Myeloma 101 and Standards of Care for Myeloma

















- Slides: 31

Myeloma 101 and Standards of Care for Myeloma Joanne Hewitt Ph. D, NP, CON(C) Nurse Practitioner Cross Cancer Institute October 13, 2018 MASS Education Conference

What Is Multiple Myeloma? Cancer of the plasma cells in bone marrow Growth of myeloma cells: Disrupts normal bone marrow function Reduces normal immune function Results in abnormal production and release of monoclonal protein into blood and/or urine Destroys and invades surrounding bone Barlogie B, et al. In: Williams Hematology; 2006. Durie BG. IMF 2007. BG. IMF 2007.

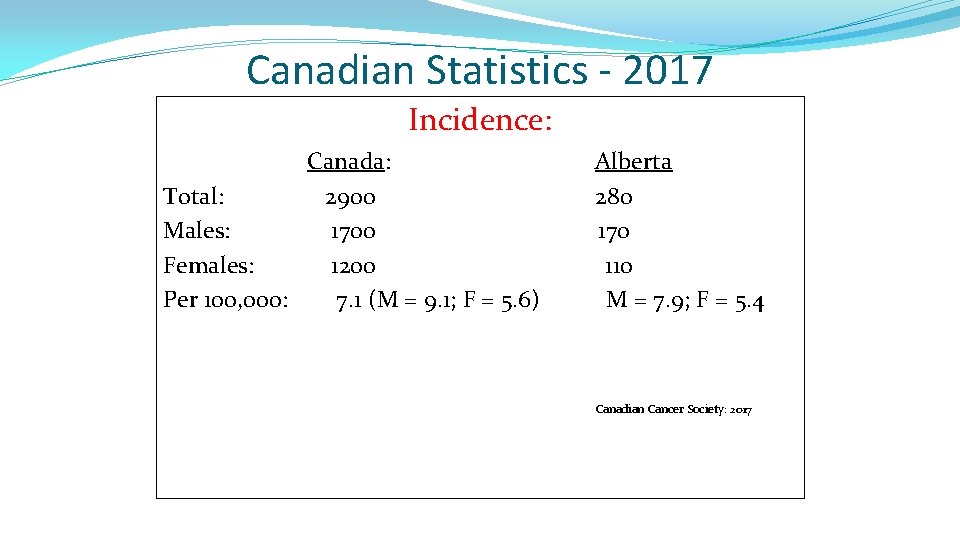
Canadian Statistics - 2017 Incidence: Canada: Total: 2900 Males: 1700 Females: 1200 Per 100, 000: 7. 1 (M = 9. 1; F = 5. 6) Alberta 280 170 110 M = 7. 9; F = 5. 4 Canadian Cancer Society: 2017


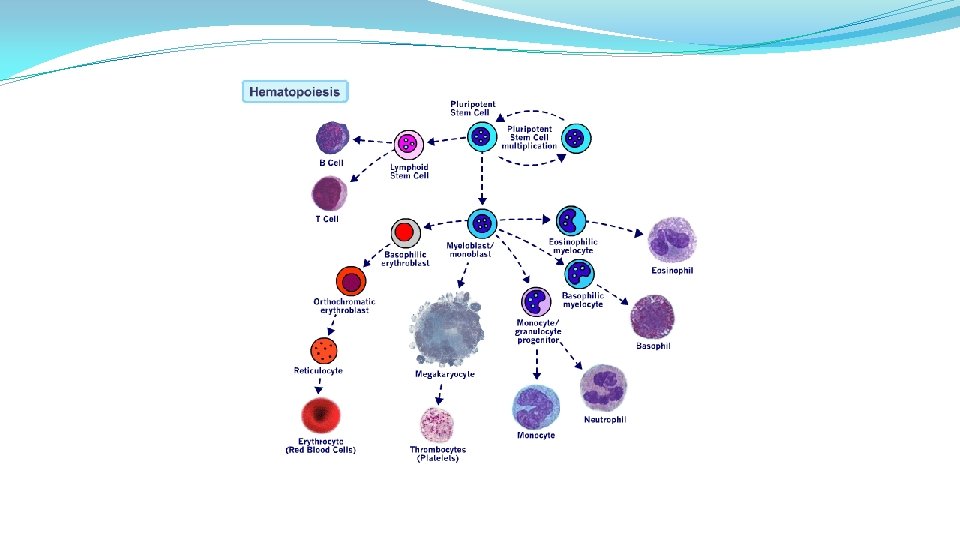
Normal Plasma Cell Function Origins of plasma cells Each mature plasma cell produces thousands if identical Ig every second Normal immunoglobulin molecule containing paired heavy chains with one smaller light chain attached to each

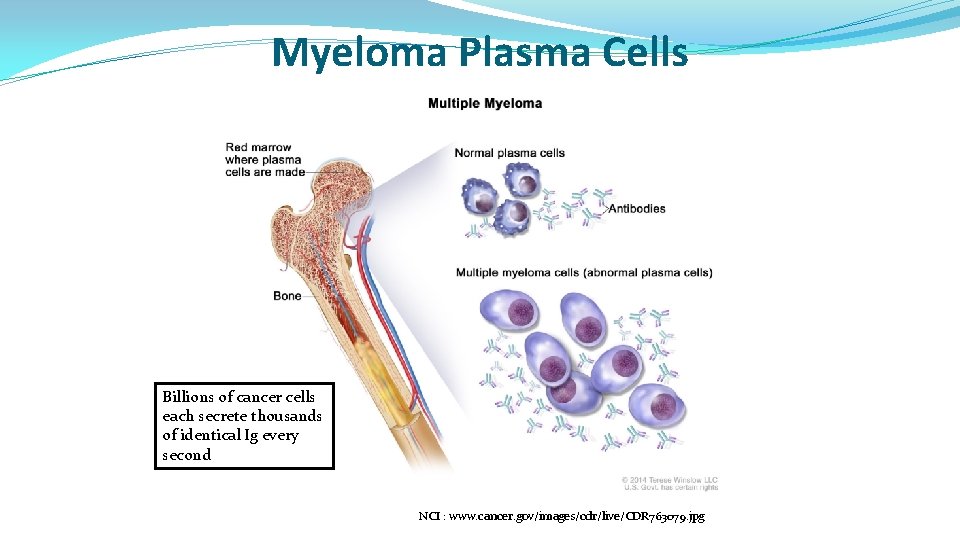
Myeloma Plasma Cells Billions of cancer cells each secrete thousands of identical Ig every second NCI : www. cancer. gov/images/cdr/live/CDR 763079. jpg

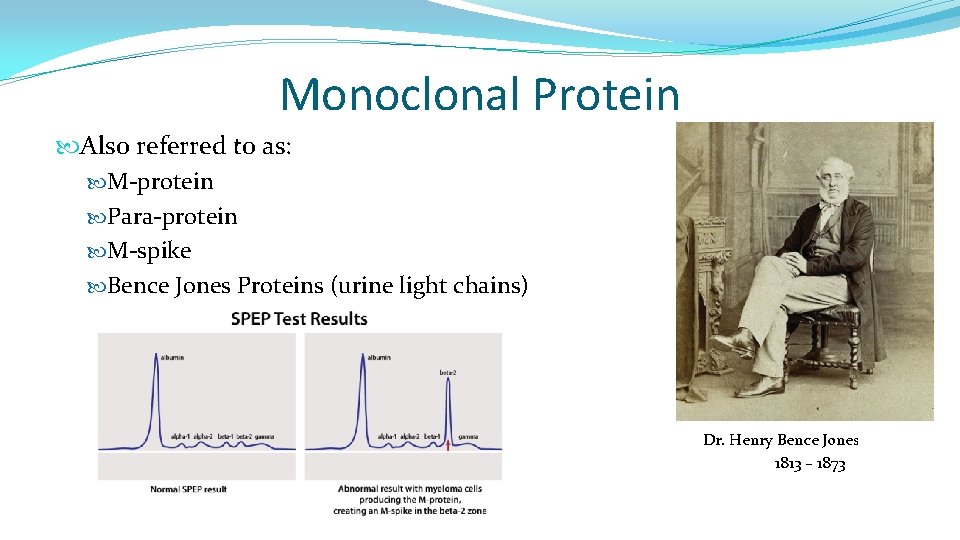
Monoclonal Protein Also referred to as: M-protein Para-protein M-spike Bence Jones Proteins (urine light chains) Dr. Henry Bence Jones 1813 – 1873


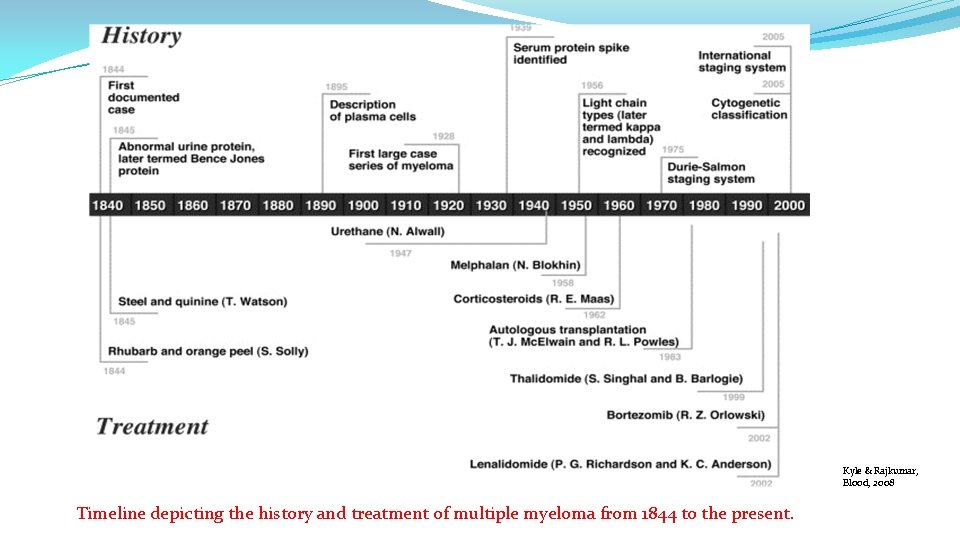
Kyle & Rajkumar, Blood, 2008 Timeline depicting the history and treatment of multiple myeloma from 1844 to the present.




Bone Destruction Types of bone cells: Osteoblasts – make bone Osteoclasts – break down & remodel bone as bones grow or if there is stress on the skeleton In normal adult bone, the activity of both of these types of cells is balanced so that bones do nto weaken or keep getting bigger


Renal Impairment Common – 20 -25 % of patients at diagnosis - up to 50% at some time during disease course Approx. half of these will have some degree of persistent renal impairment, with 2 -12% requiring dialysis Cause: damage to renal tubules by free light chains (cast nephropathy or “myeloma kidney”) Other factors can also contribute to this damage

Standard Therapy for Newly Diagnosed Multiple Myeloma

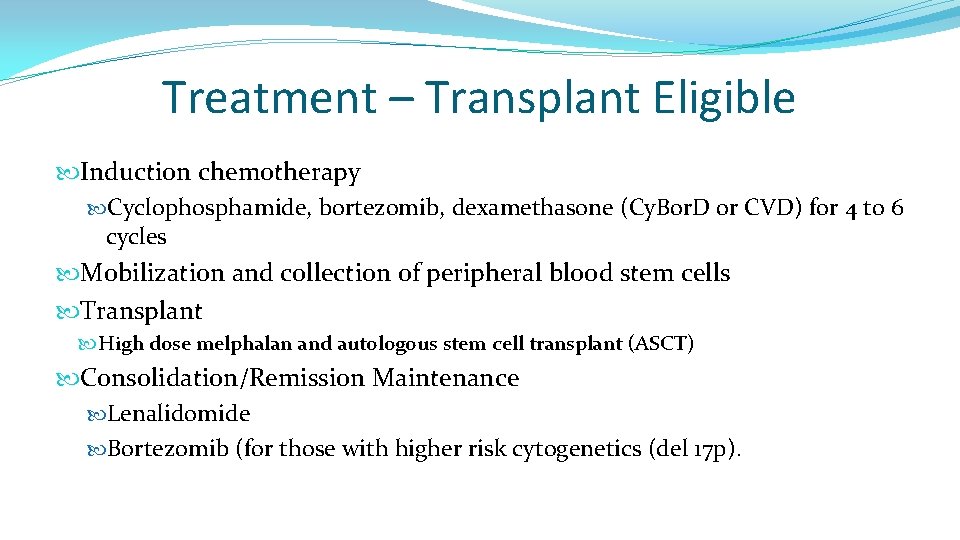
Treatment – Transplant Eligible Induction chemotherapy Cyclophosphamide, bortezomib, dexamethasone (Cy. Bor. D or CVD) for 4 to 6 cycles Mobilization and collection of peripheral blood stem cells Transplant High dose melphalan and autologous stem cell transplant (ASCT) Consolidation/Remission Maintenance Lenalidomide Bortezomib (for those with higher risk cytogenetics (del 17 p).

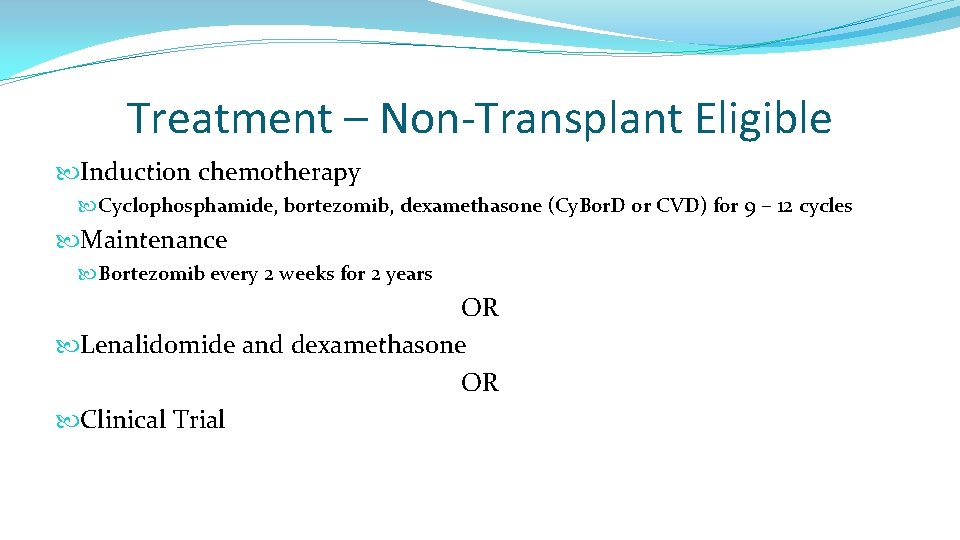
Treatment – Non-Transplant Eligible Induction chemotherapy Cyclophosphamide, bortezomib, dexamethasone (Cy. Bor. D or CVD) for 9 – 12 cycles Maintenance Bortezomib every 2 weeks for 2 years OR Lenalidomide and dexamethasone OR Clinical Trial

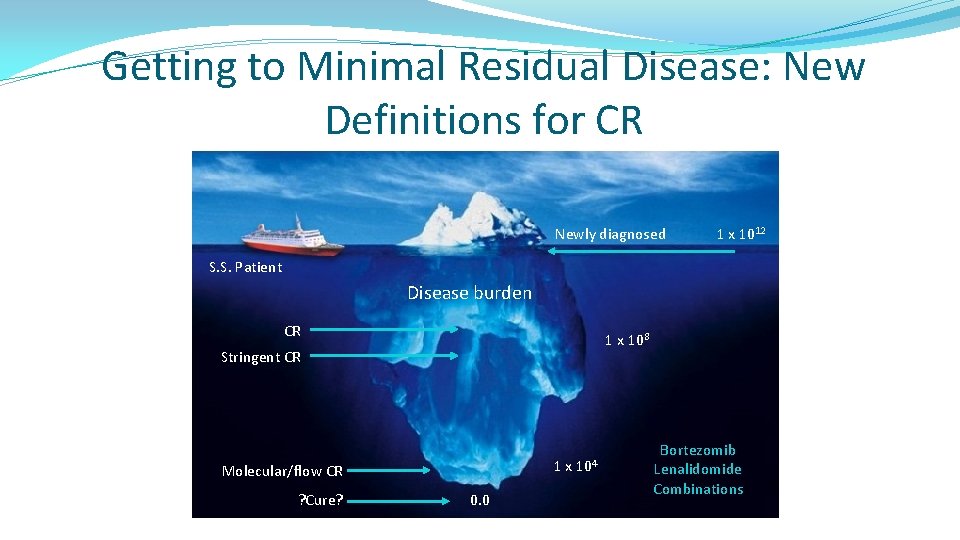
Getting to Minimal Residual Disease: New Definitions for CR Newly diagnosed 1 x 1012 S. S. Patient Disease burden CR 1 x 108 Stringent CR 1 x Molecular/flow CR ? Cure? 0. 0 104 Bortezomib Lenalidomide Combinations



Therapy for Relapsed Disease

MEDICATIONS AVAILABLE TO TREAT MYELOMA Proteasome Inhibitors (Pls) Monoclonal Antibodies Steroids • Bortezomib (s/c) • Daratumumab (iv) • Dexamethasone (po or iv) • Prednisone (po) • Carfilzomib (iv) • Ixazomib (oral) Alkylating Agents • Cyclophosphamide (po or iv) Transplant Immunomodulatory agents (IMi. Ds) • Melphalan (po or iv) • Autologous stem cell • Thalidomide (po) • Lenalidomide (po) • Other Chemotherapy Agents • Pomalidomide (po) • Doxorubicin (iv) • Etoposide (po or iv) • Cisplatin (iv) Agarwal A et al. Clinical Lymphoma Myeloma and Leukemia 2016; http: //dx. doi. org/10. 1016/j. clml. 2016. 11. 010 23

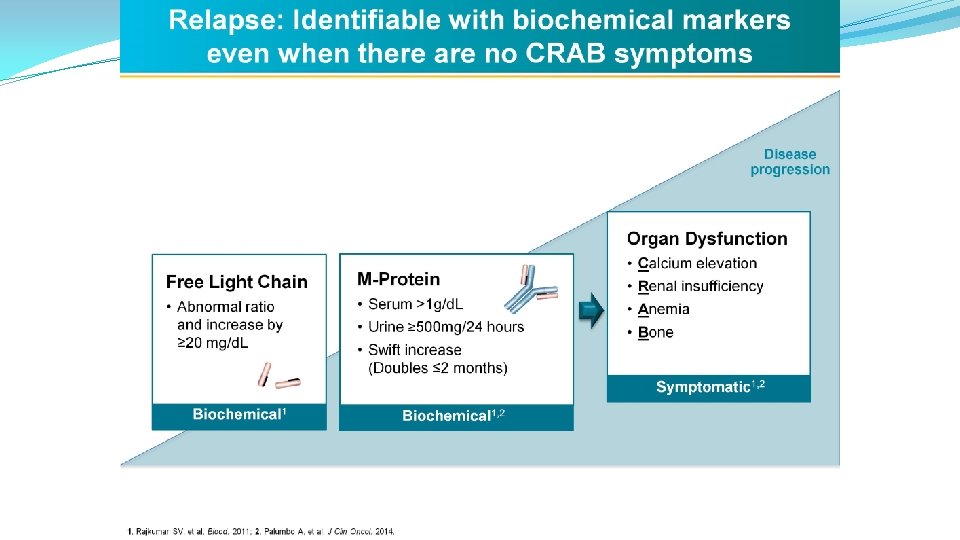
RELAPSED / REFRACTORY MULTIPLE MYELOMA MANAGEMENT • When to treat? • Treatment indicated when patients develop symptomatic relapse, a rapidly rising monoclonal protein level, or extramedullary disease • General Management: • A patient naïve to an agent (or class of agents) is generally treated with that agent • A second transplant can be considered in selected patients if the benefit from the first exceeds 18 -24 months • A patient with relapsed myeloma who has not previously undergone ASCT, can be considered for high-dose therapy (if they can tolerate high-dose therapy) • A patient who responded to a particular doublet with previous duration of response (DOR) of ≥ 6– 9 months can be retreated at relapse with similar agents • Consider adding an alkylator to a doublet regimen if the desired response is not seen • Duration of therapy: • Determined by clinical context • Treatments: • Combine treatments with different mechanisms of action • Depends on previous therapies and response durations, marrow reserve, and comorbidities Laubach L, et al. Leukemia 2016; 30: 1005– 17; doi: 10. 1038/leu. 2015. 356 Nooka AK, et al. Blood 2015; 125(20): 3085– 99. 24

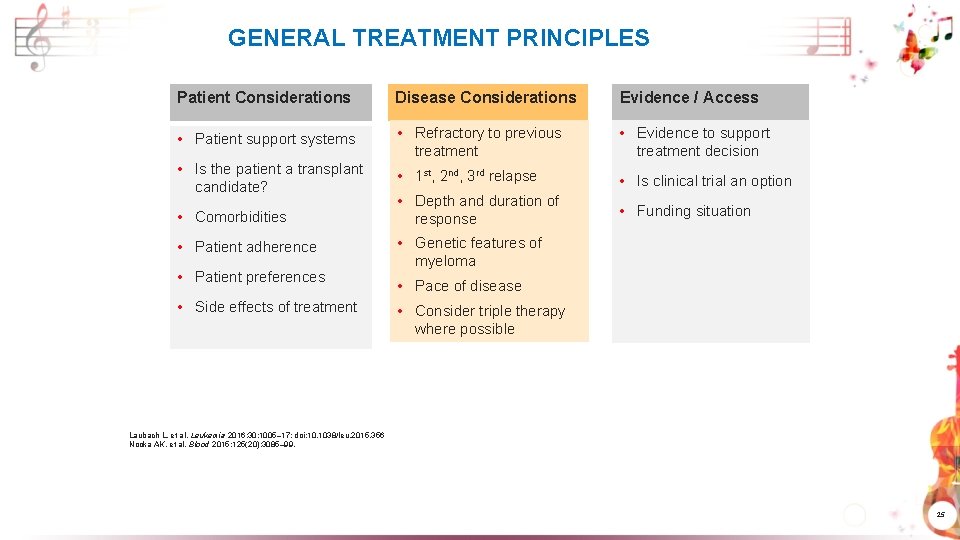
GENERAL TREATMENT PRINCIPLES Patient Considerations Disease Considerations Evidence / Access • Patient support systems • Refractory to previous treatment • Evidence to support treatment decision • Is the patient a transplant candidate? • 1 st, 2 nd, 3 rd relapse • Is clinical trial an option • Comorbidities • Depth and duration of response • Funding situation • Patient adherence • Genetic features of myeloma • Patient preferences • Side effects of treatment • Pace of disease • Consider triple therapy where possible Laubach L, et al. Leukemia 2016; 30: 1005– 17; doi: 10. 1038/leu. 2015. 356 Nooka AK, et al. Blood 2015; 125(20): 3085– 99. 25

Landgren, Medscape Education, 2016

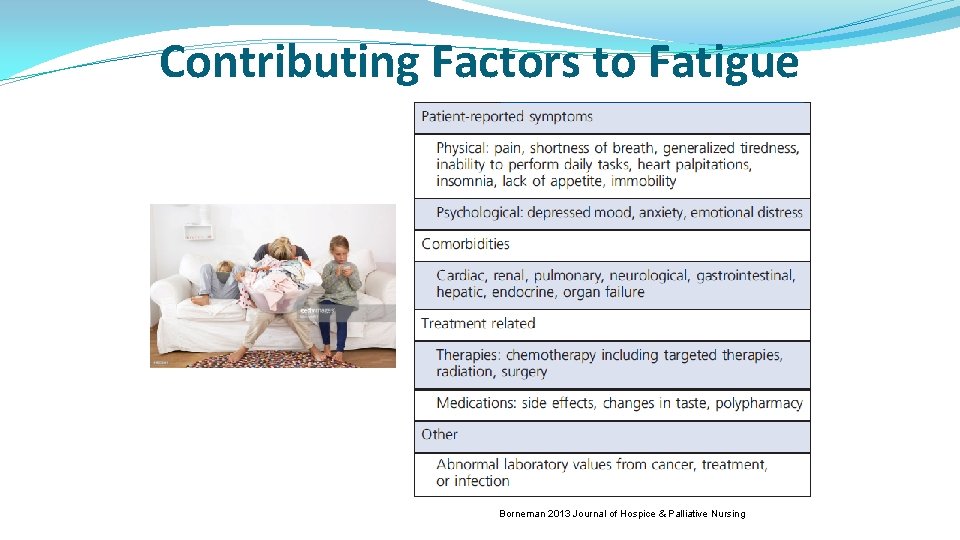
Contributing Factors to Fatigue Borneman 2013 Journal of Hospice & Palliative Nursing


Kurtin, S. , APSHO Regional Lecture Series

SUMMARY Chronic disease with need for continuous therapy & regular hospital visits, blood tests, F/U visits with various HCPs • Drug therapy monitoring: • Medication review for drug interactions • Adjust regimens as needed for renal dysfunction • Adherence: drug calendars & patient buy-in for continuous therapy • Ensure proper monitoring of blood counts requiring dose adjustments • Patient education: • Educate patients on adverse events and strategies for managing them • Education on how to take multi-drug treatments properly • Reinforce adherence with oral regimens • Supportive care: • Nausea/vomiting, anemia, pain management, etc • Prophylactic anticoagulation when required, monitoring and adjustment of doses • Viral prophylaxis • Bisphonates • Monitor for infection Ashjian E, Redic K. J Oncol Pharm Practice 2016; 22(2): 289 -302. 30

THANK YOU
 Level of care primary secondary tertiary
Level of care primary secondary tertiary Crab criteria multiple myeloma
Crab criteria multiple myeloma Mgus
Mgus Myeloma uk forum
Myeloma uk forum Vtd protocol multiple myeloma
Vtd protocol multiple myeloma European myeloma network
European myeloma network Waldenstrom's
Waldenstrom's Daratumumab macmillan
Daratumumab macmillan Rulo formasyonu yapan hastalıklar
Rulo formasyonu yapan hastalıklar Waldenstrom macroglobulinemia vs multiple myeloma
Waldenstrom macroglobulinemia vs multiple myeloma Myeloma
Myeloma Kpd multiple myeloma
Kpd multiple myeloma Mayo clinic multiple myeloma
Mayo clinic multiple myeloma Pritesh patel md
Pritesh patel md Anita waldmann
Anita waldmann Factors determining service standards
Factors determining service standards Care certificate and code of conduct standards include
Care certificate and code of conduct standards include Managed care 101
Managed care 101 L 101: introduction to health care leadership
L 101: introduction to health care leadership Managed care 101
Managed care 101 Health care 101
Health care 101 Qi 101: introduction to health care improvement
Qi 101: introduction to health care improvement Amcp-101
Amcp-101 Iso 22301 utbildning
Iso 22301 utbildning Typiska novell drag
Typiska novell drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Ekologiskt fotavtryck
Ekologiskt fotavtryck Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Underlag för särskild löneskatt på pensionskostnader
Underlag för särskild löneskatt på pensionskostnader Tidbok yrkesförare
Tidbok yrkesförare Anatomi organ reproduksi
Anatomi organ reproduksi