THE TRANSITION ELEMENTS THE TRANSITION ELEMENTS Position properties

![(b) The second Transition series From Yttrium = [Kr] 5 s 24 d 1 (b) The second Transition series From Yttrium = [Kr] 5 s 24 d 1](https://slidetodoc.com/presentation_image/d036a77e75a757192d63048b9cab3214/image-4.jpg)
![(ii) The Lanthanide elements Starts with Lanthanum = [Xe] 5 d 16 s 2. (ii) The Lanthanide elements Starts with Lanthanum = [Xe] 5 d 16 s 2.](https://slidetodoc.com/presentation_image/d036a77e75a757192d63048b9cab3214/image-5.jpg)
![(iii) The Actinide elements. Starts with Ac = [Rn]6 d 17 s 2 = (iii) The Actinide elements. Starts with Ac = [Rn]6 d 17 s 2 =](https://slidetodoc.com/presentation_image/d036a77e75a757192d63048b9cab3214/image-6.jpg)








- Slides: 17

THE TRANSITION ELEMENTS

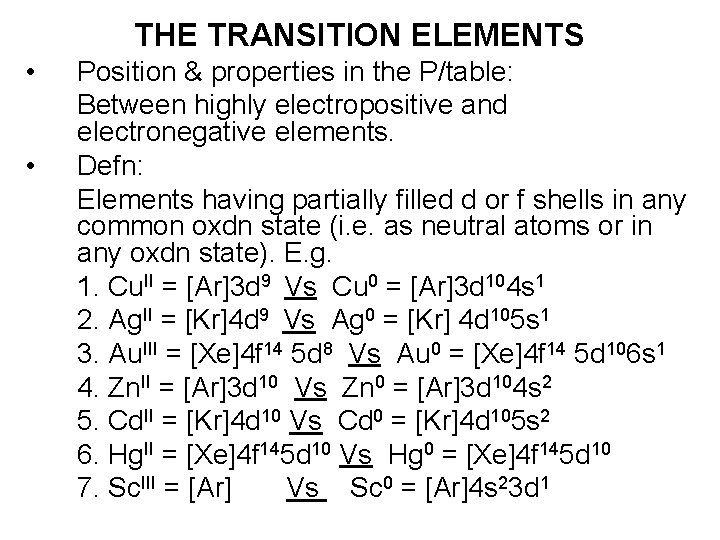
THE TRANSITION ELEMENTS • • Position & properties in the P/table: Between highly electropositive and electronegative elements. Defn: Elements having partially filled d or f shells in any common oxdn state (i. e. as neutral atoms or in any oxdn state). E. g. 1. Cu. II = [Ar]3 d 9 Vs Cu 0 = [Ar]3 d 104 s 1 2. Ag. II = [Kr]4 d 9 Vs Ag 0 = [Kr] 4 d 105 s 1 3. Au. III = [Xe]4 f 14 5 d 8 Vs Au 0 = [Xe]4 f 14 5 d 106 s 1 4. Zn. II = [Ar]3 d 10 Vs Zn 0 = [Ar]3 d 104 s 2 5. Cd. II = [Kr]4 d 10 Vs Cd 0 = [Kr]4 d 105 s 2 6. Hg. II = [Xe]4 f 145 d 10 Vs Hg 0 = [Xe]4 f 145 d 10 7. Sc. III = [Ar] Vs Sc 0 = [Ar]4 s 23 d 1

• Three groups of Transition elements are recognized: (1) The main Transition elements or d-block elements. (2) The Lanthanide elements (3) The Actinide elements. (i) The main Transition d-block elements. (a) The First Transition series Sc= [Ar]4 s 23 d 1 = Lightest member Sc Ti V Cr Mn Fe Co Ni and Cu Cu = [Ar]3 d 104 s 1 Have partially filled 3 d shells either in the ground state of the free atom [All EXCEPT Cu] or in one or more of their chemically important ions [ALL EXCEPT Sc]. Note: from Zn = 3 d 104 s 2 until next 9 elements = NONTRANSITION
![b The second Transition series From Yttrium Kr 5 s 24 d 1 (b) The second Transition series From Yttrium = [Kr] 5 s 24 d 1](https://slidetodoc.com/presentation_image/d036a77e75a757192d63048b9cab3214/image-4.jpg)
(b) The second Transition series From Yttrium = [Kr] 5 s 24 d 1 Y Zr Nb Mo Tc Ru Rh Pd Ag & [Cd non-trans] All have partially filled 4 d shells either in the free element [ALL EXCEPT Ag] or in one or more of the chemically important ions [ALL EXCEPT Y]. (c) The Third Transition series starts with Hf = [Xe] 6 s 25 d 2. [Xe]4 f 146 s 15 d 10 Hf Ta W Re Os Ir Pt Au & [Hg non-trans] All have partially filled 5 d shells in one or more of their chemically important oxidation states as well as in the neutral atom [EXCEPT Au].
![ii The Lanthanide elements Starts with Lanthanum Xe 5 d 16 s 2 (ii) The Lanthanide elements Starts with Lanthanum = [Xe] 5 d 16 s 2.](https://slidetodoc.com/presentation_image/d036a77e75a757192d63048b9cab3214/image-5.jpg)
(ii) The Lanthanide elements Starts with Lanthanum = [Xe] 5 d 16 s 2. Next 14 elements electrons enter the 4 f shell until Lu (Lutetium). Ce = [Xe]4 f 15 d 16 s 2 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu = [Xe]4 f 145 d 16 s 2. 5 d 1 electron in Ce, Gd & Lu, in all others this electron is shifted to the 4 f orbital. The elements La – Lu have very similar chemical and physical properties. Those of Lanthanum are prototypal and hence the elements are called the LANTHANIDES.
![iii The Actinide elements Starts with Ac Rn6 d 17 s 2 (iii) The Actinide elements. Starts with Ac = [Rn]6 d 17 s 2 =](https://slidetodoc.com/presentation_image/d036a77e75a757192d63048b9cab3214/image-6.jpg)
(iii) The Actinide elements. Starts with Ac = [Rn]6 d 17 s 2 = Actinium The difference in energy btn 5 f & 6 d orbitals in the beginning of the series is less than that btn the 4 f & 5 d orbitals for the lanthanides. For Elements immediately following Ac and their ions there may be electrons in 5 f or 6 d or both (i. e. 5 f & 6 d are involved in accommodating successive electrons). After FOUR or FIVE more electrons have been added to the Ac configuration, the 5 f orbitals become more stable (Plutonium is reached). Ac Th Pa U Np Pu Am cm Bk Cf Es Fm Md No Lr.

Differences between the Three classes of Transition elements. 1. D-block elements Partially filled 3 d, 4 d or 5 d orbitals. Electrons occupying them are strongly influenced by the surroundings and vice versa. ORBITALS PROJECT TO PERIPHERY OF ATOMS. 2. The Lanthanides Partially filled 4 f orbitals. Electrons occupying them are largely screened from the surroundings by the overlying shells [5 s, 5 p] of electrons. 4 f orbitals deeply buried in atoms/ions

RESULT: (i) Chemistry of Lanthanides Homologous (ii) Chemistry of d-block elements. Errate and Irregular variations in chemical properties through a series of d-block elements. 3. The Actinide elements. - Behaviour lies between d-block and the Lanthanides. - 5 f orbitals not so exposed as the d orbitals and not so well shielded as 4 f orbitals.

Factors affecting the stability of Electronic configurations: 1. Effective nuclear charge. • In hydrogen all the subshells of each principal shell are equi-energetic (one electron atom). • Multi-electron atoms Energies of subshell depend on the populations of all the other levels. The s, p, d, f etc subshells split apart and drop to lower energies. REASON: Steady increase in the Effective Nuclear charge. • Each electron is IMPERFECTLY SHIELDED from the nuclear charge by other electrons.

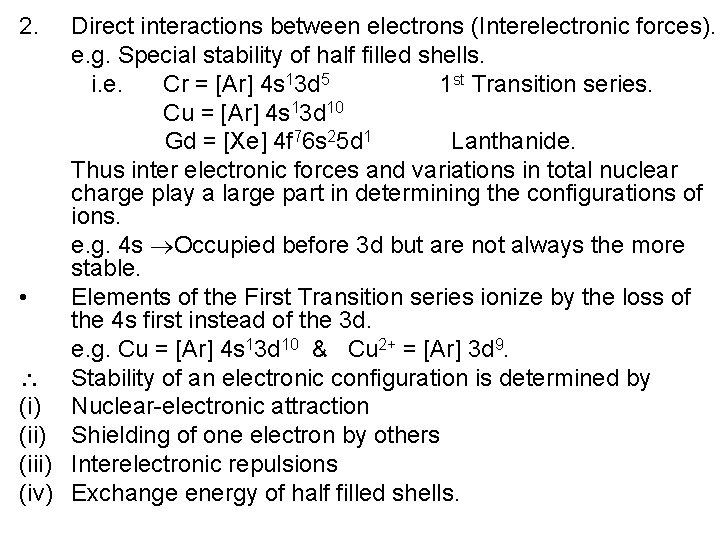
2. Direct interactions between electrons (Interelectronic forces). e. g. Special stability of half filled shells. i. e. Cr = [Ar] 4 s 13 d 5 1 st Transition series. Cu = [Ar] 4 s 13 d 10 Gd = [Xe] 4 f 76 s 25 d 1 Lanthanide. Thus inter electronic forces and variations in total nuclear charge play a large part in determining the configurations of ions. e. g. 4 s Occupied before 3 d but are not always the more stable. • Elements of the First Transition series ionize by the loss of the 4 s first instead of the 3 d. e. g. Cu = [Ar] 4 s 13 d 10 & Cu 2+ = [Ar] 3 d 9. Stability of an electronic configuration is determined by (i) Nuclear-electronic attraction (ii) Shielding of one electron by others (iii) Interelectronic repulsions (iv) Exchange energy of half filled shells.

General Properties: 1. They are all metals 2. They are almost all hard, strong, high-melting, high-boiling metals that conduct heat and electricity well. 3. They form alloys with one another and with other metallic elements. 4. Many of them are sufficiently electropositive to dissolve in mineral acids although a few are “noble” 5. With very few exceptions, they exhibit variable valence. 6. Their ions and compounds are coloured in one if not all oxidation states (with very few exceptions). 7. Due to partially filled shells, they form some paramagnetic compounds. 8. They form complex compounds (coordination compounds/ions). Periodic trends in properties: • Atomic radii, Atomic volume and density. • Ionisation energy and Electronegativity. • Melting and Boiling points.

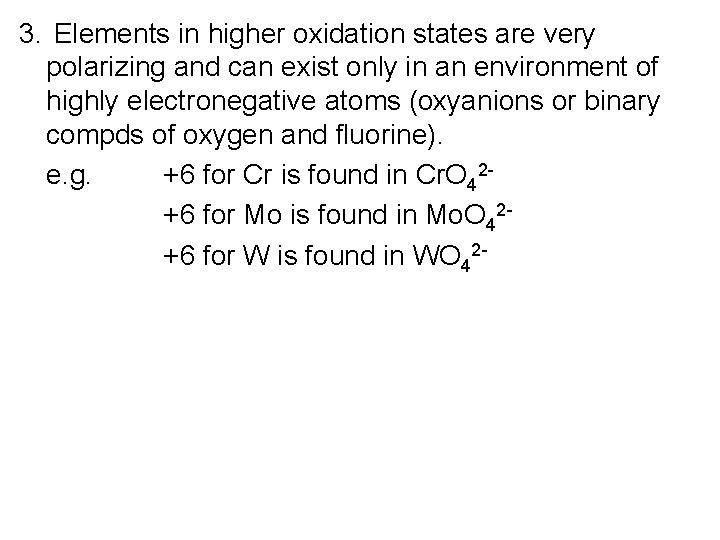
3. Elements in higher oxidation states are very polarizing and can exist only in an environment of highly electronegative atoms (oxyanions or binary compds of oxygen and fluorine). e. g. +6 for Cr is found in Cr. O 42+6 for Mo is found in Mo. O 42+6 for W is found in WO 42 -

OXIDATION STATES (i) The II state: • All the elements Ti Cu form well defined binary compds in the DIVALENT STATE. These are essentially ionic e. g. MX 2. • Aqua ions are well defined: [M(H 2 O)6]2+ except Ti. (ii) The III state: • All the elements form at least some compds in this state (Highest for Cu). e. g. Ti 2 O 3, Cr. F 3. • Fluorides and oxides are generally ionic. Chlorides may have considerable covalent character e. g. . Fe. Cl 3.

THE LANTHANIDES La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu. They are also known as Lanthanoids or Lanthanon elements. Commercially important minerals. 1. Monazine=mixed La 2. Bastnaesite= La LARGEST NATURAL OCCURING GROUPS The Lanthanides are strictly the 14 elements that follow Lanthanum in the Periodic Table, in which fourteen 4 f electrons are successively added to the Lanthanum configuration. • Prime Oxidation state = M 3+ La = [Xe]5 d 16 s 2 Lu = [Xe]4 f 145 d 16 s 2 The Lanthanide contraction This is the significant and steady decrease in the size of atoms and ions of the Lanthanides with increasing atomic number (z). Due to imperfect shielding of one electron by another in the

Note: The largest decrease occurs with the first f electrons added and also after f 7. • Elements highly electropositive. Prime Ox. State = +3 sum of first THREE ionization energies is relatively Low. • They form [M(H 2 O)n]3+ Ce can form Ce 4+ Sm, Eu, Yb and Tm can form M 2+. Some properties of the Lanthanides. • The metals are very reactive. • Heating with Halogens give Ln. X 3. • They all react directly with water, slowly in the cold, rapidly on heating liberating H 2 • React exothermically with hydrogen though heating to 300 -400 o. C is often required to initiate the reaction • Eu and Yb dissolve in liquid ammonia at – 78 o. C giving blue solutions. These solutions are those expected for M 2+ ions and solvated electrons.

THE TRIVALENT STATE • This is the characteristic oxidation state for all the lanthanides. • They form (i) Oxides and hydroxides resembling the Ca Ba group ie M 2 O 3 and M(OH)3. - Basicity decrease with increasing atomic number. (ii) MX 3 ionic halides (iii) M 2 S 3 and MX (X = N, P, As, Sb or Bi).

THE TETRAVALENT STATE (1) Ce(IV) is the only +4 Lanthanide ion that exist in aqueous solution as well as in the solid state. • Compounds: Ce. O 2 (Ceric Oxide), Ce. O 2. n. H 3 O, (Hydrous Ceric Oxide), Ce. F 4. 2. Pr (Praseodymium), Tb (Terbium) and Eu (Europium) solid compounds of oxidation state IV are known. eg. Na. Pr. F 5, Pr. F 4, Tb. O 2, Tb. F 4 Cs 3 Nd. F 7. THE DIVALENT STATE Aqueous solutions and solid compounds of Sm 2+, Eu 2+ and Yb 2+ are known. Solid compounds of M 3+ OF Nd, Sm, Dy, Yb and Tm are known but are unstablein water. Eg. Nd. Br 2, Sm. Br 2. Oxides Nd. O and Sm. O ( both golden yellow) and Eu. O (dark red 0 Yb. O SOME USES OF THE LANTHANIDES/LANTHANOIDS 1. Additives in special purposes steels. Eg making pipes 2. Catalysis(mixed metal oxides are used)