Solutions Intermolecular Forces Liquids and Solids Intermolecular Forces






























































- Slides: 140

Solutions Intermolecular Forces, Liquids, and Solids Intermolecular Forces

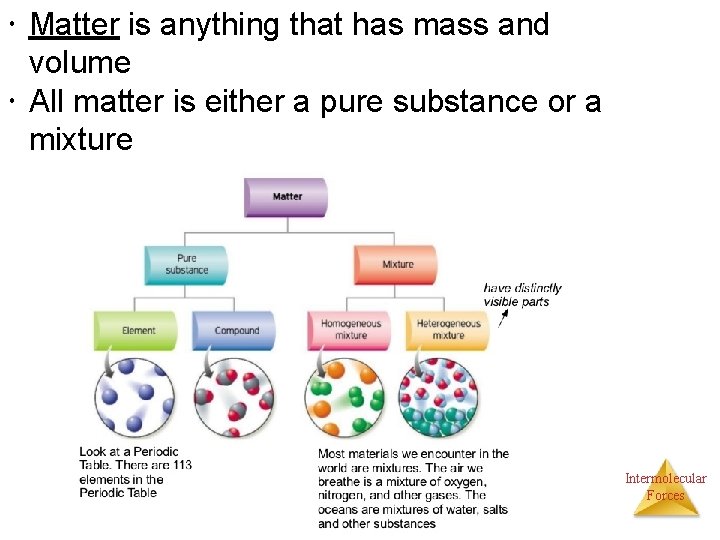
Matter is anything that has mass and volume All matter is either a pure substance or a mixture Intermolecular Forces

Classifications of Matter • Recall that matter could either be a pure substance (element or compound) or a mixture • Mixtures are just mingled together, not chemically combined. They can either be Ø Heterogeneous – does not appear the same throughout Ø Homogenous – appears the same throughout. Depending on the size of particles, homogenous mixtures can be a • Colloid – with large enough particles to scatter light • Solution – with particles too small to be seen in a microscope Intermolecular Forces

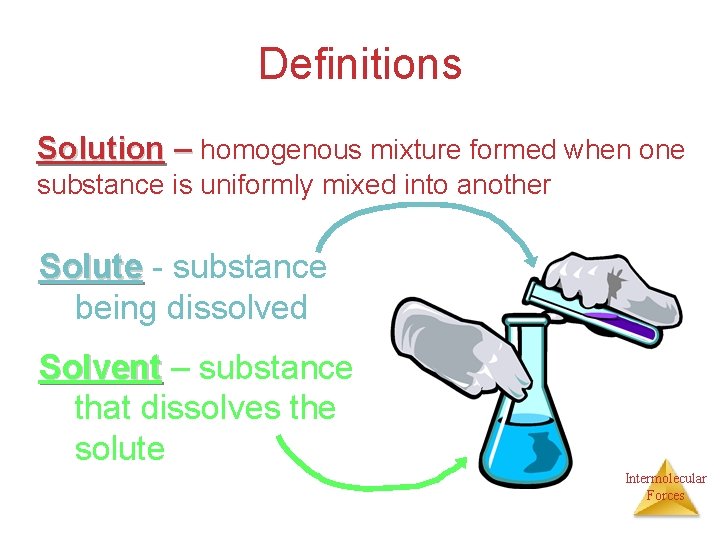
Definitions Solution – homogenous mixture formed when one substance is uniformly mixed into another Solute - substance being dissolved Solvent – substance that dissolves the solute Intermolecular Forces

Types of Solutions • Based on state of solvent. • “Universal solvent” is water (H 2 O) as many things dissolve in water • All solid-liquid-gas combos are possible. • EX: Ø Tooth filling: mercury (liquid) dissolved in silver (solid) Ø Adult 21+ beverages: alcohol (liquid) dissolved in water (liquid) Ø Soda: carbon dioxide (gas) dissolved in water (liquid) Intermolecular Forces

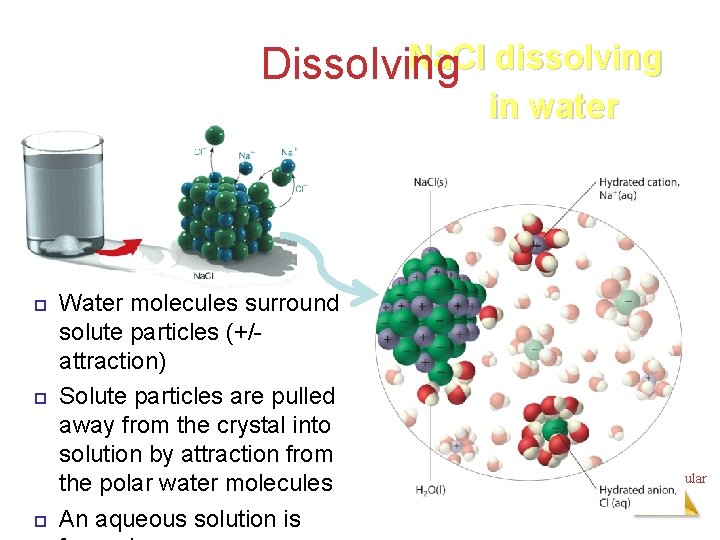
Dissolving: Salt Water Solute – Na. Cl Solvent - H 2 OIntermolecular Forces

Na. Cl dissolving Dissolving in water Water molecules surround solute particles (+/attraction) Solute particles are pulled away from the crystal into solution by attraction from the polar water molecules An aqueous solution is Intermolecular Forces

Dissolving Because of this attraction, Polar solutes can be dissolved by polar solvents Nonpolar solutes are dissolved by nonpolar solvents “Like dissolves like” Detergents are long molecules with a polar “head” and long nonpolar “tail” Dissolve and are attracted to both types Attract both dirt/oil and water Intermolecular Forces

Solubility is a measure of how much solute will dissolve in a given amount of solvent at a given temperature EX: 8 g of water will dissolve in 100 m. L of water at 25˚C Soluble – substance can dissolve in another substance Insoluble – substance cannot dissolve in another substance Intermolecular Forces

Concentration – ratio of the amount of solute dissolved per quantity of solvent Dilute solution – contains a large amount of solvent and a small amount of solute Example: A tablespoon of sugar in a bucket of water Concentrated solution – contains a large amount of solute and a small amount of solvent Example: Two cups of sugar in a glass of lemonade Intermolecular Forces

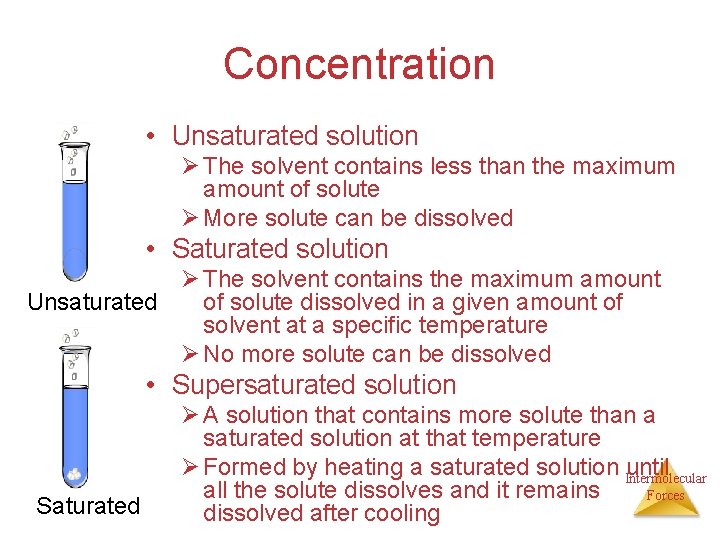
Concentration Unsaturated solution: solution Saturated solution: The solvent contains less Contains the maximum amount of solute than the maximum amount dissolved in a given of solute amount of solvent at a More solute can be specific temperature dissolved No more solute can be dissolved Supersaturated solution A solution that contains more solute than a saturated solution at that temperature Formed by heating a saturated solution until all of the Intermolecular solute dissolves and it remains dissolved after cooling Forces

Concentration • Unsaturated solution Ø The solvent contains less than the maximum amount of solute Ø More solute can be dissolved • Saturated solution Ø The solvent contains the maximum amount Unsaturated of solute dissolved in a given amount of solvent at a specific temperature Ø No more solute can be dissolved • Supersaturated solution Saturated Ø A solution that contains more solute than a saturated solution at that temperature Ø Formed by heating a saturated solution Intermolecular until all the solute dissolves and it remains Forces dissolved after cooling

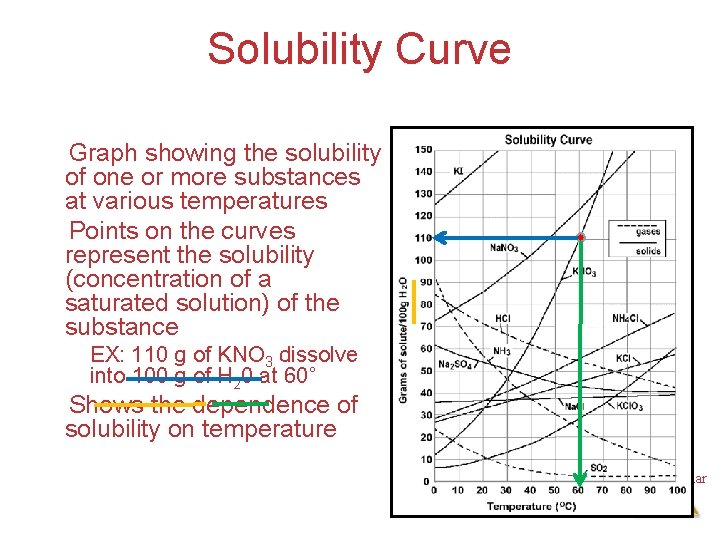
Solubility Curve Graph showing the solubility of one or more substances at various temperatures Points on the curves represent the solubility (concentration of a saturated solution) of the substance EX: 110 g of KNO 3 dissolve into 100 g of H 20 at 60˚ Shows the dependence of solubility on temperature Intermolecular Forces

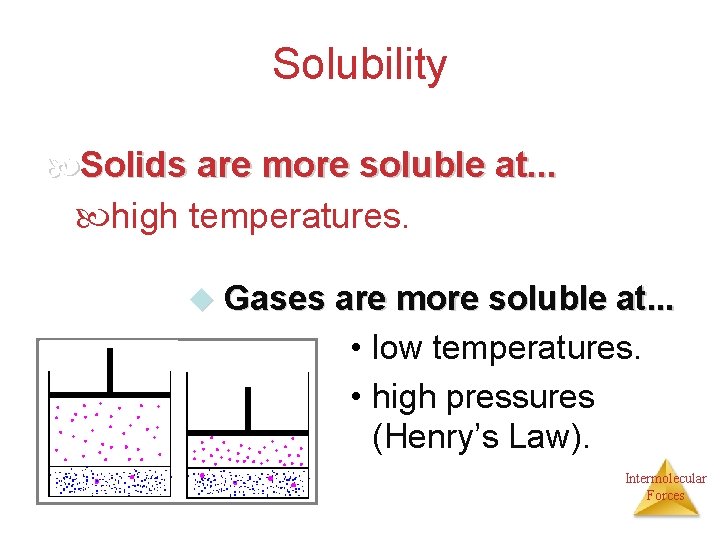
Solubility Solids are more soluble at. . . high temperatures. u Gases are more soluble at. . . • low temperatures. • high pressures (Henry’s Law). Intermolecular Forces

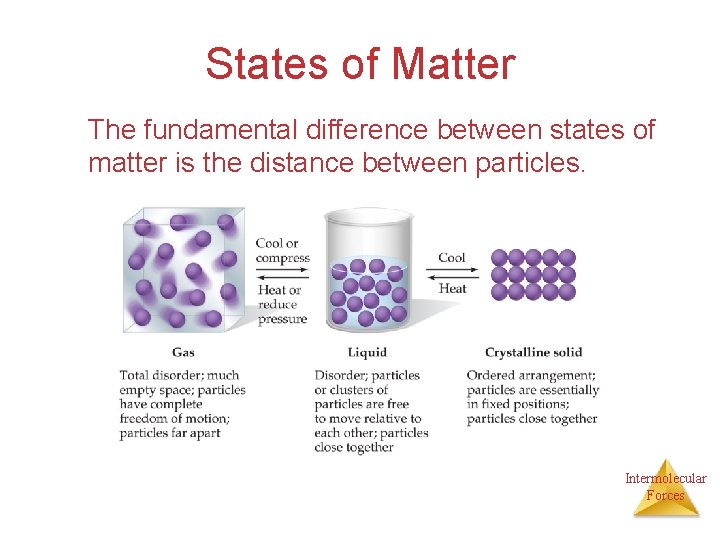
States of Matter The fundamental difference between states of matter is the distance between particles. Intermolecular Forces

States of Matter Because in the solid and liquid states particles are closer together, we refer to them as condensed phases. Intermolecular Forces

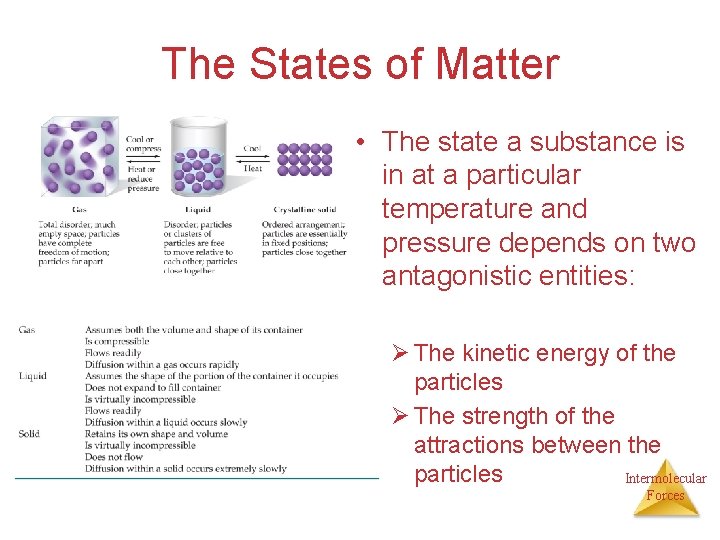
The States of Matter • The state a substance is in at a particular temperature and pressure depends on two antagonistic entities: Ø The kinetic energy of the particles Ø The strength of the attractions between the particles Intermolecular Forces

• Converting a gas into a liquid or solid requires the molecules to get closer to each other: – cool or compress. • Converting a solid into a liquid or gas requires the molecules to move further apart: – heat or reduce pressure. • The forces holding solids and liquids together are called intermolecular forces. Intermolecular Forces

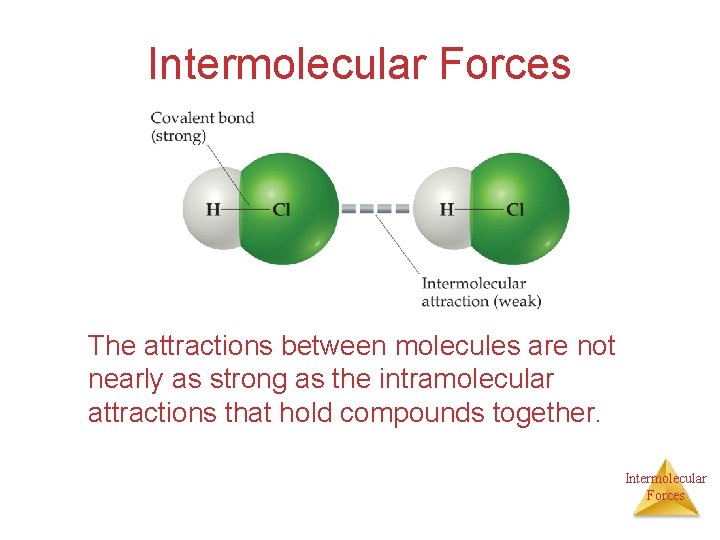
• The covalent bond holding a molecule together is an intramolecular force. • The attraction between molecules is an intermolecular force. • Intermolecular forces are much weaker than intramolecular forces (e. g. 16 k. J/mol vs. 431 k. J/mol for HCl). • When a substance melts or boils the intermolecular forces are broken (not the covalent bonds). Intermolecular Forces

Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions that hold compounds together. Intermolecular Forces

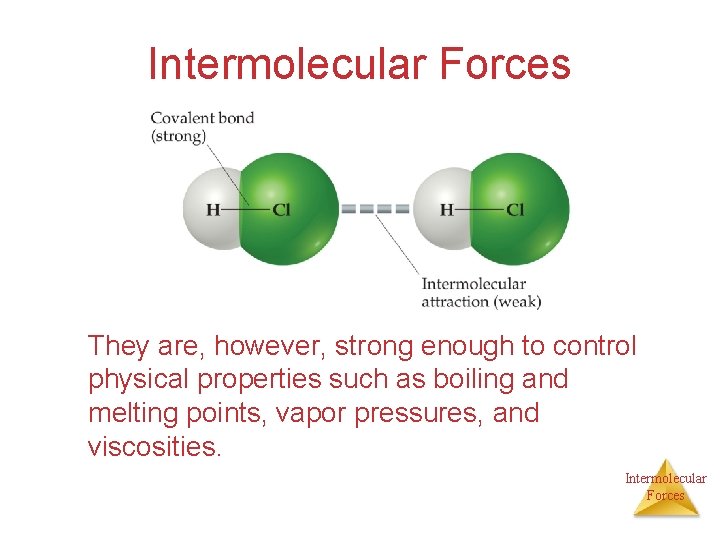
Intermolecular Forces They are, however, strong enough to control physical properties such as boiling and melting points, vapor pressures, and viscosities. Intermolecular Forces

Intermolecular Forces These intermolecular forces as a group are referred to as van der Waals forces. Intermolecular Forces

Van der Waals Forces (Forces of attraction between molecules) • Dipole-dipole interactions • Hydrogen bonding • London dispersion forces Intermolecular Forces

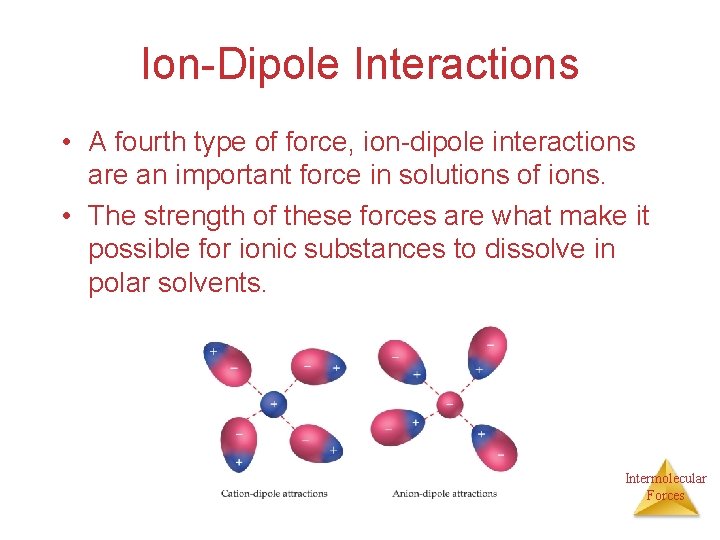
Ion-Dipole Interactions • A fourth type of force, ion-dipole interactions are an important force in solutions of ions. • The strength of these forces are what make it possible for ionic substances to dissolve in polar solvents. Intermolecular Forces

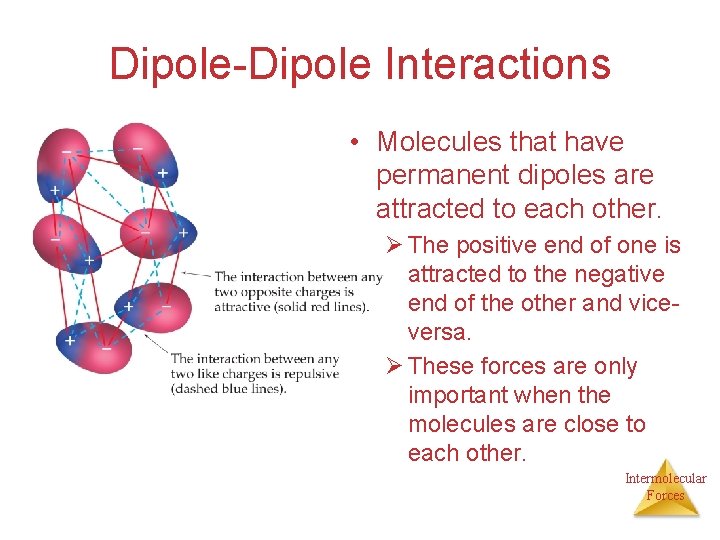
Dipole-Dipole Interactions • Molecules that have permanent dipoles are attracted to each other. Ø The positive end of one is attracted to the negative end of the other and viceversa. Ø These forces are only important when the molecules are close to each other. Intermolecular Forces

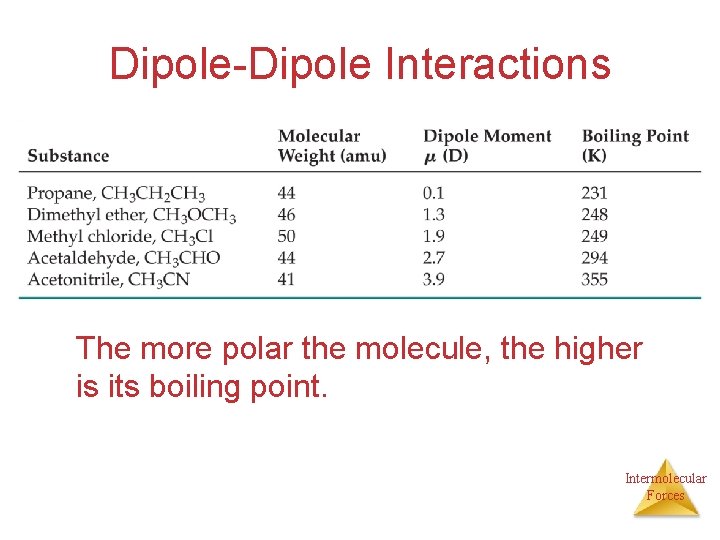
Dipole-Dipole Interactions The more polar the molecule, the higher is its boiling point. Intermolecular Forces

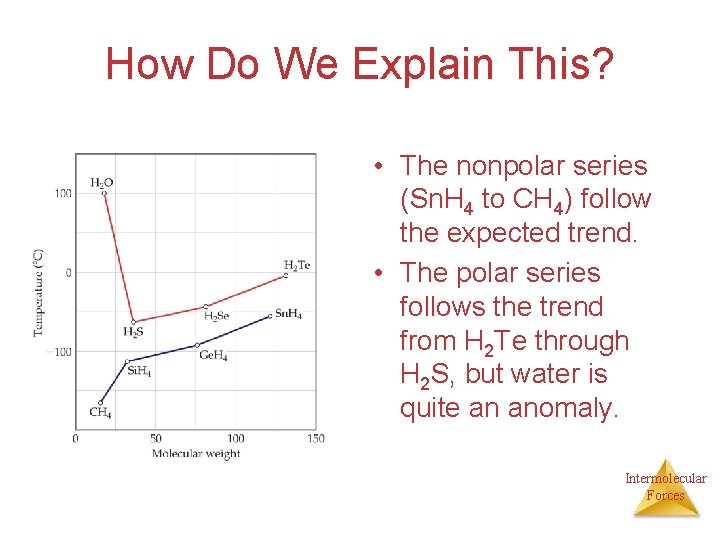
How Do We Explain This? • The nonpolar series (Sn. H 4 to CH 4) follow the expected trend. • The polar series follows the trend from H 2 Te through H 2 S, but water is quite an anomaly. Intermolecular Forces

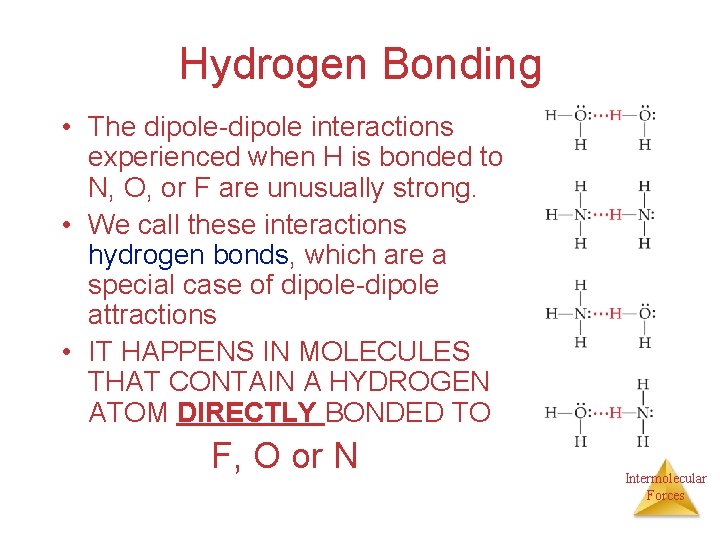
Hydrogen Bonding • The dipole-dipole interactions experienced when H is bonded to N, O, or F are unusually strong. • We call these interactions hydrogen bonds, which are a special case of dipole-dipole attractions • IT HAPPENS IN MOLECULES THAT CONTAIN A HYDROGEN ATOM DIRECTLY BONDED TO F, O or N Intermolecular Forces

Why H bonds form? • The Hydrogen atom consists of one proton and one electron. When its electron is attracted by the most electronegative elements of the table, what is left is an almost “naked proton” which in turns attracts the negative part of the molecule. • The energies of H bonds range 4 to 25 k. J/mol. Regular covalent bond strength range between 150 and 1100 k. J/mol. • H bonds are of fundamental importance in biological molecules, they help to stabilize the structure of proteins, and are responsible for the way DNA carries genetic information Intermolecular Forces

• Hydrogen bonds are also responsible for – Ice floating, fish surviving winter and many broken pipes! • Solids are usually more closely packed than liquids; therefore, solids are more dense than liquids. • Ice is ordered with an open structure to optimize Hbonding, therefore, ice is less dense than water. • Ice density = 0. 917 g/ml • Water density = 1 g/ml • Ice is an unusual solid because it has a larger volume in the solid state than in the liquid state! Intermolecular Forces

Hydrogen Bonding • In water the H-O bond length is 1. 0 Å. • The O…H hydrogen bond length is 1. 8 Å. • Ice has waters arranged in an open, regular hexagon. • Each + H points towards a lone pair on O. • Each water forms H bonds with 4 other molecules When water melts the structure collapses and the molecules move closer, then the liquid is less dense Intermolecular Forces

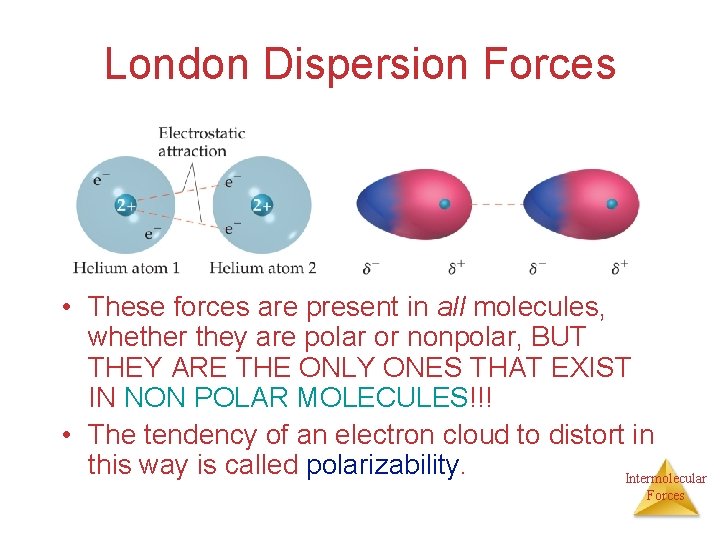
London Dispersion Forces Are due to momentary or instantaneous dipoles in a molecule While the electrons in the 1 s orbital of helium would repel each other (and, therefore, tend to stay far away from each other), it does happen that they occasionally wind up on the Intermolecular same side of the atom. Forces

London Dispersion Forces At that instant, then, the helium atom is polar, with an excess of electrons on the left side and a shortage on the right side. Intermolecular Forces

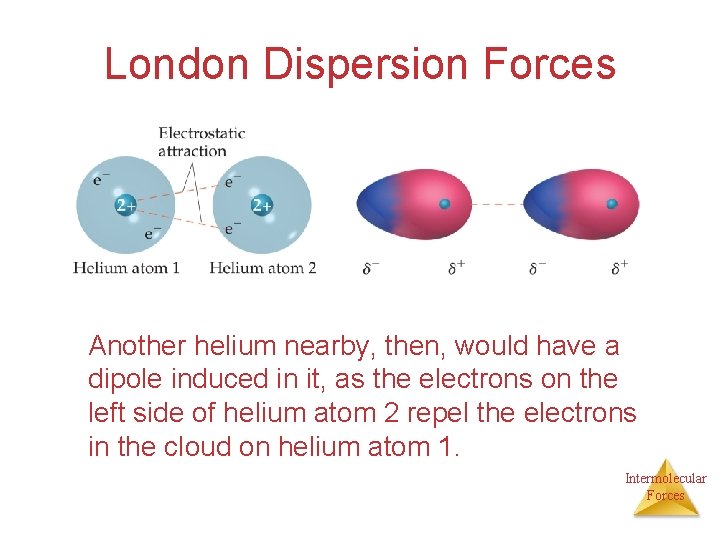
London Dispersion Forces Another helium nearby, then, would have a dipole induced in it, as the electrons on the left side of helium atom 2 repel the electrons in the cloud on helium atom 1. Intermolecular Forces

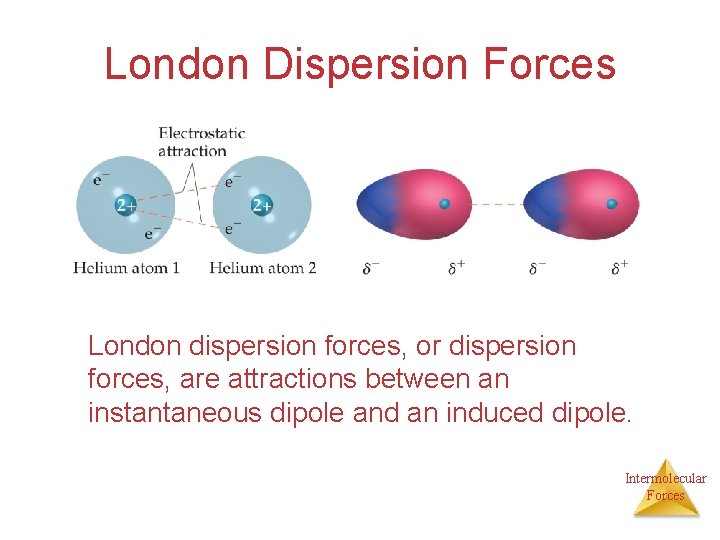
London Dispersion Forces London dispersion forces, or dispersion forces, are attractions between an instantaneous dipole and an induced dipole. Intermolecular Forces

London Dispersion Forces • These forces are present in all molecules, whether they are polar or nonpolar, BUT THEY ARE THE ONLY ONES THAT EXIST IN NON POLAR MOLECULES!!! • The tendency of an electron cloud to distort in this way is called polarizability. Intermolecular Forces

Factors Affecting London Forces • The “polarizability” of a molecule can be seen as a measure of the “squishiness” of its electron cloud. The greater the polarizability of the molecule, the more easily its electron cloud can be distorted to give a MOMENTARY dipole. **LARGER MOLECULES TEND TO HAVE GREATER POLARIZABILITIES. Polarizability and dispersion forces increases with molecular mass Intermolecular Forces

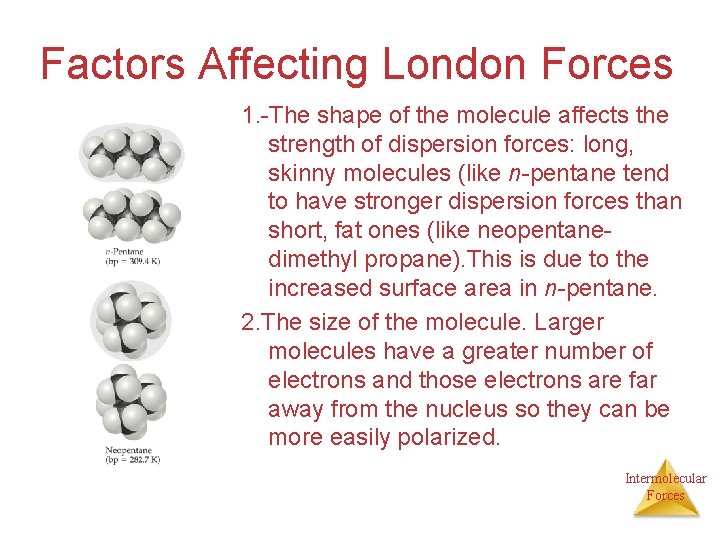
Factors Affecting London Forces 1. -The shape of the molecule affects the strength of dispersion forces: long, skinny molecules (like n-pentane tend to have stronger dispersion forces than short, fat ones (like neopentanedimethyl propane). This is due to the increased surface area in n-pentane. 2. The size of the molecule. Larger molecules have a greater number of electrons and those electrons are far away from the nucleus so they can be more easily polarized. Intermolecular Forces

Summary of London Dispersion Forces • Polarizability is the ease with which an electron cloud can be deformed. • The larger the molecule (the greater the number of electrons) the more polarizable. • London dispersion forces increase as molecular weight increases. • London dispersion forces exist between all molecules. • London dispersion forces depend on the shape of the molecule. • Like all dipole-dipole forces are significant only if Intermolecular Forces molecules are very close together.

• Predict the boiling point of Halogens and Noble Gases based on the molecular mass. Intermolecular Forces

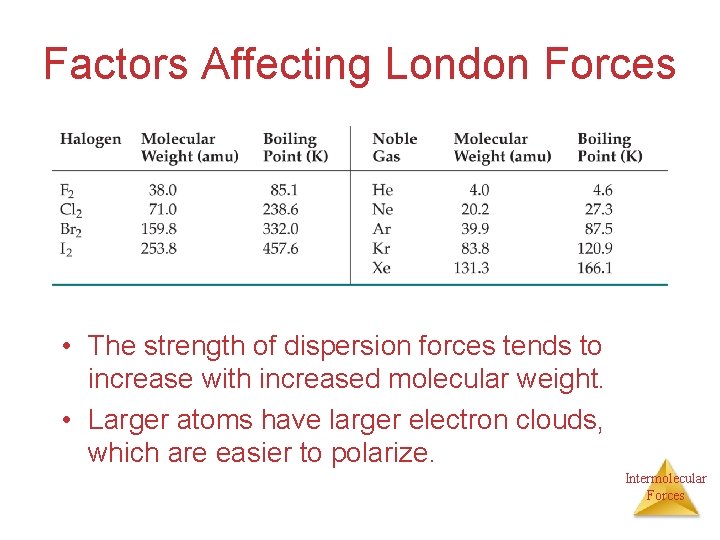
Factors Affecting London Forces • The strength of dispersion forces tends to increase with increased molecular weight. • Larger atoms have larger electron clouds, which are easier to polarize. Intermolecular Forces

Which Have a Greater Effect: Dipole-Dipole Interactions or Dispersion Forces? • If two molecules are of comparable size and shape, dipole-dipole interactions will likely be the dominating force. • If one molecule is much larger than another, dispersion forces will likely determine its physical properties (the > molecular mass the stronger the attractions). Intermolecular Forces

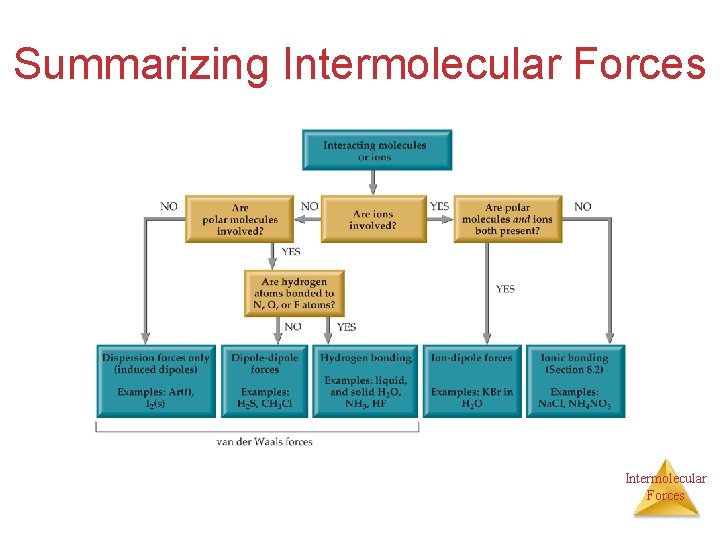
Summarizing Intermolecular Forces

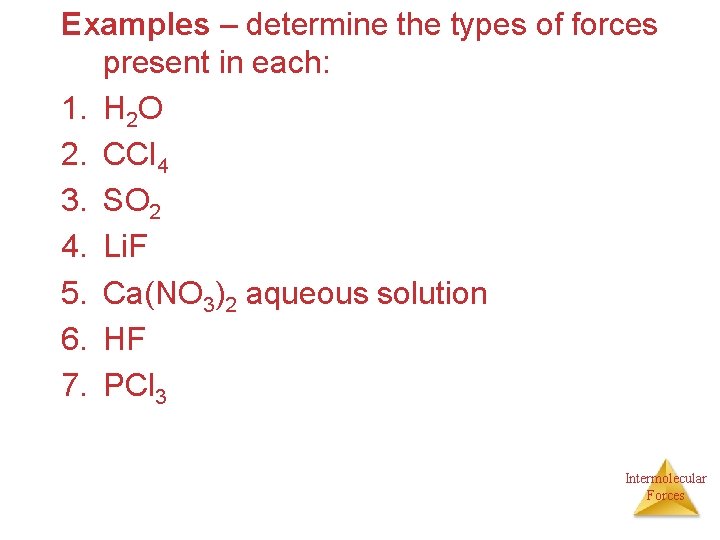
Examples – determine the types of forces present in each: 1. H 2 O 2. CCl 4 3. SO 2 4. Li. F 5. Ca(NO 3)2 aqueous solution 6. HF 7. PCl 3 Intermolecular Forces

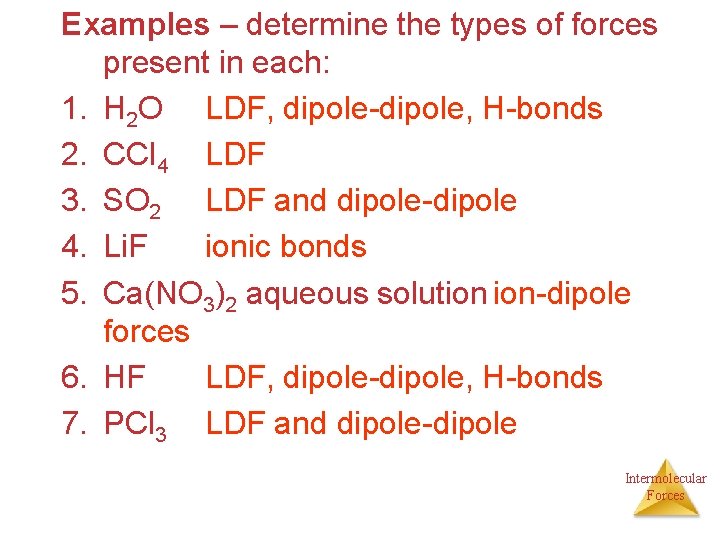
Examples – determine the types of forces present in each: 1. H 2 O LDF, dipole-dipole, H-bonds 2. CCl 4 LDF 3. SO 2 LDF and dipole-dipole 4. Li. F ionic bonds 5. Ca(NO 3)2 aqueous solution ion-dipole forces 6. HF LDF, dipole-dipole, H-bonds 7. PCl 3 LDF and dipole-dipole Intermolecular Forces

Relative Strengths of Forces 1. 2. 3. 4. 5. Bonds ( ionic, covalent, metallic) Ion-dipole forces Hydrogen bonds Dipole-dipole forces London dispersion forces Intermolecular Forces

SAMPLE EXERCISE 11. 1 Comparing Intermolecular Forces The dipole moments of acetonitrile, CH 3 CN, and methyl iodide, CH 3 I, are 3. 9 D and 1. 62 D, respectively. (a) Which of these substances will have the greater dipole-dipole attractions among its molecules? (b) Which of these substances will have the greater London dispersion attractions? (c) The boiling points of CH 3 CN and CH 3 I are 354. 8 K and 315. 6 K, respectively. Which substance has the greater overall attractive forces? Solution (a) Dipole-dipole attractions increase in magnitude as the dipole moment of the molecule increases. Thus, CH 3 CN molecules attract each other by stronger dipole-dipole forces than CH 3 I molecules do. (b) When molecules differ in their molecular weights, the more massive molecule generally has the stronger dispersion attractions. In this case CH 3 I (142. 0 amu) is much more massive than CH 3 CN (41. 0 amu), so the dispersion forces will be stronger for CH 3 I. (c) Because CH 3 CN has the higher boiling point, we can conclude that more energy is required to overcome attractive forces between CH 3 CN molecules. Thus, the total intermolecular attractions are stronger for CH 3 CN, suggesting that dipole-dipole forces are decisive when comparing these two substances. Nevertheless, dispersion forces play an important role in determining the properties of CH 3 I. PRACTICE EXERCISE Of Br 2, Ne, HCl, HBr, and N 2, which is likely to have (a) the largest intermolecular dispersion forces, (b) the largest dipole-dipole attractive forces? Intermolecular Forces Answers: (a) Br 2 (largest molecular weight), (b) HCl (largest polarity)

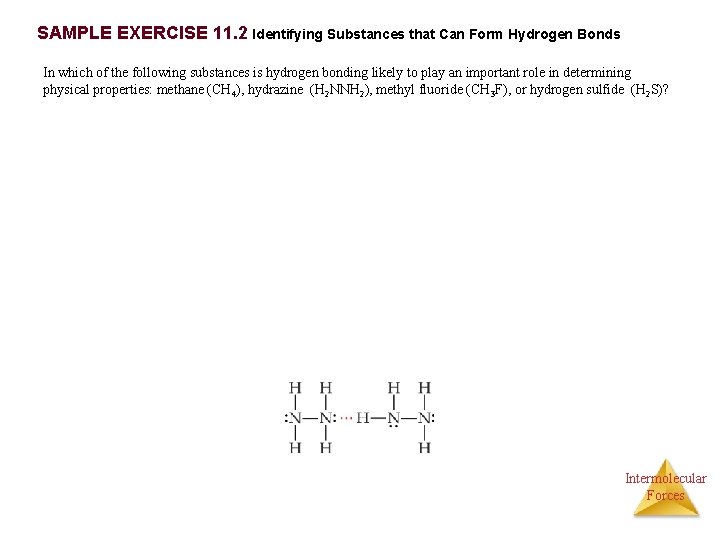
SAMPLE EXERCISE 11. 2 Identifying Substances that Can Form Hydrogen Bonds In which of the following substances is hydrogen bonding likely to play an important role in determining physical properties: methane (CH 4), hydrazine (H 2 NNH 2), methyl fluoride (CH 3 F), or hydrogen sulfide (H 2 S)? Intermolecular Forces

PRACTICE EXERCISE In which of the following substances is significant hydrogen bonding possible: methylene chloride (CH 2 Cl 2) phosphine (PH 3) hydrogen peroxide (HOOH), or acetone (CH 3 COCH 3)? Answer: HOOH Intermolecular Forces

SAMPLE EXERCISE 11. 3 Predicting the Types and Relative Strengths of Intermolecular Forces List the substances Ba. Cl 2, H 2, CO, HF, and Ne in order of increasing boiling points. Solution Analyze: We need to relate the properties of the listed substances to boiling point. Plan: The boiling point depends in part on the attractive forces in the liquid. We need to order these according to the relative strengths of the different kinds of forces. Solve: The attractive forces are stronger for ionic substances than for molecular ones, so Ba. Cl 2 should have the highest boiling point. The intermolecular forces of the remaining substances depend on molecular weight, polarity, and hydrogen bonding. The molecular weights are H 2 (2), CO (28), HF (20), and Ne (20). The boiling point of H 2 should be the lowest because it is nonpolar and has the lowest molecular weight. The molecular weights of CO, HF, and Ne are roughly the same. Because HF can hydrogen bond, however, it should have the highest boiling point of the three. Next is CO, which is slightly polar and has the highest molecular weight. Finally, Ne, which is nonpolar, should have the lowest boiling point of these three. The predicted order of boiling points is therefore Check: The actual normal boiling points are H 2 (20 K), Ne (27 K), CO (83 K), HF (293 K), and Ba. Cl 2 (1813 K), in agreement with our predictions. PRACTICE EXERCISE (a) Identify the intermolecular forces present in the following substances, and (b) select the substance with the highest boiling point: CH 3, CH 3 OH, and CH 3 CH 2 OH. Answers: (a) CH 3 has only dispersion forces, whereas the other two substances have both dispersion. Intermolecular forces and hydrogen bonds; (b) CH 3 CH 2 OH Forces

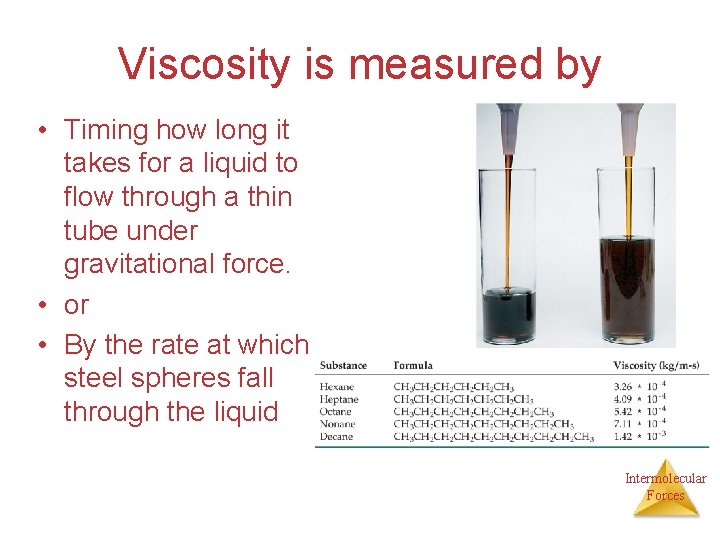
Some Properties of Liquids Viscosity • Viscosity is the resistance of a liquid to flow. • A liquid flows by sliding molecules over each other. • The stronger the intermolecular forces, the higher the viscosity. At higher temperatures viscosity decreases. Intermolecular Forces

Viscosity is measured by • Timing how long it takes for a liquid to flow through a thin tube under gravitational force. • or • By the rate at which steel spheres fall through the liquid Intermolecular Forces

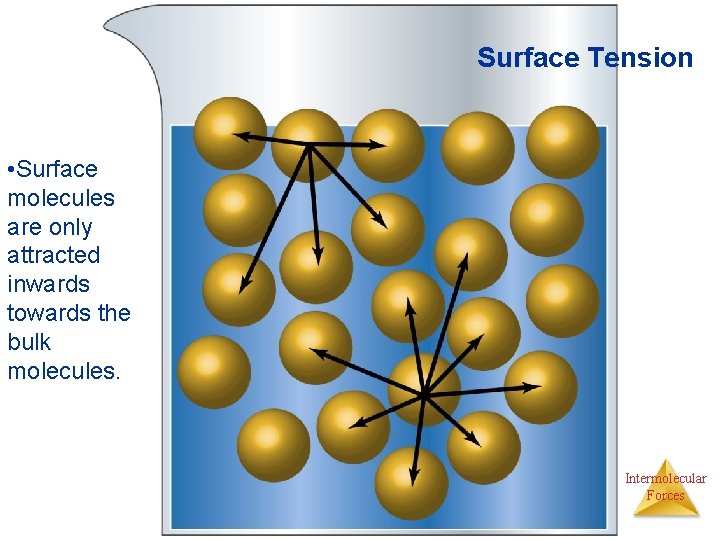
Surface Tension Surface tension results from the net inward force experienced by the molecules on the surface of a liquid. Intermolecular Forces

Surface Tension • Surface molecules are only attracted inwards towards the bulk molecules. Intermolecular Forces

Surface Tension • Is due to an unbalance of intermolecular forces at the surface of the liquid. Molecules in the interior are attracted equally in all directions, but at the surface they experience an inward force. • The inward pull reduces the surface area, making the molecules at the surface pack closely. This reduces the surface area. Spheres have the smaller surface per volume, then water drops asume spherical shape. . Surface tension is the amount of energy required to increase the surface area of a liquid. • Water has high surface tension due to H bonding. Intermolecular Forces

Cohesive forces bind molecules to each other (intermolecular forces). Adhesive forces bind molecules to a surface. (water to glass or to paper) • Meniscus is the shape of the liquid surface. – If adhesive forces are greater than cohesive forces, the liquid surface is attracted to its container more than the bulk molecules. Therefore, the meniscus is U-shaped (e. g. water in glass). – If cohesive forces are greater than adhesive forces, the meniscus is curved downwards (Hg) Intermolecular Forces

Intermolecular Forces

Capillary Action Is the rise of a liquid up very narrow tubes. This phenomenon occurs because the adhesive forces between the liquid and the walls of the container (glass) tend to increase the surface area. The surface tension of the liquid pulls the molecules up in order to reduce the area and the liquid climbs until the adhesive and cohesive forces are balanced by the force of gravity. Intermolecular Forces

October 24 Section 4 Phase changes Section 5 Vapor pressure • HW P 479 Q 33 -35 -39 -37 • QUIZ TOMORROW !!! *Specific Heat of a Substance *Energy Changes Accompanying Phase Changes /Heating and Cooling Curves *Supercooled liquids Intermolecular Forces

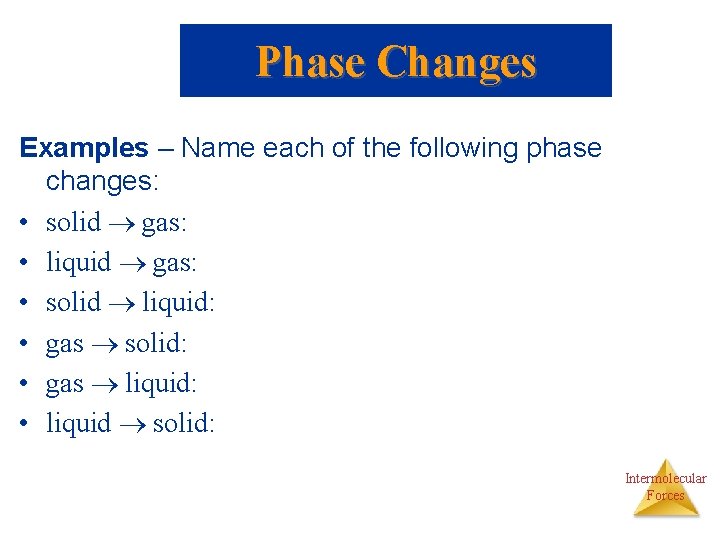
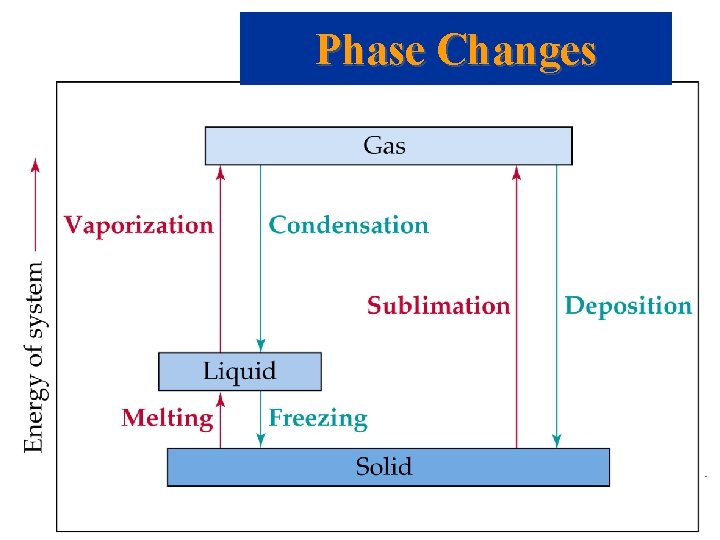
Phase Changes Examples – Name each of the following phase changes: • solid gas: • liquid gas: • solid liquid: • gas solid: • gas liquid: • liquid solid: Intermolecular Forces

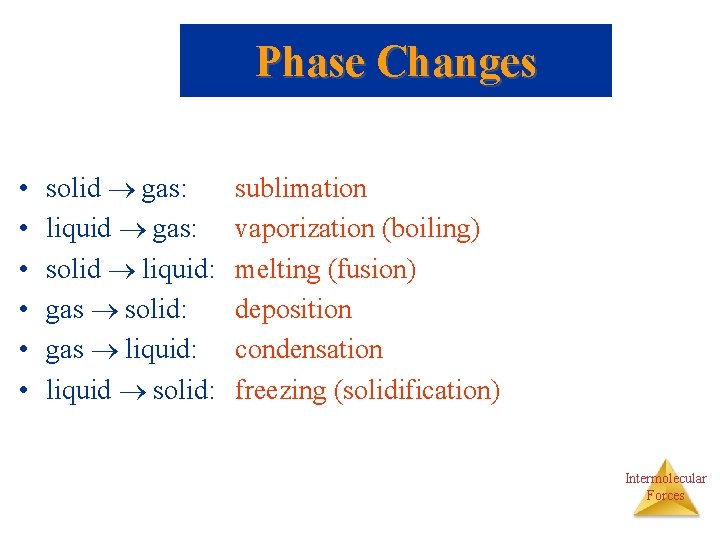
Phase Changes • • • solid gas: liquid gas: solid liquid: gas solid: gas liquid: liquid solid: sublimation vaporization (boiling) melting (fusion) deposition condensation freezing (solidification) Intermolecular Forces

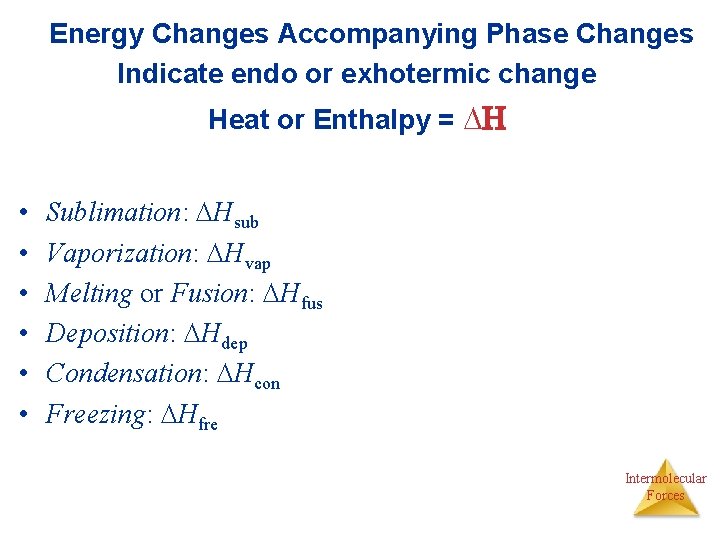
Energy Changes Accompanying Phase Changes Indicate endo or exhotermic change Heat or Enthalpy = H • • • Sublimation: Hsub Vaporization: Hvap Melting or Fusion: Hfus Deposition: Hdep Condensation: Hcon Freezing: Hfre Intermolecular Forces

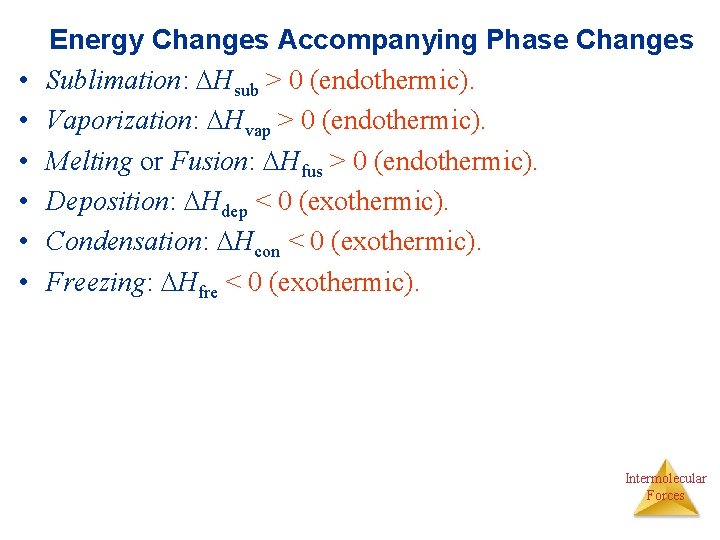
• • • Energy Changes Accompanying Phase Changes Sublimation: Hsub > 0 (endothermic). Vaporization: Hvap > 0 (endothermic). Melting or Fusion: Hfus > 0 (endothermic). Deposition: Hdep < 0 (exothermic). Condensation: Hcon < 0 (exothermic). Freezing: Hfre < 0 (exothermic). Intermolecular Forces

VAPORIZATION IS ENDOTHERMIC • In hot climates drinking water is cooled by evaporating water from the surfaces of porous clay pots. As water evaporates it ABSORBS heat from the water inside the container which is maintained cool. • Like cooling yourself off on a hot day by pouring water over your body. As water evaporates it absorbs heat Intermolecular Forces

Intermolecular Forces

Intermolecular Forces

FREEZING IS EXOTHERMIC • In freezing weather, citrus crops are sprayed with water to protect the fruit from frost damage. As the water freezes (around the fruit-outside the fruit!) it releases heat, which helps to prevent the fruit from freezing. Intermolecular Forces

Intermolecular Forces

Phase Changes Intermolecular Forces

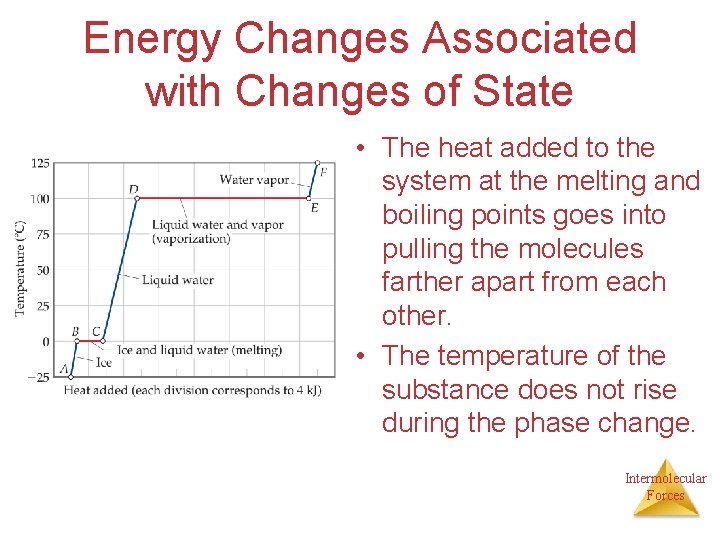
Energy Changes Associated with Changes of State • The heat added to the system at the melting and boiling points goes into pulling the molecules farther apart from each other. • The temperature of the substance does not rise during the phase change. Intermolecular Forces

• POTENTIAL ENERGY : STORED ENERGY. The energy inside the substance. • KINETIC ENERGY : Associated with motion. • Average KE = TEMPERATURE Intermolecular Forces

Endothermic Phase Changes • If the substance is melting or boiling, heat is being absorbed, and is being used to change the state of matter. • THE AVERAGE KINETIC ENERGY DOES NOT CHANGE!!! THE POTENTIAL ENERGY INCREASES. Intermolecular Forces

Exothermic changes • If the substance is undergoing condensation or freezing then heat energy is being released. The potential energy is decreasing and the TEMPERATURE REMAINS CONSTANT!!! Intermolecular Forces

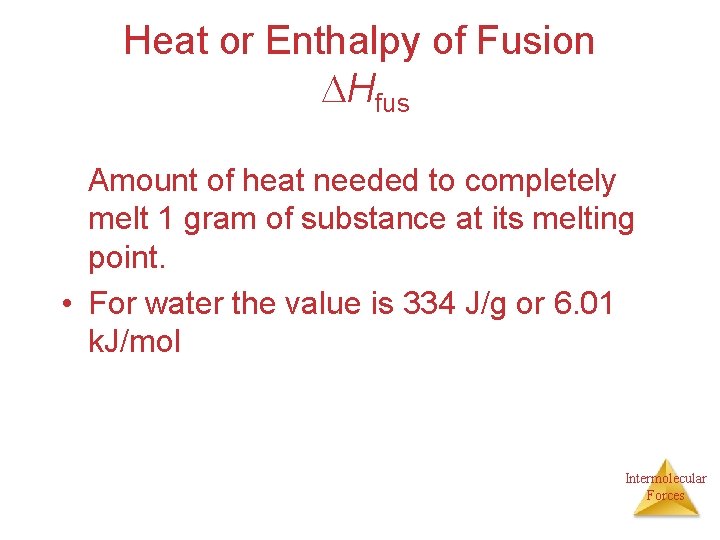
Heat or Enthalpy of Fusion Hfus Amount of heat needed to completely melt 1 gram of substance at its melting point. • For water the value is 334 J/g or 6. 01 k. J/mol Intermolecular Forces

Heat or Enthalpy of Vaporization Hvap • Heat of vaporization: amount of heat needed to completely convert 1 g of liquid to gas. • For water the value is • 2260 J/g or 40. 7 k. J/mol Intermolecular Forces

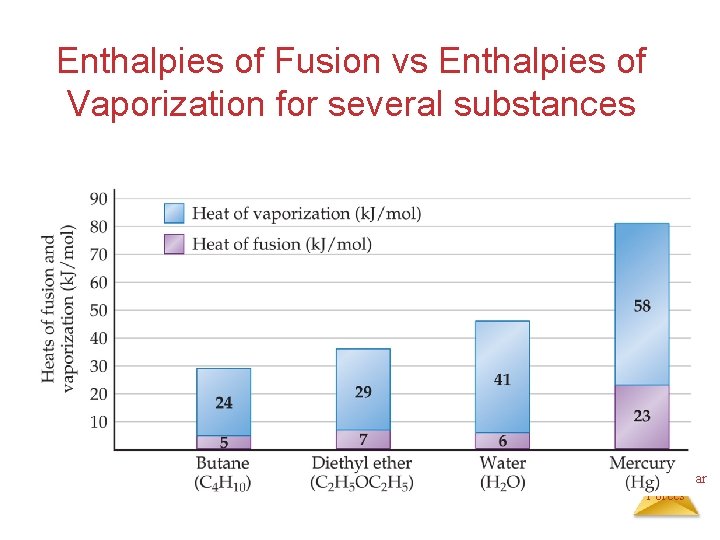
Enthalpies of Fusion vs Enthalpies of Vaporization for several substances Intermolecular Forces

• Generally heat of fusion (enthalpy of fusion) is less than heat of vaporization. – it takes more energy to completely separate molecules (from liquid to gas), than partially separate them (from solid to liquid). Intermolecular Forces

Problems How to calculate heat • • • Review of basic calorimetry problems Amount of Heat = Q = H= enthalphy C = Specific Heat Q = mass x T x C This formula can be used when there is a change in T. • During a phase change use the heat of fusion or the heat of vaporization Intermolecular Forces

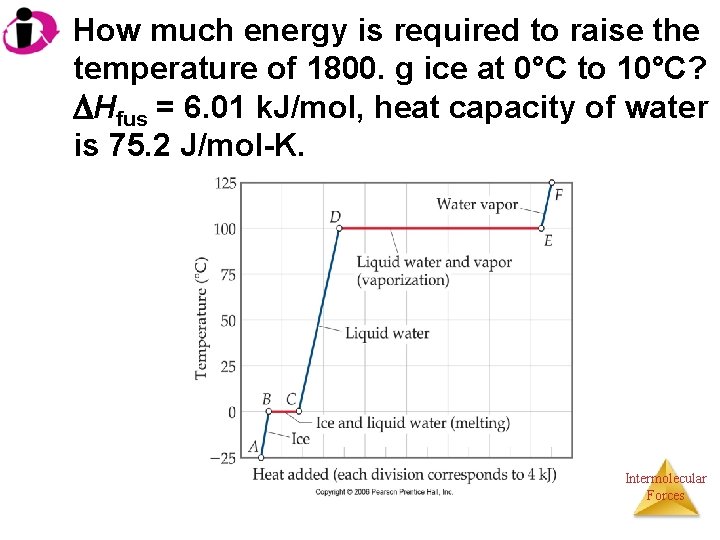
How much energy is required to raise the temperature of 1800. g ice at 0°C to 10°C? DHfus = 6. 01 k. J/mol, heat capacity of water is 75. 2 J/mol-K. Intermolecular Forces

1800 g = 100 moles of ice The enthalpy of fusion (or heat of fusion) DH =(6. 01 k. J/mol)(100 mol) = 601 k. J. To raise the water temperature 10°C requires q = (75. 2 J/mol-K)(100 mol)(10°C ) = 75. 2 k. J. Total energy = 601 k. J +75 k. J = 676 k. J Intermolecular Forces

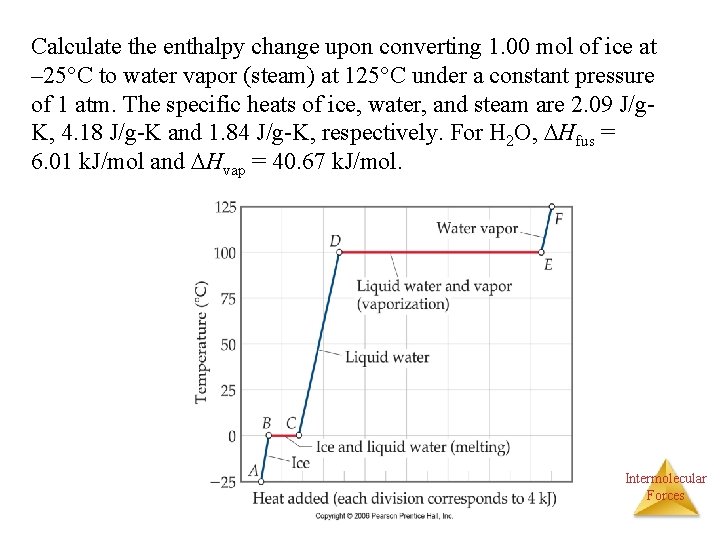
Calculate the enthalpy change upon converting 1. 00 mol of ice at – 25°C to water vapor (steam) at 125°C under a constant pressure of 1 atm. The specific heats of ice, water, and steam are 2. 09 J/g. K, 4. 18 J/g-K and 1. 84 J/g-K, respectively. For H 2 O, Hfus = 6. 01 k. J/mol and Hvap = 40. 67 k. J/mol. Intermolecular Forces

Problem # 38 p 479 • Chlorofluorocarbons of CFCs were used as refrigerants. • The heat of vaporization of CCl 2 F 2 is 289 J/g. What mass of this substance must evaporate in order to freeze 100 g of water initially at 18 0 C? Heat of fusion for water is 334 J/g and its specific heat is 4. 18 J/g. C Intermolecular Forces

Plan • 1. Find the heat that water needs to release to cool down from 18 0 C to 0 0 C • 2. Find the heat the water needs to loose to freeze. • 3. Add the results, that is the total amount of heat the water has to lose. • 4. Use the heat of evaporation of CCl 2 F 2 to find the mass needed to absorb the heat found in 3. Intermolecular Forces

Supercooling • When a liquid is cooled down so fast that the molecules do not have time to accommodate to their regular structure we get a supercooled liquid. • It is basically a liquid below the FP of it. It is unstable and it freezes suddenly if a dust particle enters the liquid or by shaking or stirring it. Intermolecular Forces

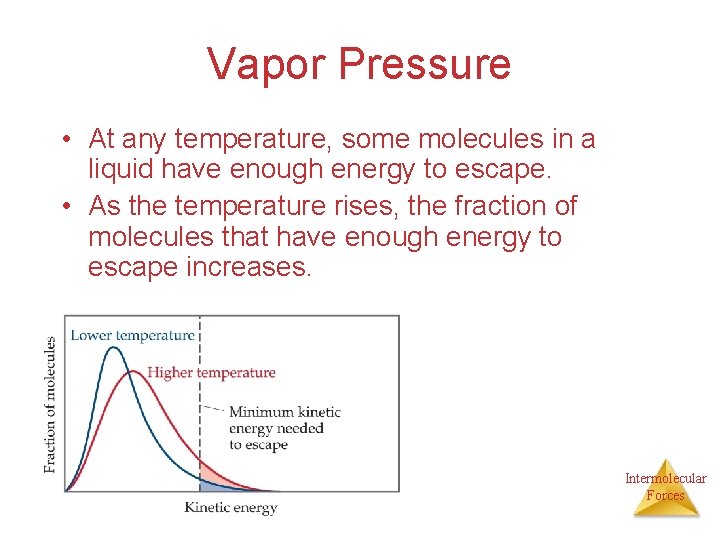
Vapor Pressure • At any temperature, some molecules in a liquid have enough energy to escape. • As the temperature rises, the fraction of molecules that have enough energy to escape increases. Intermolecular Forces

Vapor Pressure • The pressure exerted by the vapor of a liquid when vapor and liquid states are in dynamic equilibrium. • Dynamic equilibrium: 2 opposing processes occurring simultaneously with NO NET CHANGE OBSERVED!!! Intermolecular Forces

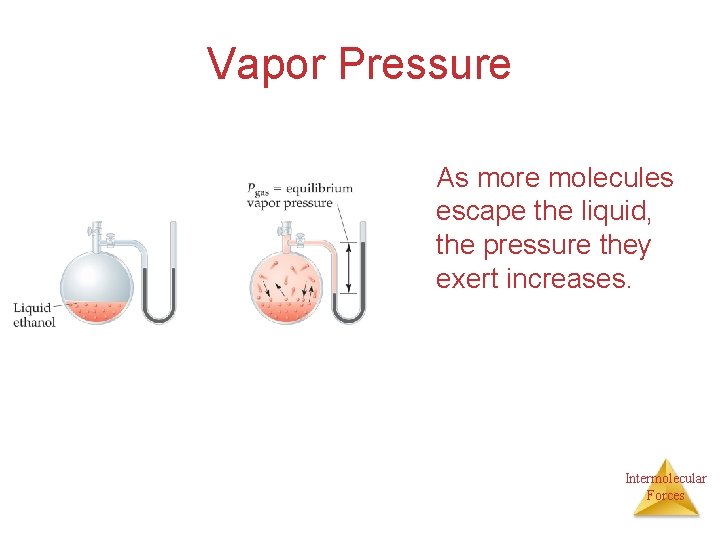
Vapor Pressure As more molecules escape the liquid, the pressure they exert increases. Intermolecular Forces

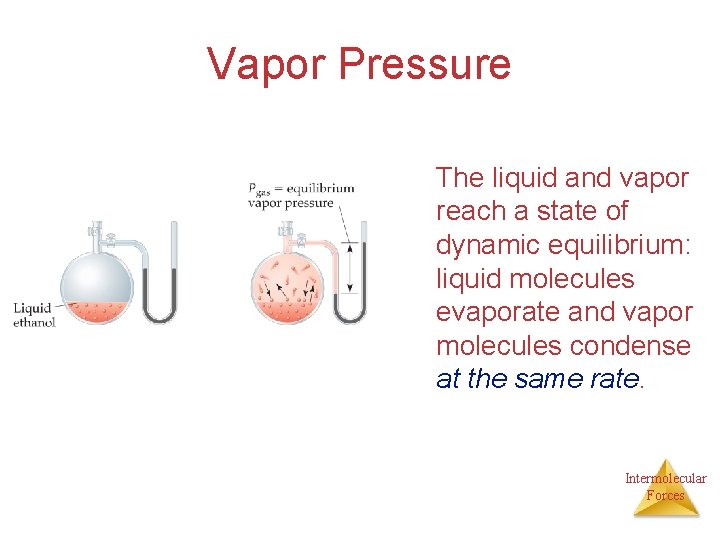
Vapor Pressure The liquid and vapor reach a state of dynamic equilibrium: liquid molecules evaporate and vapor molecules condense at the same rate. Intermolecular Forces

Vapor Pressure Explaining Vapor Pressure on the Molecular Level • Some of the molecules on the surface of a liquid have enough energy to escape the attraction of the bulk liquid. • These molecules move into the gas phase. • As the number of molecules in the gas phase increases, some of the gas phase molecules strike the surface and return to the liquid. • After some time the pressure of the gas will be constant at the vapor pressure. Intermolecular Forces

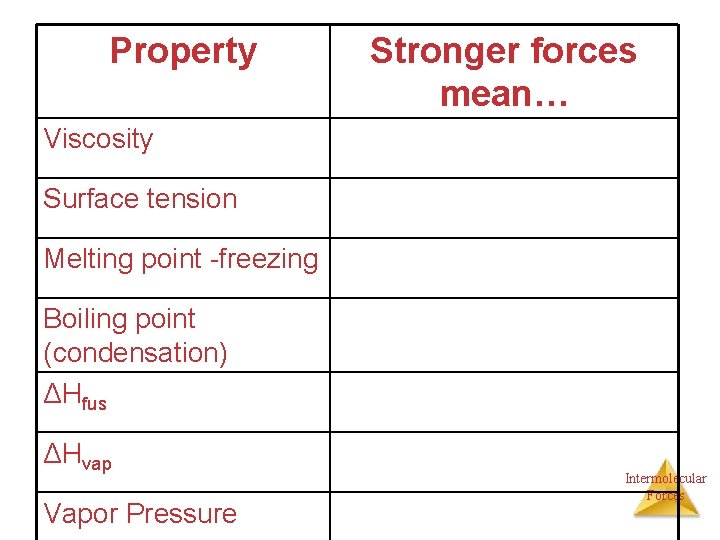
Property Stronger forces mean… Viscosity Surface tension Melting point -freezing Boiling point (condensation) ΔHfus ΔHvap Vapor Pressure Intermolecular Forces

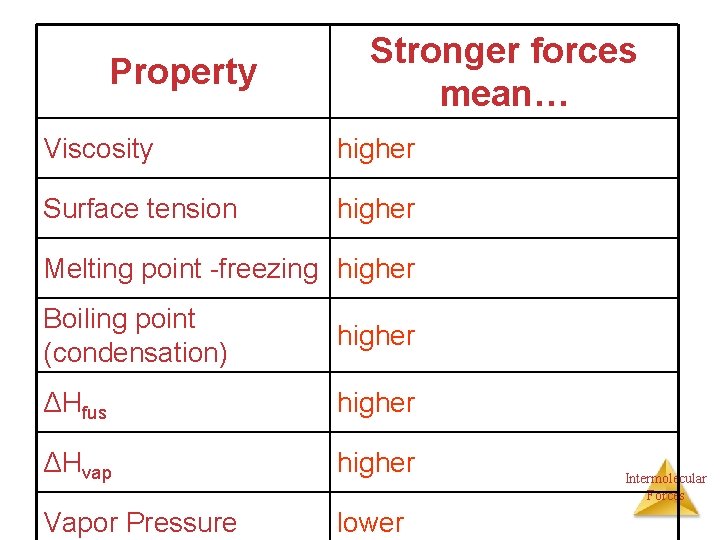
Property Stronger forces mean… Viscosity higher Surface tension higher Melting point -freezing higher Boiling point (condensation) higher ΔHfus higher ΔHvap higher Vapor Pressure lower Intermolecular Forces

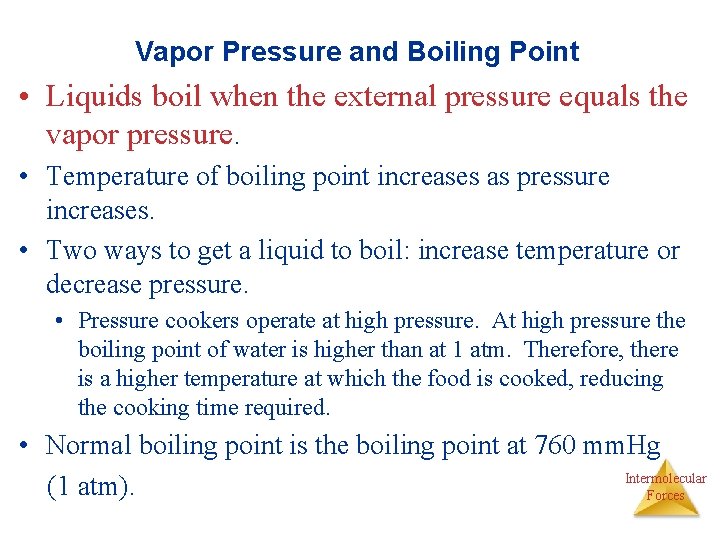
Vapor Pressure and Boiling Point • Liquids boil when the external pressure equals the vapor pressure. • Temperature of boiling point increases as pressure increases. • Two ways to get a liquid to boil: increase temperature or decrease pressure. • Pressure cookers operate at high pressure. At high pressure the boiling point of water is higher than at 1 atm. Therefore, there is a higher temperature at which the food is cooked, reducing the cooking time required. • Normal boiling point is the boiling point at 760 mm. Hg Intermolecular (1 atm). Forces

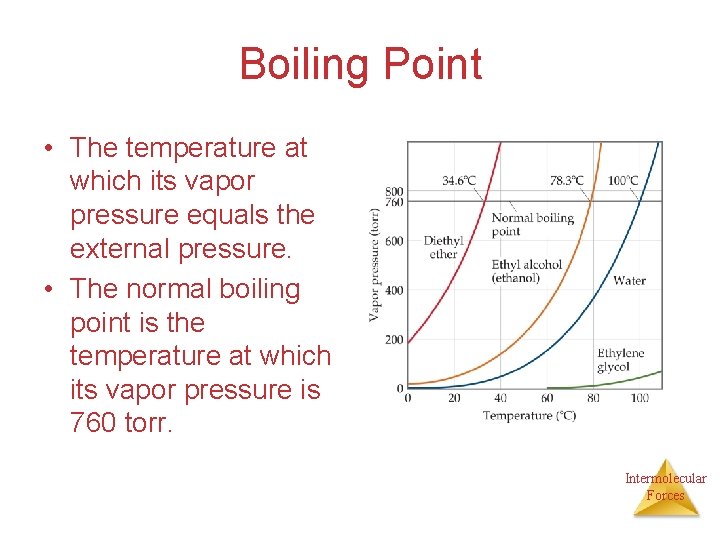
Boiling Point • The temperature at which its vapor pressure equals the external pressure. • The normal boiling point is the temperature at which its vapor pressure is 760 torr. Intermolecular Forces

Volatile Liquids • High vapor pressure – Low attraction between molecules • REMEMBER VAPOR PRESSURE DEPENDS ON TEMPERATURE! Intermolecular Forces

October 25 Section 6 • Phase diagrams P 480 Q 41 -42 *Vapor Pressure and Boiling Point P 480 43 to 55 odd only Critical Temperature and Pressure • HW Q 41 -42 51 to 55 odd only Intermolecular Forces

Phase Diagrams • Phase diagram: plot of pressure vs. Temperature summarizing all equilibria between phases. • Given a temperature and pressure, phase diagrams tell us which phase will exist. • Any temperature and pressure combination not on a curve represents a single phase. Intermolecular Forces

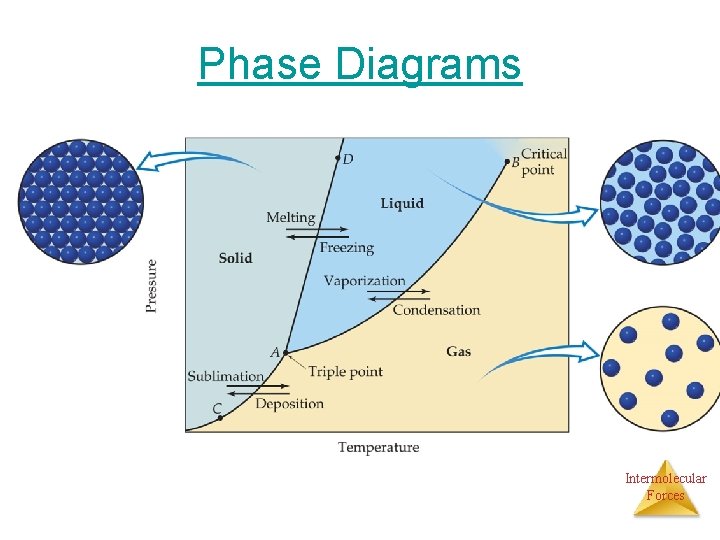
• Features of a phase diagram: – Triple point: temperature and pressure at which all three phases are in equilibrium. – Vapor-pressure curve: generally as pressure increases, temperature increases. – Critical point: critical temperature and pressure for the gas. – Melting point curve: as pressure increases, the solid phase is favored if the solid is more dense than the liquid. Intermolecular – Normal melting point: melting point at 1 atm. Forces

Phase Diagrams Intermolecular Forces

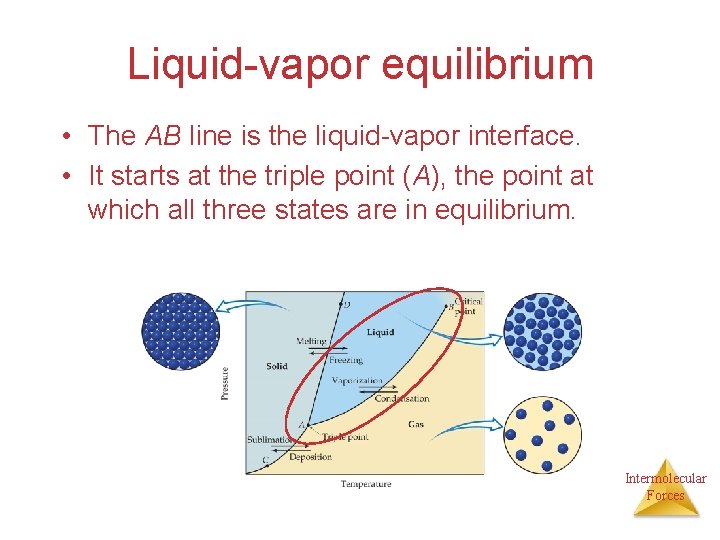
Liquid-vapor equilibrium • The AB line is the liquid-vapor interface. • It starts at the triple point (A), the point at which all three states are in equilibrium. Intermolecular Forces

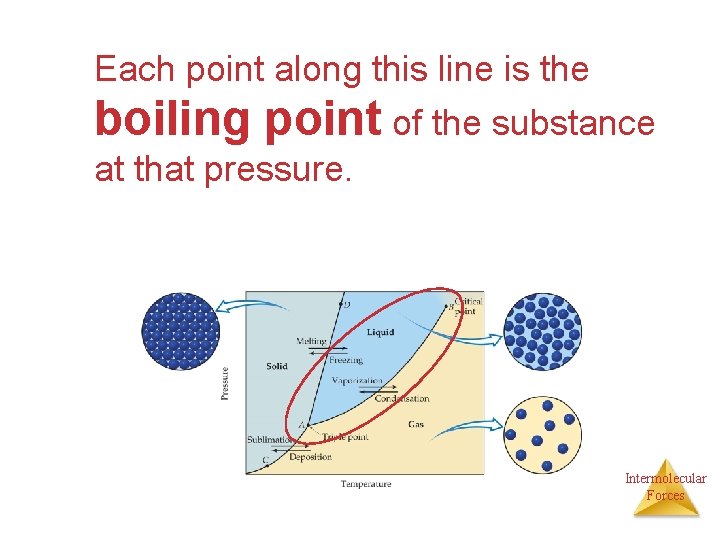
Each point along this line is the boiling point of the substance at that pressure. Intermolecular Forces

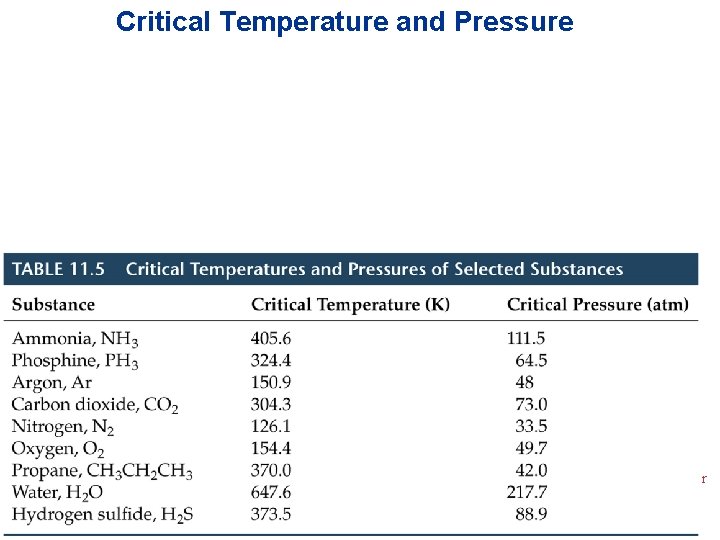
Critical Temperature • The highest temperature at which a liquid can form/exist. • Above the critical temperature no liquid can exist. • The greater the intermolecular forces the greater the Tcrit Intermolecular Forces

Critical Pressure • The pressure required to liquify the gas at the critical temperature. • When doing problems with Critical Temperatures and Pressure CHECK THE UNITS!!!! Intermolecular Forces

Supercritical Fluid Extraction • Substances at temperature and pressures higher than the critical T and P( P several hundred atm) are supercritical fluids. At these conditions they behave like special gases because their densities are similar to the liquids. Supercritical fluids can be good solvents and lowering the P or increasing T changes the solubility and that can be used to separate mixtures. • Supercritical CO 2 is used to decaffeinate coffee. Intermolecular Forces

Critical Temperature and Pressure Intermolecular Forces

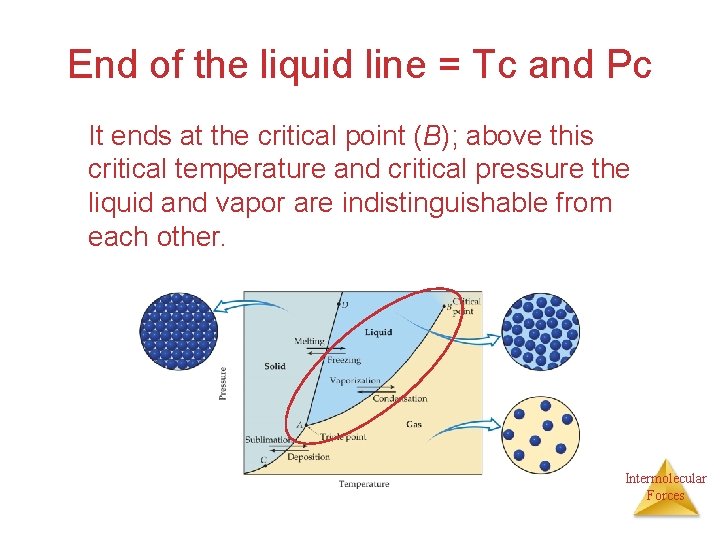
End of the liquid line = Tc and Pc It ends at the critical point (B); above this critical temperature and critical pressure the liquid and vapor are indistinguishable from each other. Intermolecular Forces

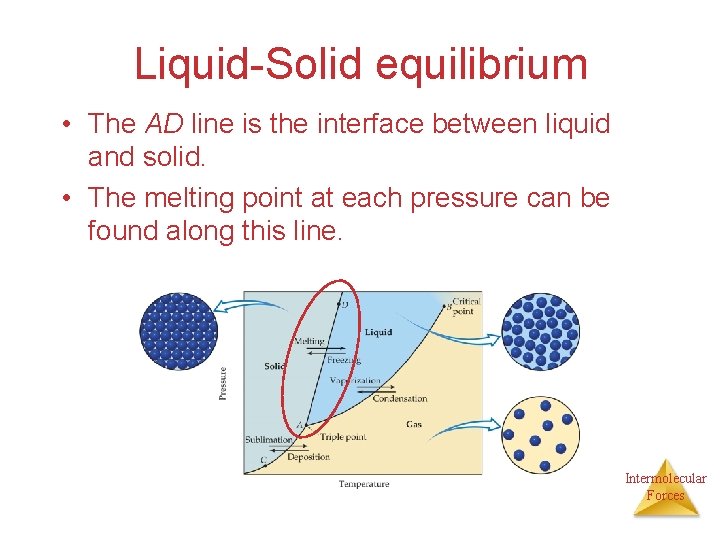
Liquid-Solid equilibrium • The AD line is the interface between liquid and solid. • The melting point at each pressure can be found along this line. Intermolecular Forces

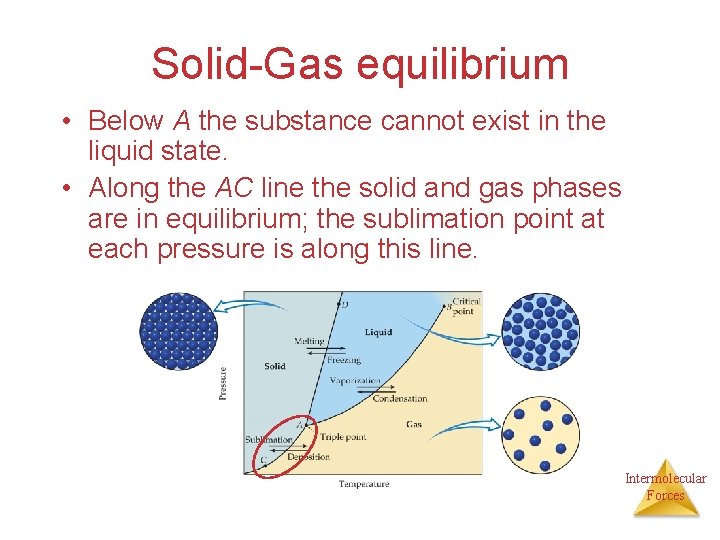
Solid-Gas equilibrium • Below A the substance cannot exist in the liquid state. • Along the AC line the solid and gas phases are in equilibrium; the sublimation point at each pressure is along this line. Intermolecular Forces

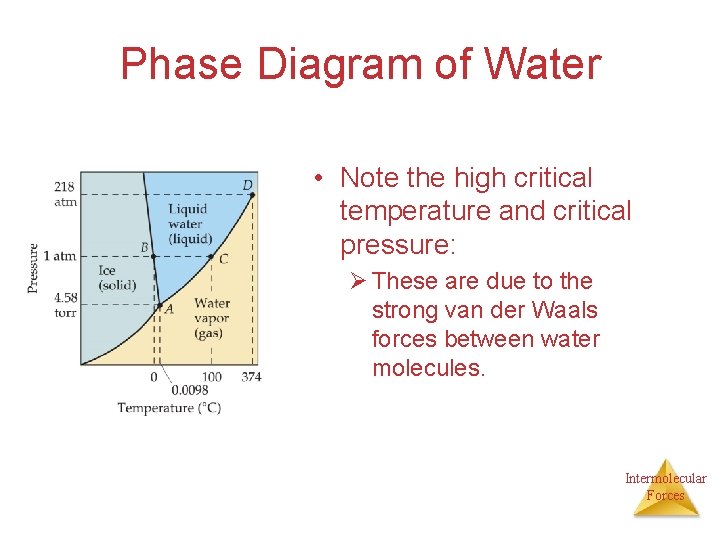
Phase Diagram of Water • Note the high critical temperature and critical pressure: Ø These are due to the strong van der Waals forces between water molecules. Intermolecular Forces

Remember Le Chatelier’s principle, increasing the pressure shifts the equilibrium to the side that occupies less volume. Since ice occupies more volume than liquid when we increase the pressure ice melts! (the equilibrium shifts to the water). Most substances are the opposite because the solid phase is more dense that the liquid phase. Intermolecular Forces

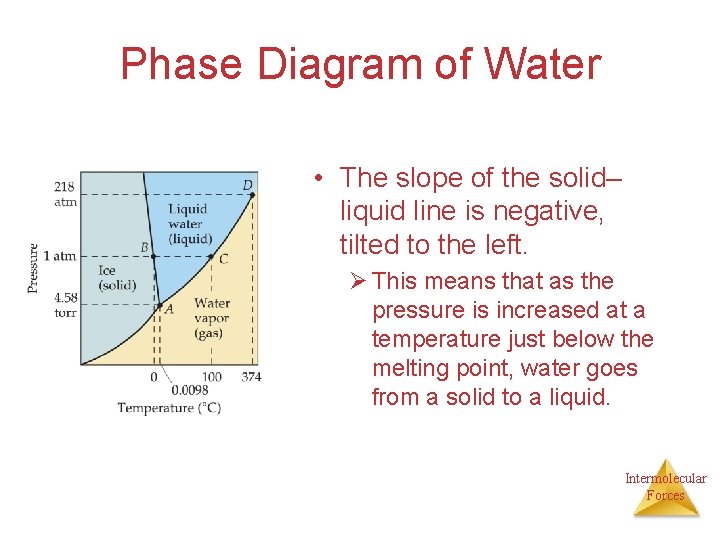
Phase Diagram of Water • The slope of the solid– liquid line is negative, tilted to the left. Ø This means that as the pressure is increased at a temperature just below the melting point, water goes from a solid to a liquid. Intermolecular Forces

• For most substances the solid form is denser than the liquid form and an increase in P favors the solid, so to change it to liquid more T is needed. This results in an slope towards the right (positive slope). Intermolecular Forces

• The slope to the left is abnormal and is due to the fact that water is less dense in the solid state (why? ? ? ), so increasing the pressure favors the liquid state. Intermolecular Forces

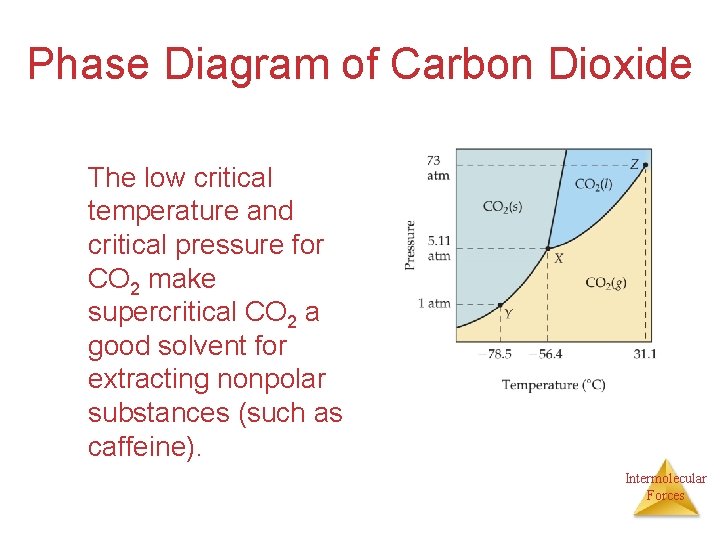
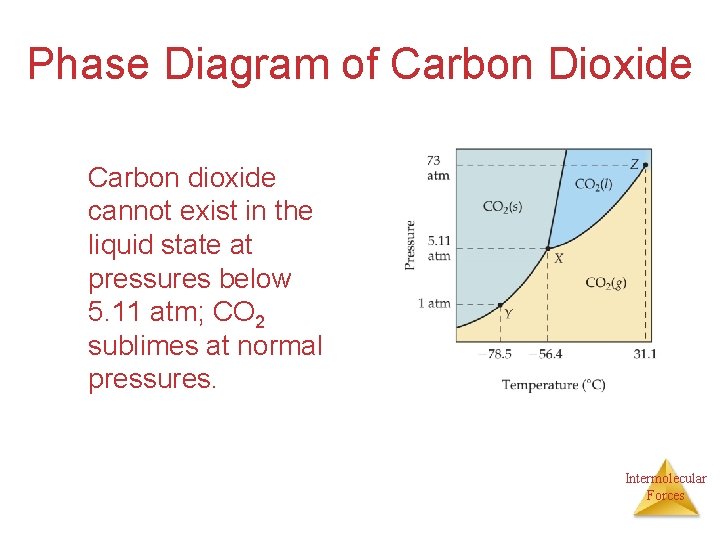
Phase Diagram of Carbon Dioxide The low critical temperature and critical pressure for CO 2 make supercritical CO 2 a good solvent for extracting nonpolar substances (such as caffeine). Intermolecular Forces

Phase Diagram of Carbon Dioxide Carbon dioxide cannot exist in the liquid state at pressures below 5. 11 atm; CO 2 sublimes at normal pressures. Intermolecular Forces

• Water: – Why does the melting point curve slope to the left? – What are the temperature and pressure at the triple point? – What are the normal freezing and boiling points? – What are the critical temperature and pressure? – What change occurs at 50 C as the pressure is decreased from 1. 0 Intermolecular atm to 0. 0010 atm? Forces

• Water: – Why does the melting point curve slope to the left? ice is less dense than water – What are the temperature and pressure at the triple point? 0. 0098 C and 4. 58 mm. Hg – What are the normal freezing and boiling points? Freezing = 0 C and Boiling = 100 C – What are the critical temperature and pressure? 374 C and 218 atm – What change occurs at 50 C as the pressure is decreased from 1. 0 Intermolecular atm to 0. 0010 atm? Forces vaporization

• Carbon Dioxide: – At what temperature and pressure does the triple point occur? – What is the normal sublimation point? – What is the critical point? – What change occurs at 30. atm as you move from -60˚C to 0˚C? Intermolecular Forces

• Carbon Dioxide: – At what temperature and pressure does the triple point occur? -56. 4 C and 5. 11 atm – What is the normal sublimation point? -78. 5 C – What is the critical point? 31. 1 C and 73 atm – What change occurs at 30. atm as you move from -60˚C to 0˚C? melting Intermolecular Forces

October 26 SECTION 7 & 8 • Tomorrow two period test on chapter 10 & 11 • We’ll begin with chapter 6 – Atomic Structure next Monday. Print slides on Sunday. Structure of solids • Bonding in solids • HW 57, 71, 73, 75, 77 Intermolecular Forces

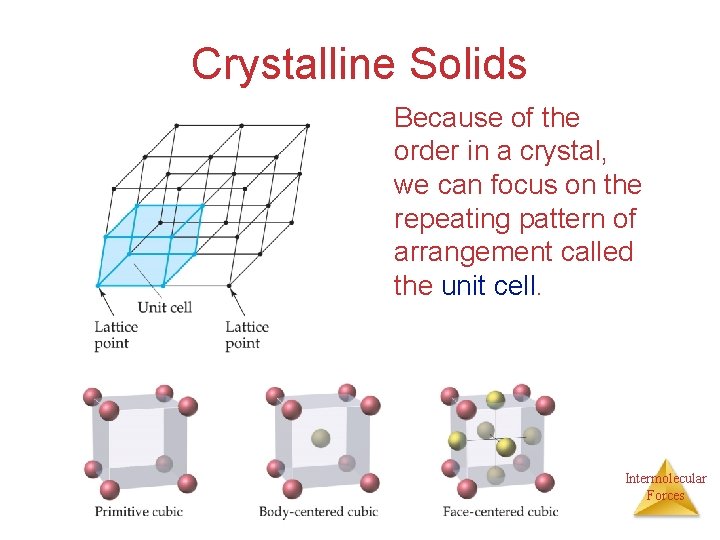
Structures of Solids • • • Unit Cells Crystalline solid: well-ordered, definite arrangements of molecules, atoms or ions. Crystals have an ordered, repeated structure. The smallest repeating unit in a crystal is a unit cell. Unit cell is the smallest unit with all the symmetry of the entire crystal. Three-dimensional stacking of unit cells is the crystal lattice. Intermolecular Forces

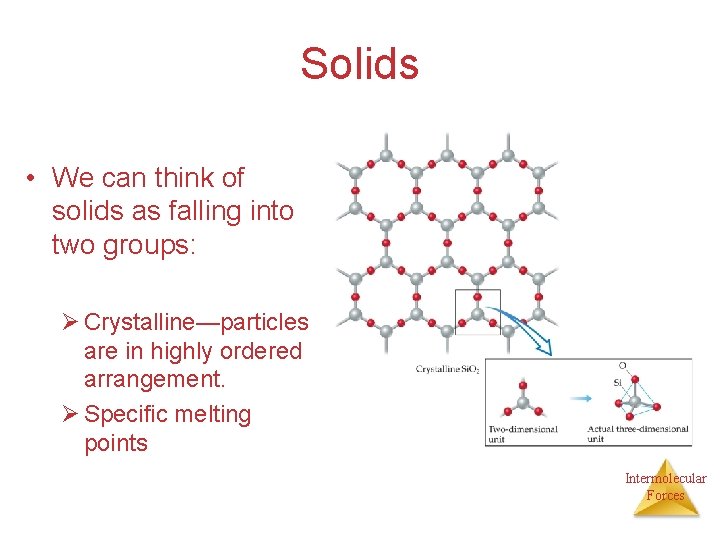
Solids • We can think of solids as falling into two groups: Ø Crystalline—particles are in highly ordered arrangement. Ø Specific melting points Intermolecular Forces

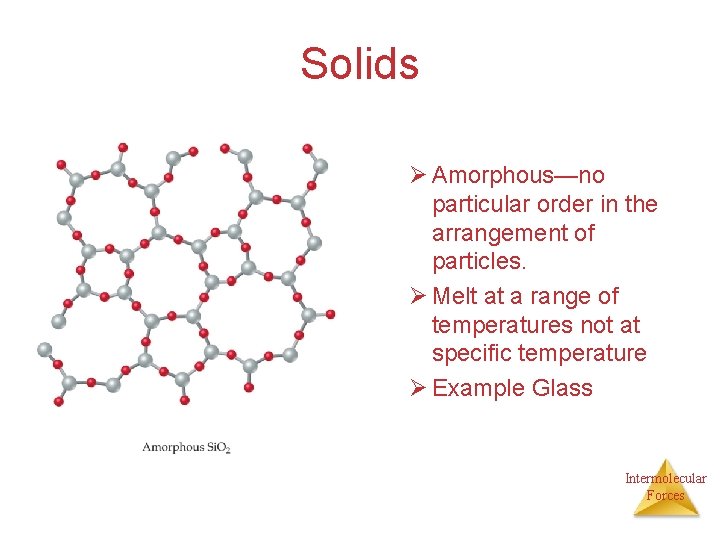
Solids Ø Amorphous—no particular order in the arrangement of particles. Ø Melt at a range of temperatures not at specific temperature Ø Example Glass Intermolecular Forces

Crystalline Solids Because of the order in a crystal, we can focus on the repeating pattern of arrangement called the unit cell. Intermolecular Forces

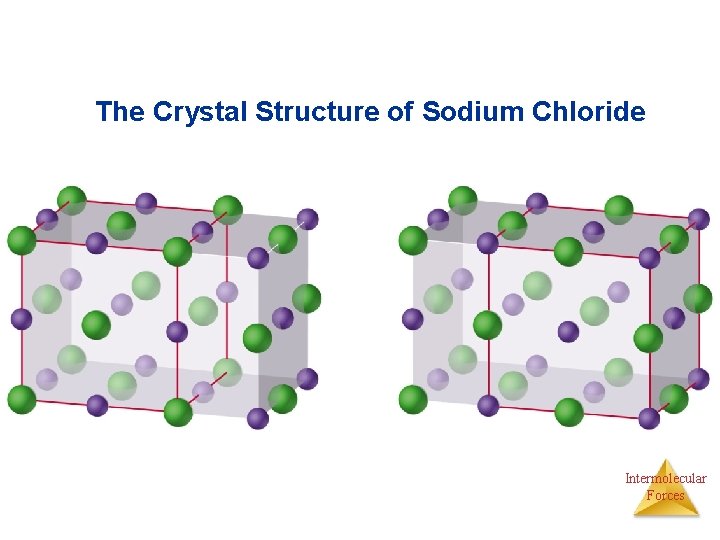
The Crystal Structure of Sodium Chloride Intermolecular Forces

• The unit cell is the smallest repeating unit that has all of the symmetry characteristic of the way atoms/ions or molecules are arranged in the crystal. • It reflects the Stoichiometry of the solid. Intermolecular Forces

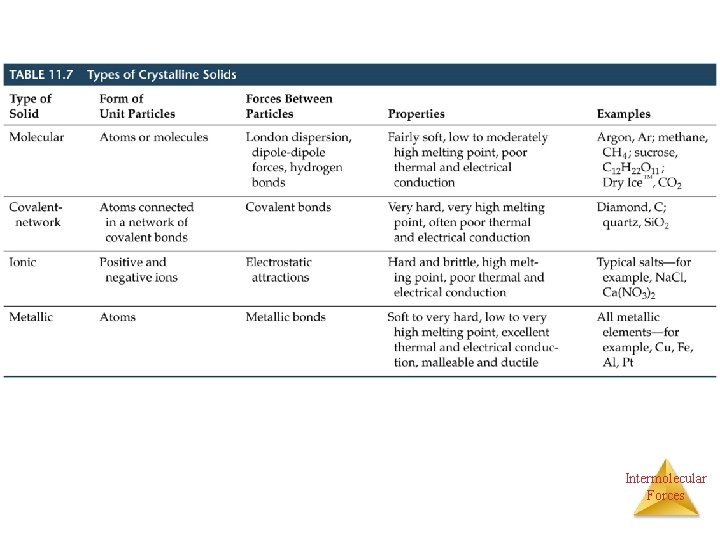
Bonding in Solids • There are four types of solid: – Molecular (formed from molecules) - usually soft with low melting points and poor conductivity. – Covalent network (formed from atoms) - very hard with very high melting points and poor conductivity. – Ionic (formed from ions) - hard, brittle, high melting points and poor conductivity. – Metallic (formed from metal atoms) - soft or hard, high melting points, good conductivity, malleable and ductile. Intermolecular Forces

Intermolecular Forces

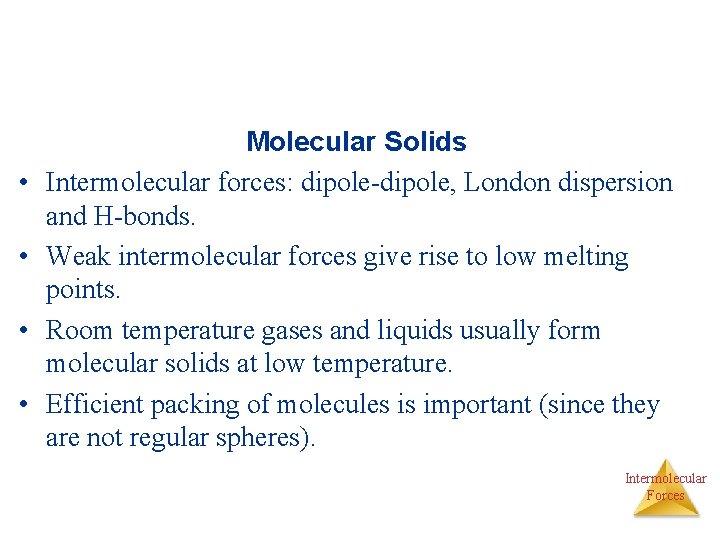
• • Molecular Solids Intermolecular forces: dipole-dipole, London dispersion and H-bonds. Weak intermolecular forces give rise to low melting points. Room temperature gases and liquids usually form molecular solids at low temperature. Efficient packing of molecules is important (since they are not regular spheres). Intermolecular Forces

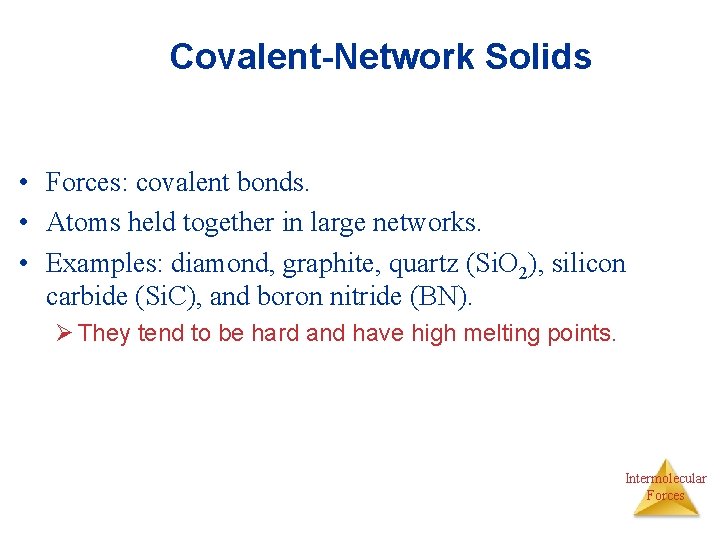
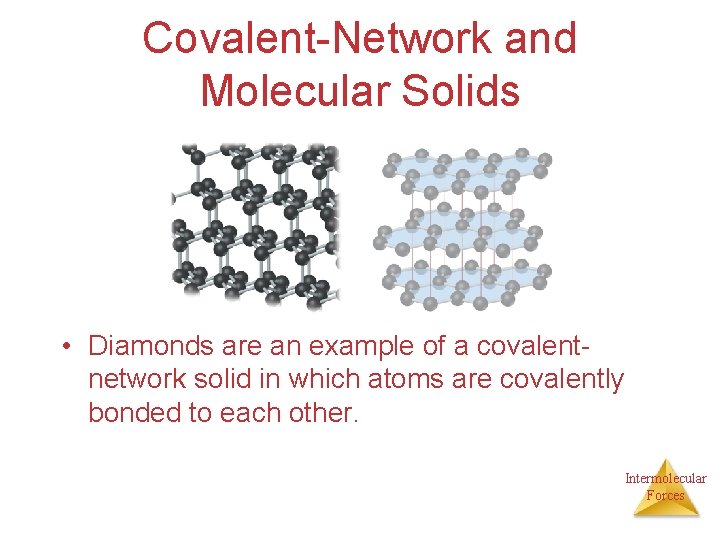
Covalent-Network Solids • Forces: covalent bonds. • Atoms held together in large networks. • Examples: diamond, graphite, quartz (Si. O 2), silicon carbide (Si. C), and boron nitride (BN). Ø They tend to be hard and have high melting points. Intermolecular Forces

Covalent-Network and Molecular Solids • Diamonds are an example of a covalentnetwork solid in which atoms are covalently bonded to each other. Intermolecular Forces

Diamond Øeach C atom has a coordination number of 4; each C atom is tetrahedral; there is a three-dimensional array of atoms. ØDiamond is hard, and has a high melting point (3550 C). Øcontain orbitals or bands of delocalized electrons that belong not to single atoms but to each crystal as a whole Intermolecular Forces

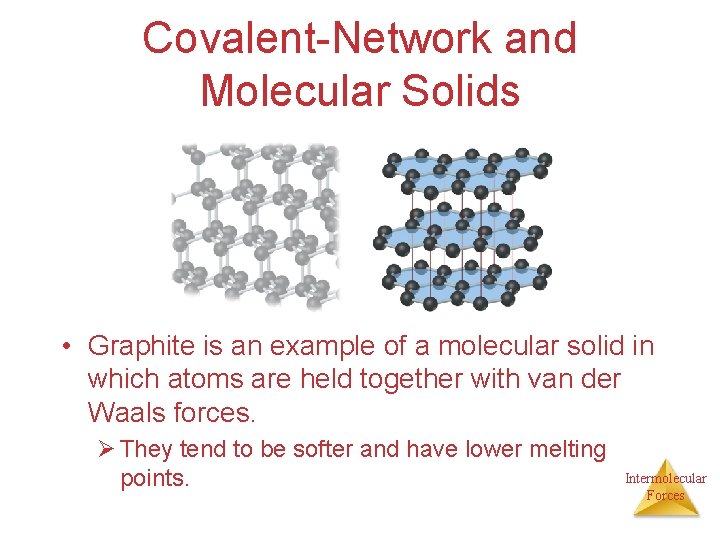
Covalent-Network and Molecular Solids • Graphite is an example of a molecular solid in which atoms are held together with van der Waals forces. Ø They tend to be softer and have lower melting points. Intermolecular Forces

• In graphite – each C atom is arranged in a planar hexagonal ring; – layers of interconnected rings are placed on top of each other; – the distance between C atoms is close to benzene (1. 42 Å vs. 1. 395 Å in benzene); – the distance between layers is large (3. 41 Å); – electrons move in delocalized orbitals (good conductor). Intermolecular Forces

Silicon dioxide • Has a high melting point - around 1700°C. Very strong silicon-oxygen covalent bonds have to be broken throughout the structure before melting occurs. • It is hard. This is due to the need to break the very strong covalent bonds. • Doesn't conduct electricity. There aren't any delocalized electrons. All the electrons are held tightly between the atoms, and aren't free to move. • It is insoluble in water and organic solvents. There are no possible attractions which could occur between solvent molecules and the silicon or oxygen atoms which could overcome the covalent bonds in the giant structure. • Giant covalent structures are arranged in a continuous lattice. This structure is very strong because of the Intermolecular Forces strong forces between the molecules.

Ionic Solids • Ions (spherical) held together by electrostatic forces of attraction. • There are some simple classifications for ionic lattice types. Intermolecular Forces

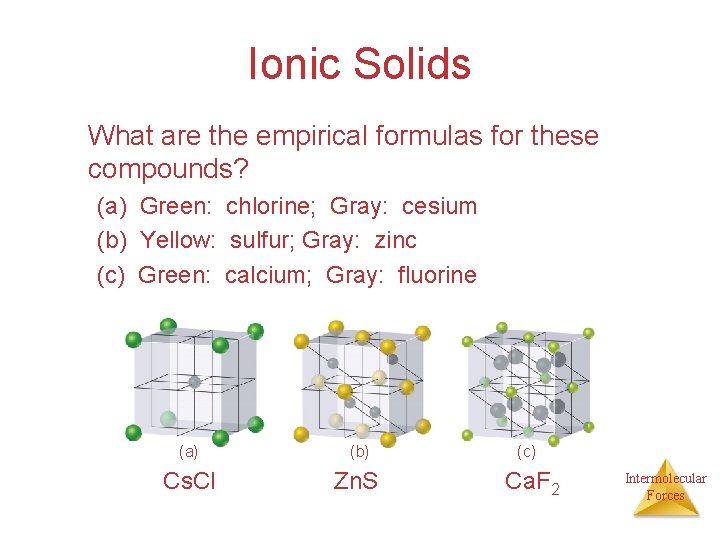
Ionic Solids What are the empirical formulas for these compounds? (a) Green: chlorine; Gray: cesium (b) Yellow: sulfur; Gray: zinc (c) Green: calcium; Gray: fluorine (a) (b) Cs. Cl Zn. S (c) Ca. F 2 Intermolecular Forces

• Na. Cl Structure • Each ion has a coordination number of 6. • Face-centered cubic lattice. • Cation to anion ratio is 1: 1. • Examples: Li. F, KCl, Ag. Cl and Ca. O. • Cs. Cl Structure • Cs+ has a coordination number of 8. • Different from the Na. Cl structure (Cs+ is larger than Na+). • Cation to anion ratio is 1: 1. Intermolecular Forces

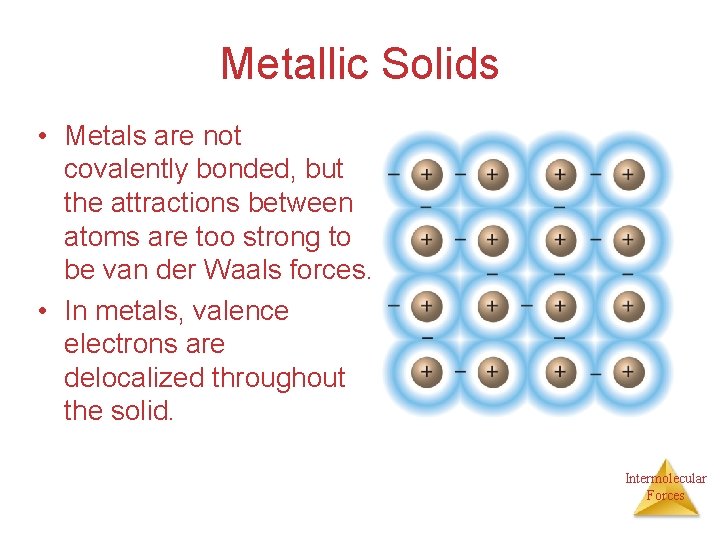
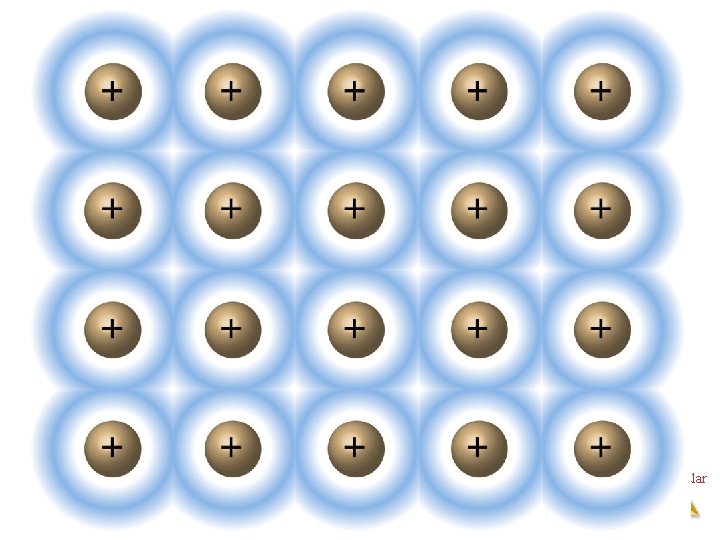
Metallic Solids • Metals are not covalently bonded, but the attractions between atoms are too strong to be van der Waals forces. • In metals, valence electrons are delocalized throughout the solid. Intermolecular Forces

Metals • Closely packed lattice with delocalize electrons throughout • The metal nuclei float in a sea of electrons. • Metals conduct because the electrons are delocalized and are mobile Intermolecular Forces

Intermolecular Forces