Exercise and Physiotherapy in Myeloma UK Myeloma Forum













- Slides: 20

Exercise and Physiotherapy in Myeloma UK Myeloma Forum Spring Meeting 2016 Orla Mc. Court Team Lead Physiotherapist | Inpatient Haematology | UCH


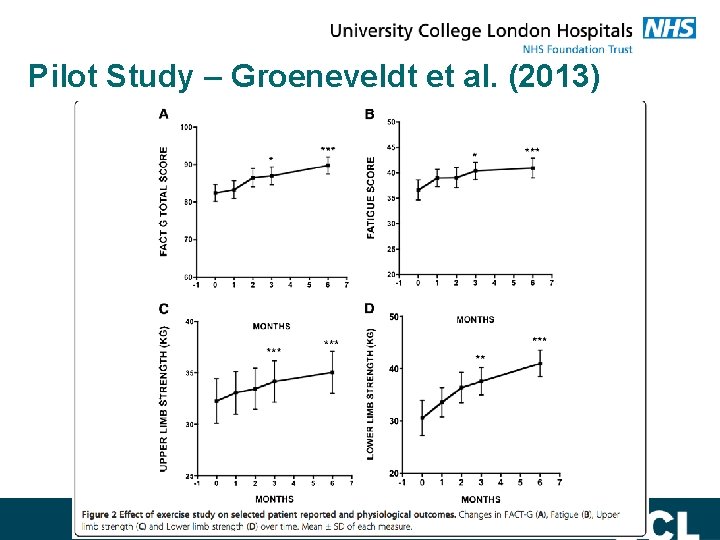
Pilot Study – Groeneveldt et al. (2013) Single arm phase II study Physiotherapist-led tailored exercise programme - Aerobic, resistance and stretching exercise programme. - Exercising 3 times per week for 6 months. First 3 months: - 1 supervised, group based session per week - 2 independent sessions at home 3 – 6 months: - 3 independent sessions at home per week - 1 monthly supervised group session

Pilot Study – Groeneveldt et al. (2013)

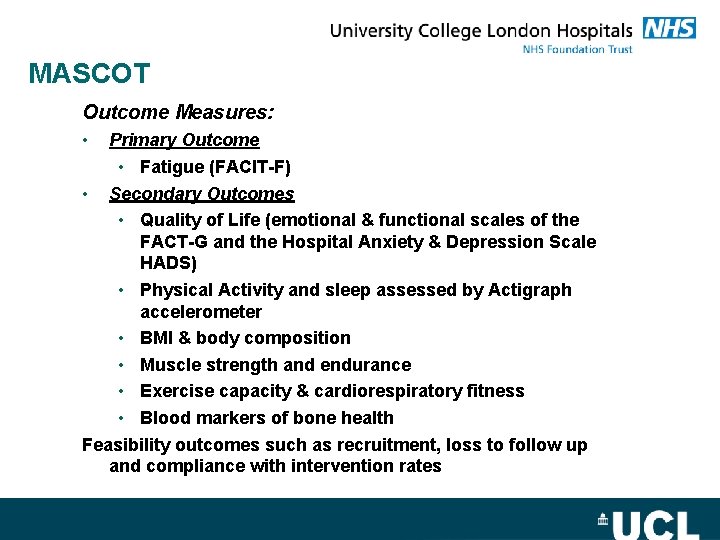
MASCOT Myeloma – Advancing Survivorship after Cancer diagnosis: Outcomes Trial

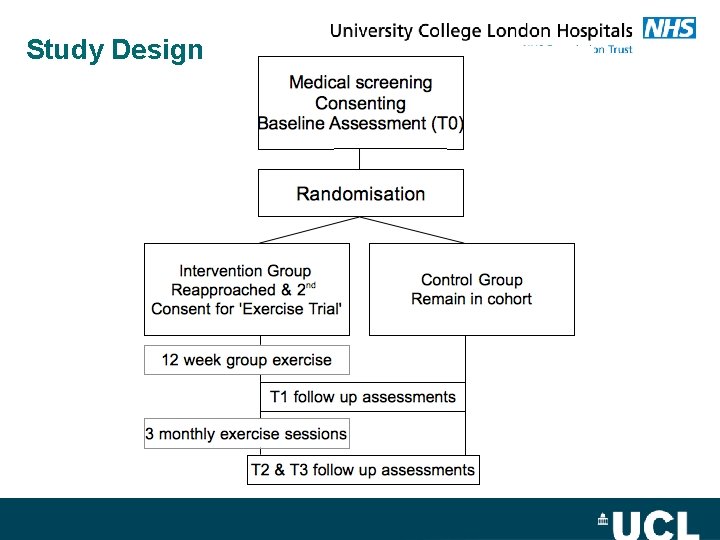
MASCOT Design: Randomised controlled trial embedded within a cohort study. Intervention: a 6 month physiotherapist-led exercise programme and behaviour change intervention versus usual care. Patient group: Patients who have completed chemotherapy or in stable plateau phase on maintenance only Asessment Timepoints: Baseline (T 0), three (T 1), six (T 2) & twelve (T 3) months.

MASCOT Outcome Measures: • Primary Outcome • Fatigue (FACIT-F) • Secondary Outcomes • Quality of Life (emotional & functional scales of the FACT-G and the Hospital Anxiety & Depression Scale HADS) • Physical Activity and sleep assessed by Actigraph accelerometer • BMI & body composition • Muscle strength and endurance • Exercise capacity & cardiorespiratory fitness • Blood markers of bone health Feasibility outcomes such as recruitment, loss to follow up and compliance with intervention rates

MASCOT Participants Inclusion criteria: • Stable disease for at least 6 weeks, off treatment or on maintenance treatment • Ability to give informed consent • Good performance status (ECOG 0 -2) • Clinically able to carry out an exercise training programme on a regular basis Exclusion criteria: • • Spinal Instability (as determined by MDT with appropriate radiology) Those who have had recent spinal or other surgery for pathological fractures (within 4 weeks) Abnormal resting ECG, unexplained by further cardiology work-up At risk of pathological fracture Already participating in an exercise programme as part of a research study Unstable angina MSK conditions that limit mobility

Study Design

Thoughts on the use of the 'adapted Zelen' design Blinding of participants to intervention study at baseline assessment – large number of participants ask about exercise and its benefits after their baseline physical assessment. 'Teachable Moment' for behaviour change? Double consent process – participants declining to take part in exercise trial but wish to continue in cohort

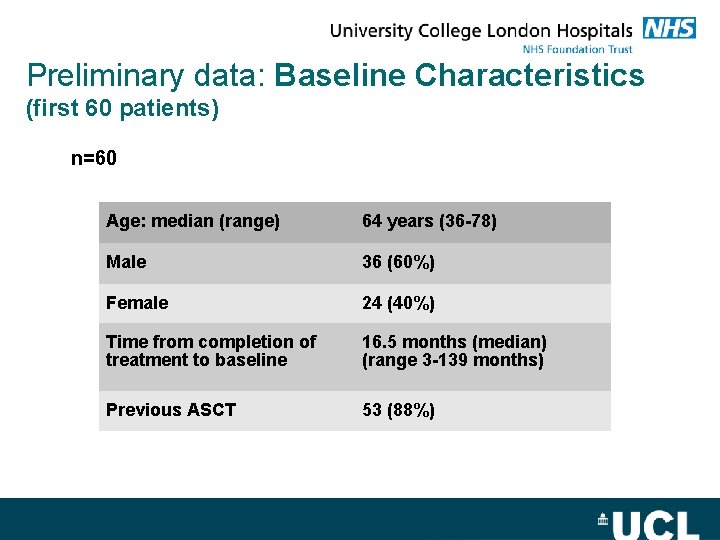
Preliminary data: Baseline Characteristics (first 60 patients) n=60 Age: median (range) 64 years (36 -78) Male 36 (60%) Female 24 (40%) Time from completion of treatment to baseline 16. 5 months (median) (range 3 -139 months) Previous ASCT 53 (88%)

Preliminary data: Fatigue, Quality of Life & Fitness n=51 Age: median (range) 64 years (42 -78) Male 59% Time from completion of last treatment 19 months (median) (range 3 -139 months) Mean Fatigue – FACIT-F score 40. 94 (median 45, range 8 -52) Mean Quality of Life – FACT-G score 40. 58 (median 42, range 19. 4 -52) Mean VO 2 peak 17. 59 ml/kg/min (± 4. 94, range 10 -28 ml/kg/min)

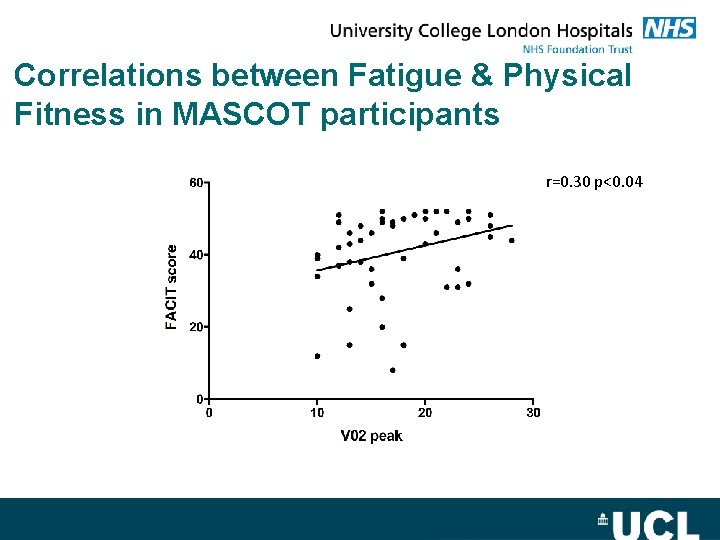
Correlations between Fatigue & Physical Fitness in MASCOT participants r=0. 30 p<0. 04

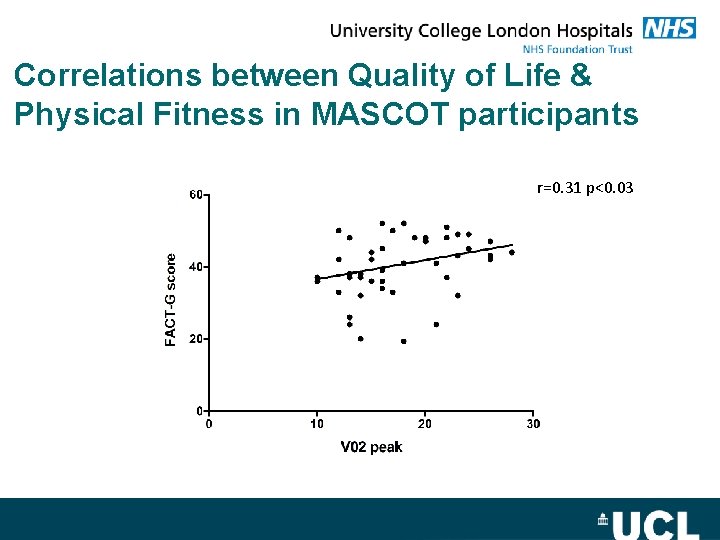
Correlations between Quality of Life & Physical Fitness in MASCOT participants r=0. 31 p<0. 03

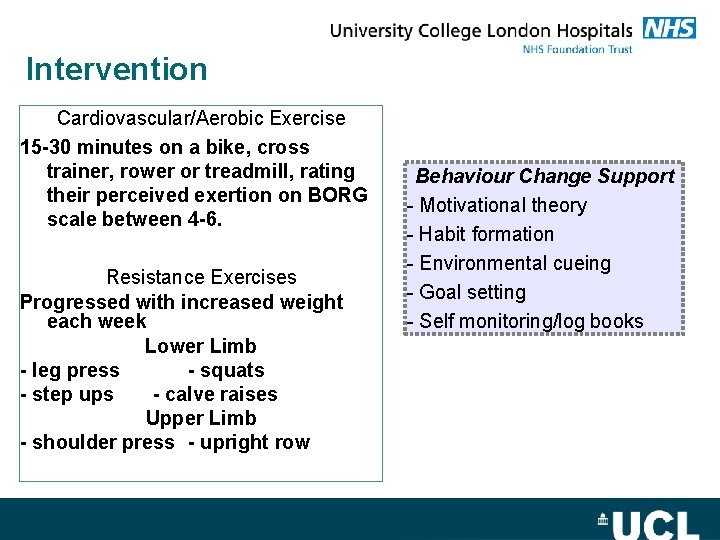
Intervention Cardiovascular/Aerobic Exercise 15 -30 minutes on a bike, cross trainer, rower or treadmill, rating their perceived exertion on BORG scale between 4 -6. Resistance Exercises Progressed with increased weight each week Lower Limb - leg press - squats - step ups - calve raises Upper Limb - shoulder press - upright row Behaviour Change Support - Motivational theory - Habit formation - Environmental cueing - Goal setting - Self monitoring/log books

Early Anecdotal Feedback from Exercise Participants Benefit of group sessions in meeting other myeloma patients, often for the first time, and discovering common experiences of treatment. Feelings of gratitude to the haematology team – participation in study is a way of 'giving back' Wish for earlier information/advice about physical activity/exercise during or soon after treatment.

Early Anecdotal Feedback from Exercise Participants Improvement in quality of life – return to physical activities that hadn't been carried out since pre-diagnosis. 78 year old lady who, after 6 weeks of intervention, no longer had to call her husband to lift her out of the bath as she could do it independently. “If I'd have done this study years ago, I might have been able to return to work”

Future Considerations for Supportive Care and Research • Dedicated physiotherapist for providing exercise programmes and advice during inpatient stay at UCLH. • Development of a 'prehab' pathway for patients undergoing stem cell transplant to include physiotherapy and dietetic input. • Idea of a 'cancer rehabilitation plan' that the patient takes through their treatment and into recovery to improve self management and integrated care.

Acknowledgements Dr Abigail Fisher – UCL Health Behaviour Research Centre Dr Maggie Heinrich – UCL Health Behaviour Research Centre Dr Rebecca Beeken – UCL Health Behaviour Research Centre Dr Bruce Paton – Institute of Sport, Exercise & Health Dr Shirley D'Sa – UCLH Department of Haematology Dr Ali Rismani – UCLH Department of Haematology Professor Allan Hackshaw – UCL Cancer Institute Professor Kwee Yong – UCL Cancer Institute Dedicated to Professor Jane Wardle Study funded by Cancer Research UK and Celgene

Fatigue and QOL in myeloma patients Bone destruction Pain, fractures, deformity muscle loss Impaired mobility Reduced physical function Fatigue Loss of confidence Reduced quality of life Altered social and economic status Fear of disease relapse Anxiety over access to treatments
 Myeloma uk forum
Myeloma uk forum Crab criteria multiple myeloma
Crab criteria multiple myeloma Crab mm
Crab mm Vtd protocol multiple myeloma
Vtd protocol multiple myeloma European myeloma network
European myeloma network Smoldering myeloma
Smoldering myeloma Myeloma cure on the horizon
Myeloma cure on the horizon üig
üig Waldenstrom macroglobulinemia vs multiple myeloma
Waldenstrom macroglobulinemia vs multiple myeloma Myeloma
Myeloma Kpd myeloma
Kpd myeloma Mayo clinic multiple myeloma
Mayo clinic multiple myeloma Multiple myeloma cure
Multiple myeloma cure Anita waldmann
Anita waldmann Solent msk physiotherapy
Solent msk physiotherapy Gibbs reflective cycle physiotherapy
Gibbs reflective cycle physiotherapy Ahpra physiotherapy registration
Ahpra physiotherapy registration Transverse myelitis physiotherapy
Transverse myelitis physiotherapy Physiotherapy degree apprenticeship
Physiotherapy degree apprenticeship What is the mains supply
What is the mains supply Physiotherapy introduction
Physiotherapy introduction