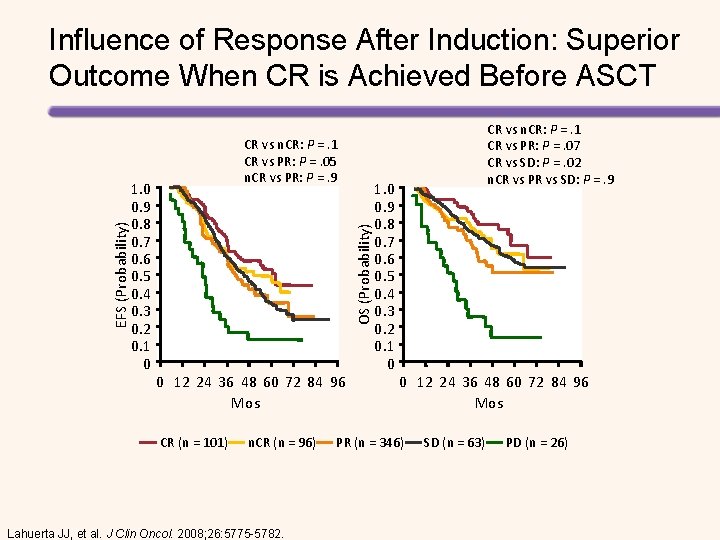
1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 CR vs n. CR: P =. 1 CR vs PR: P =. 05 n. CR vs PR: P =. 9 OS (Probability) EFS (Probability) Influence of Response After Induction: Superior Outcome When CR is Achieved Before ASCT 0 12 24 36 48 60 72 84 96 Mos CR (n = 101) n. CR (n = 96) Lahuerta JJ, et al. J Clin Oncol. 2008; 26: 5775 -5782. 1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 CR vs n. CR: P =. 1 CR vs PR: P =. 07 CR vs SD: P =. 02 n. CR vs PR vs SD: P =. 9 0 12 24 36 48 60 72 84 96 Mos PR (n = 346) SD (n = 63) PD (n = 26)

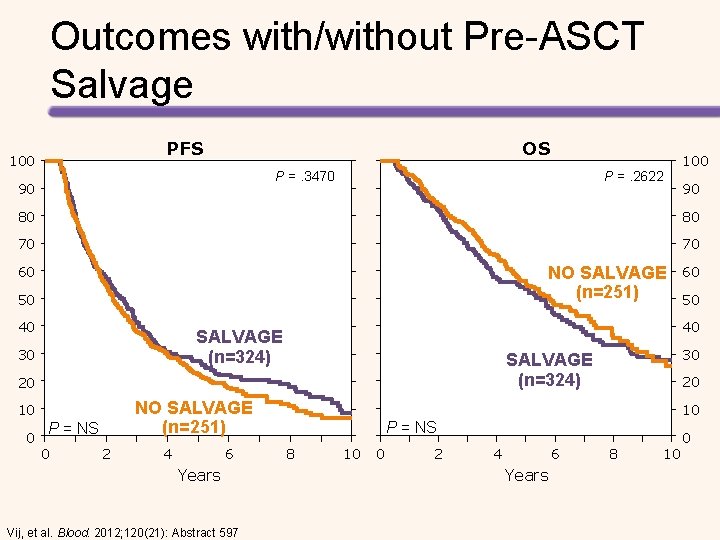
Outcomes with/without Pre-ASCT Salvage PFS 100 OS P =. 3470 90 P =. 2622 100 90 80 80 70 70 NO SALVAGE (n=251) 60 50 40 0 NO SALVAGE (n=251) P = NS 0 2 4 6 Years Vij, et al. Blood. 2012; 120(21): Abstract 597 30 SALVAGE (n=324) 20 10 50 40 SALVAGE (n=324) 30 60 20 10 P = NS 8 10 0 2 4 6 Years 8 10 0

International Myeloma Working Group (IMWG) Consensus Recommendations for Cell Doses • Is there an optimum CD 34+ cell dose to be infused? – Cell doses > 3 × 106 CD 34+ cells/kg associated with better outcomes[1 -3] – Studies primarily retrospective – Recommendation: the issue of optimal CD 34+ cell dosing in a. HSCT for MM requires a prospective clinical trial • Is there an optimal dose of CD 34+ cells to be collected? – Recommendations[1] • Minimum target of 4 × 106 CD 34+ cells/kg should be collected • If feasible an average of 8 -10 × 106 CD 34+ cells/kg should be collected – These targets allow most patients to undergo at least 2 a. HSCT, each with an optimal cell dose a. HSCT, autologous hematopoietic stem cell transplantation; MM, multiple myeloma. [1] Desikan JR, et al. Br J Haematol. 2001; 112: 242 -247. [2]Bensinger W, et al. J Clin Oncol. 1995; 13: 2547 -2555. [3] Weaver CH, et al. Blood. 1995; 86: 3961 -3969. [4] Giralt S, et al. Leukemia. 2009; 23: 1904 -1912

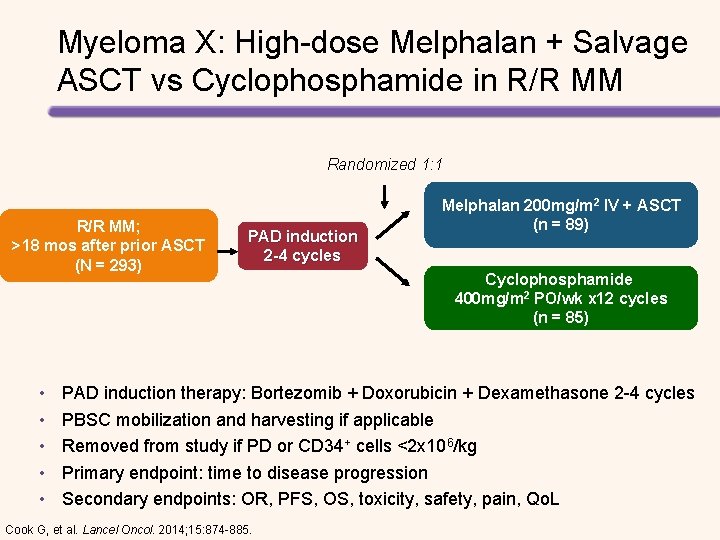
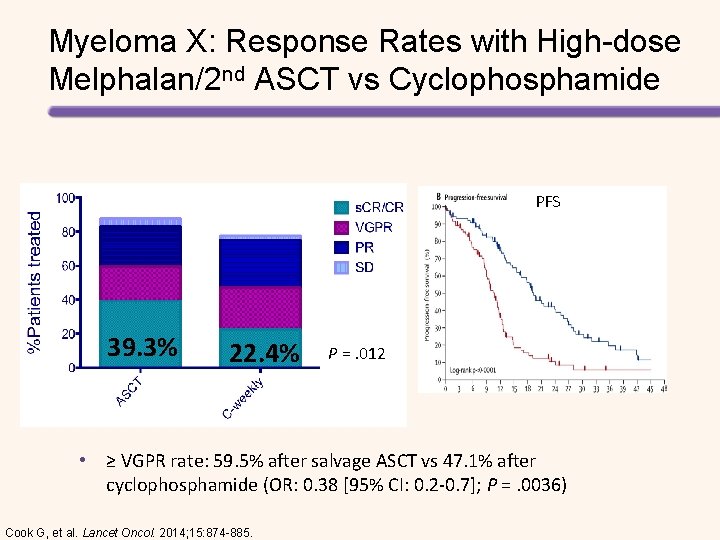
Myeloma X: High-dose Melphalan + Salvage ASCT vs Cyclophosphamide in R/R MM Randomized 1: 1 R/R MM; >18 mos after prior ASCT (N = 293) • • • PAD induction 2 -4 cycles Melphalan 200 mg/m 2 IV + ASCT (n = 89) Cyclophosphamide 400 mg/m 2 PO/wk x 12 cycles (n = 85) PAD induction therapy: Bortezomib + Doxorubicin + Dexamethasone 2 -4 cycles PBSC mobilization and harvesting if applicable Removed from study if PD or CD 34+ cells <2 x 106/kg Primary endpoint: time to disease progression Secondary endpoints: OR, PFS, OS, toxicity, safety, pain, Qo. L Cook G, et al. Lancel Oncol. 2014; 15: 874 -885.

Myeloma X: Response Rates with High-dose Melphalan/2 nd ASCT vs Cyclophosphamide PFS 39. 3% 22. 4% P =. 012 • ≥ VGPR rate: 59. 5% after salvage ASCT vs 47. 1% after cyclophosphamide (OR: 0. 38 [95% CI: 0. 2 -0. 7]; P =. 0036) Cook G, et al. Lancet Oncol. 2014; 15: 874 -885.

Barriers to Autologous Transplant Access SOCIAL ECONOMIC Age Ethnicity and race Language Culture Health literacy Patient/family attitudes Caregiver availability Socioeconomic status Education Number of wage earners Employment status Insurance coverage Place of residence Transportation ACCESS TO TRANSPLANT PROVIDER Physician referral Provider attitudes/biases Provider expertise Provider diversity Adapted from: Majhail NS, et al. Biol Blood Marrow Transplant. 2010; 16(8): 1070 -1075. HEALTH CARE SYSTEM Limited number of HCT centers Workforce shortage Capacity limitations Infrastructure issues

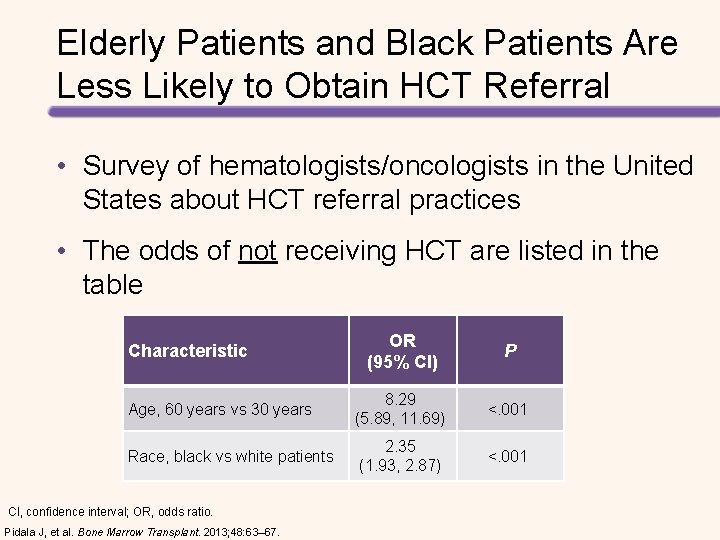
Elderly Patients and Black Patients Are Less Likely to Obtain HCT Referral • Survey of hematologists/oncologists in the United States about HCT referral practices • The odds of not receiving HCT are listed in the table OR (95% CI) P Age, 60 years vs 30 years 8. 29 (5. 89, 11. 69) <. 001 Race, black vs white patients 2. 35 (1. 93, 2. 87) <. 001 Characteristic CI, confidence interval; OR, odds ratio. Pidala J, et al. Bone Marrow Transplant. 2013; 48: 63– 67.

Factors to be Considered in the Treatment of Elderly Patients • Lower functional capacity (performance status, activities of daily living [ADL score], cognitive function) • Comorbidities (renal, pulmonary, hepatic, cardiac, bone marrow) • Disability • Frailty (weakness, poor endurance, weight loss, low physical activity, slow gait speed) • A higher prevalence of unfavorable prognostic factors (β 2 -microglobulin ≥ 3. 5 μg/m. L, albumin <3. 5 g/d. L, hemoblobin <10 g/d. L, International Staging System [ISS] stage III) • Polypharmacy • Lower capacity to tolerate toxicity • Therapy should be adjusted according to risk groups defined by age, comorbidity, organ function, disability, and frailty Ludwig, et al. Oncologist. 2012; 17: 592– 606

ROLE OF MAINTENANCE THERAPY
![Maintenance in Myeloma PFS advantage1 3 OS improvements 2 Toxicities of Maintenance in Myeloma • PFS advantage[1 -3] • OS improvements? [2] • Toxicities of](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-40.jpg)
Maintenance in Myeloma • PFS advantage[1 -3] • OS improvements? [2] • Toxicities of treatment – Myelosuppression[3] – Second primary malignancies[3, 4] – Quality of life • Unclear whether all patients benefit from maintenance • Unclear which agent and duration of therapy [1] Attal M, et al. ASH 2013. Abstract 406. [2] Mc. Carthy PL, et al. N Engl J Med. 2012; 366: 1770 -1781. [3] Attal M, et al. N Engl J Med. 2012; 366: 1782 -1791. [4] Palumbo A, et al. Lancet Oncol. 2014; 15: 333 -342.

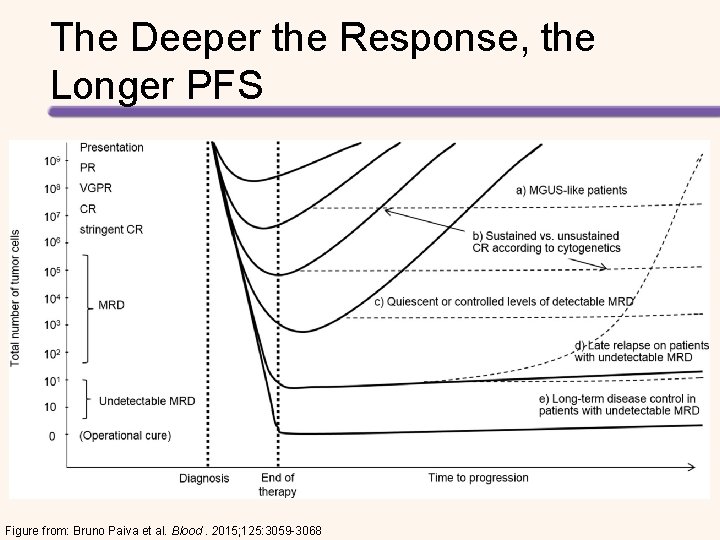
The Deeper the Response, the Longer PFS Figure from: Bruno Paiva et al. Blood. 2015; 125: 3059 -3068

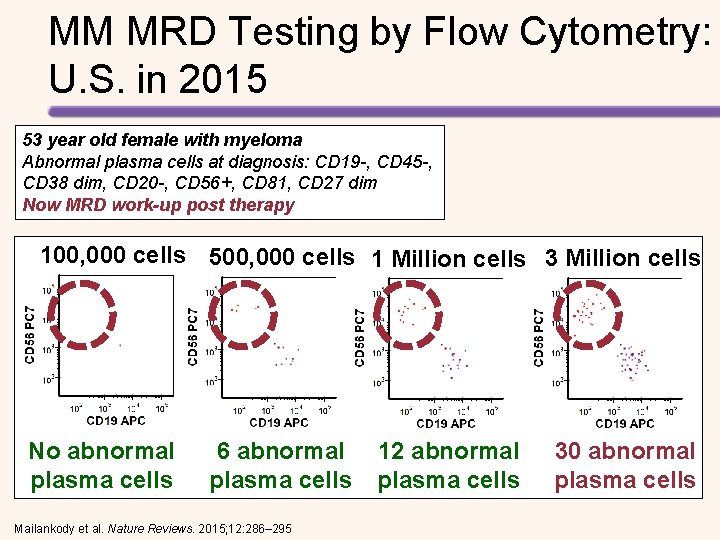
MM MRD Testing by Flow Cytometry: U. S. in 2015 53 year old female with myeloma Abnormal plasma cells at diagnosis: CD 19 -, CD 45 -, CD 38 dim, CD 20 -, CD 56+, CD 81, CD 27 dim Now MRD work-up post therapy 100, 000 cells 500, 000 cells 1 Million cells 3 Million cells No abnormal plasma cells 6 abnormal plasma cells Mailankody et al. Nature Reviews. 2015; 12: 286– 295 12 abnormal plasma cells 30 abnormal plasma cells

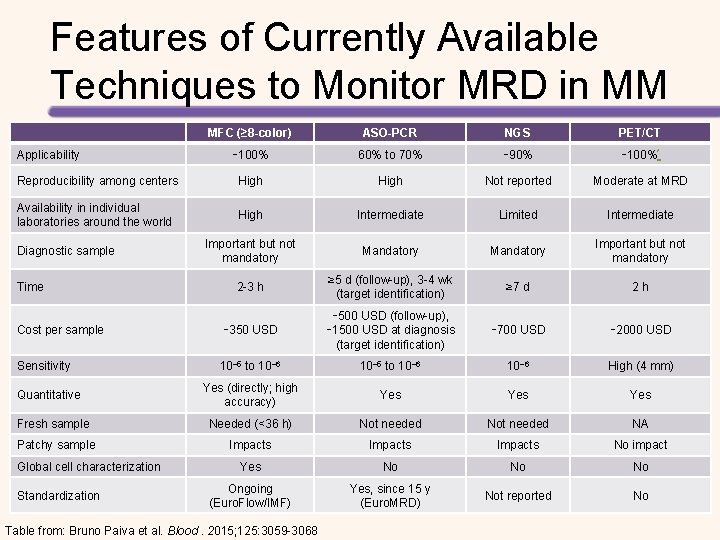
Features of Currently Available Techniques to Monitor MRD in MM MFC (≥ 8 -color) ASO-PCR NGS PET/CT ∼ 100% 60% to 70% ∼ 90% ∼ 100%* Reproducibility among centers High Not reported Moderate at MRD Availability in individual laboratories around the world High Intermediate Limited Intermediate Important but not mandatory Mandatory Important but not mandatory 2 -3 h ≥ 5 d (follow-up), 3 -4 wk (target identification) ≥ 7 d 2 h Cost per sample ∼ 350 USD ∼ 500 USD (follow-up), ∼ 1500 USD at diagnosis (target identification) ∼ 700 USD ∼ 2000 USD Sensitivity 10− 5 to 10− 6 High (4 mm) Yes (directly; high accuracy) Yes Yes Fresh sample Needed (<36 h) Not needed NA Patchy sample Impacts No impact Yes No No No Ongoing (Euro. Flow/IMF) Yes, since 15 y (Euro. MRD) Not reported No Applicability Diagnostic sample Time Quantitative Global cell characterization Standardization Table from: Bruno Paiva et al. Blood. 2015; 125: 3059 -3068
![Imaging of Residual Disease in MM PETCT1 2 PET may be useful for Imaging of Residual Disease in MM PET/CT[1, 2] • PET may be useful for](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-44.jpg)
Imaging of Residual Disease in MM PET/CT[1, 2] • PET may be useful for some, but not all, patients 1. 0 PFS (Proportion) • Variability in PET approaches across different studies and institutions PFS (CR Pts After First-line Therapy) PET CR median: 90 mos 0. 8 0. 6 0. 4 NO PET CR median: 50 mos 0. 2 P =. 010 0 0 12 24 [1] Zamagni E, et al. Blood. 2011; 118: 5989 -95. [2] Zamagni E, et al. ASH 2013. Abstract 1936. 36 Mos 48 60 72

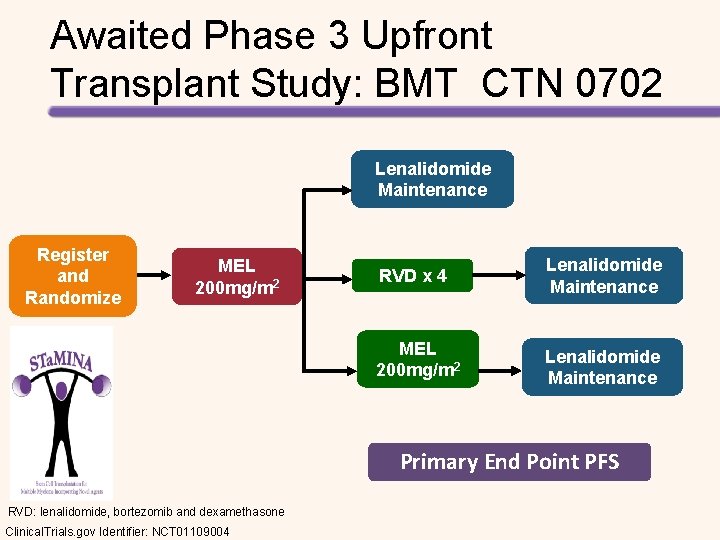
Awaited Phase 3 Upfront Transplant Study: BMT CTN 0702 Lenalidomide Maintenance Register and Randomize MEL 200 mg/m 2 RVD x 4 Lenalidomide Maintenance MEL 200 mg/m 2 Lenalidomide Maintenance Primary End Point PFS RVD: lenalidomide, bortezomib and dexamethasone Clinical. Trials. gov Identifier: NCT 01109004

CAN WE CHANGE CONDITIONING?

Conditioning Regimens for MM • Anti Myeloma activity – Standard • MELPHALAN; TBI (total body radiation) – Investigational • TOXICITY – – Mortality (TRM) – very low for MEL 200 mg/m 2 GI toxicity – dose limiting toxicity Pulmonary Syndrome Arrhythmias • COST – Days in Hospital – Cost of procurement – Timely availability – e. g TBI / Thiotepa in the USA

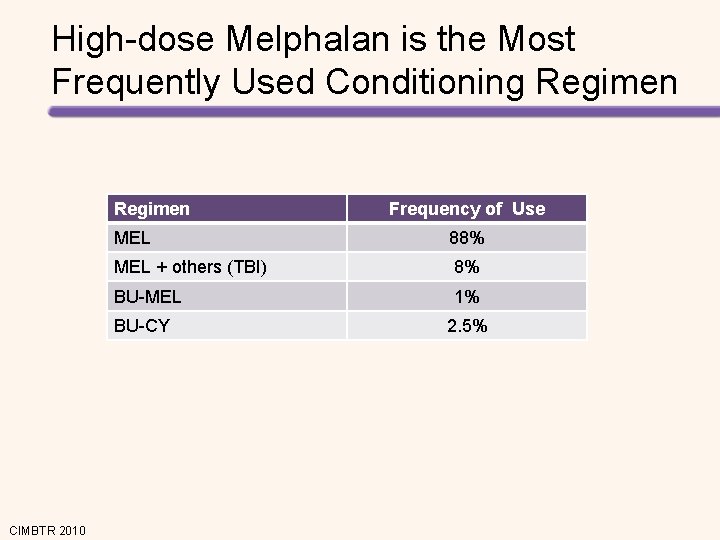
High-dose Melphalan is the Most Frequently Used Conditioning Regimen MEL 88% MEL + others (TBI) 8% BU-MEL 1% BU-CY CIMBTR 2010 Frequency of Use 2. 5%

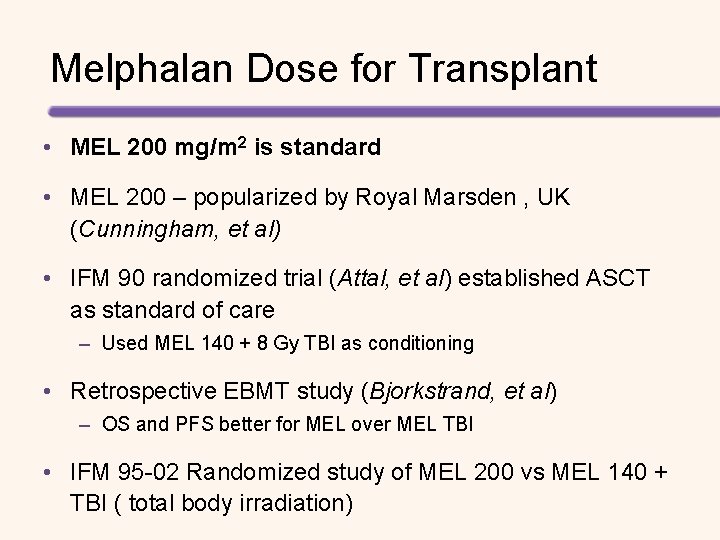
Melphalan Dose for Transplant • MEL 200 mg/m 2 is standard • MEL 200 – popularized by Royal Marsden , UK (Cunningham, et al) • IFM 90 randomized trial (Attal, et al) established ASCT as standard of care – Used MEL 140 + 8 Gy TBI as conditioning • Retrospective EBMT study (Bjorkstrand, et al) – OS and PFS better for MEL over MEL TBI • IFM 95 -02 Randomized study of MEL 200 vs MEL 140 + TBI ( total body irradiation)

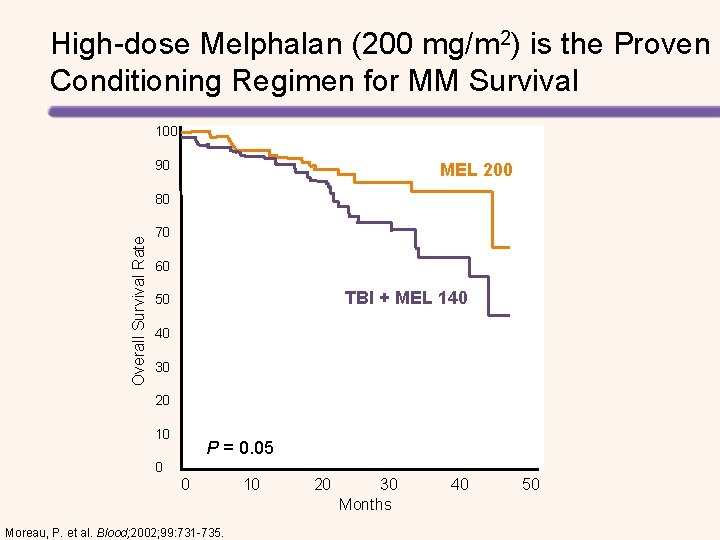
High-dose Melphalan (200 mg/m 2) is the Proven Conditioning Regimen for MM Survival 100 90 MEL 200 Overall Survival Rate 80 70 60 TBI + MEL 140 50 40 30 20 10 P = 0. 05 0 0 10 20 30 40 50 Months Moreau, P. et al. Blood; 2002; 99: 731 -735.

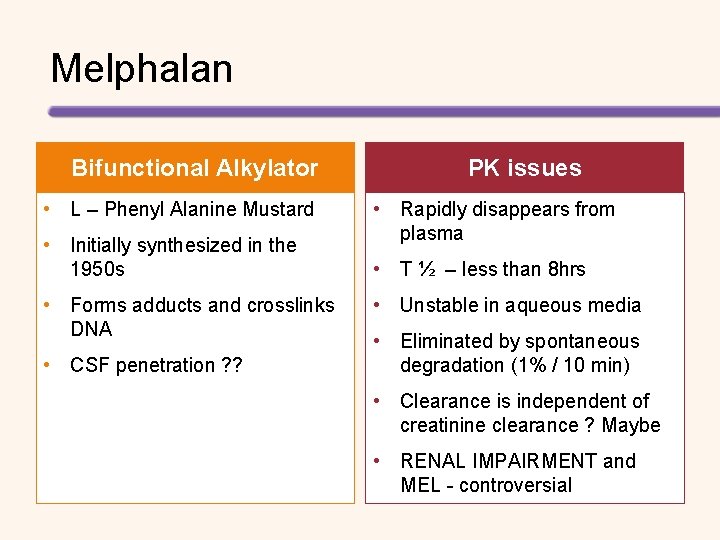
Melphalan Bifunctional Alkylator • L – Phenyl Alanine Mustard • Initially synthesized in the 1950 s • Forms adducts and crosslinks DNA • CSF penetration ? ? PK issues • Rapidly disappears from plasma • T ½ – less than 8 hrs • Unstable in aqueous media • Eliminated by spontaneous degradation (1% / 10 min) • Clearance is independent of creatinine clearance ? Maybe • RENAL IMPAIRMENT and MEL - controversial
![Intensification of MEL 220 mgm 2 French single arm study1 Escalating MEL to 300 Intensification of MEL 220 mg/m 2 (French single arm study)[1] Escalating MEL to 300](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-52.jpg)
Intensification of MEL 220 mg/m 2 (French single arm study)[1] Escalating MEL to 300 mg/m 2 [2] • Cardiac toxicity reported • Amifostine for protection • No obvious superiority over MEL 200 (historical) • MTD was MEL 280 mg/m 2 • At least 2 other MEL 280 studies in progress or completed • At higher MEL doses – – – Atrial Fibrillation Hepatic Necrosis Cardiac Death Severe Mucositis Increased deaths [1] Moreau, et al. Bone Marrow Transplant. 1999; 23: 1003 -1006. [2] Philips, et al. Biol Blood Marrow Transplant. 2004; 10: 473 -483.
![Riskadapted Melphalan Dosing Suggested Melphalan Doseadjustment for Patients with Renal Impairment1 Cr Cl 15 Risk-adapted Melphalan Dosing Suggested Melphalan Dose-adjustment for Patients with Renal Impairment[1] Cr. Cl >15](https://slidetodoc.com/presentation_image/8e1418a2e28fa0c75d388e2e40026154/image-53.jpg)
Risk-adapted Melphalan Dosing Suggested Melphalan Dose-adjustment for Patients with Renal Impairment[1] Cr. Cl >15 < 60 m. L/min Cr. Cl < 15 m. L/min or the patient is on hemodialysis 140 mg/m 2 High-dose Melphalan Dose-Reductions in Obese Patients[3] Median MEL 200 cases MEL TBI cases Normal Overweight Obese Severe Obese Total MEL 340 370 376 Dose / m 2 198 193 167 172 Total MEL 245 250 276 272 Dose/m 2 140 137 131 125 • Melphalan dose may also be adjusted due to comorbidities [1] Dimopoulos, et al. JCO. 2010; 28: 4976 -4984. [2] Palumbo A, Anderson K. N Engl J Med. 2011; 364: 1046 -1060. 3. Vogl, et al. ASH 2008. Abstract 3333. ; BBMT 2011 Dec; 17(12): 1765

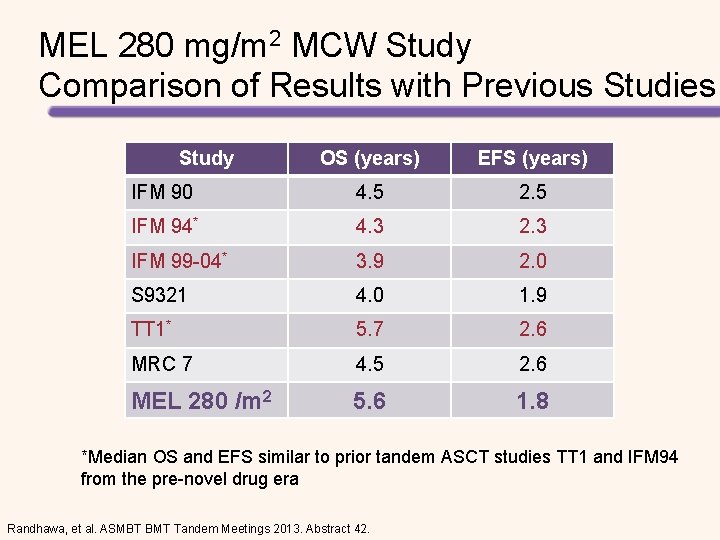
MEL 280 mg/m 2 MCW Study Comparison of Results with Previous Studies Study OS (years) EFS (years) IFM 90 4. 5 2. 5 IFM 94* 4. 3 2. 3 IFM 99 -04* 3. 9 2. 0 S 9321 4. 0 1. 9 TT 1* 5. 7 2. 6 MRC 7 4. 5 2. 6 MEL 280 /m 2 5. 6 1. 8 *Median OS and EFS similar to prior tandem ASCT studies TT 1 and IFM 94 from the pre-novel drug era Randhawa, et al. ASMBT BMT Tandem Meetings 2013. Abstract 42.

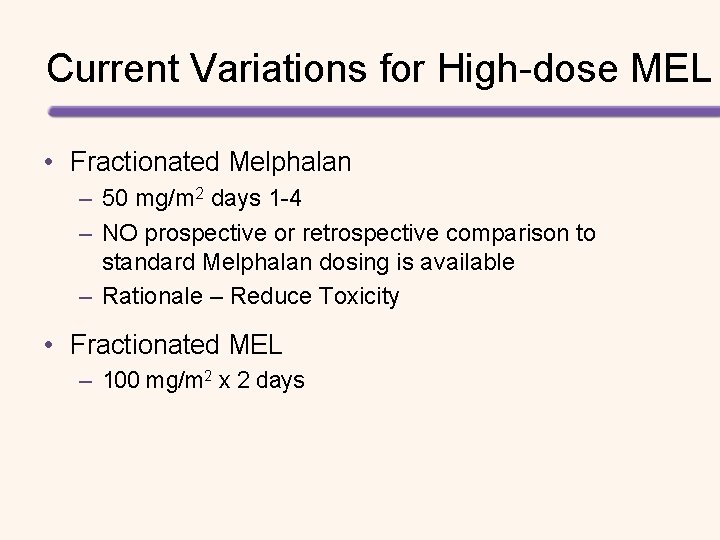
Current Variations for High-dose MEL • Fractionated Melphalan – 50 mg/m 2 days 1 -4 – NO prospective or retrospective comparison to standard Melphalan dosing is available – Rationale – Reduce Toxicity • Fractionated MEL – 100 mg/m 2 x 2 days

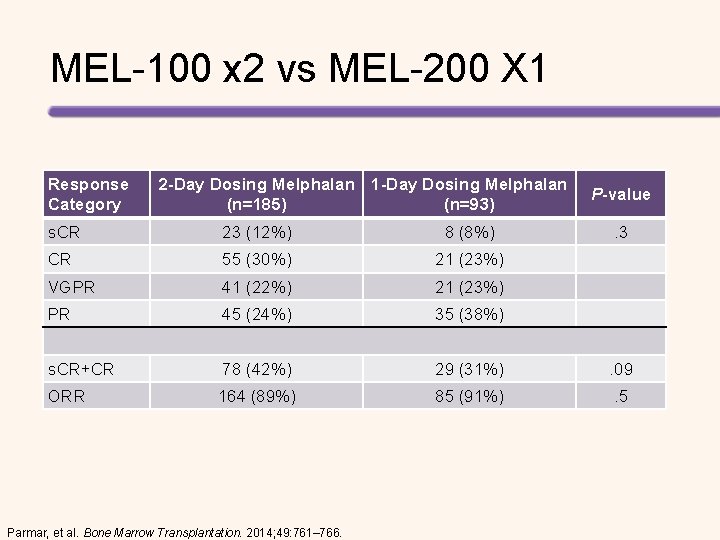
MEL-100 x 2 vs MEL-200 X 1 Response Category 2 -Day Dosing Melphalan 1 -Day Dosing Melphalan (n=185) (n=93) P-value s. CR 23 (12%) 8 (8%) . 3 CR 55 (30%) 21 (23%) VGPR 41 (22%) 21 (23%) PR 45 (24%) 35 (38%) s. CR+CR 78 (42%) 29 (31%) . 09 ORR 164 (89%) 85 (91%) . 5 Parmar, et al. Bone Marrow Transplantation. 2014; 49: 761– 766.

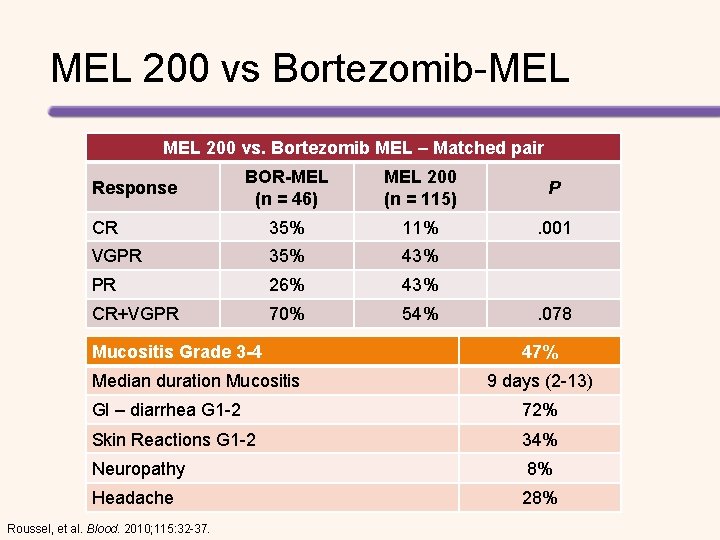
MEL 200 vs Bortezomib-MEL 200 vs. Bortezomib MEL – Matched pair BOR-MEL (n = 46) MEL 200 (n = 115) P CR 35% 11% . 001 VGPR 35% 43% PR 26% 43% CR+VGPR 70% 54% Response Mucositis Grade 3 -4 Median duration Mucositis . 078 47% 9 days (2 -13) GI – diarrhea G 1 -2 72% Skin Reactions G 1 -2 34% Neuropathy 8% Headache 28% Roussel, et al. Blood. 2010; 115: 32 -37.

Other Conditioning Regimens Currently in Use • Classic – Busulfan Cyclophosphamide – Busulfan – Melphalan – Busulfan – Cyclophosphamide – Thiotepa • Variations of MEL – Escalated doses – Fractionated MEL • Newer – – Targeted Marrow Radiation + MEL + Bortezomib MEL + Arsenic Trioxide MEL + Carfilzomib

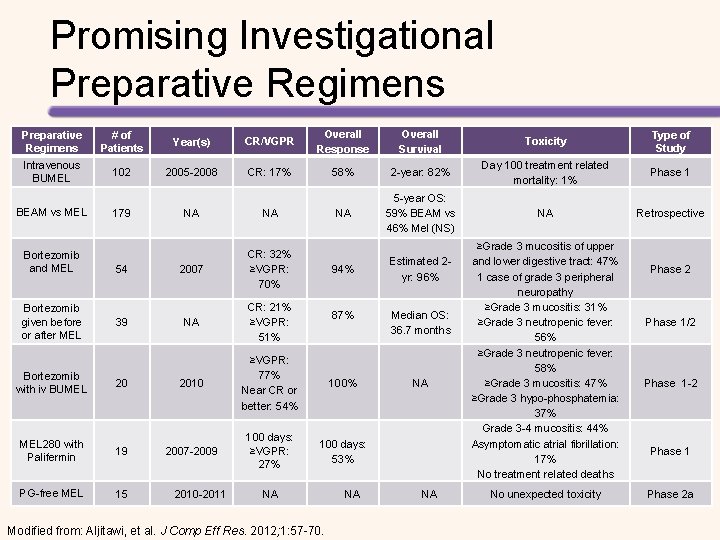
Promising Investigational Preparative Regimens # of Patients Year(s) CR/VGPR Overall Response Overall Survival Toxicity Type of Study Intravenous BUMEL 102 2005 -2008 CR: 17% 58% 2 -year: 82% Day 100 treatment related mortality: 1% Phase 1 NA 5 -year OS: 59% BEAM vs 46% Mel (NS) NA Retrospective BEAM vs MEL Bortezomib and MEL Bortezomib given before or after MEL 179 54 39 NA NA 2007 CR: 32% ≥VGPR: 70% 94% Estimated 2 yr: 96% NA CR: 21% ≥VGPR: 51% 87% Median OS: 36. 7 months 100% NA 100 days: 53% Bortezomib with iv BUMEL 20 2010 ≥VGPR: 77% Near CR or better: 54% MEL 280 with Palifermin 19 2007 -2009 100 days: ≥VGPR: 27% PG-free MEL 15 2010 -2011 NA Modified from: Aljitawi, et al. J Comp Eff Res. 2012; 1: 57 -70. NA NA ≥Grade 3 mucositis of upper and lower digestive tract: 47% 1 case of grade 3 peripheral neuropathy ≥Grade 3 mucositis: 31% ≥Grade 3 neutropenic fever: 56% ≥Grade 3 neutropenic fever: 58% ≥Grade 3 mucositis: 47% ≥Grade 3 hypo-phosphatemia: 37% Grade 3 -4 mucositis: 44% Asymptomatic atrial fibrillation: 17% No treatment related deaths No unexpected toxicity Phase 2 Phase 1/2 Phase 1 -2 Phase 1 Phase 2 a

Some other newer regimens • CAR-MEL – Carfilzomib + Melphalan • TMI – total marrow irradiation 60

MELPHALAN CHALLENGES PK Variability Propylene Glycol Special Issues

MEL Pharmacokinetics • Inter-individual variability – Creatinine Clearance – Fat free mass – Hematocrit • Higher MEL exposure—increased toxicity and efficacy • Unbound MEL—sensitive predictor of toxicity and efficacy Nath, et al. Br J Clin Pharmacol. 2010; 69: 484 -497.

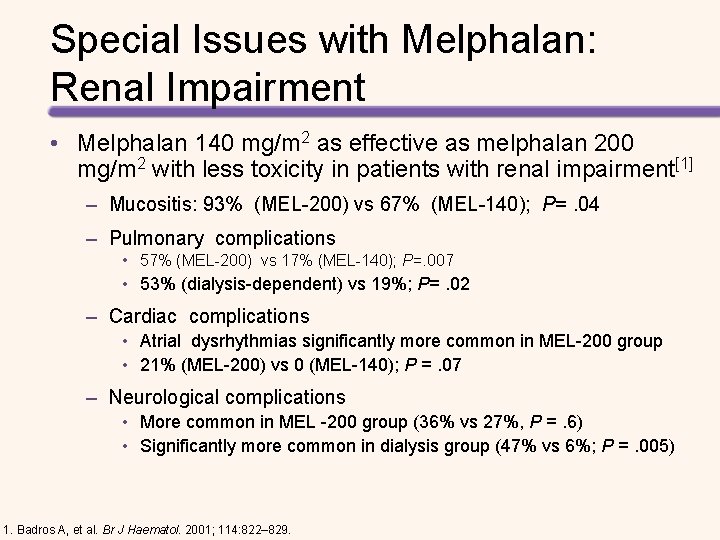
Special Issues with Melphalan: Renal Impairment • Melphalan 140 mg/m 2 as effective as melphalan 200 mg/m 2 with less toxicity in patients with renal impairment[1] – Mucositis: 93% (MEL-200) vs 67% (MEL-140); P=. 04 – Pulmonary complications • 57% (MEL-200) vs 17% (MEL-140); P=. 007 • 53% (dialysis-dependent) vs 19%; P=. 02 – Cardiac complications • Atrial dysrhythmias significantly more common in MEL-200 group • 21% (MEL-200) vs 0 (MEL-140); P =. 07 – Neurological complications • More common in MEL -200 group (36% vs 27%, P =. 6) • Significantly more common in dialysis group (47% vs 6%; P =. 005) 1. Badros A, et al. Br J Haematol. 2001; 114: 822– 829.

Special Issues with Melphalan: Renal Impairment • Pharmacokinetics of MEL are no different in renal-impaired patients BUT – More leukopenia noted in patients with renal impairment in early studies – MEL-200 poorly tolerated by patients with renal dysfunction – 60% MEL can be recovered from urine • Auto-SCT should be performed early in the disease course before renal failure becomes irreversible 1 – MEL-140 is associated with less toxicity and equal efficacy to MEL 200 in patients with renal failure – Renal failure patients with low albumin had a higher treatment-related mortality and may do better with even lower doses of MEL (70 ± 100 mg/m 2) 1. Badros A, et al. Br J Haematol. 2001; 114: 822– 829.

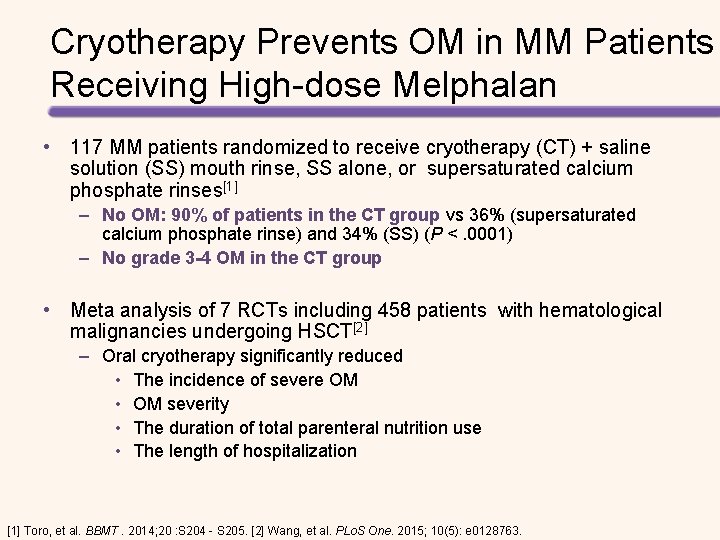
Cryotherapy Prevents OM in MM Patients Receiving High-dose Melphalan • 117 MM patients randomized to receive cryotherapy (CT) + saline solution (SS) mouth rinse, SS alone, or supersaturated calcium phosphate rinses[1] – No OM: 90% of patients in the CT group vs 36% (supersaturated calcium phosphate rinse) and 34% (SS) (P <. 0001) – No grade 3 -4 OM in the CT group • Meta analysis of 7 RCTs including 458 patients with hematological malignancies undergoing HSCT[2] – Oral cryotherapy significantly reduced • The incidence of severe OM • OM severity • The duration of total parenteral nutrition use • The length of hospitalization [1] Toro, et al. BBMT. 2014; 20 : S 204 - S 205. [2] Wang, et al. PLo. S One. 2015; 10(5): e 0128763.

Special Issues with Melphalan: Administration • When reconstituted, Melphalan rapidly hydrolyzes ~1% every 10 minutes • Manufacturer recommendations: – Dilute dose in NS to </= 0. 45 mg/m. L and infuse over at least 15 minutes – Complete the infusion within 60 minutes of reconstitution of the vial • BMT programs should verify that infusions have ended before the Melphalan expiration time/date

Special Issues with Melphalan: Administration • Example: Patient 2. 1 m 2 ordered 200 mg/m 2 • A dose of 420 mg diluted in 933 m. L NS (0. 45 mg/m. L) must infuse @ 1867 m. L/h administer the dose over 30 minutes • The dose is prepared as 2 bags (466. 5 m. L each) to infuse simultaneously with each pump @ 933 m. L/h – A typical infusion pump has a maximum infusion rate of 999 m. L/h

Special Issues with Melphalan: Stability • Highly unstable in solution • 10% per loss of activity/ hr • Propylene Glycol (PG) – Additive to MEL – Toxic in the ICU setting when given as continuous infusion – Rate of PG infusion exceeds FDA guidelines when MEL bolus given currently

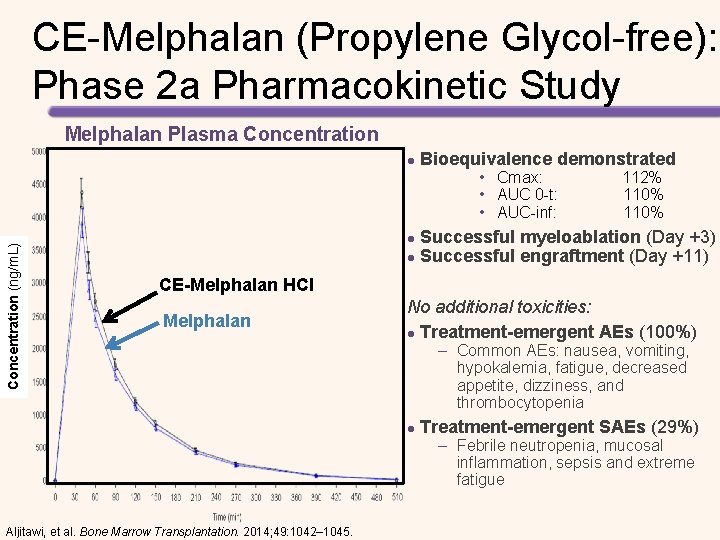
CE-Melphalan (Propylene Glycol-free): Phase 2 a Pharmacokinetic Study Melphalan Plasma Concentration l Bioequivalence demonstrated Concentration (ng/m. L) • Cmax: 112% • AUC 0 -t: 110% • AUC-inf: 110% Successful myeloablation (Day +3) l Successful engraftment (Day +11) l CE-Melphalan HCl Melphalan No additional toxicities: l Treatment-emergent AEs (100%) – Common AEs: nausea, vomiting, hypokalemia, fatigue, decreased appetite, dizziness, and thrombocytopenia l Treatment-emergent SAEs (29%) – Febrile neutropenia, mucosal inflammation, sepsis and extreme fatigue Aljitawi, et al. Bone Marrow Transplantation. 2014; 49: 1042– 1045.

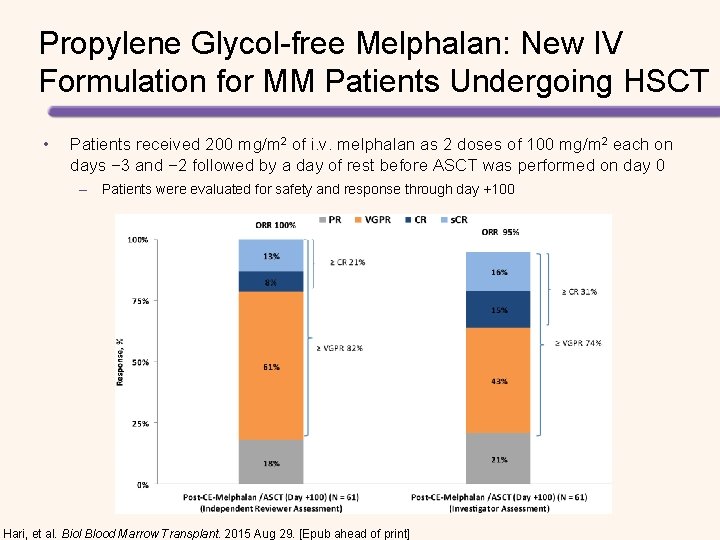
Propylene Glycol-free Melphalan: New IV Formulation for MM Patients Undergoing HSCT • Patients received 200 mg/m 2 of i. v. melphalan as 2 doses of 100 mg/m 2 each on days − 3 and − 2 followed by a day of rest before ASCT was performed on day 0 – Patients were evaluated for safety and response through day +100 Hari, et al. Biol Blood Marrow Transplant. 2015 Aug 29. [Epub ahead of print]

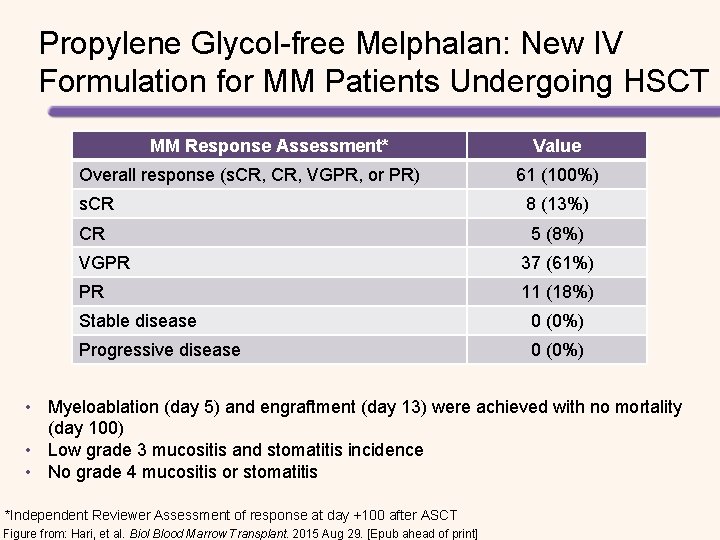
Propylene Glycol-free Melphalan: New IV Formulation for MM Patients Undergoing HSCT MM Response Assessment* Overall response (s. CR, VGPR, or PR) Value 61 (100%) s. CR 8 (13%) CR 5 (8%) VGPR 37 (61%) PR 11 (18%) Stable disease 0 (0%) Progressive disease 0 (0%) • Myeloablation (day 5) and engraftment (day 13) were achieved with no mortality (day 100) • Low grade 3 mucositis and stomatitis incidence • No grade 4 mucositis or stomatitis *Independent Reviewer Assessment of response at day +100 after ASCT Figure from: Hari, et al. Biol Blood Marrow Transplant. 2015 Aug 29. [Epub ahead of print]

ALLOGENEIC TRANSPLANTATION

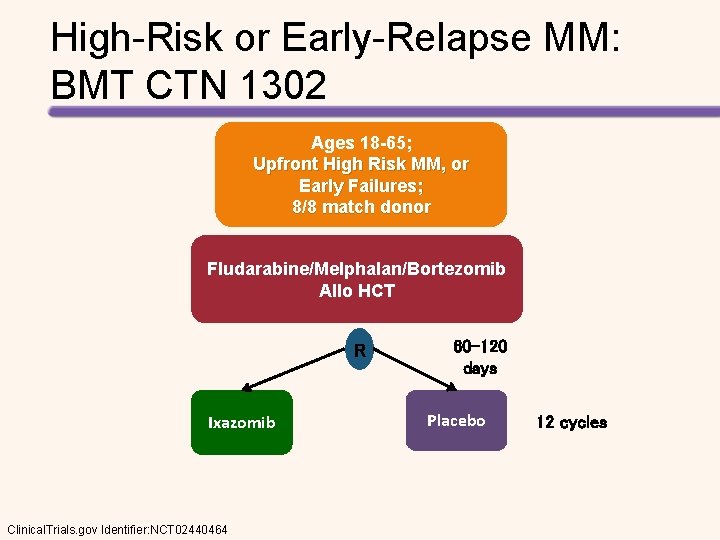
High-Risk or Early-Relapse MM: BMT CTN 1302 Ages 18 -65; Upfront High Risk MM, or Early Failures; 8/8 match donor Fludarabine/Melphalan/Bortezomib Allo HCT R Ixazomib Clinical. Trials. gov Identifier: NCT 02440464 60 -120 days Placebo 12 cycles

CONCLUSIONS

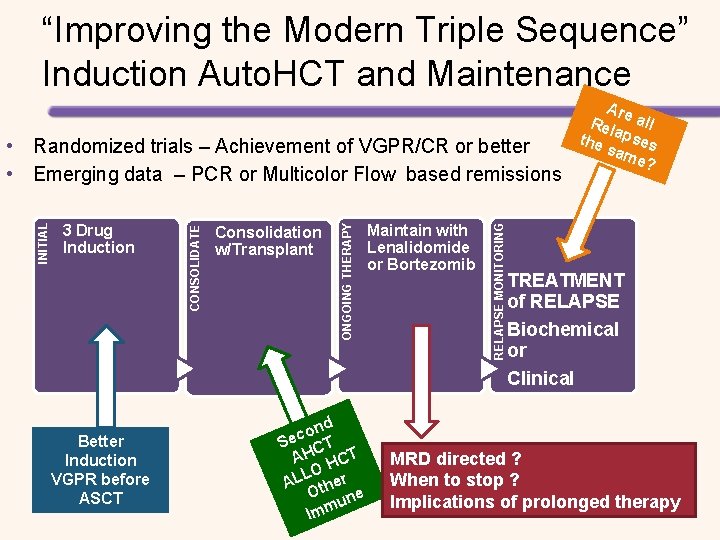
“Improving the Modern Triple Sequence” Induction Auto. HCT and Maintenance Better Induction VGPR before ASCT Maintain with Lenalidomide or Bortezomib d n o Sec CT AH HCT LO AL ther O ne u Imm RELAPSE MONITORING Consolidation w/Transplant ONGOING THERAPY 3 Drug Induction CONSOLIDATE INITIAL • Randomized trials – Achievement of VGPR/CR or better • Emerging data – PCR or Multicolor Flow based remissions Are Rel all the apses sam e? TREATMENT of RELAPSE Biochemical or Clinical MRD directed ? When to stop ? Implications of prolonged therapy

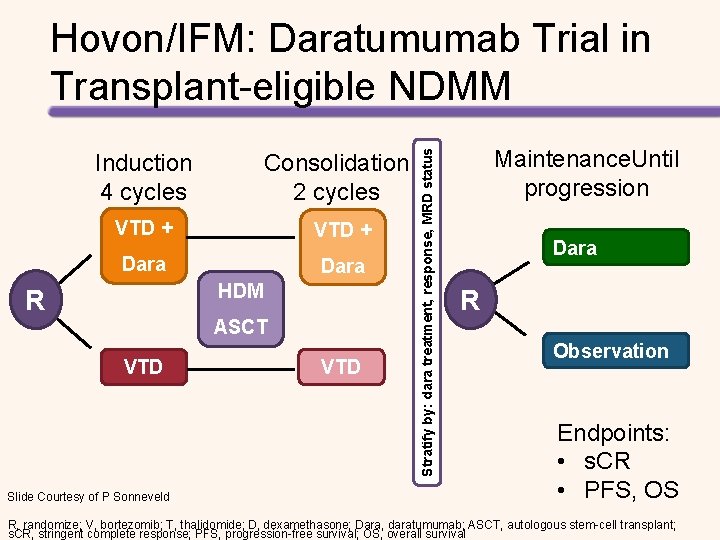
Induction 4 cycles Consolidation 2 cycles VTD + Dara HDM R ASCT VTD Slide Courtesy of P Sonneveld VTD Stratify by: dara treatment, response, MRD status Hovon/IFM: Daratumumab Trial in Transplant-eligible NDMM Maintenance. Until progression Dara R Observation Endpoints: • s. CR • PFS, OS R, randomize; V, bortezomib; T, thalidomide; D, dexamethasone; Dara, daratumumab; ASCT, autologous stem-cell transplant; s. CR, stringent complete response; PFS, progression-free survival; OS, overall survival

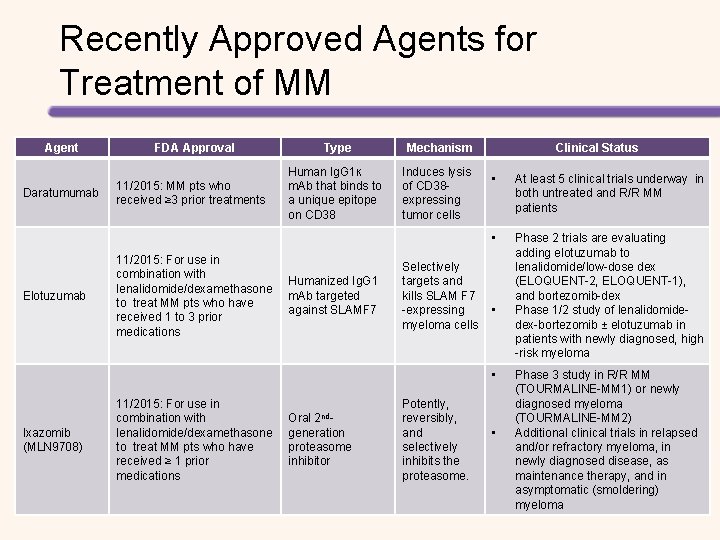
Recently Approved Agents for Treatment of MM Agent Daratumumab Elotuzumab FDA Approval 11/2015: MM pts who received ≥ 3 prior treatments 11/2015: For use in combination with lenalidomide/dexamethasone to treat MM pts who have received 1 to 3 prior medications Type Mechanism Human Ig. G 1ĸ m. Ab that binds to a unique epitope on CD 38 Induces lysis of CD 38 expressing tumor cells Humanized Ig. G 1 m. Ab targeted against SLAMF 7 Clinical Status • At least 5 clinical trials underway in both untreated and R/R MM patients • Phase 2 trials are evaluating adding elotuzumab to lenalidomide/low-dose dex (ELOQUENT-2, ELOQUENT-1), and bortezomib-dex Phase 1/2 study of lenalidomidedex-bortezomib ± elotuzumab in patients with newly diagnosed, high -risk myeloma Selectively targets and kills SLAM F 7 -expressing • myeloma cells • Ixazomib (MLN 9708) 11/2015: For use in combination with lenalidomide/dexamethasone to treat MM pts who have received ≥ 1 prior medications Oral 2 ndgeneration proteasome inhibitor Potently, reversibly, and selectively inhibits the proteasome. • Phase 3 study in R/R MM (TOURMALINE-MM 1) or newly diagnosed myeloma (TOURMALINE-MM 2) Additional clinical trials in relapsed and/or refractory myeloma, in newly diagnosed disease, as maintenance therapy, and in asymptomatic (smoldering) myeloma