CHAPTER 7 PERIODIC PROPERTIES OF THE ELEMENTS Prof
















































- Slides: 102

CHAPTER 7 – PERIODIC PROPERTIES OF THE ELEMENTS Prof. Dr. Nizam M. El-Ashgar Chemistry Department, IUG

DEVELOPMENT OF THE PERIODIC TABLE The periodic nature of the table arises from the repeating patterns in the electron configurations of the elements. Elements in the same column of the table contain the same number of valence electrons.

MENDELEEV’S TABLE The number of known elements in 1800 was 31, but had increased to 63 by 1865. In 1869 Mendeleev and Lothar Meyer published almost identical classification schemes. Both noticed that similar chemical properties recurred when elements were placed in order of increasing atomic weight. Mendeleev advanced his ideas more vigorously. Mendeleev created the “first” periodic table by arranging the elements with increasing atomic weight. Mendeleev leave blank spaces and predict the existence of yet undiscovered elements based on their location on his periodic table.

Group 4 b Group 5 b Group 6 b Missing Element =eka-silicon Group 1 b Group 2 b Group 3 Group 4 Group 5 Group 6 Group 7 Group 1 Group 2

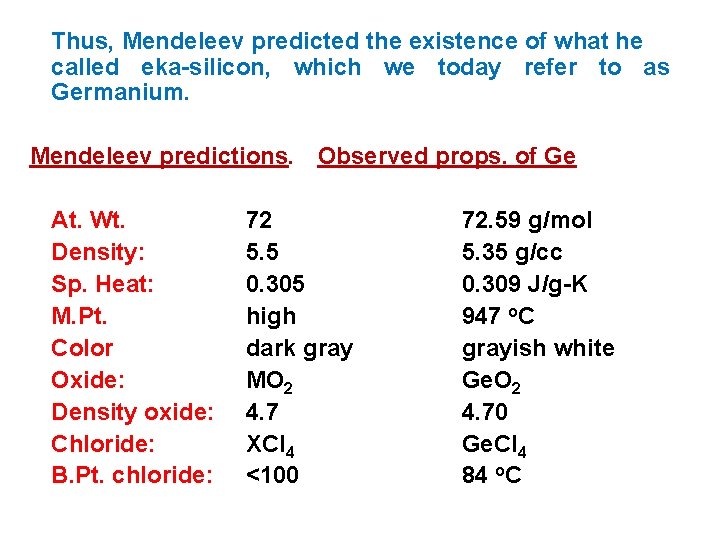
Thus, Mendeleev predicted the existence of what he called eka-silicon, which we today refer to as Germanium. Mendeleev predictions. Observed props. of Ge At. Wt. Density: Sp. Heat: M. Pt. Color Oxide: Density oxide: Chloride: B. Pt. chloride: 72 5. 5 0. 305 high dark gray MO 2 4. 7 XCl 4 <100 72. 59 g/mol 5. 35 g/cc 0. 309 J/g-K 947 o. C grayish white Ge. O 2 4. 70 Ge. Cl 4 84 o. C

PROPERTIES AND ELECTRON CONFIGURATION The properties of the elements follow a periodic pattern. Elements in the same column have similar properties. The elements in a period show a pattern that repeats. The quantum-mechanical model explains this because the number of valence electrons and the types of orbitals they occupy are also periodic.

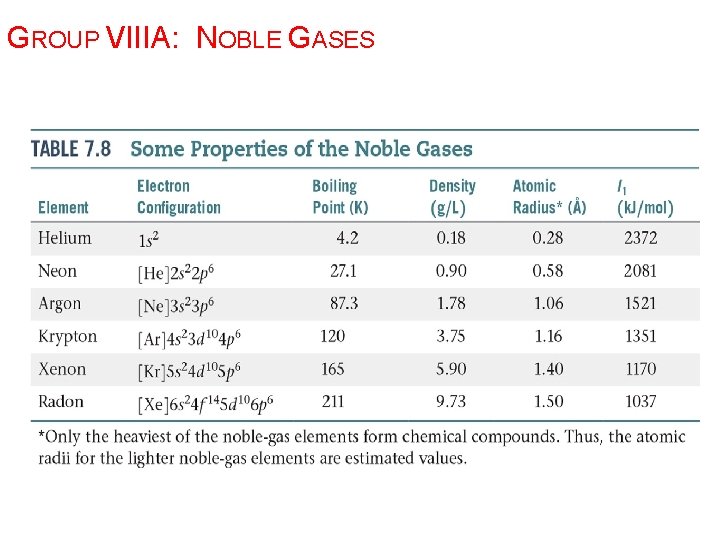
THE NOBLE GAS ELECTRON CONFIGURATION: NS 2 NP 6 The noble gases have eight valence electrons. Except for He, which has only two electrons They are especially nonreactive. He and Ne are practically inert. The reason the noble gases are so nonreactive is that the electron configuration of the noble gases is especially stable: full valence level.

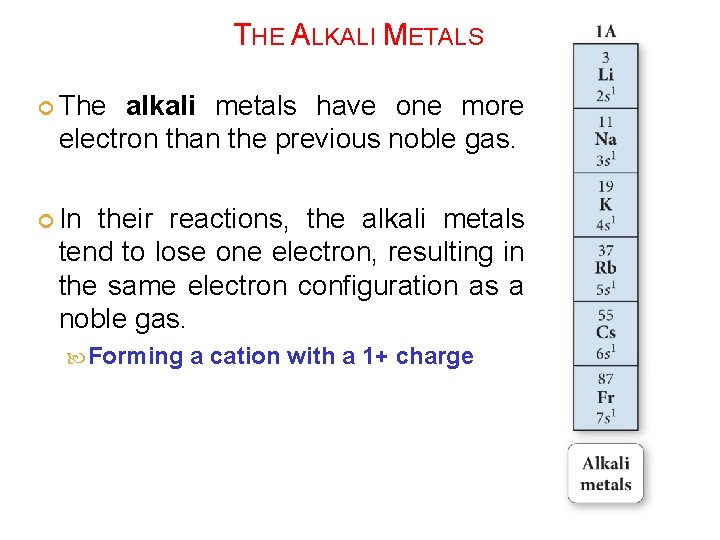
THE ALKALI METALS The alkali metals have one more electron than the previous noble gas. In their reactions, the alkali metals tend to lose one electron, resulting in the same electron configuration as a noble gas. Forming a cation with a 1+ charge

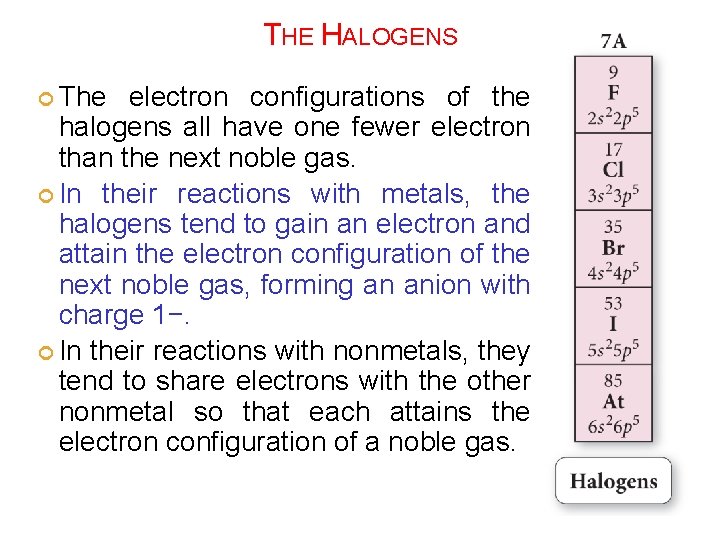
THE HALOGENS The electron configurations of the halogens all have one fewer electron than the next noble gas. In their reactions with metals, the halogens tend to gain an electron and attain the electron configuration of the next noble gas, forming an anion with charge 1−. In their reactions with nonmetals, they tend to share electrons with the other nonmetal so that each attains the electron configuration of a noble gas.

EIGHT VALENCE ELECTRONS Quantum mechanical calculations show that eight valence electrons should result in a very unreactive atom. an atom that is very stable the noble gases have eight valence electrons and are all very stable and unreactive. He has two valence electrons, but that fills its valence shell. Conversely, elements that have either one more or one less electron should be very reactive. the halogen atoms have seven valence electrons and are the most reactive nonmetals. the alkali metals have one more electron than a noble gas atom and are the most reactive group of metals.

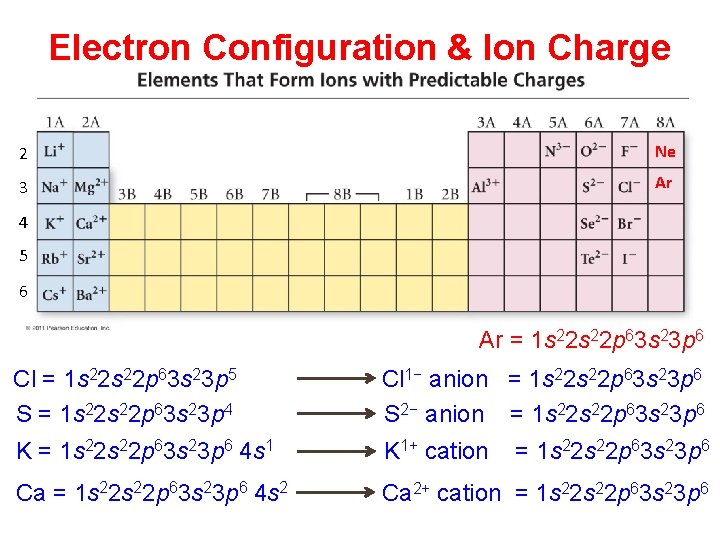
Electron Configuration & Ion Charge 2 Ne 3 Ar 4 5 6 Ar = 1 s 22 p 63 s 23 p 6 Cl = 1 s 22 p 63 s 23 p 5 S = 1 s 22 p 63 s 23 p 4 Cl 1− anion = 1 s 22 p 63 s 23 p 6 S 2− anion = 1 s 22 p 63 s 23 p 6 K = 1 s 22 p 63 s 23 p 6 4 s 1 K 1+ cation = 1 s 22 p 63 s 23 p 6 Ca = 1 s 22 p 63 s 23 p 6 4 s 2 Ca 2+ cation = 1 s 22 p 63 s 23 p 6


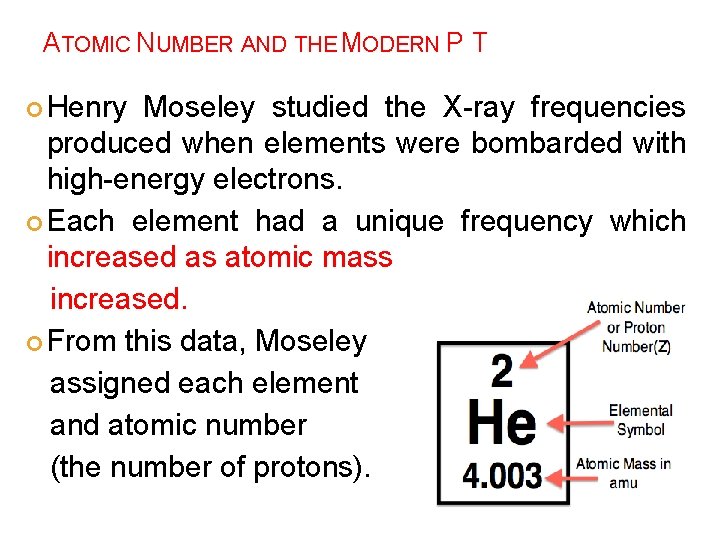
ATOMIC NUMBER AND THE MODERN P T Henry Moseley studied the X-ray frequencies produced when elements were bombarded with high-energy electrons. Each element had a unique frequency which increased as atomic mass increased. From this data, Moseley assigned each element and atomic number (the number of protons).

PERIODICITY Periodicity is the repetitive pattern of a property for elements based on atomic number. The following properties are discussed in this chapter: Sizes of atoms and ions – Ionization energy – Electron affinity – Some group chemical property trends – First, we will discuss a fundamental property that leads to many nuclear charge. of the trends, effective

EFFECTIVE NUCLEAR CHARGE Since electrons are negatively charged, they are attracted to nuclei, which are positively charged. Many of the properties of atoms depend on their electron configurations and on how strongly their outer electrons are attracted to the nucleus.

ELECTRONS AND THE NUCLEUS The force of attraction between an electron and the nucleus is based on Coulomb’s law and depends on two amounts: 1. The magnitude of the net nuclear charge acting on the electron 2. The average distance between the nucleus and the electron. In large atoms: The electrons in outer shells do not experience the full charge of the nucleus because inner shells of electrons “shield” them from the nucleus.

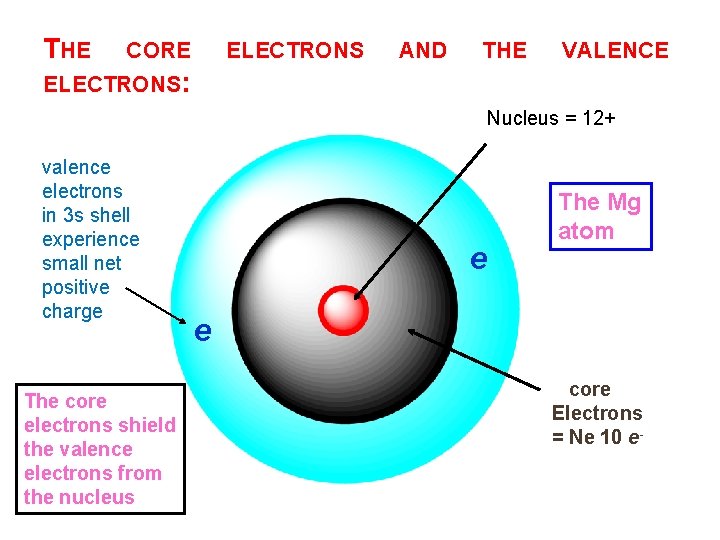
THE CORE ELECTRONS AND THE VALENCE ELECTRONS: Nucleus = 12+ valence electrons in 3 s shell experience small net positive charge The core electrons shield the valence electrons from the nucleus e The Mg atom e core Electrons = Ne 10 e-

SHIELDING • Outer valence electrons are shielded from the nucleus by the core electrons. üScreening or shielding effect üOuter electrons do not effectively screen for each other. • The shielding causes the outer electrons to not experience the full strength of the nuclear charge.

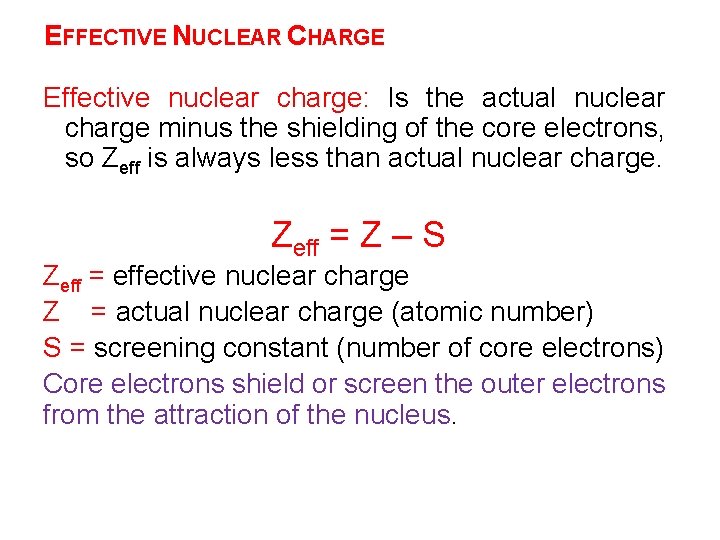
EFFECTIVE NUCLEAR CHARGE Effective nuclear charge: Is the actual nuclear charge minus the shielding of the core electrons, so Zeff is always less than actual nuclear charge. Zeff = Z – S Zeff = effective nuclear charge Z = actual nuclear charge (atomic number) S = screening constant (number of core electrons) Core electrons shield or screen the outer electrons from the attraction of the nucleus.

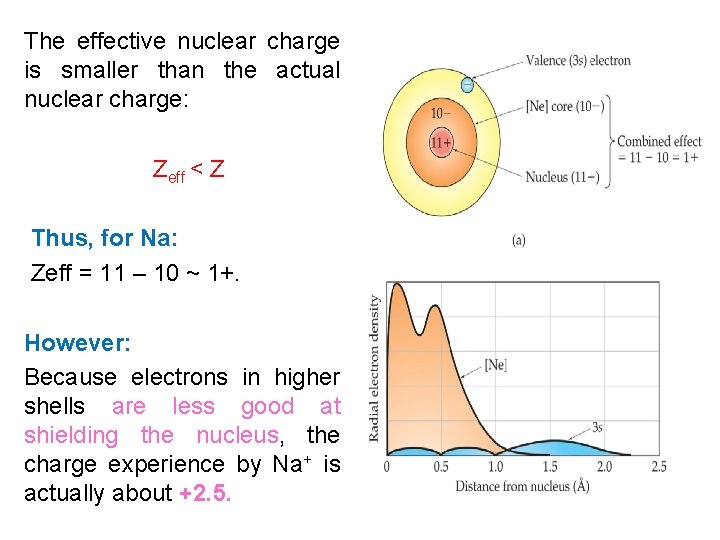
The effective nuclear charge is smaller than the actual nuclear charge: Zeff < Z Thus, for Na: Zeff = 11 – 10 ~ 1+. However: Because electrons in higher shells are less good at shielding the nucleus, the charge experience by Na+ is actually about +2. 5.

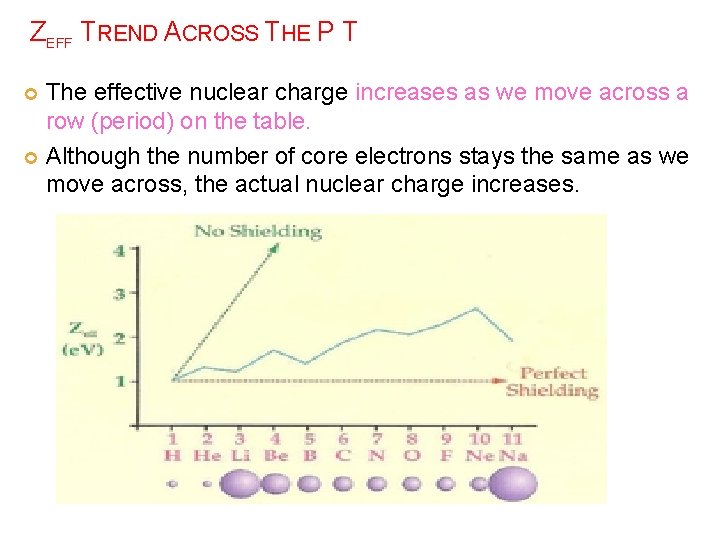
ZEFF TREND ACROSS THE P T The effective nuclear charge increases as we move across a row (period) on the table. Although the number of core electrons stays the same as we move across, the actual nuclear charge increases.

ZEFF TREND DOWN THE P T Going down a column, the effective nuclear charge increases slightly. Actual nuclear charge is increases as you move down a group, but the larger electron cores are less able the screen the valence electrons.

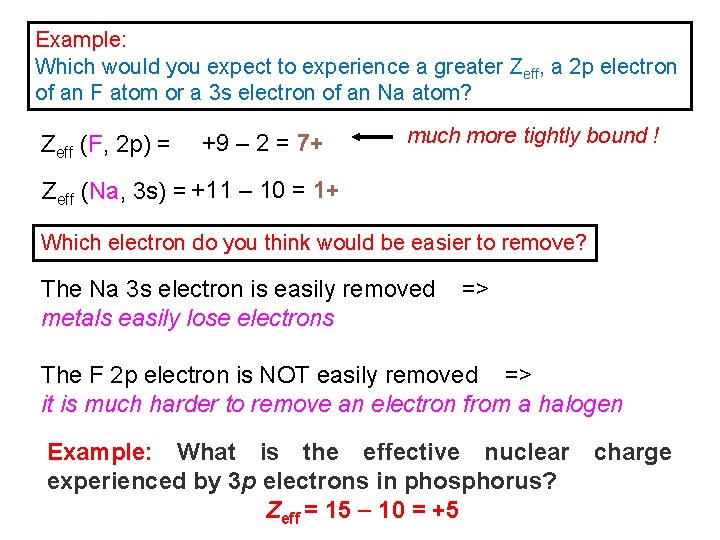
Example: Which would you expect to experience a greater Zeff, a 2 p electron of an F atom or a 3 s electron of an Na atom? Zeff (F, 2 p) = +9 – 2 = 7+ much more tightly bound ! Zeff (Na, 3 s) = +11 – 10 = 1+ Which electron do you think would be easier to remove? The Na 3 s electron is easily removed => metals easily lose electrons The F 2 p electron is NOT easily removed => it is much harder to remove an electron from a halogen Example: What is the effective nuclear charge experienced by 3 p electrons in phosphorus? Zeff = 15 10 = +5


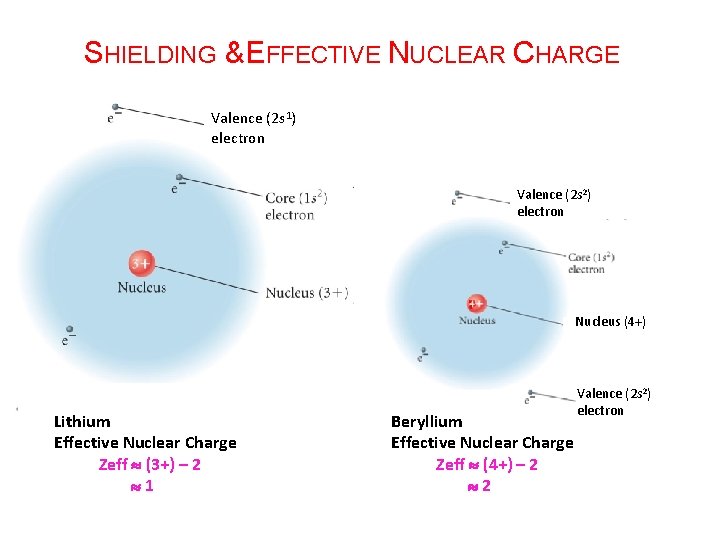
SHIELDING & EFFECTIVE NUCLEAR CHARGE Valence (2 s 1) electron Valence (2 s 2) electron Nucleus (4+) Lithium Effective Nuclear Charge Zeff (3+) – 2 1 Beryllium Effective Nuclear Charge Zeff (4+) – 2 2 Valence (2 s 2) electron

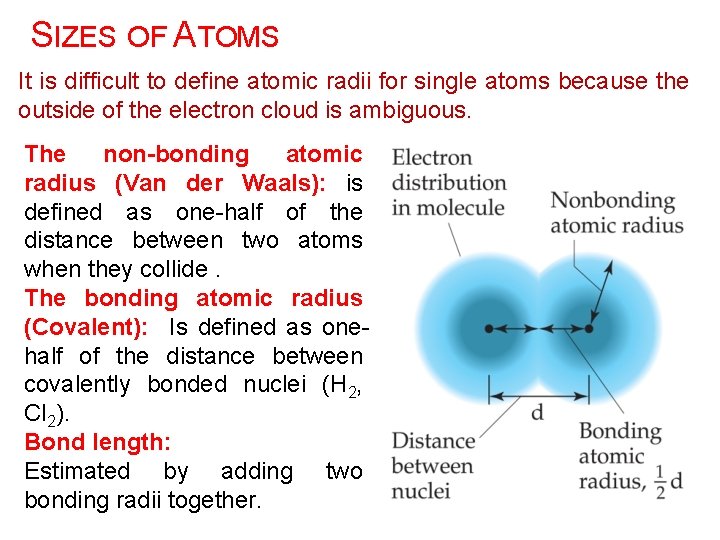
SIZES OF ATOMS It is difficult to define atomic radii for single atoms because the outside of the electron cloud is ambiguous. The non-bonding atomic radius (Van der Waals): is defined as one-half of the distance between two atoms when they collide. The bonding atomic radius (Covalent): Is defined as onehalf of the distance between covalently bonded nuclei (H 2, Cl 2). Bond length: Estimated by adding two bonding radii together.


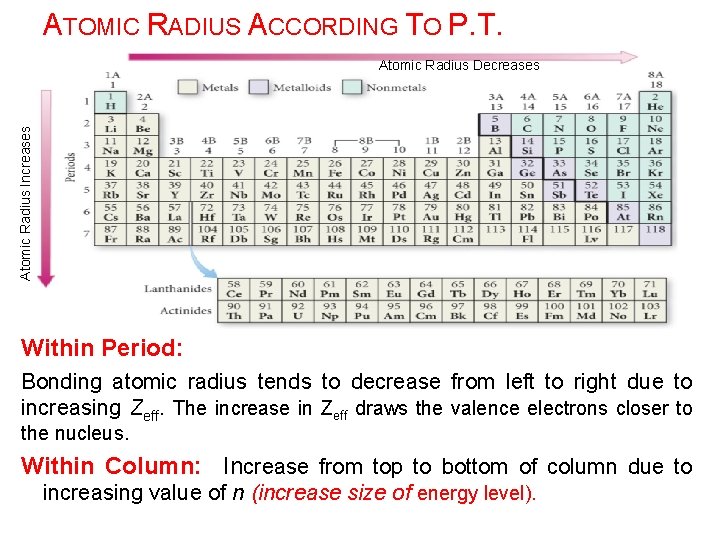
ATOMIC RADIUS ACCORDING TO P. T. Atomic Radius Increases Atomic Radius Decreases Within Period: Bonding atomic radius tends to decrease from left to right due to increasing Zeff. The increase in Zeff draws the valence electrons closer to the nucleus. Within Column: Increase from top to bottom of column due to increasing value of n (increase size of energy level).


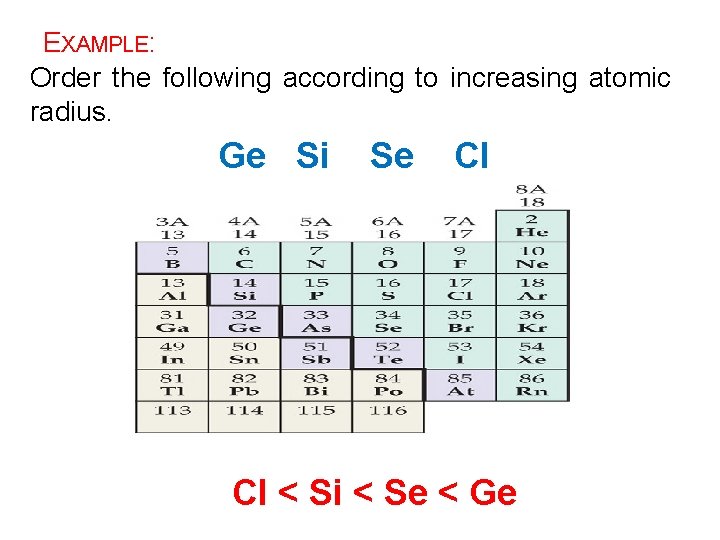
EXAMPLE: Order the following according to increasing atomic radius. Ge Si Se Cl Cl < Si < Se < Ge

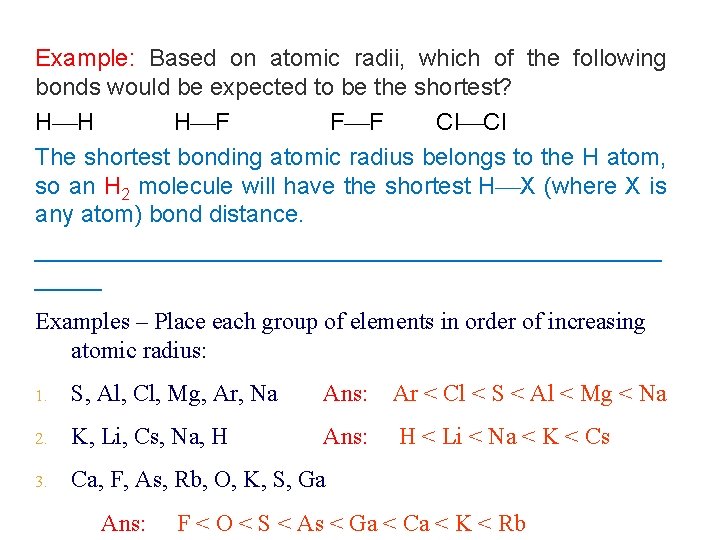
Example: Based on atomic radii, which of the following bonds would be expected to be the shortest? H H H F F F Cl Cl The shortest bonding atomic radius belongs to the H atom, so an H 2 molecule will have the shortest H X (where X is any atom) bond distance. ________________________ Examples – Place each group of elements in order of increasing atomic radius: 1. S, Al, Cl, Mg, Ar, Na Ans: Ar < Cl < S < Al < Mg < Na 2. K, Li, Cs, Na, H Ans: H < Li < Na < K < Cs 3. Ca, F, As, Rb, O, K, S, Ga Ans: F < O < S < As < Ga < Ca < K < Rb

SAMPLE EXERCISE 7. 1 Methyl mercaptan, CH 3 SH, is used to help detect natural gas leaks. Use Figure 7. 6 (text book) to predict the lengths of the C-S, C-H, and S-H bonds in this molecule. Solution: C—S bond length = bonding atomic radius of C + bonding atomic radius of S = 0. 77Å + 1. 02Å = 1. 79Å C—H bond length S—H bond length = 0. 77Å + 0. 37Å = 1. 14Å = 1. 02Å + 0. 37Å = 1. 39Å

PRACTICE EXERCISE Using Figure 7. 6 (in text book), predict which will be greater, the P-Br bond length in PBr 3 or the As-Cl bond length in As. Cl 3. Answer: P—Br

SAMPLE EXERCISE 7. 2 Arrange the following atoms in order of increasing atomic size: P, S, As, and Se. Solution: S < P < Se < As 1. 02 1. 06 1. 19 Practice Exercise Arrange the following atoms in order of increasing atomic radius: Na, Be, and Mg. Answer: Be < Mg < Na

SIZES OF IONS Determined by interatomic distances in ionic compounds Ionic size depends upon: 1 -Nuclear charge. 2 -Number of electrons. 3 -Orbitals in which electrons reside.

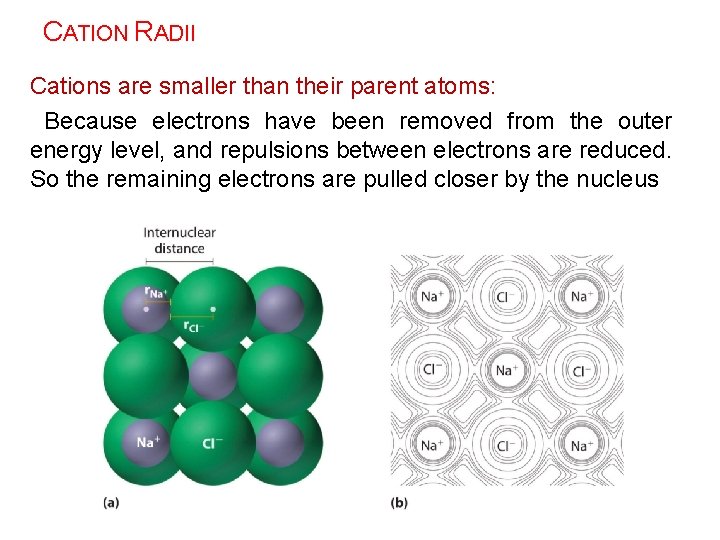
CATION RADII Cations are smaller than their parent atoms: Because electrons have been removed from the outer energy level, and repulsions between electrons are reduced. So the remaining electrons are pulled closer by the nucleus

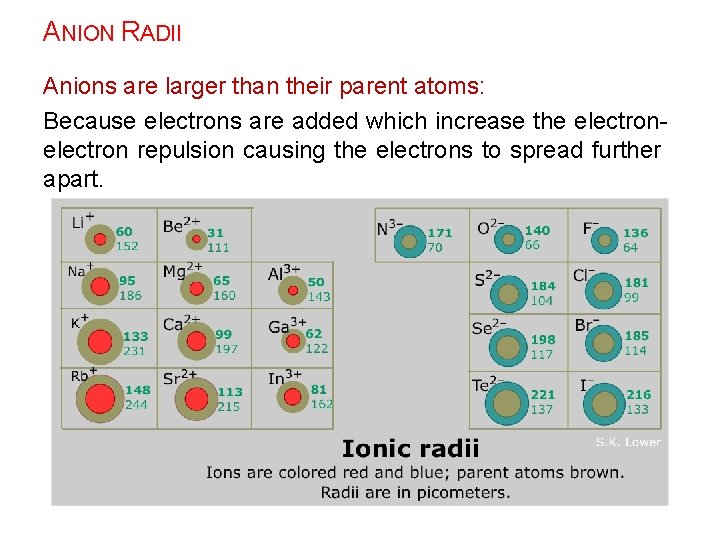
ANION RADII Anions are larger than their parent atoms: Because electrons are added which increase the electron repulsion causing the electrons to spread further apart.

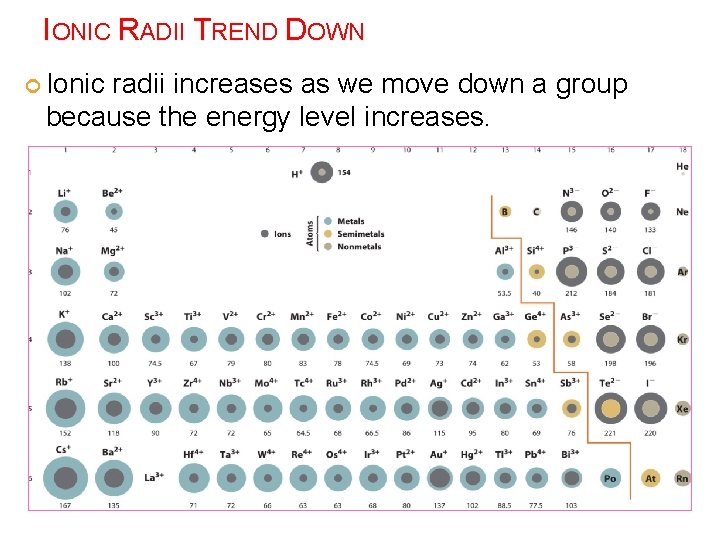
IONIC RADII TREND DOWN Ionic radii increases as we move down a group because the energy level increases.

SAMPLE EXERCISE 7. 3 Arrange these atoms in order of decreasing size: Mg 2+, Ca 2+, and Ca. Solution: Cations are smaller than their parent atoms, and so Ca 2+ < Ca. Because Ca is below Mg in group 2 A, Ca 2+ is larger than Mg 2+. Consequently, Ca > Ca 2+ > Mg 2+. Practice Exercise: Which of the following atoms and ions is largest: S 2 -, S, O 2 -? Answer: S 2 -

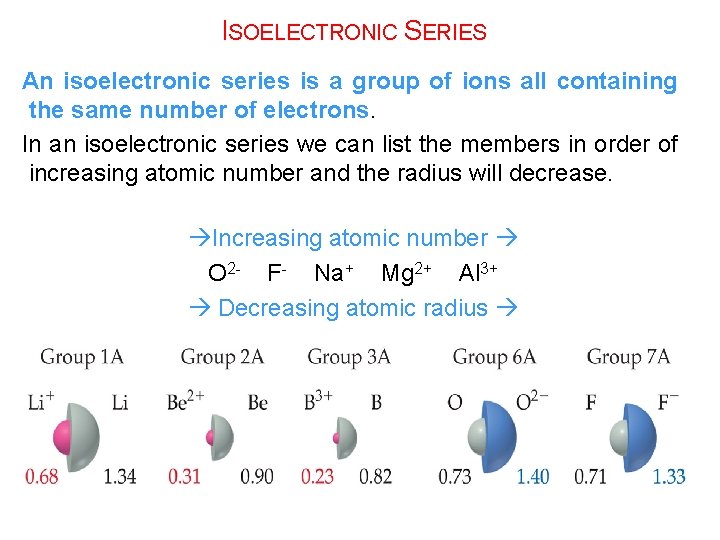
ISOELECTRONIC SERIES An isoelectronic series is a group of ions all containing the same number of electrons. In an isoelectronic series we can list the members in order of increasing atomic number and the radius will decrease. Increasing atomic number O 2 - F- Na+ Mg 2+ Al 3+ Decreasing atomic radius

ISOELECTRONIC SERIES: EXAMPLES

Example: Order the following according to increasing atomic/ionic radius: N 3 - Li+ C O 2 Answer: Li+ < C < O 2 - < N 3 -

SAMPLE EXERCISE 7. 4 Arrange the ions K+, Cl-, Ca 2+, and S 2 - in order of decreasing size. All ions having 18 electrons. Size decreases as nuclear charge (atomic number) increases. The atomic numbers of the ions are: S 16 Cl 17 K 19 Ca 20 Thus, the ions decrease in size in the order S 2– > Cl– > K+ > Ca 2+ _______________________________________ Practice Exercise: Which of the following ions is largest, Rb+, Sr 2+, or Y 3+? Answer: Rb+

Examples : Choose the larger species in each case: 1. Na or Na+ 2. Br or Br 3. N or N 34. O- or O 25. Mg 2+ or Sr 2+ 6. Mg 2+ or O 27. Fe 2+ or Fe 3+

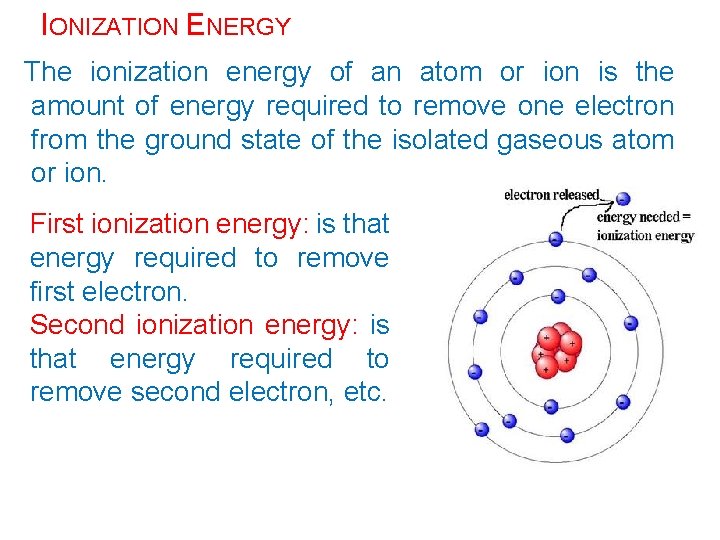
IONIZATION ENERGY The ionization energy of an atom or ion is the amount of energy required to remove one electron from the ground state of the isolated gaseous atom or ion. First ionization energy: is that energy required to remove first electron. Second ionization energy: is that energy required to remove second electron, etc.

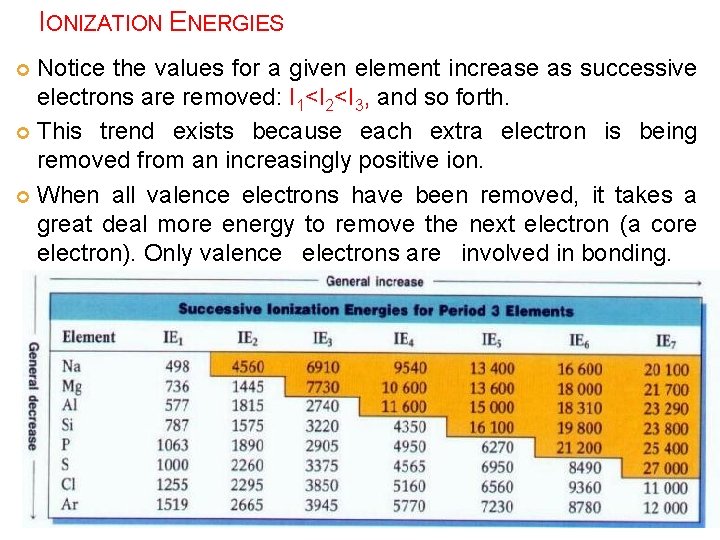
IONIZATION ENERGIES Notice the values for a given element increase as successive electrons are removed: I 1<I 2<I 3, and so forth. This trend exists because each extra electron is being removed from an increasingly positive ion. When all valence electrons have been removed, it takes a great deal more energy to remove the next electron (a core electron). Only valence electrons are involved in bonding.

SAMPLE EXERCISE 7. 5 Based on the locations of sodium, calcium, and sulfur, predict the one with the largest second ionization energy. Na (Z = 11) [Ne] 3 s 1 Highest second IE. S (Z = 16) [Ne] 3 s 2 3 p 4 Ca (Z = 20) [Ar] 4 s 2 (IE 2) of the respective elements: Ca (1, 145 k. J/mol) < S (2, 252 k. J/mol) < Na (4, 562 k. J/mol). ____________________________________ Practice Exercise: Which will have the greater third ionization energy, Ca or S? Answer: Ca

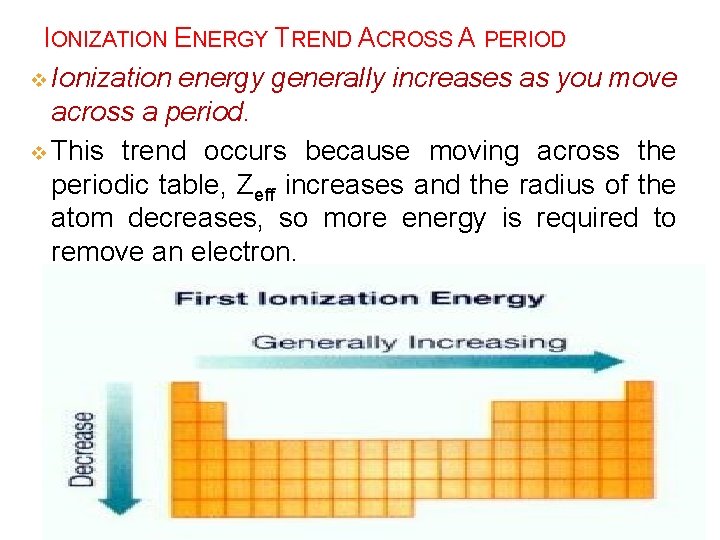
IONIZATION ENERGY TREND ACROSS A PERIOD v Ionization energy generally increases as you move across a period. v This trend occurs because moving across the periodic table, Zeff increases and the radius of the atom decreases, so more energy is required to remove an electron.

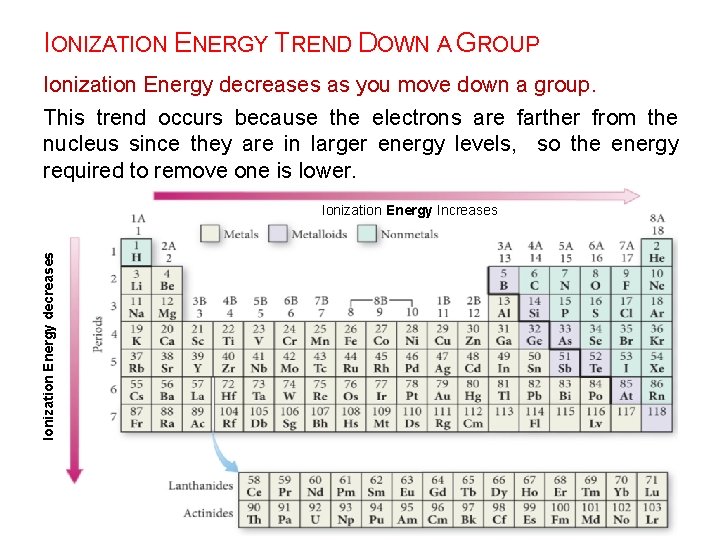
IONIZATION ENERGY TREND DOWN A GROUP Ionization Energy decreases as you move down a group. This trend occurs because the electrons are farther from the nucleus since they are in larger energy levels, so the energy required to remove one is lower. Ionization Energy decreases Ionization Energy Increases

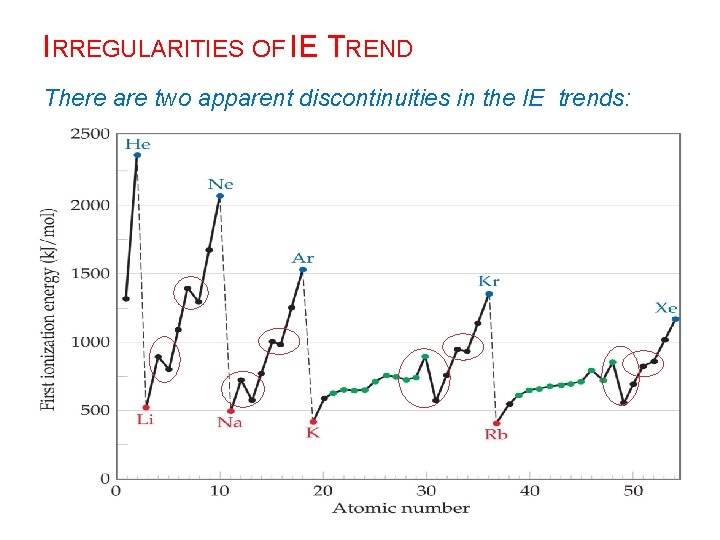
IRREGULARITIES OF IE TREND There are two apparent discontinuities in the IE trends:

The first occurs between Groups IIA and IIIA: Electron removed from p-orbital rather than s-orbital Ø Electron farther from nucleus Ø Small amount of repulsion by s electrons. GIIA GIIIA Be (Z=4) B (Z=5) [He] 2 s 2 [He] 2 s 2 2 p 1 Explanation: The outer np 1 electron is in a p orbital is slightly further away form the nucleus and experiences slightly more shielding from the full ns 2 sub-shell in addition to the inner complete shells.

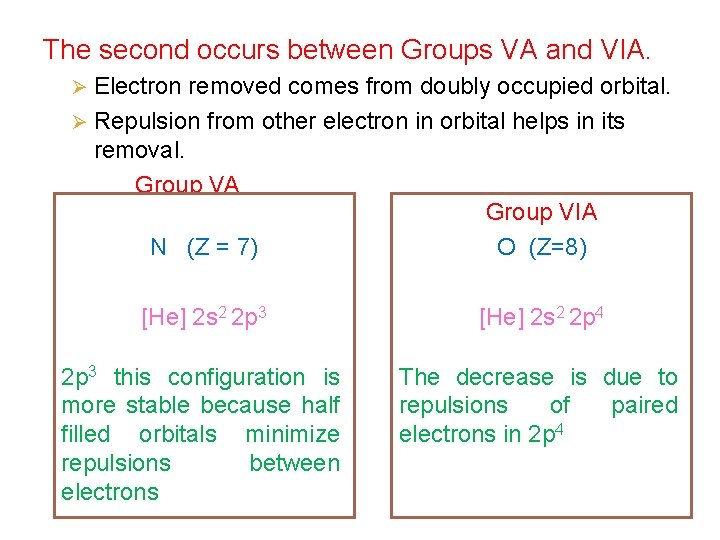
The second occurs between Groups VA and VIA. Ø Electron removed comes from doubly occupied orbital. Ø Repulsion from other electron in orbital helps in its removal. Group VA N (Z = 7) Group VIA O (Z=8) [He] 2 s 2 2 p 3 [He] 2 s 2 2 p 4 2 p 3 this configuration is more stable because half filled orbitals minimize repulsions between electrons The decrease is due to repulsions of paired electrons in 2 p 4

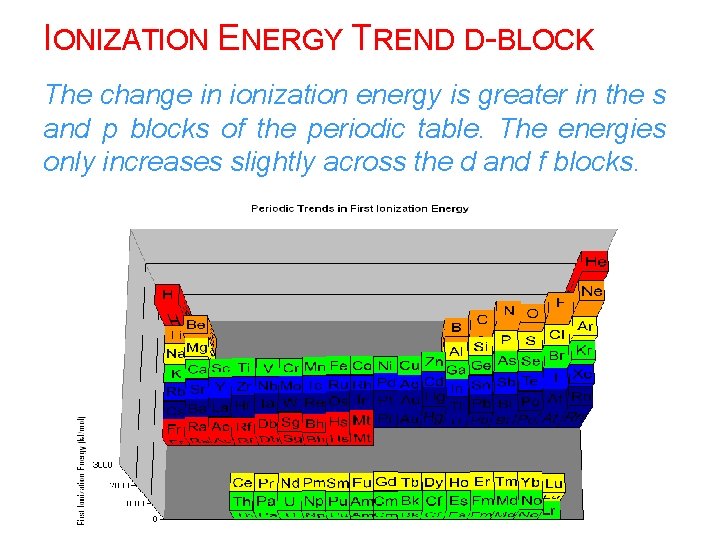
IONIZATION ENERGY TREND D-BLOCK The change in ionization energy is greater in the s and p blocks of the periodic table. The energies only increases slightly across the d and f blocks.


Examples: Put each set in order of increasing first ionization energy: 1. P, Cl, Al, Na, S, Mg Na < Al < Mg < S < P < Cl 2. Ca, Be, Ba, Mg, Sr Ba < Sr < Ca < Mg < Be 3. Ca, F, As, Rb, O, K, S, Ga Rb < K < Ga < Ca < As < S < O < F

SAMPLE EXERCISE 7. 6 Arrange the following atoms in order of increasing first ionization energy: Ne, Na, P, Ar, K. Answer: K < Na < P < Ar < Ne ____________________________________ Practice Exercise: Which has the lowest first ionization energy, B, Al, C, or Si? Which has the highest ionization energy? Answer: Al lowest, C highest

ELECTRON CONFIGURATION FOR IONS ØWhen electrons are removed from a cation, they are removed from the highest sublevel in the highest principle energy level. Li: [He]2 s 1 becomes Li+: [He] Na: [He]2 s 22 p 6 3 s 1 becomes Na+: [He]2 s 22 p 6 = [Ne]

Ø When electrons are added to an anion, they are added to the last partially filled orbital or the next sublevel. Example F (Z = 9)

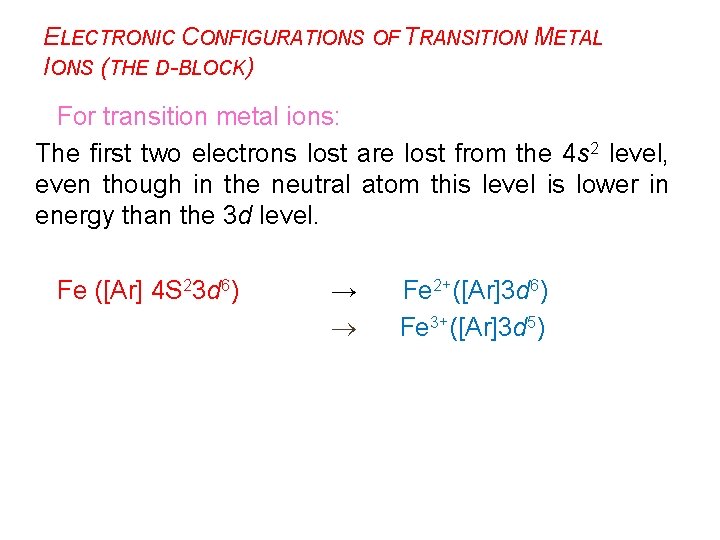
ELECTRONIC CONFIGURATIONS OF TRANSITION METAL IONS (THE D-BLOCK) For transition metal ions: The first two electrons lost are lost from the 4 s 2 level, even though in the neutral atom this level is lower in energy than the 3 d level. Fe ([Ar] 4 S 23 d 6) → Fe 2+([Ar]3 d 6) Fe 3+([Ar]3 d 5)

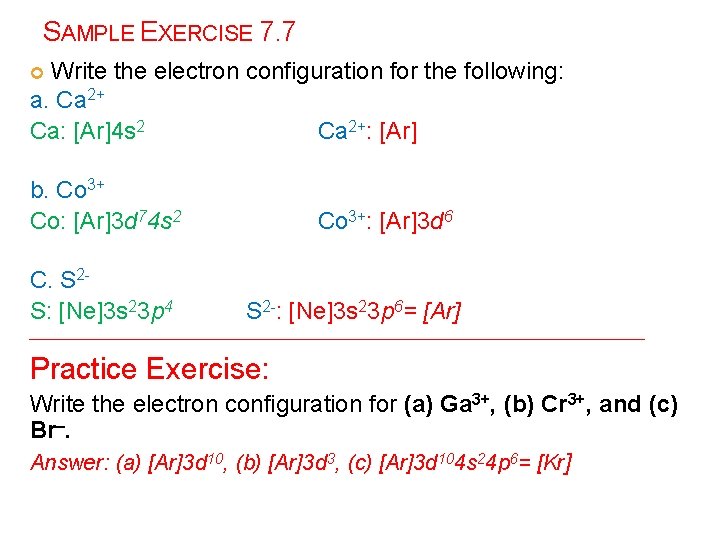
SAMPLE EXERCISE 7. 7 Write the electron configuration for the following: a. Ca 2+ Ca: [Ar]4 s 2 Ca 2+: [Ar] b. Co 3+ Co: [Ar]3 d 74 s 2 C. S 2 S: [Ne]3 s 23 p 4 Co 3+: [Ar]3 d 6 S 2 -: [Ne]3 s 23 p 6= [Ar] ___________________________________ Practice Exercise: Write the electron configuration for (a) Ga 3+, (b) Cr 3+, and (c) Br–. Answer: (a) [Ar]3 d 10, (b) [Ar]3 d 3, (c) [Ar]3 d 104 s 24 p 6= [Kr]

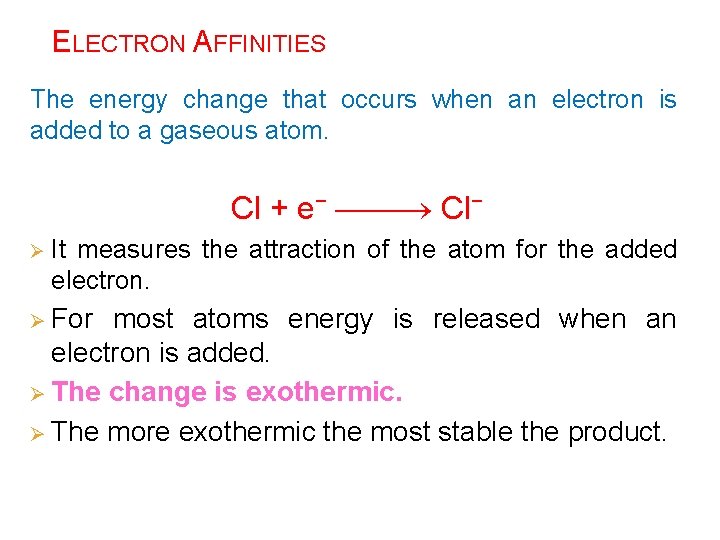
ELECTRON AFFINITIES The energy change that occurs when an electron is added to a gaseous atom. Cl + e− Cl− Ø It measures the attraction of the atom for the added electron. Ø For most atoms energy is released when an electron is added. Ø The change is exothermic. Ø The more exothermic the most stable the product.

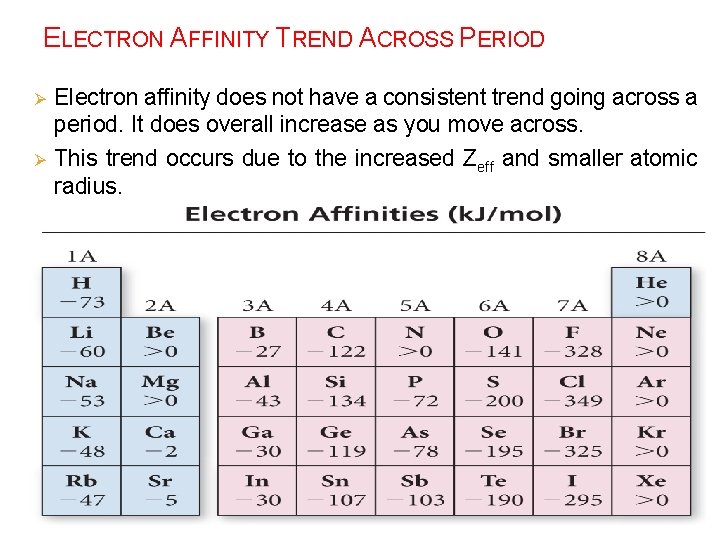
ELECTRON AFFINITY TREND ACROSS PERIOD Ø Ø Electron affinity does not have a consistent trend going across a period. It does overall increase as you move across. This trend occurs due to the increased Zeff and smaller atomic radius.

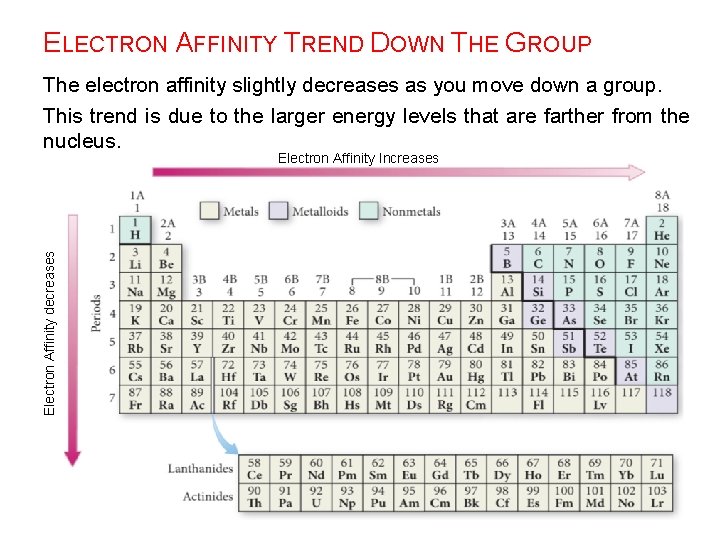
ELECTRON AFFINITY TREND DOWN THE GROUP The electron affinity slightly decreases as you move down a group. This trend is due to the larger energy levels that are farther from the nucleus. Electron Affinity decreases Electron Affinity Increases

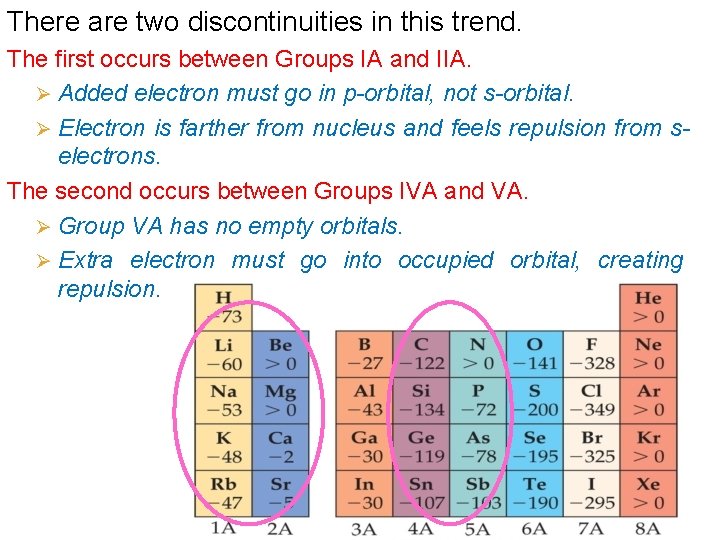
There are two discontinuities in this trend. The first occurs between Groups IA and IIA. Ø Added electron must go in p-orbital, not s-orbital. Ø Electron is farther from nucleus and feels repulsion from selectrons. The second occurs between Groups IVA and VA. Ø Group VA has no empty orbitals. Ø Extra electron must go into occupied orbital, creating repulsion.

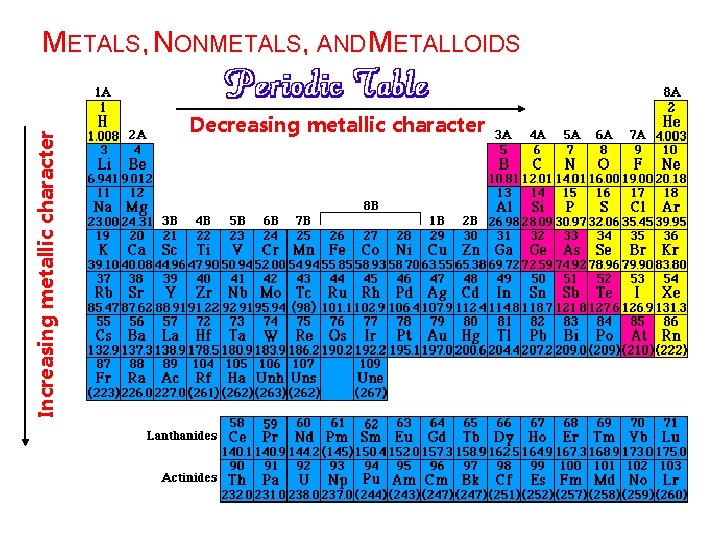
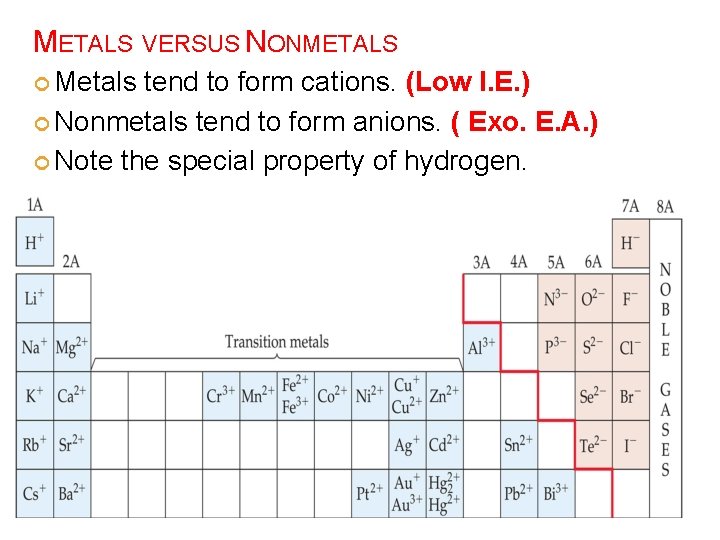
Increasing metallic character METALS, NONMETALS, AND METALLOIDS Decreasing metallic character

METALS VERSUS NONMETALS Metals tend to form cations. (Low I. E. ) Nonmetals tend to form anions. ( Exo. E. A. ) Note the special property of hydrogen.

PROPERTIES OF METALS Ø Ø Ø Ø Solids at room temperature (except Hg) Low ionization energies, so they form cations Metals are oxidized (lost electrons) in reactions Malleable and Ductile Shiny luster Good conductors Form ionic compounds with nonmetals Metal oxides are basic (ex. Na 2 O)

METALLIC TREND Ø Metals react by losing electrons and forming cations. Ø Metallic character increases with the easiness of losing electrons. Ø The trend is opposite to the ionization energy. Ø Decreases across a period and increases down a group.

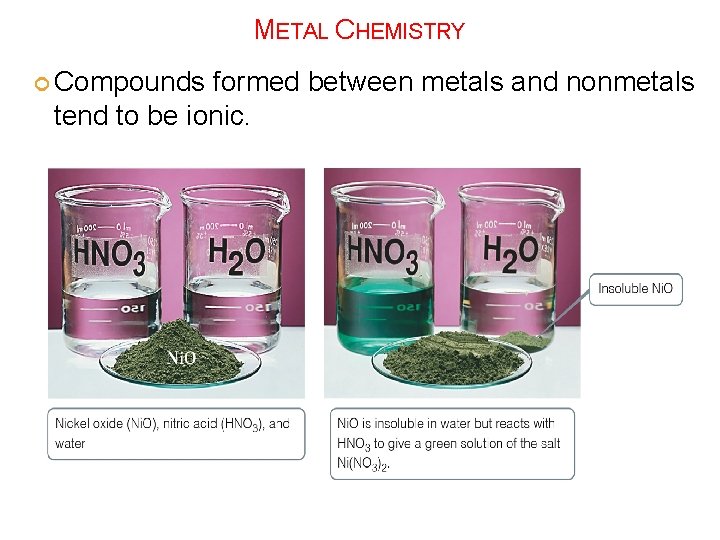
METAL CHEMISTRY Compounds formed between metals and nonmetals tend to be ionic.

SAMPLE EXERCISE 7. 8 Would you expect scandium oxide to be a solid, liquid, or gas at room temperature? b) Because scandium oxide is the oxide of a metal, we would expect it to be an ionic solid. Indeed it is, with the very high melting point of 2485 o. C. b)Write the balanced equation for the reaction of scandium oxide (Sc 3+) with nitric acid. Sc 2 O 3(s) + 6 HNO 3(aq) → 2 Sc(NO 3)3 (aq) + 3 H 2 O(l) a) _____________________________________ Practice Exercise: Write the balance equation for the reaction between copper (II) oxide and sulfuric acid. Answer:

PRACTICE EXERCISE Write the balance equation for the reaction between copper (II) oxide and sulfuric acid. Answer: Cu. O(s) + H 2 SO 4(aq) →Cu. SO 4(aq) + H 2 O(l)

PROPERTIES OF NONMETALS Ø Ø Ø Ø Solid, liquid, or gas (depends on element) High electron affinities, so they form anions Nonmetals are reduced (gain electrons) in reactions Solids are dull, brittle Poor conductors Form molecular compounds with nonmetals Nonmetal oxides are acidic.

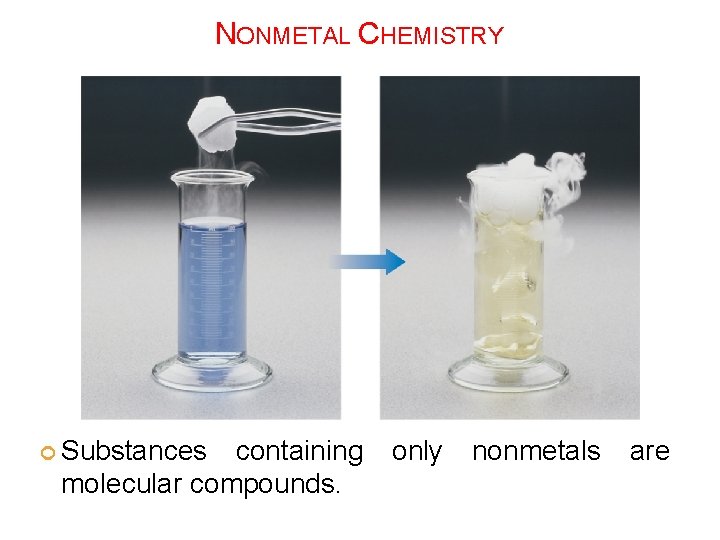
NONMETAL CHEMISTRY Substances containing only nonmetals are molecular compounds.

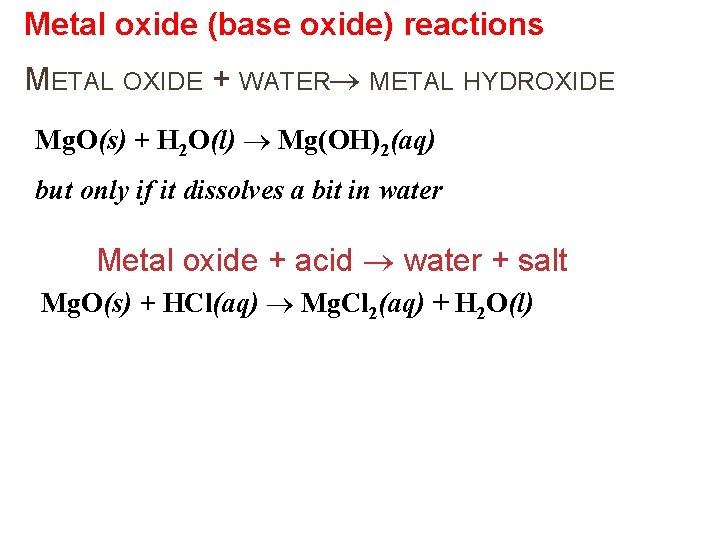
Metal oxide (base oxide) reactions METAL OXIDE + WATER METAL HYDROXIDE Mg. O(s) + H 2 O(l) Mg(OH)2(aq) but only if it dissolves a bit in water Metal oxide + acid water + salt Mg. O(s) + HCl(aq) Mg. Cl 2(aq) + H 2 O(l)

nonmetal oxide (acid oxide) reactions NONMETAL OXIDE + WATER ACID SO 3(g) + H 2 O(l) H 2 SO 4(aq) nonmetal oxide + base water + salt CO 2(g) + 2 Na. OH(aq) Na 2 CO 3(aq) + H 2 O(l)

SAMPLE EXERCISE 7. 9 Write the balanced equation for the reactions of solid selenium dioxide and the following: a) Water. Selenium dioxide is Se. O 2. Its reaction with water is like that of carbon dioxide: Se. O 2(s) + H 2 O(l) → H 2 Se. O 3(aq) b) Aqueous sodium hydroxide. Se. O 2(s) + 2 Na. OH(aq) →Na 2 Se. O 3(aq) + H 2 O(l) _____________________________________ ___ Practice Exercise: Write the balanced equation for the reaction of solid tetraphosphorus hexoxide with water. Answer: P 4 O 6(s) + 6 H 2 O(l) → 4 H 3 PO 3(aq)

METALLOIDS Metalloids have properties intermediate between those of metals and nonmetals. For instance, silicon looks shiny, but is brittle and fairly poor conductor and electrically semiconductors (computer chips)

GROUP TRENDS FOR THE ACTIVE METALS The alkali metals (group 1) and the alkaline earth metals (group 2) are considered the active metals.

PROPERTIES OF ALKALI METALS Ø Ø Ø Soft metallic solids with low melting points. Found only as compounds in nature. Very reactive!!! When bonded with hydrogen, the hydrogen has a -1 charge (hydride). Ex: Li. H React vigorously with water. When bonded with oxygen, they can form the following: a. Oxide (O 2 -) = Li 2 O b. Peroxide (O 22 -) = H 2 O 2 c. Superoxide (O 2 -) = KO 2

ALKALI METALS They have low densities and melting points. They also have low ionization energies.

ALKALI METAL CHEMISTRY Ø Ø Their reactions with water are famously exothermic. They react vigorously with water and have to be stored in mineral oil or kerosene. 2 M (s) + 2 H 2 O (l) 2 MOH (aq) + H 2 (g) https: //youtu. be/m 55 kgy. Ap. Yr. Y

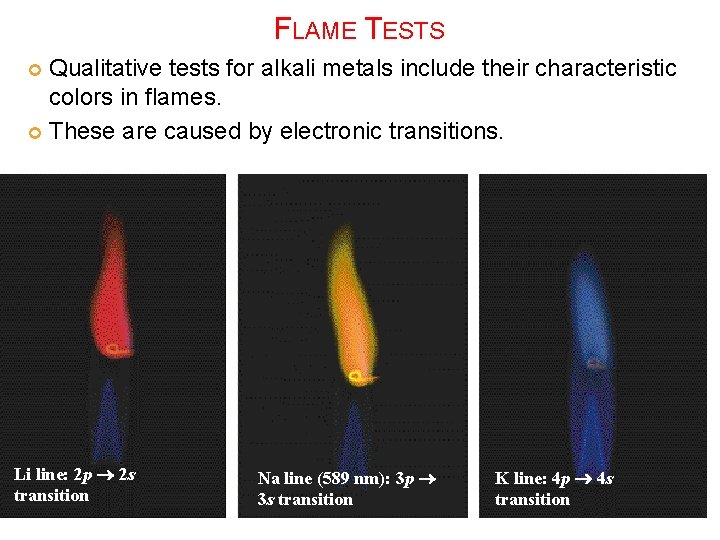
FLAME TESTS Qualitative tests for alkali metals include their characteristic colors in flames. These are caused by electronic transitions. Li line: 2 p 2 s transition Na line (589 nm): 3 p 3 s transition K line: 4 p 4 s transition

ALKALI METALS REACTIONS Alkali metals combine directly with most nonmetals Alkali metals (except Li) react with oxygen to form peroxides. Ø K, Rb, and Cs also form superoxides: K + O 2 KO 2 Ø Alkali metals produce different oxides when reacting with O 2: 4 Li(s) + O 2(g) 2 Li 2 O(s) (oxide) 2 Na(s) + O 2(g) Na 2 O 2(s) (peroxide) K(s) + O 2(g) KO 2(s) (superoxide) Ø

SAMPLE EXERCISE 7. 10 Write a balanced equation that predicts the reaction of cesium metal with the following: a. Cl 2 b. H 2 O c. H 2

PRACTICE EXERCISE Write a balanced equation for the reaction between potassium metal and sulfur. Answer: 2 K(s) + S(s) →K 2 S(s)

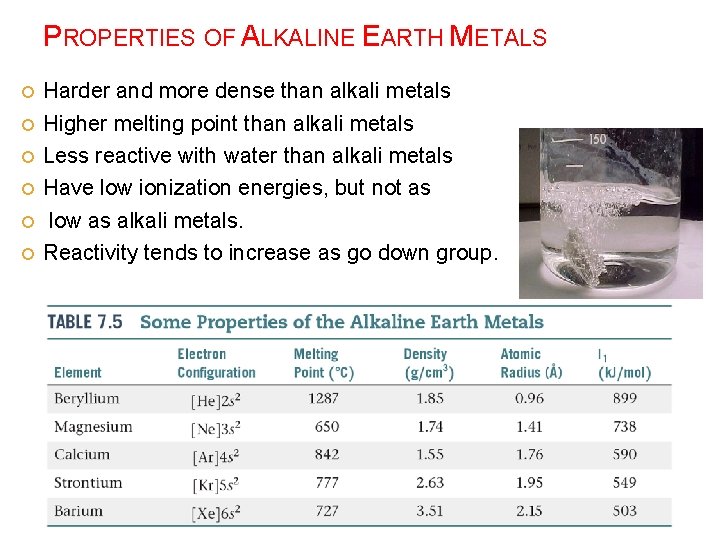
PROPERTIES OF ALKALINE EARTH METALS Harder and more dense than alkali metals Higher melting point than alkali metals Less reactive with water than alkali metals Have low ionization energies, but not as low as alkali metals. Reactivity tends to increase as go down group.

FLAME TEST COLORS Ca 2+ Orange-red Sr 2+ Crimson Red Ba 2+ Pale Green They are used in firework!

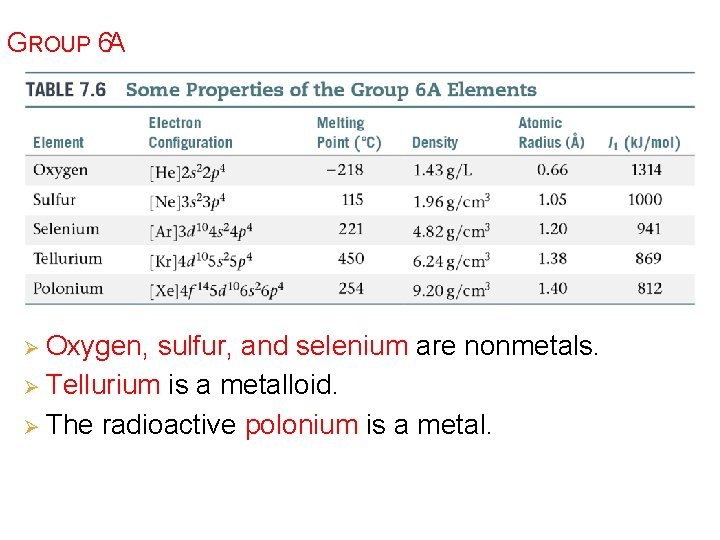
GROUP 6 A Ø Oxygen, sulfur, and selenium are nonmetals. Ø Tellurium is a metalloid. Ø The radioactive polonium is a metal.

OXYGEN Ø Two allotropes: O 2 § O 3, ozone § Ø Three anions: O 2−, oxide § O 22−, peroxide § O 21−, superoxide § Ø Tends to take electrons from other elements (oxidation)

SULFUR Weaker oxidizing agent than oxygen. Most stable allotrope is S 8, a ringed molecule.

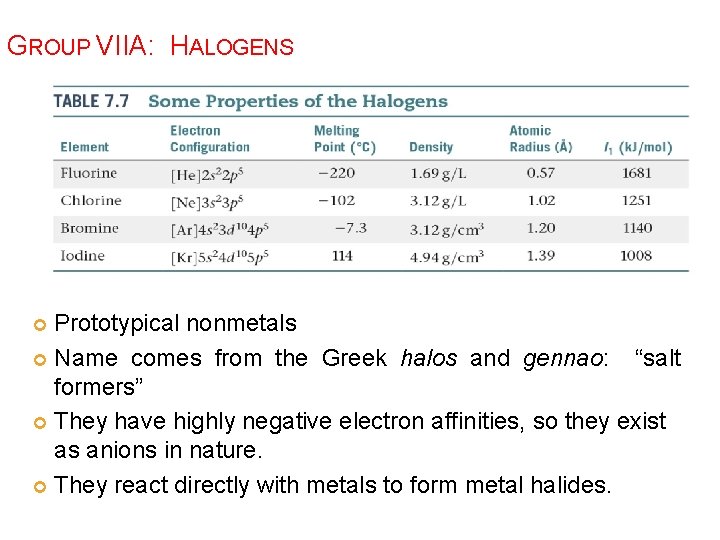
GROUP VIIA: HALOGENS Prototypical nonmetals Name comes from the Greek halos and gennao: “salt formers” They have highly negative electron affinities, so they exist as anions in nature. They react directly with metals to form metal halides.

GROUP VIIA: HALOGENS Ø Large, negative electron affinities § Therefore, tend to oxidize other elements easily Ø React directly with metals to form metal halides Ø Chlorine added to water supplies to serve as disinfectant

HALOGENS COLORS Fluorine is a pale yellow gas. Chlorine is a greenish-yellow gas. Bromine is a reddish-brown liquid. Iodine is a gray/black solid that forms purple vapors. Halogens are very reactive.

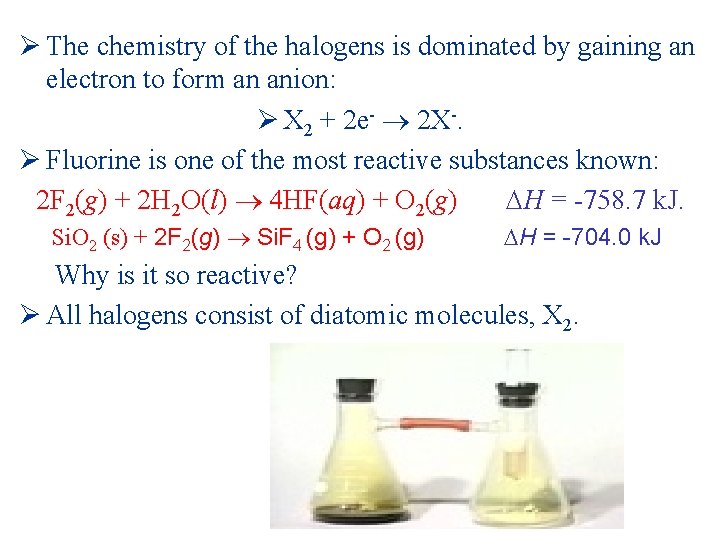
Ø The chemistry of the halogens is dominated by gaining an electron to form an anion: Ø X 2 + 2 e- 2 X-. Ø Fluorine is one of the most reactive substances known: 2 F 2(g) + 2 H 2 O(l) 4 HF(aq) + O 2(g) H = -758. 7 k. J. Si. O 2 (s) + 2 F 2(g) Si. F 4 (g) + O 2 (g) H = -704. 0 k. J Why is it so reactive? Ø All halogens consist of diatomic molecules, X 2.

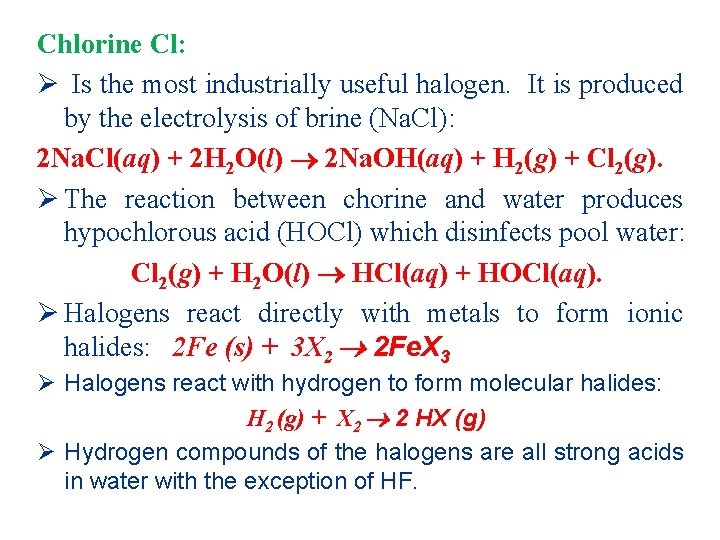
Chlorine Cl: Ø Is the most industrially useful halogen. It is produced by the electrolysis of brine (Na. Cl): 2 Na. Cl(aq) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g) + Cl 2(g). Ø The reaction between chorine and water produces hypochlorous acid (HOCl) which disinfects pool water: Cl 2(g) + H 2 O(l) HCl(aq) + HOCl(aq). Ø Halogens react directly with metals to form ionic halides: 2 Fe (s) + 3 X 2 2 Fe. X 3 Ø Halogens react with hydrogen to form molecular halides: H 2 (g) + X 2 2 HX (g) Ø Hydrogen compounds of the halogens are all strong acids in water with the exception of HF.

GROUP VIIIA: NOBLE GASES

Ø Properties of Noble Gases: Ø Astronomical ionization energies. Ø Noble gases have full outer energy levels, and positive electron affinities. § Therefore, relatively unreactive. Ø Monatomic gases. Ø They rarely form compounds, but compounds have been formed with xenon, krypton, and argon. Ø Most of these compounds contain fluorine, since it is highly reactive.

GROUP VIIIA: NOBLE GASES Ø Xe forms compounds: three Xe. F 2 § Xe. F 4 (at right) § Xe. F 6 § Ø Kr forms only one stable compound: § Kr. F 2 Ø The unstable HAr. F was synthesized in 2000.

GROUP TRENDS FOR SELECTED NONMETALS Hydrogen: Hydrogen is in the alkali metal group even though it is a nonmetal. It can be metallic under extremely high pressures. Ø It belongs in group 1 because it has 1 valence electron and can form a +1 charge. Ø It belongs in group 17 because it is a nonmetal, it can form a -1 charge, and it only needs 1 more electron to achieve noble gas confiuguration. Ø

NONMETAL GROUPS When going down a group of nonmetals, the elements go from nonmetallic to metallic in nature.

HW EXERCISES: 1 3 32 62 99 5 8 34 36 66 71 106 12 42 74 16 44 78 21 48 82 24 54 88 28 56 96
