Aim How are Elements Organized in the Periodic









- Slides: 10

Aim: How are Elements Organized in the Periodic Table? DO Now: 1. How would you organize these buttons? 2. How do you think elements are organized in the periodic table? 3. Write at least one trend you notice about the elements in the periodic table.

Do Now: Read the text. Then answer the following questions. 1. 2. 3. 4. How did Mendeleev organize the elements in the periodic table? How are the elements organized in the modern periodic table? What are the rows called? Columns? What are valence electrons? How do you determine the number of valence electrons from the periodic table? 5. What do elements in the same group have in common?

While you’re reading, highlight the following information 1. How are the elements arranged in the modern periodic table? 2. What are rows called? Columns? 3. How are elements in the same group similar?

History of the Periodic Table • The first periodic table was developed by chemist Dmitri Mendeleev; arranged elements in order of increasing atomic mass. (look at Te and I; not in order of increasing mass) • The modern periodic law states: elements arranged in order of increasing atomic numbers. • Elements are sorted into groups based on similar properties.

Periods and Groups Periods • Horizontal rows • The # of the period indicates the # of energy levels (shells) in the element • The # of valence electrons (electrons in outer shell) increases from left to right • Properties of elements change across a period Groups or Families • Vertical columns • Most elements within the same group have the same number of valence electrons (electrons in outermost shell); therefore, have similar chemical properties

Three Classes of Elements 1. Metals • Alkaline Earth Metals • Transition Metals • Inner Transition Metals 2. Metalloids 3. Nonmetals • Halogen • Noble Gases


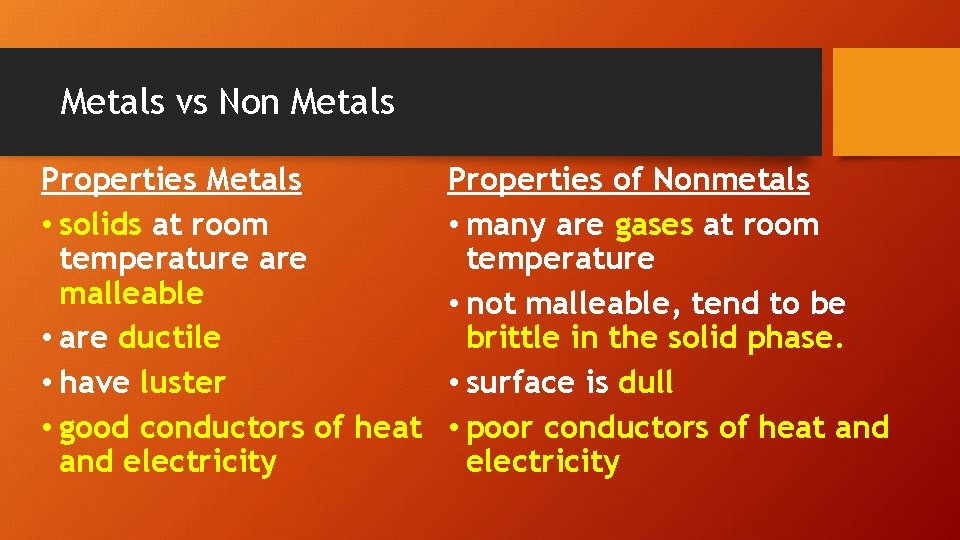
Metals vs Non Metals Properties Metals • solids at room temperature are malleable • are ductile • have luster • good conductors of heat and electricity Properties of Nonmetals • many are gases at room temperature • not malleable, tend to be brittle in the solid phase. • surface is dull • poor conductors of heat and electricity

Metalloids • an element (e. g. , germanium or silicon) whose properties are intermediate between those of metals and solid nonmetals

Question 1. Explain in terms of valence electrons why beryllium and magnesium have similar chemical properties. 2. Which one of the following is not a property of metals? a. ductility b. malleability c. good electrical conductivity d. having a dull appearance e. good heat conductivity