Group 7 the halogens The elements in group


- Slides: 9

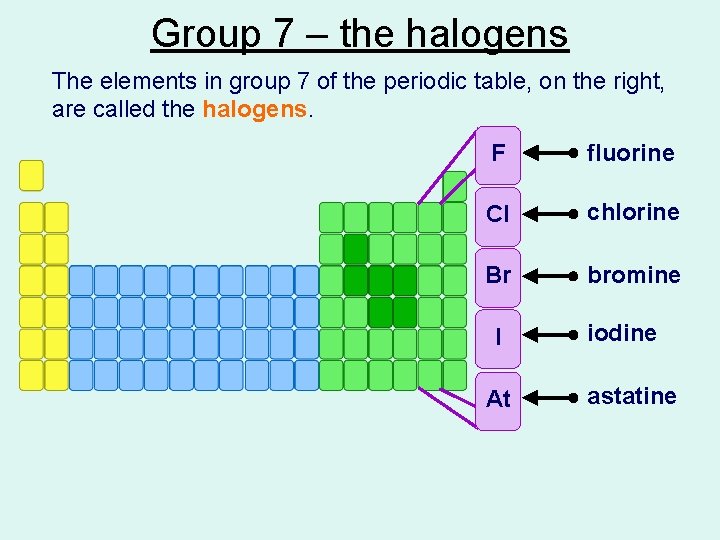
Group 7 – the halogens The elements in group 7 of the periodic table, on the right, are called the halogens. F fluorine Cl chlorine Br bromine I At iodine astatine

Complete page 1 of worksheet Use page 66 to help you.

Halogens – what do they look like? Chlorine Bromine Iodine

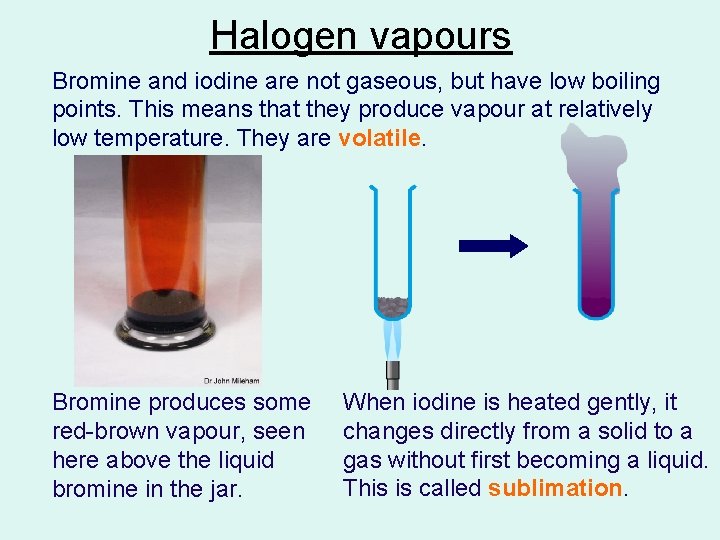
Halogen vapours Bromine and iodine are not gaseous, but have low boiling points. This means that they produce vapour at relatively low temperature. They are volatile. Bromine produces some red-brown vapour, seen here above the liquid bromine in the jar. When iodine is heated gently, it changes directly from a solid to a gas without first becoming a liquid. This is called sublimation.

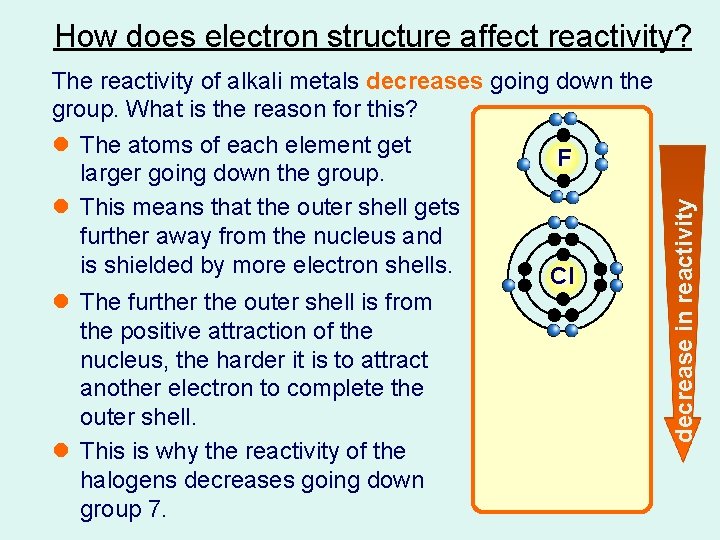
The reactivity of alkali metals decreases going down the group. What is the reason for this? l The atoms of each element get F larger going down the group. l This means that the outer shell gets further away from the nucleus and is shielded by more electron shells. Cl l The further the outer shell is from the positive attraction of the nucleus, the harder it is to attract another electron to complete the outer shell. l This is why the reactivity of the halogens decreases going down group 7. decrease in reactivity How does electron structure affect reactivity?

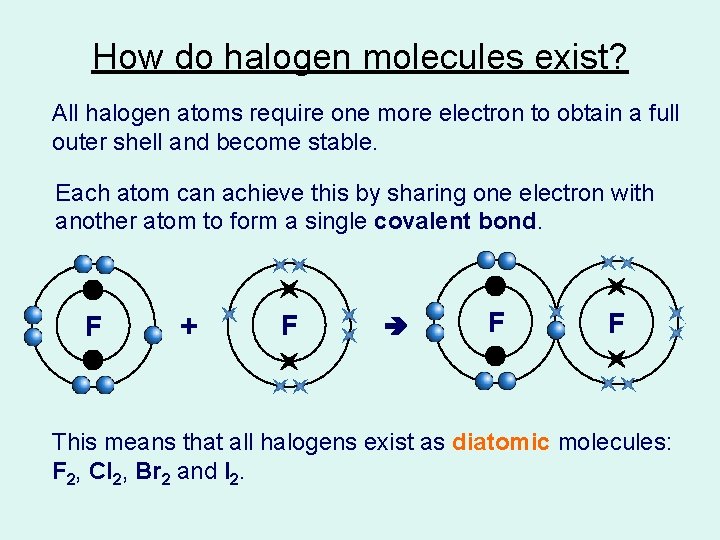
How do halogen molecules exist? All halogen atoms require one more electron to obtain a full outer shell and become stable. Each atom can achieve this by sharing one electron with another atom to form a single covalent bond. F + F F F This means that all halogens exist as diatomic molecules: F 2, Cl 2, Br 2 and I 2.

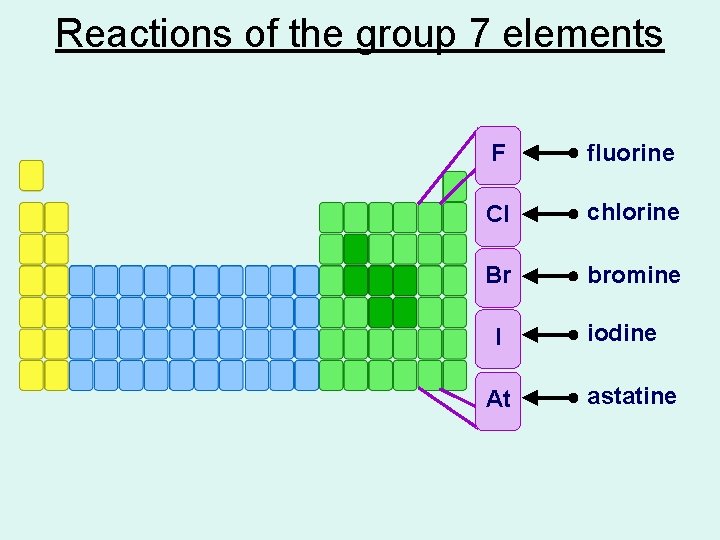
Reactions of the group 7 elements F fluorine Cl chlorine Br bromine I At iodine astatine

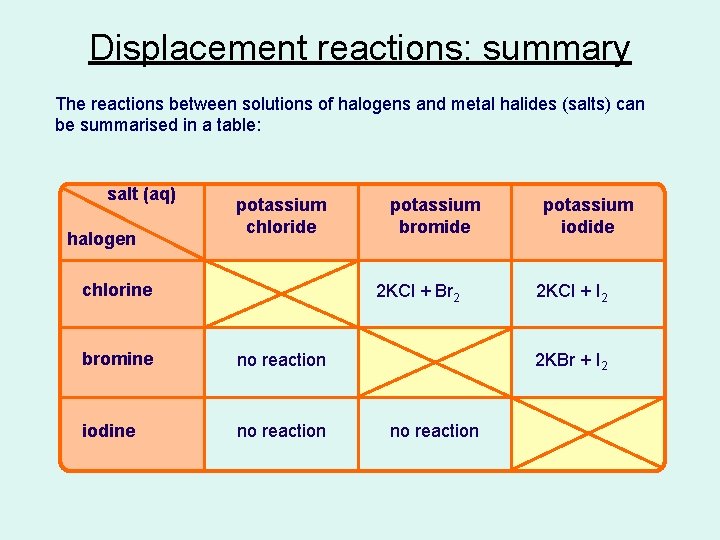
Displacement reactions: summary The reactions between solutions of halogens and metal halides (salts) can be summarised in a table: salt (aq) halogen potassium chloride chlorine potassium bromide 2 KCl + Br 2 bromine no reaction iodine no reaction potassium iodide 2 KCl + I 2 2 KBr + I 2 no reaction

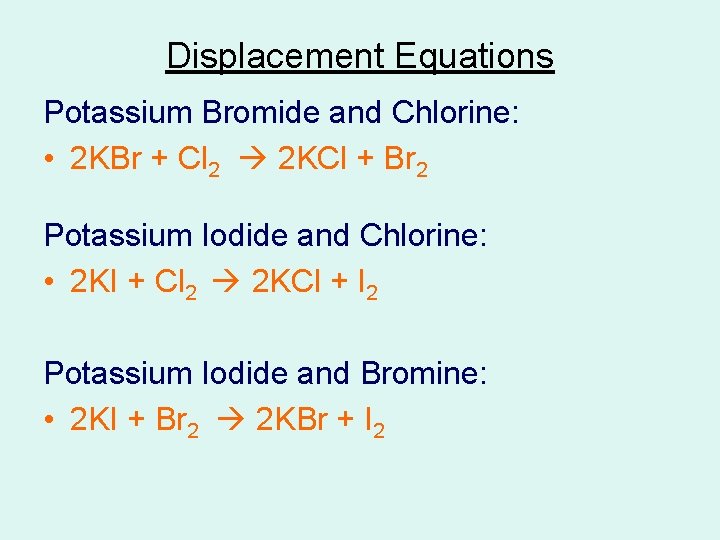
Displacement Equations Potassium Bromide and Chlorine: • 2 KBr + Cl 2 2 KCl + Br 2 Potassium Iodide and Chlorine: • 2 KI + Cl 2 2 KCl + I 2 Potassium Iodide and Bromine: • 2 KI + Br 2 2 KBr + I 2