Please note these are the actual videorecorded proceedings












- Slides: 25

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

Rafael Fonseca MD Chair, Department of Medicine Mayo Clinic in AZ Multiple Myeloma 2018 Phoenix, Arizona Mayo Clinic College of Medicine Mayo Clinic Comprehensive Cancer Center Rochester, Minnesota Jacksonville, Florida

Disclosures • Consulting: AMGEN, BMS, Celgene, Takeda, Bayer, Jansen, Abb. Vie, Pharmacyclics, Merck, Sanofi, Kite, and Juno. • • • SAB: Adaptive Biotechnologies Patent for FISH in MM - ~$2000/year Registered independent Believe in stem cell transplant The COI science is weak and flawed Dislike wasting your time with this slide @rfonsi 1, fonseca. rafael@mayo. edu

Regulatory and reimbursement issues aside, do you plan to administer daratumumab outside of a clinical trial in the up-front setting? No 12 In combination with a proteasome inhibitor/ lenalidomide/dexamethasone (KRD or RVD) 5 In combination with MPV 3 In combination with RD or VD 3 N = 23

Regulatory and reimbursement issues aside, what is the optimal induction regimen prior to transplant for a 60 -year-old otherwise healthy patient with newly diagnosed MM, normal renal function and no high-risk features who presents with rib and back pain as a result of lytic lesions? RVD KRd N = 23 20 3

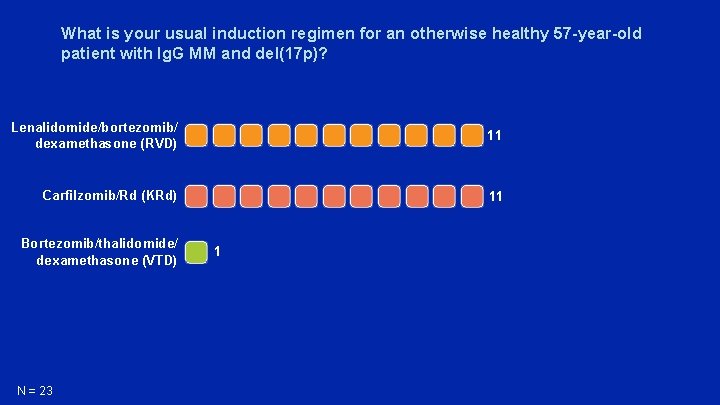
What is your usual induction regimen for an otherwise healthy 57 -year-old patient with Ig. G MM and del(17 p)? Lenalidomide/bortezomib/ dexamethasone (RVD) 11 Carfilzomib/Rd (KRd) 11 Bortezomib/thalidomide/ dexamethasone (VTD) N = 23 1

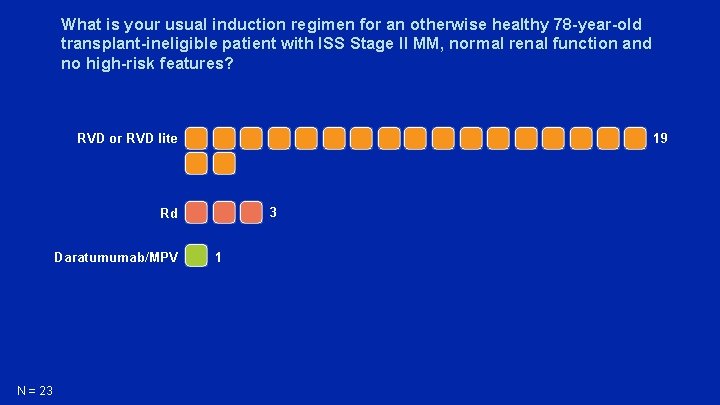
What is your usual induction regimen for an otherwise healthy 78 -year-old transplant-ineligible patient with ISS Stage II MM, normal renal function and no high-risk features? 19 RVD or RVD lite 3 Rd Daratumumab/MPV N = 23 1

Regulatory and reimbursement issues aside, are there situations outside of a clinical trial in which you believe the use of MRD assessment is clinically useful? Yes No N = 23 15 8

What is your usual recommendation for post-ASCT maintenance in patients with MM and del(17 p)? Lenalidomide + bortezomib +/dexamethasone 14 Bortezomib +/dexamethasone 4 Lenalidomide +/dexamethasone N = 23 3 Lenalidomide + ixazomib +/- dexamethasone 1 Carfilzomib/lenalidomide ± dexamethasone 1

Multiple Myeloma • • • @rfonsi 1, fonseca. rafael@mayo. edu Thalidomide Lenalidomide Pomalidomide Bortezomib Carfilzomib Ixazomib Panobinostat Daratumumab Elotuzumab Liposomal doxorubicin • Denosumab

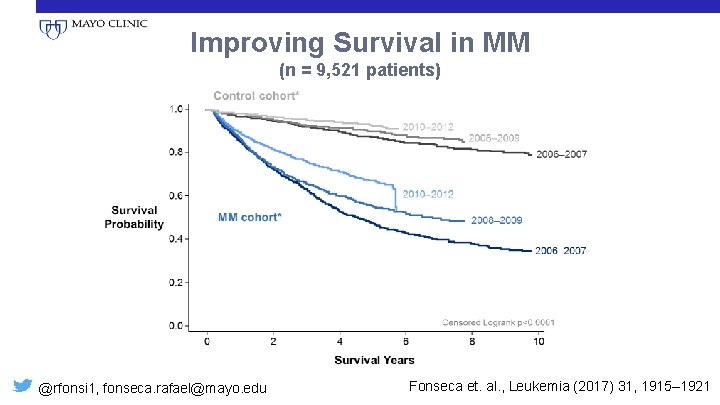
Improving Survival in MM (n = 9, 521 patients) @rfonsi 1, fonseca. rafael@mayo. edu Fonseca et. al. , Leukemia (2017) 31, 1915– 1921

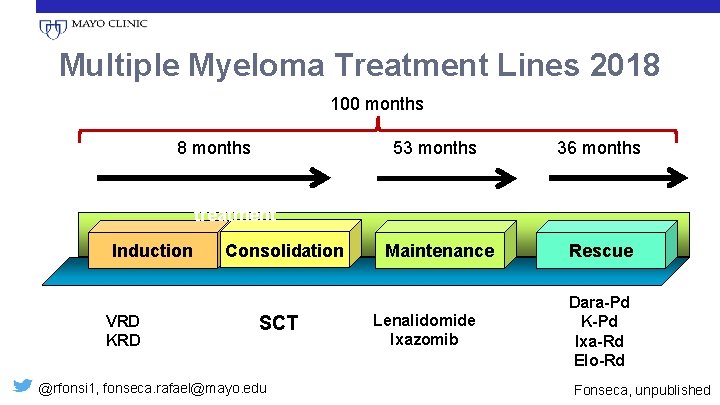
Multiple Myeloma Treatment Lines 2018 100 months 8 months 53 months Front-line treatment Induction VRD KRD Consolidation SCT @rfonsi 1, fonseca. rafael@mayo. edu Maintenance Lenalidomide Ixazomib 36 months Relapsed Rescue Dara-Pd K-Pd Ixa-Rd Elo-Rd Fonseca, unpublished

What is the best strategy for myeloma? @rfonsi 1, fonseca. rafael@mayo. edu Fonseca, unpublished

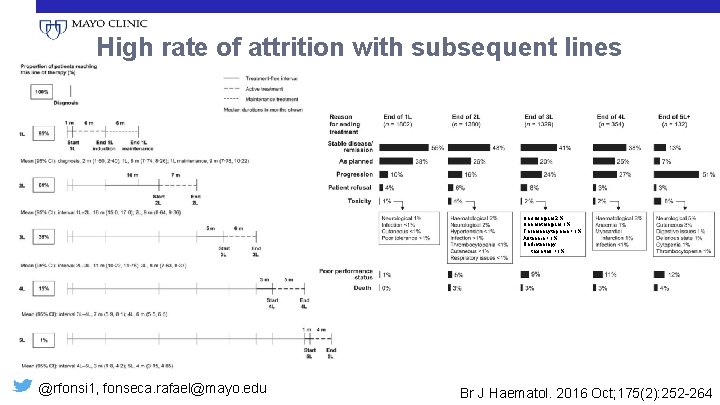
High rate of attrition with subsequent lines Neurological 2% Haematological 1% Thrombocytopenia <1% Asthenia <1% Radiotherapy toxicities <1% @rfonsi 1, fonseca. rafael@mayo. edu Br J Haematol. 2016 Oct; 175(2): 252 -264

CR vs. n. CR/VGPR/PR vs. less @rfonsi 1, fonseca. rafael@mayo. edu Martinez-Lopez et al Blood. 2011; 118(3): 529 -534

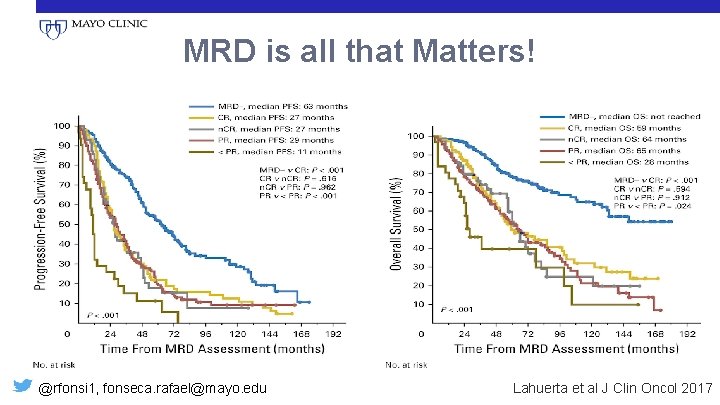
MRD is all that Matters! @rfonsi 1, fonseca. rafael@mayo. edu Lahuerta et al J Clin Oncol 2017

MRD is not everything, it’s the only thing! @rfonsi 1, fonseca. rafael@mayo. edu R Fonseca, personal information April 2018

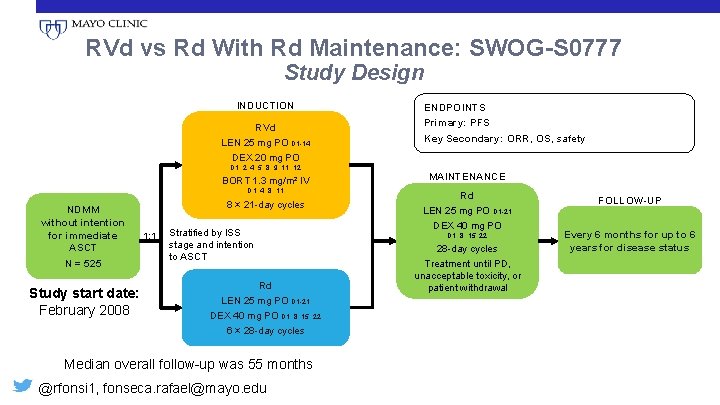
RVd vs Rd With Rd Maintenance: SWOG-S 0777 Study Design INDUCTION RVd LEN 25 mg PO D 1 -14 DEX 20 mg PO D 1, 2, 4, 5, 8, 9, 11, 12 BORT 1. 3 mg/m² IV D 1, 4, 8, 11 NDMM without intention for immediate ASCT N = 525 Study start date: February 2008 • 8 × 21 -day cycles 1: 1 Stratified by ISS stage and intention to ASCT Rd LEN 25 mg PO D 1 -21 DEX 40 mg PO D 1, 8, 15, 22 6 × 28 -day cycles Median overall follow-up was 55 months @rfonsi 1, fonseca. rafael@mayo. edu ENDPOINTS Primary: PFS Key Secondary: ORR, OS, safety MAINTENANCE Rd LEN 25 mg PO D 1 -21 DEX 40 mg PO D 1, 8, 15, 22 28 -day cycles Treatment until PD, unacceptable toxicity, or patient withdrawal FOLLOW-UP Every 6 months for up to 6 years for disease status

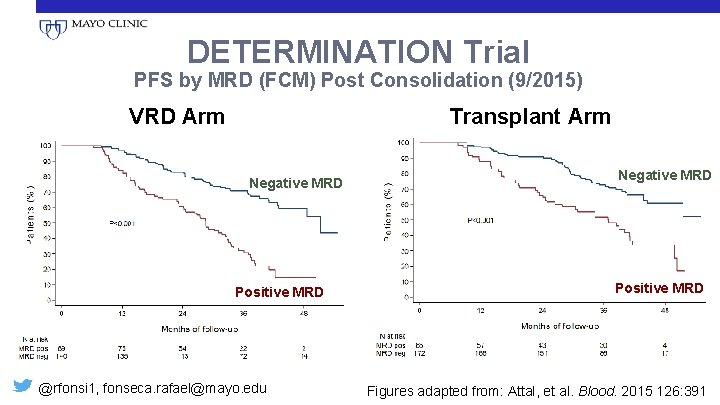
DETERMINATION Trial PFS by MRD (FCM) Post Consolidation (9/2015) VRD Arm Transplant Arm Negative MRD Positive MRD @rfonsi 1, fonseca. rafael@mayo. edu Negative MRD Positive MRD Figures adapted from: Attal, et al. Blood. 2015 126: 391

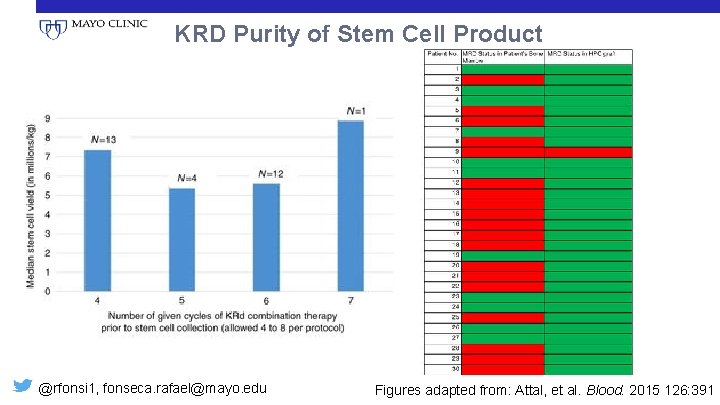
KRD Purity of Stem Cell Product @rfonsi 1, fonseca. rafael@mayo. edu Figures adapted from: Attal, et al. Blood. 2015 126: 391

ALCYONE Study Design • Transplantineligible NDMM • ECOG 0 -2 • Creatinine clearance ≥ 40 m. L/min • No peripheral neuropathy grade ≥ 2 VMP × 9 cycles (n = 356) 1: 1 Randomization (N = 706) Key eligibility criteria: Primary endpoint: Bortezomib: 1. 3 mg/m 2 SC Cycle 1: twice weekly Cycles 2 -9: once weekly Melphalan: 9 mg/m 2 PO on Days 1 -4 Prednisone: 60 mg/m 2 PO on Days 1 -4 D-VMP × 9 cycles (n = 350) • PFS D Cycles 10+ Daratumumab: 16 mg/kg IV Cycle 1: once weekly Cycles 2 -9: every 3 weeks + Same VMP schedule Stratification factors • ISS (I vs III) • Region (EU vs other) • Age (<75 vs ≥ 75 years) Mateos M-V et al. Proc ASH 2017; Abstract LBA-4. 16 mg/kg IV Every 4 weeks: until PD Followup for PD and survival Secondary endpoints: • • • ORR ≥VGPR rate ≥CR rate MRD (NGS; 10– 5) OS Safety Statistical analyses • 360 PFS events: 85% power for 8 -month PFS improvementa • Interim analysis: ~216 PFS events

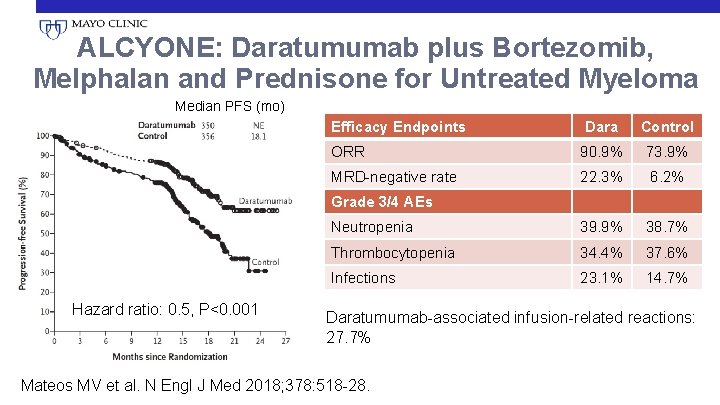
ALCYONE: Daratumumab plus Bortezomib, Melphalan and Prednisone for Untreated Myeloma Median PFS (mo) Efficacy Endpoints Dara Control ORR 90. 9% 73. 9% MRD-negative rate 22. 3% 6. 2% Neutropenia 39. 9% 38. 7% Thrombocytopenia 34. 4% 37. 6% Infections 23. 1% 14. 7% Grade 3/4 AEs Hazard ratio: 0. 5, P<0. 001 Daratumumab-associated infusion-related reactions: 27. 7% Mateos MV et al. N Engl J Med 2018; 378: 518 -28.

Daratumumab plus bortezomib-melphalanprednisone (VMP) in elderly (≥ 75 y) patients (Pts) with newly diagnosed multiple myeloma (NDMM) ineligible for transplantation (ALCYONE) Michele Cavo et al. ASCO 2018; Abstract 8031. Monday, June 4, 2018 8: 00 AM – 11: 30 AM Poster Session Hall A

Select Ongoing Phase III Trials of Novel Agents in Untreated Myeloma Trial Identifier Target # pts Randomization 1, 080 • • Daratumumab + VTD daratumumab x 2 years VTD observation NCT 02252172 745 • • Daratumumab + lenalidomide, dexamethasone Lenalidomide, dexamethasone IMROZ 440 • • Isatuximab + VRD ELOQUENT-1 750 • • Elotuzumab + lenalidomide, dexamethasone Lenalidomide, dexamethasone Cassiopeia Clinicaltrials. gov, May 31, 2018

Thank you! @rfonsi 1, fonseca. rafael@mayo. edu
 Antigentest åre
Antigentest åre To stop proceedings temporarily; move to another place
To stop proceedings temporarily; move to another place Divorce law in india
Divorce law in india Ra 9344 cases
Ra 9344 cases Adjournment of meeting
Adjournment of meeting Theoretical yeild
Theoretical yeild Sanctifying grace definition
Sanctifying grace definition Will you please be quiet please themes
Will you please be quiet please themes Please note that comma
Please note that comma Signal phrases
Signal phrases Note taking definition
Note taking definition What is a debit memo
What is a debit memo Difference between note making and note taking
Difference between note making and note taking Simple discount rate formula
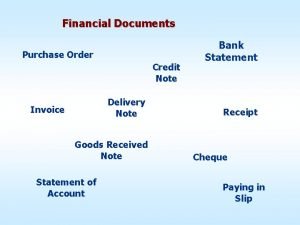
Simple discount rate formula Goods received note vs delivery note
Goods received note vs delivery note Difference between note making and note taking
Difference between note making and note taking Debit note
Debit note Các số nguyên tố là gì
Các số nguyên tố là gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thang điểm glasgow
Thang điểm glasgow ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Cái miệng nó xinh thế
Cái miệng nó xinh thế