MULTIPLE MYELOMA Dtype cyclin and PI 3 k




- Slides: 41

MULTIPLE MYELOMA: D-type cyclin and PI 3 k pathways UKMF Spring meeting 2015 Kwee Yong, UCL Cancer Institute

Not one, but many myelomas

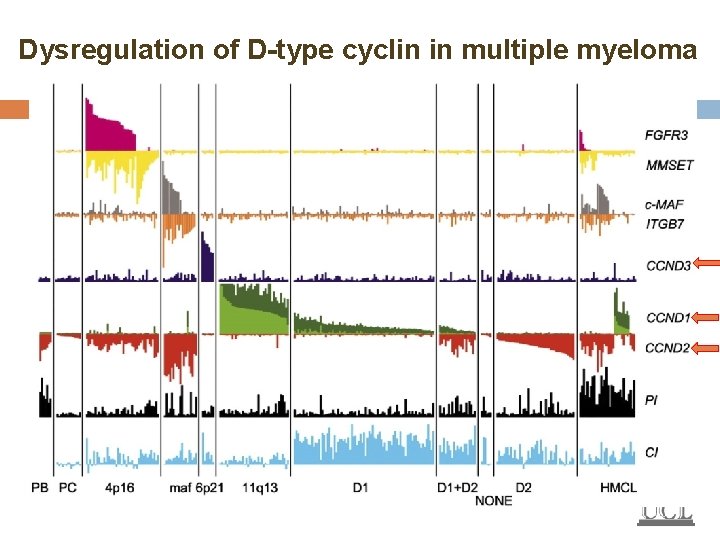
Dysregulation of D-type cyclin in multiple myeloma

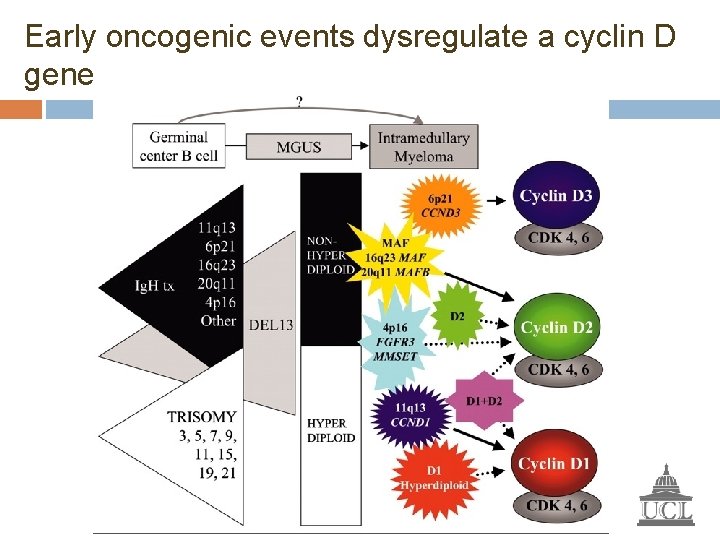
Early oncogenic events dysregulate a cyclin D gene

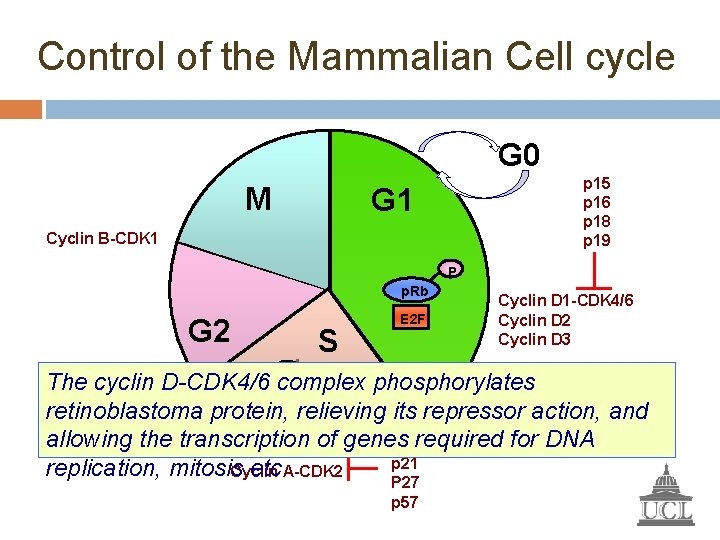
Control of the Mammalian Cell cycle G 0 M p 15 p 16 p 18 p 19 G 1 Cyclin B-CDK 1 P p. Rb G 2 S E 2 F Cyclin D 1 -CDK 4/6 Cyclin D 2 Cyclin D 3 P The cyclin D-CDK 4/6 phosphorylates P complex P E 2 F p 21 p. Rb Cyclin E-CDK 2 retinoblastoma protein, relieving its repressor action, and p 27 allowing the transcription of genes required for DNA p 57 p 21 replication, mitosis etc. A-CDK 2 Cyclin P 27 p 57

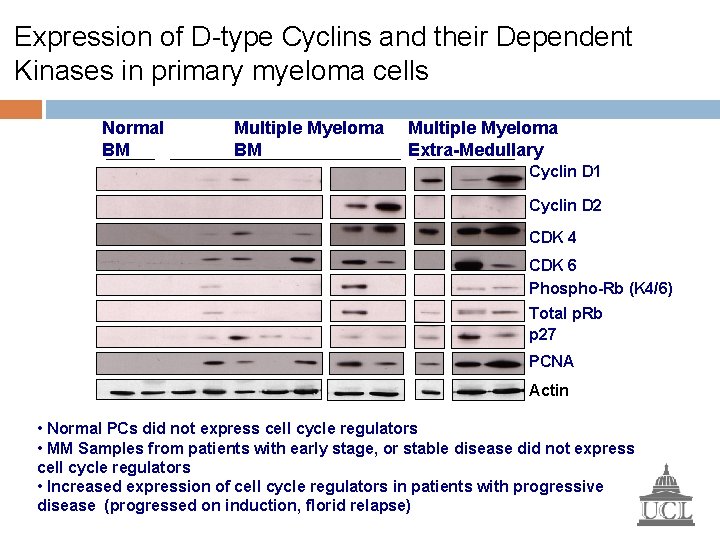
Expression of D-type Cyclins and their Dependent Kinases in primary myeloma cells Normal BM Multiple Myeloma Extra-Medullary Cyclin D 1 Cyclin D 2 CDK 4 CDK 6 Phospho-Rb (K 4/6) Total p. Rb p 27 PCNA Actin • Normal PCs did not express cell cycle regulators • MM Samples from patients with early stage, or stable disease did not express cell cycle regulators • Increased expression of cell cycle regulators in patients with progressive disease (progressed on induction, florid relapse)

What is the significance of cyclin D expression in myeloma? Contribution of cell cycle dysregulation to disease pathogenesis Does cyclin D 1 -expressing myeloma behave differently from D 2 -expressing disease?

What is the significance of cyclin D expression in myeloma? Contribution of cell cycle dysregulation to disease pathogenesis Does cyclin D 1 -expressing myeloma behave differently from D 2 disease?

Proliferative rate is prognostic Thomas et al, Eur J Hematol, 2010

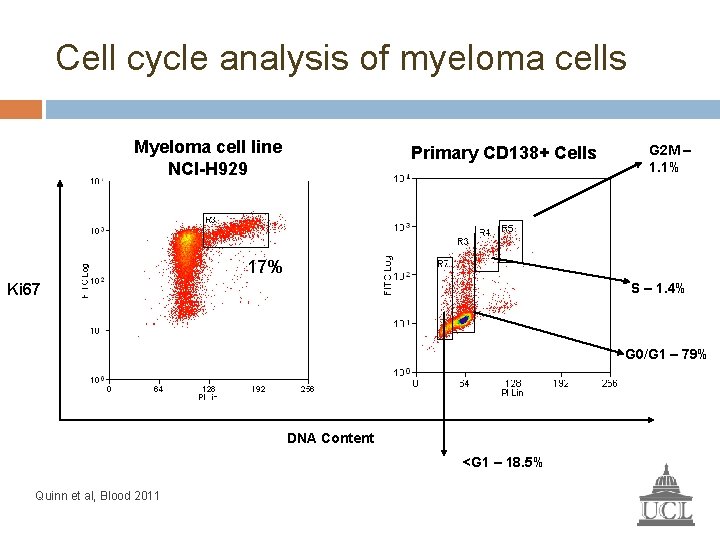
Cell cycle analysis of myeloma cells Myeloma cell line NCI-H 929 Primary CD 138+ Cells G 2 M – 1. 1% 17% S – 1. 4% Ki 67 G 0/G 1 – 79% DNA Content <G 1 – 18. 5% Quinn et al, Blood 2011

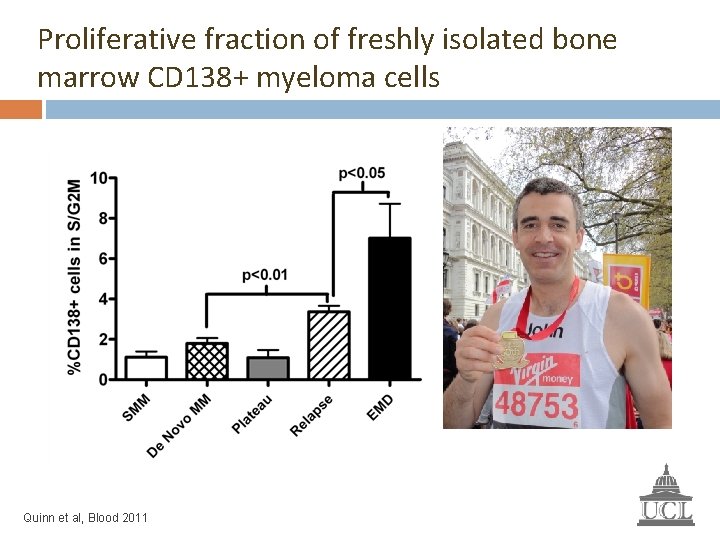
Proliferative fraction of freshly isolated bone marrow CD 138+ myeloma cells Smouldering MM = 4 De Novo MM = 30 Plateau = 8 Relapse = 88 Extramedullary disease = 9 Total = 139 Quinn et al, Blood 2011

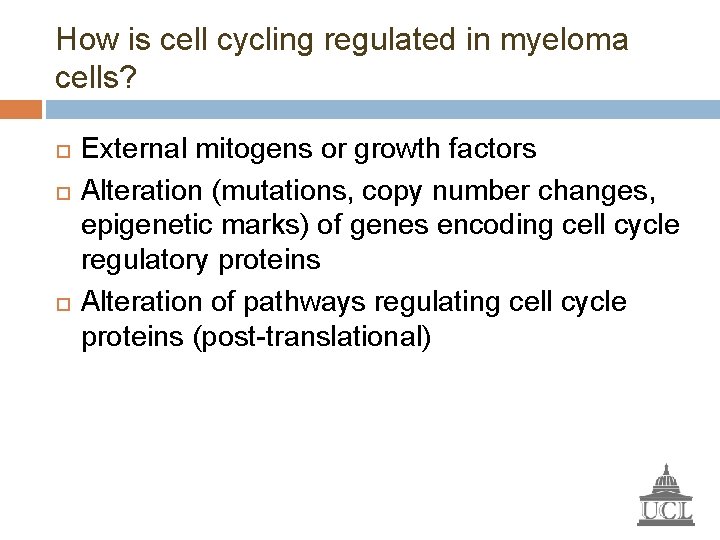
How is cell cycling regulated in myeloma cells? External mitogens or growth factors Alteration (mutations, copy number changes, epigenetic marks) of genes encoding cell cycle regulatory proteins Alteration of pathways regulating cell cycle proteins (post-translational)

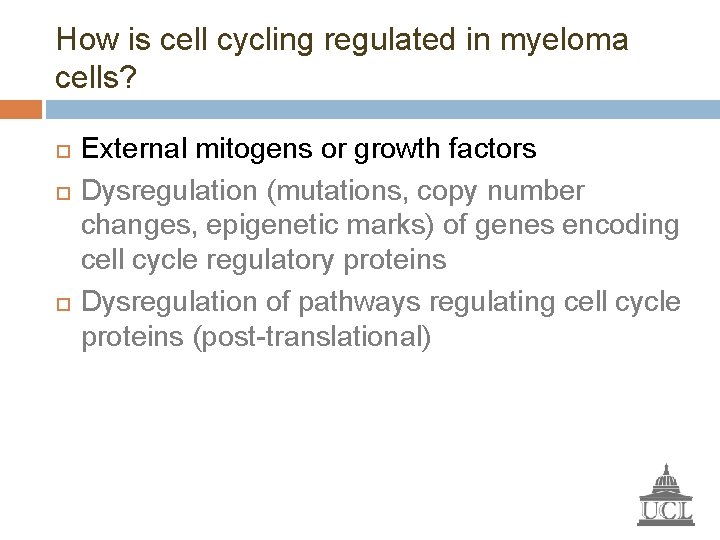
How is cell cycling regulated in myeloma cells? External mitogens or growth factors Dysregulation (mutations, copy number changes, epigenetic marks) of genes encoding cell cycle regulatory proteins Dysregulation of pathways regulating cell cycle proteins (post-translational)

What is the significance of cyclin D expression in myeloma? Contribution of cell cycle dysregulation to disease pathogenesis Does cyclin D 1 -expressing myeloma behave differently from D 2 disease?

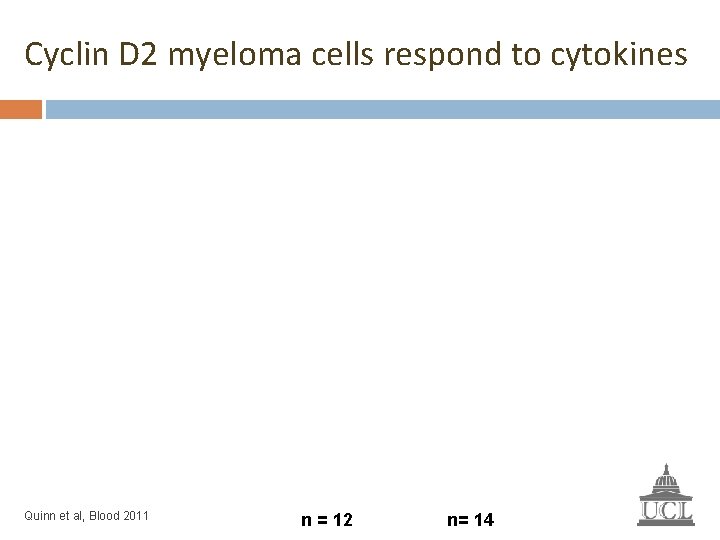
Cyclin D 2 myeloma cells respond to cytokines Quinn et al, Blood 2011 n = 12 n= 14

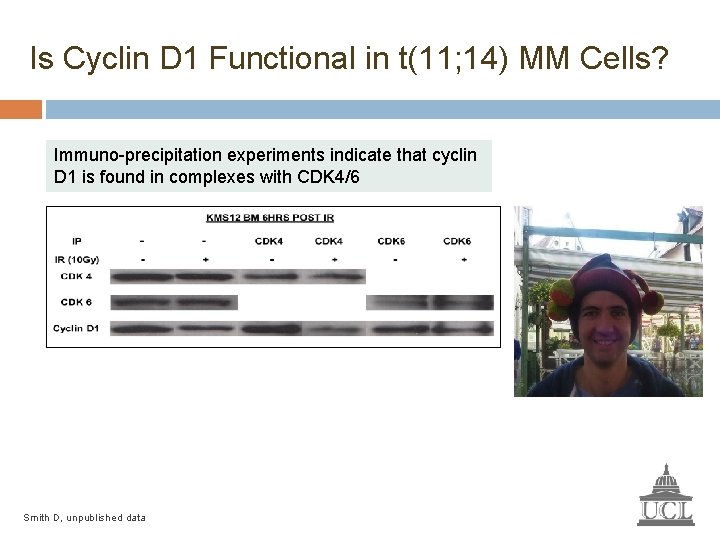
Is Cyclin D 1 Functional in t(11; 14) MM Cells? Immuno-precipitation experiments indicate that cyclin D 1 is found in complexes with CDK 4/6 Smith D, unpublished data

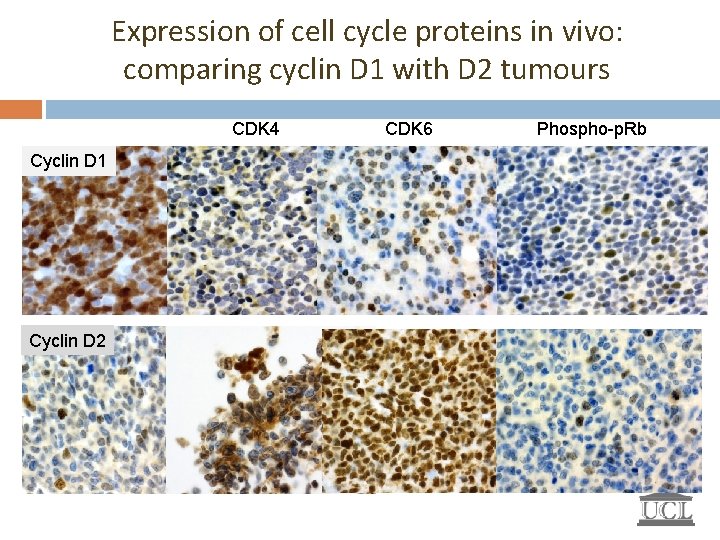
Expression of cell cycle proteins in vivo: comparing cyclin D 1 with D 2 tumours CDK 4 Cyclin D 1 Cyclin D 2 CDK 6 Phospho-p. Rb

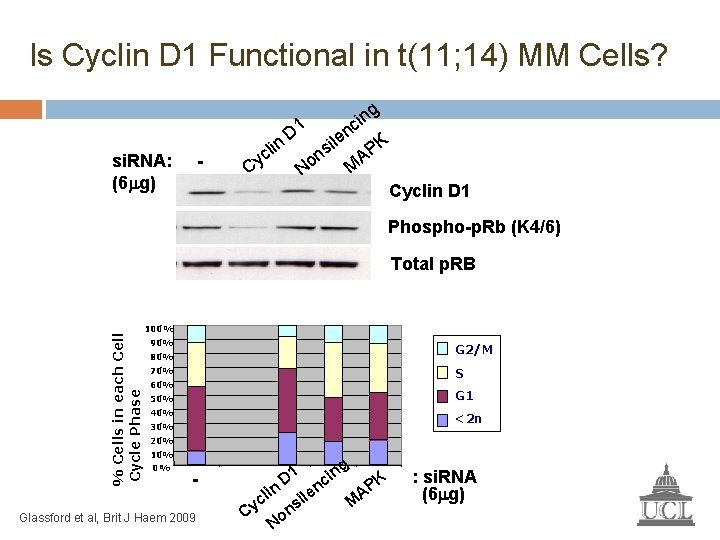
Is Cyclin D 1 Functional in t(11; 14) MM Cells? si. RNA: (6 g) - g n i c n D 1 e l K i n li s P c n A M Cy No Cyclin D 1 Phospho-p. Rb (K 4/6) % Cells in each Cell Cycle Phase Total p. RB 100% 90% G 2/M 80% 70% S 60% 50% G 1 40% <2 n 30% 20% 10% 0% - Glassford et al, Brit J Haem 2009 c Cy lin ng D 1 No i ns le i nc M K AP : si. RNA (6 g)

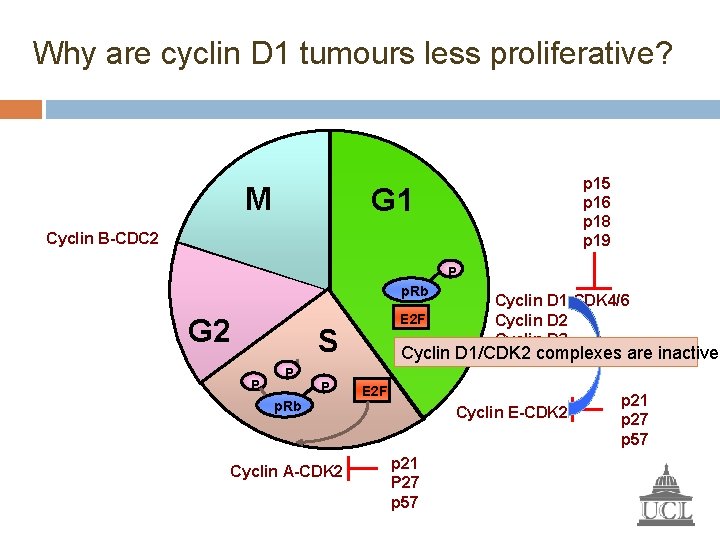
Why are cyclin D 1 tumours less proliferative? M p 15 p 16 p 18 p 19 G 1 Cyclin B-CDC 2 P p. Rb G 2 E 2 F S P P P p. Rb Cyclin A-CDK 2 Cyclin D 1 -CDK 4/6 Cyclin D 2 Cyclin D 3 Cyclin D 1/CDK 2 complexes are inactive E 2 F Cyclin E-CDK 2 p 21 P 27 p 57 p 21 p 27 p 57

Non-cell cycle functions of cyclin D 1? DNA repair Survival Jirawatnotai et al, Nature 2011 Beltran et al, PNAS 2011

How is cell cycling regulated in myeloma cells? External mitogens or growth factors Dysregulation (mutations, copy number changes, epigenetic marks) of genes encoding cell cycle regulatory proteins Dysregulation of pathways regulating cell cycle proteins (post-translational)

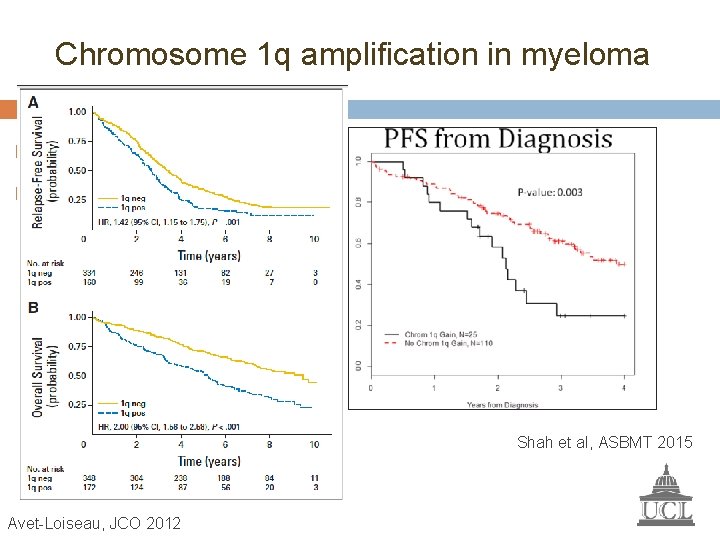
Chromosome 1 q amplification in myeloma Frequency increases with disease progression Associated with shorter survival Shah et al, ASBMT 2015 Avet-Loiseau, JCO 2012

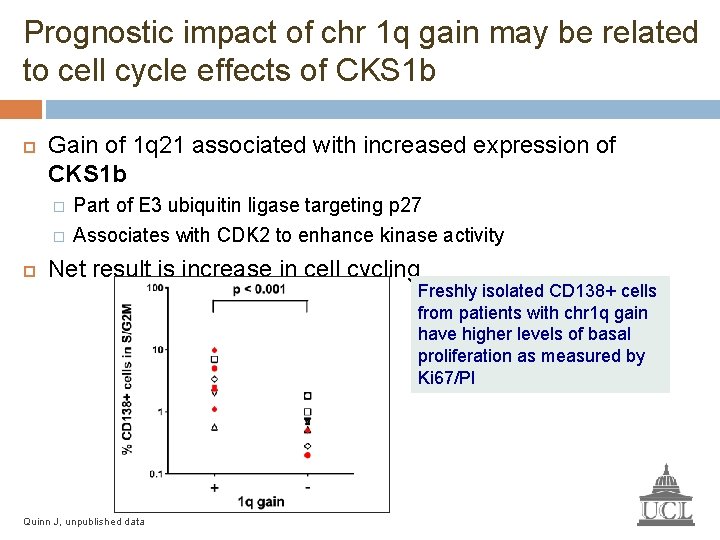
Prognostic impact of chr 1 q gain may be related to cell cycle effects of CKS 1 b Gain of 1 q 21 associated with increased expression of CKS 1 b � � Part of E 3 ubiquitin ligase targeting p 27 Associates with CDK 2 to enhance kinase activity Net result is increase in cell cycling Freshly isolated CD 138+ cells from patients with chr 1 q gain have higher levels of basal proliferation as measured by Ki 67/PI Quinn J, unpublished data

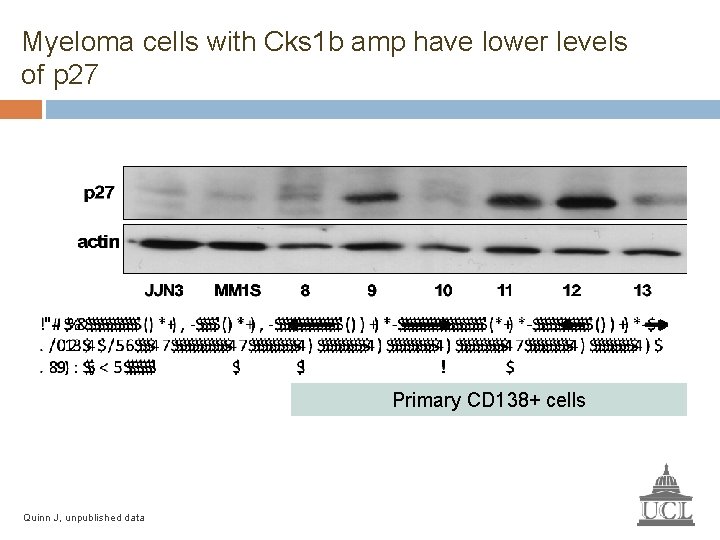
Myeloma cells with Cks 1 b amp have lower levels of p 27 Primary CD 138+ cells Quinn J, unpublished data

Therapeutic implications

Cell cycle Inhibitors � CDK inhibitors Small molecule inhibitor of CDK 4 and CDK 6 In vitro and in vivo pre-clinical data Phase 1 study with Bortezomib & Dexamethasone Smith D, unpublished data

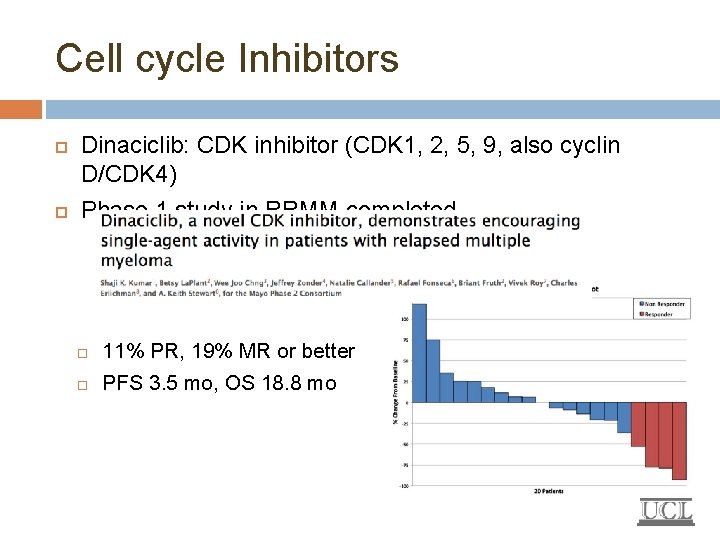
Cell cycle Inhibitors Dinaciclib: CDK inhibitor (CDK 1, 2, 5, 9, also cyclin D/CDK 4) Phase 1 study in RRMM completed 11% PR, 19% MR or better PFS 3. 5 mo, OS 18. 8 mo

How is cell cycling regulated in myeloma cells? External mitogens or growth factors Alteration (mutations, copy number changes, epigenetic marks) of genes encoding cell cycle regulatory proteins Alteration of pathways regulating cell cycle proteins (post-translational)

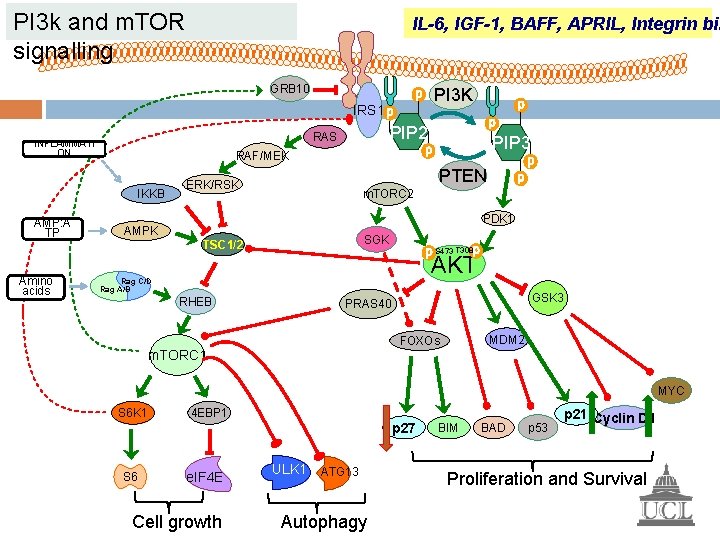
What does PI 3 k signalling have to do with D -type cyclins and cell cycle control?

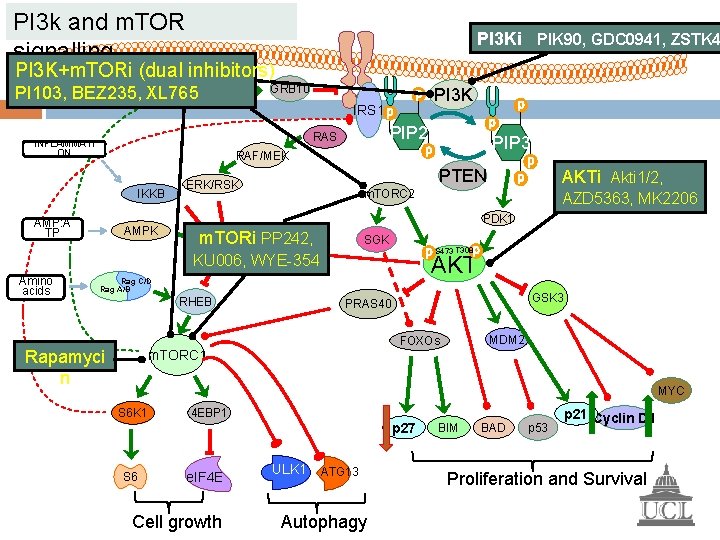
PI 3 k and m. TOR signalling IL-6, IGF-1, BAFF, APRIL, Integrin bin GRB 10 PI 3 K IRS 1 Amino acids PIP 3 RAF/MEK IKKB AMP: A TP PIP 2 RAS INFLAMMATI ON PTEN ERK/RSK m. TORC 2 PDK 1 AMPK SGK TSC 1/2 S 473 T 308 AKT Rag C/D Rag A/B RHEB GSK 3 PRAS 40 MDM 2 FOXOs m. TORC 1 MYC S 6 K 1 4 EBP 1 p 27 S 6 e. IF 4 E Cell growth ULK 1 ATG 13 Autophagy BIM BAD p 53 p 21 Cyclin D 1 Proliferation and Survival

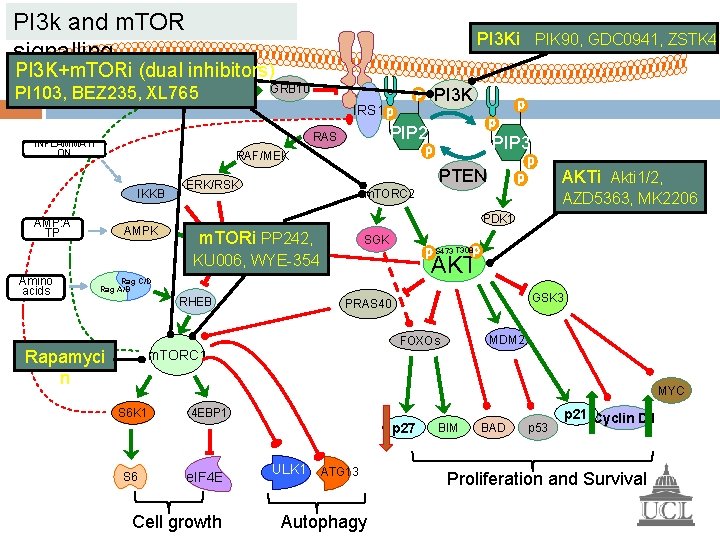
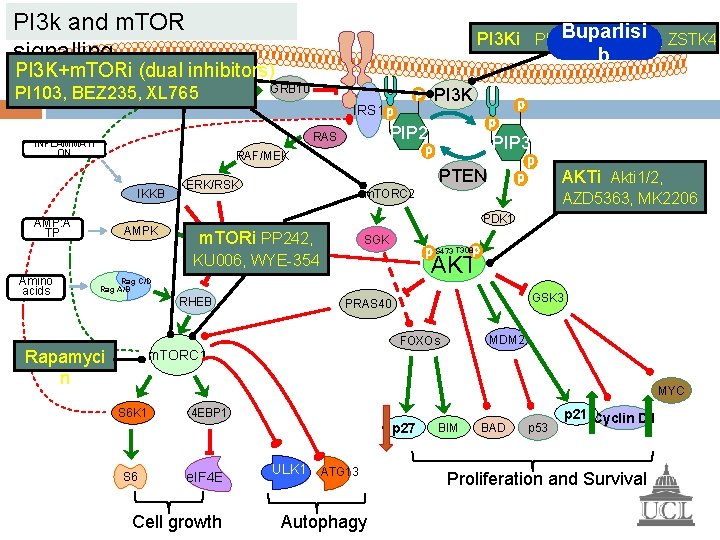
PI 3 k and m. TOR signalling PI 3 Ki PIK 90, GDC 0941, ZSTK 47 PI 3 K+m. TORi (dual inhibitors) GRB 10 PI 103, BEZ 235, XL 765 PI 3 K IRS 1 PIP 2 RAS INFLAMMATI ON IKKB AMP: A TP AMPK PTEN ERK/RSK AZD 5363, MK 2206 PDK 1 m. TORi TSC 1/2 PP 242, SGK S 473 T 308 AKT Rag C/D Rag A/B RHEB Rapamyci n AKTi Akti 1/2, m. TORC 2 KU 006, WYE-354 Amino acids PIP 3 RAF/MEK GSK 3 PRAS 40 MDM 2 FOXOs m. TORC 1 MYC S 6 K 1 4 EBP 1 p 27 S 6 e. IF 4 E Cell growth ULK 1 ATG 13 Autophagy BIM BAD p 53 p 21 Cyclin D 1 Proliferation and Survival

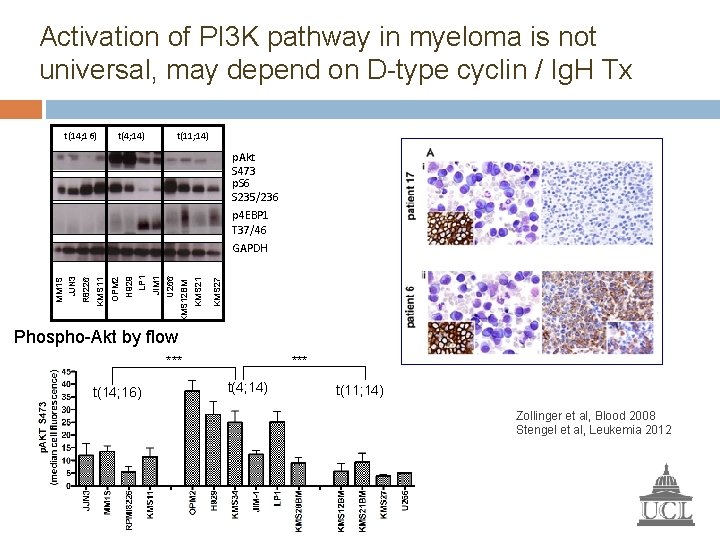
Activation of PI 3 K pathway in myeloma is not universal, may depend on D-type cyclin / Ig. H Tx t(14; 16) t(4; 14) t(11; 14) p. Akt S 473 p. S 6 S 235/236 p 4 EBP 1 T 37/46 KMS 27 KMS 21 U 266 KMS 12 BM JIM 1 H 929 LP 1 OPM 2 R 8226 KMS 11 MM 1 S JJN 3 GAPDH Phospho-Akt by flow *** t(14; 16) *** t(4; 14) t(11; 14) Zollinger et al, Blood 2008 Stengel et al, Leukemia 2012

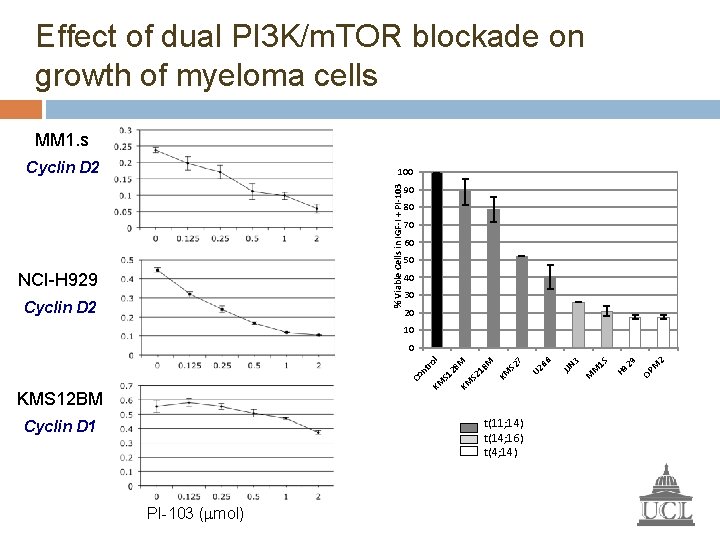
Effect of dual PI 3 K/m. TOR blockade on growth of myeloma cells MM 1. s Cyclin D 2 % Viable Cells in IGF-I + PI-103 100 NCI-H 929 Cyclin D 2 90 80 70 60 50 40 30 20 10 t(11; 14) t(14; 16) t(4; 14) Cyclin D 1 PI-103 (mmol) 2 M OP H 9 29 M 1 S M 3 JJN U 2 66 7 S 2 M 1 B KM M S 2 S 1 2 B KM KMS 12 BM KM Co nt ro l 0

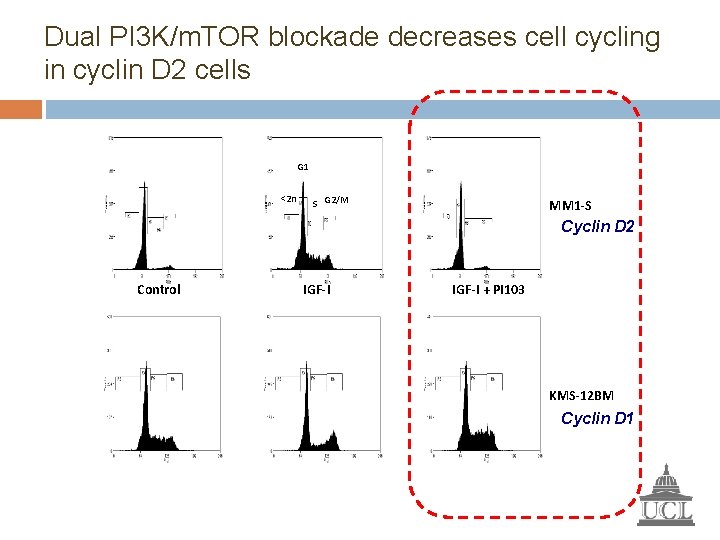
Dual PI 3 K/m. TOR blockade decreases cell cycling in cyclin D 2 cells G 1 <2 n S G 2/M MM 1 -S Cyclin D 2 Control IGF-I + PI 103 KMS-12 BM Cyclin D 1

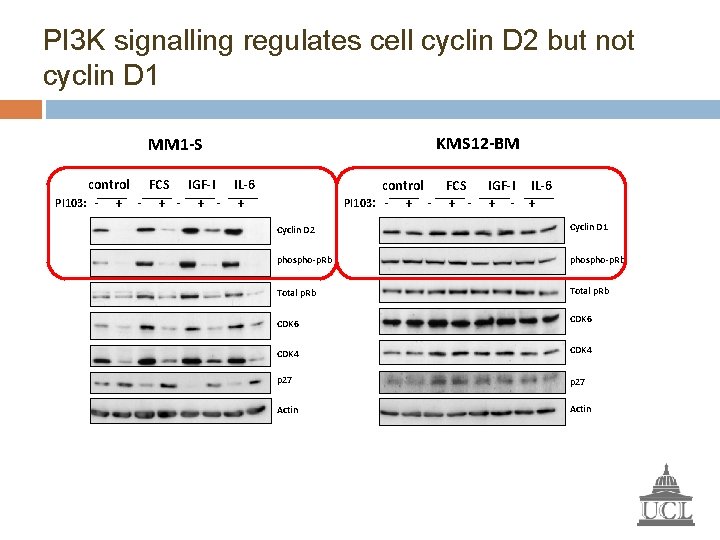
PI 3 K signalling regulates cell cyclin D 2 but not cyclin D 1 KMS 12 -BM MM 1 -S control FCS IGF-I IL-6 PI 103: - + - + control FCS PI 103: - + - IGF-I IL-6 + - + Cyclin D 2 Cyclin D 1 phospho-p. Rb Total p. Rb CDK 6 CDK 4 p 27 Actin

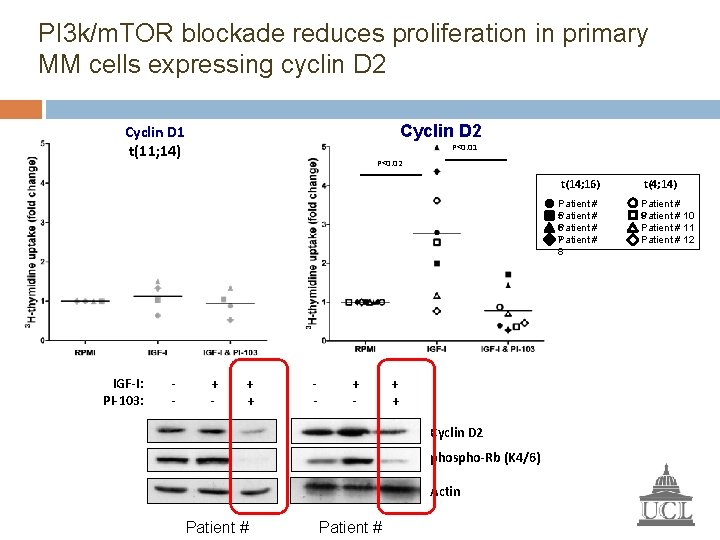
PI 3 k/m. TOR blockade reduces proliferation in primary MM cells expressing cyclin D 2 Cyclin D 1 t(11; 14) IGF-I: PI-103: - P<0. 01 P<0. 02 + - + - + + Cyclin D 2 phospho-Rb (K 4/6) Actin Patient # t(14; 16) t(4; 14) Patient # 5 Patient # 6 Patient # 7 8 Patient # P 9 atient # 10 Patient # 11 Patient # 12

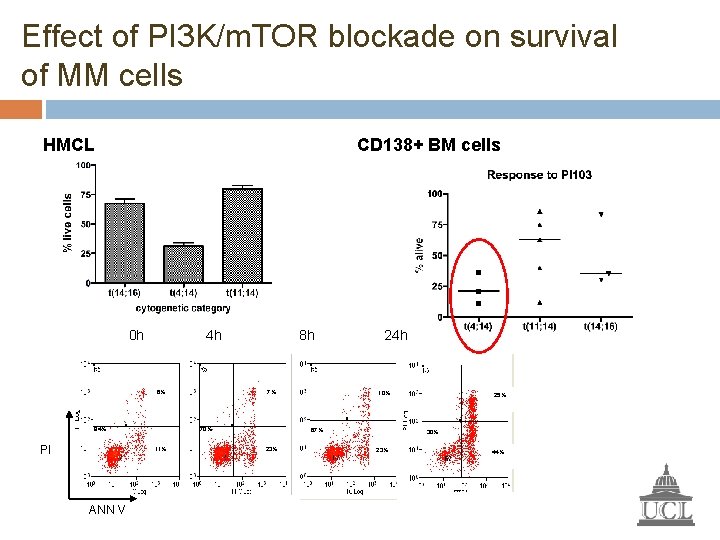
Effect of PI 3 K/m. TOR blockade on survival of MM cells HMCL CD 138+ BM cells 0 h 4 h 6% 84% PI 7% 70% 11% ANN V 8 h 24 h 10% 67% 23% 25% 30% 23% 44%

PI 3 k and m. TOR signalling PI 3 Ki PIK 90, GDC 0941, ZSTK 4 PI 3 K+m. TORi (dual inhibitors) GRB 10 PI 103, BEZ 235, XL 765 PI 3 K IRS 1 PIP 2 RAS INFLAMMATI ON IKKB AMP: A TP AMPK PTEN ERK/RSK AZD 5363, MK 2206 PDK 1 m. TORi TSC 1/2 PP 242, SGK S 473 T 308 AKT Rag C/D Rag A/B RHEB Rapamyci n AKTi Akti 1/2, m. TORC 2 KU 006, WYE-354 Amino acids PIP 3 RAF/MEK GSK 3 PRAS 40 MDM 2 FOXOs m. TORC 1 MYC S 6 K 1 4 EBP 1 p 27 S 6 e. IF 4 E Cell growth ULK 1 ATG 13 Autophagy BIM BAD p 53 p 21 Cyclin D 1 Proliferation and Survival

PI 3 k and m. TOR signalling Buparlisi PI 3 Ki PIK 90, GDC 0941, ZSTK 47 b PI 3 K+m. TORi (dual inhibitors) GRB 10 PI 103, BEZ 235, XL 765 PI 3 K IRS 1 PIP 2 RAS INFLAMMATI ON IKKB AMP: A TP AMPK PTEN ERK/RSK AZD 5363, MK 2206 PDK 1 m. TORi TSC 1/2 PP 242, SGK S 473 T 308 AKT Rag C/D Rag A/B RHEB Rapamyci n AKTi Akti 1/2, m. TORC 2 KU 006, WYE-354 Amino acids PIP 3 RAF/MEK GSK 3 PRAS 40 MDM 2 FOXOs m. TORC 1 MYC S 6 K 1 4 EBP 1 p 27 S 6 e. IF 4 E Cell growth ULK 1 ATG 13 Autophagy BIM BAD p 53 p 21 Cyclin D 1 Proliferation and Survival

Phase 1 B Study of Buparlisib with Bortezomib in Defined Genetic Subgroups of Patients with Relapsed or Refractory Multiple Myeloma Primary OBJECTIVES To determine the MTD (and/or RP 2 D, recommended phase 2 dose) of buparlisib with bortezomib (BKM-Bz) in relapsed/refractory multiple myeloma (MM) patients (dose escalation part) To evaluate the safety of the combination of BKM-BZ in patients with relapsed/refractory MM (dose expansion part)

Summary and Conclusions Cyclin D 1 and D 2 tumours differ � In cell cycle response to mitogens � Dependence on PI 3 k pathway for proliferation and survival Exploit such differences by tailoring therapies to particular genetic sub-types Understand how newly acquired genetic lesions impact on the cellular wiring imposed by type of D cyclin