Chapter 11 Liquids solids and intermolecular forces Intermolecular












- Slides: 41

Chapter 11 Liquids, solids, and intermolecular forces

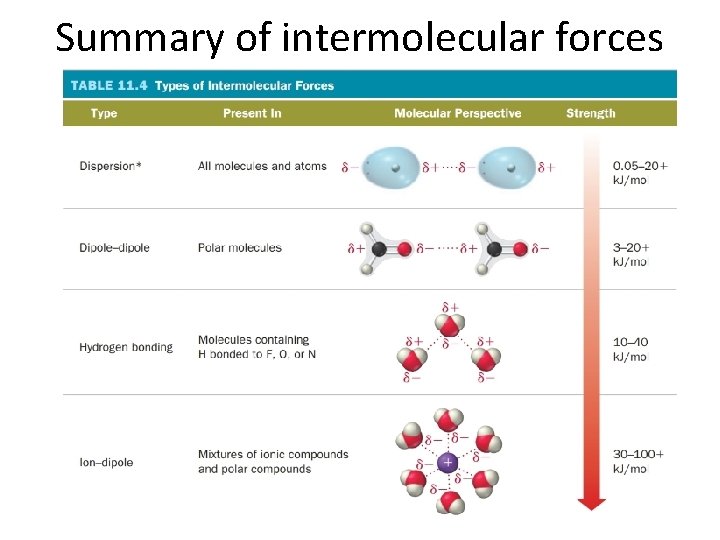
Intermolecular forces • Intermolecular forces – attractive forces between particles composed of matter (atoms, ions, and molecules) • These forces dictate many properties of an atom – Melting point, boiling point, pattern in a lattice, miscibility, solubility, etc. • Driving factor for intermolecular forces is Coulomb’s law!!!! – Positive and negative charges attract!!! • Depending on the magnitude of the negative or positve charge, the intermolecular force that attracts the particles will be stronger or weaker

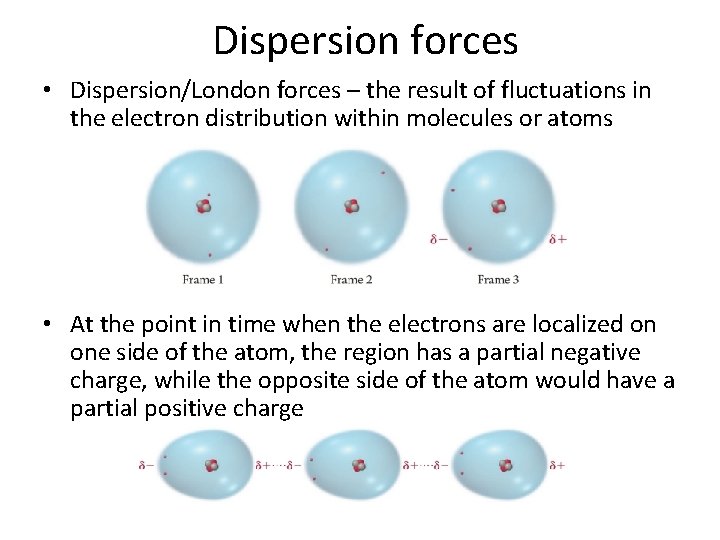
Dispersion forces • Dispersion/London forces – the result of fluctuations in the electron distribution within molecules or atoms • At the point in time when the electrons are localized on one side of the atom, the region has a partial negative charge, while the opposite side of the atom would have a partial positive charge

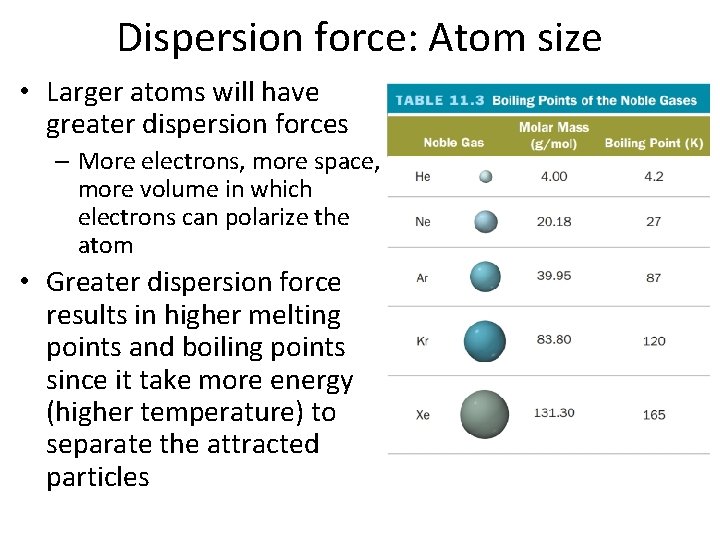
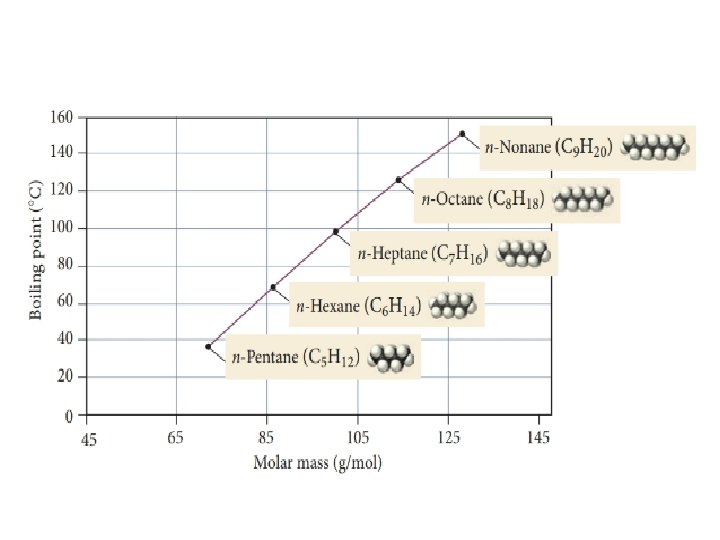
Dispersion force: Atom size • Larger atoms will have greater dispersion forces – More electrons, more space, more volume in which electrons can polarize the atom • Greater dispersion force results in higher melting points and boiling points since it take more energy (higher temperature) to separate the attracted particles

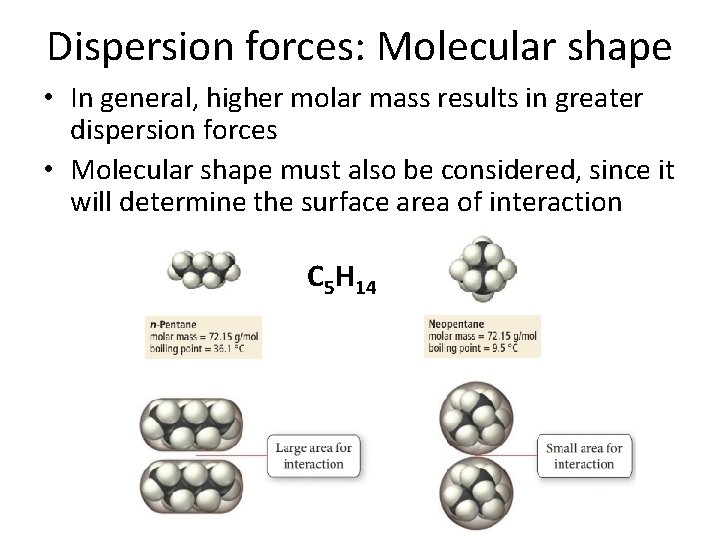
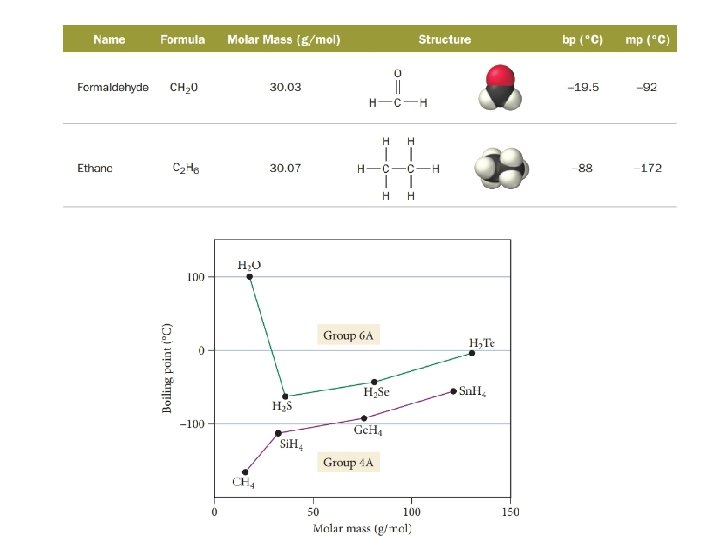
Dispersion forces: Molecular shape • In general, higher molar mass results in greater dispersion forces • Molecular shape must also be considered, since it will determine the surface area of interaction C 5 H 14


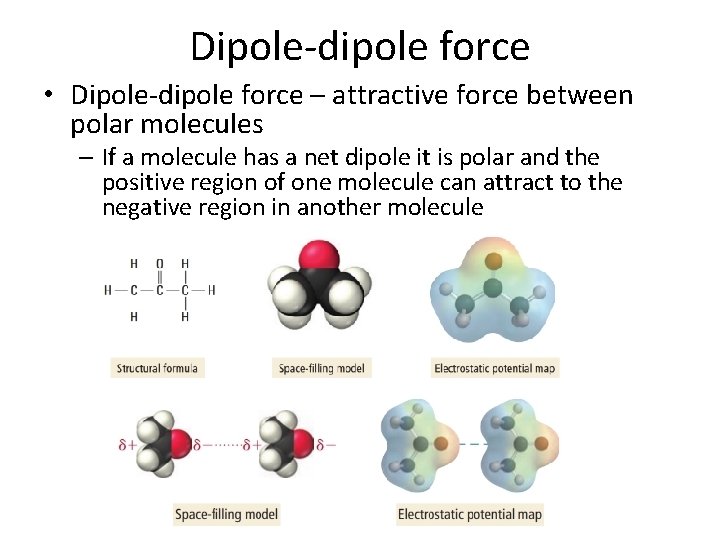
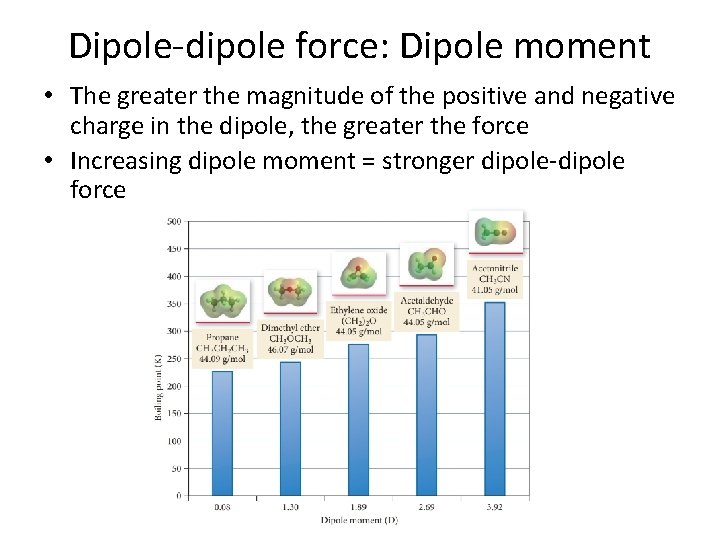
Dipole-dipole force • Dipole-dipole force – attractive force between polar molecules – If a molecule has a net dipole it is polar and the positive region of one molecule can attract to the negative region in another molecule

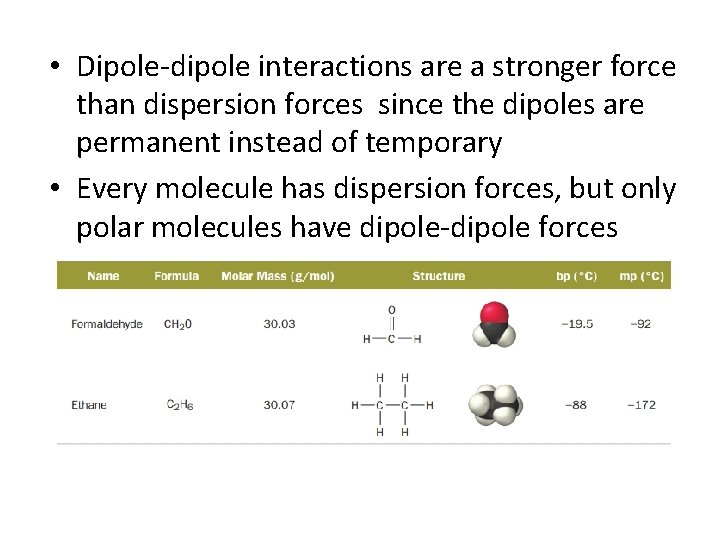
• Dipole-dipole interactions are a stronger force than dispersion forces since the dipoles are permanent instead of temporary • Every molecule has dispersion forces, but only polar molecules have dipole-dipole forces

Dipole-dipole force: Dipole moment • The greater the magnitude of the positive and negative charge in the dipole, the greater the force • Increasing dipole moment = stronger dipole-dipole force

Dipole-dipole force: Miscibility • Miscibility – the ability to mix without separating into two states • Dipole-dipole forces are present between one polar molecule and another, so they will be miscible • Dipole-dipole forces are not present between polar molecules and nonpolar molecules – Water is a polar molecule – Oil is a non polar molecule – Water and oil are not miscible, they don’t mix

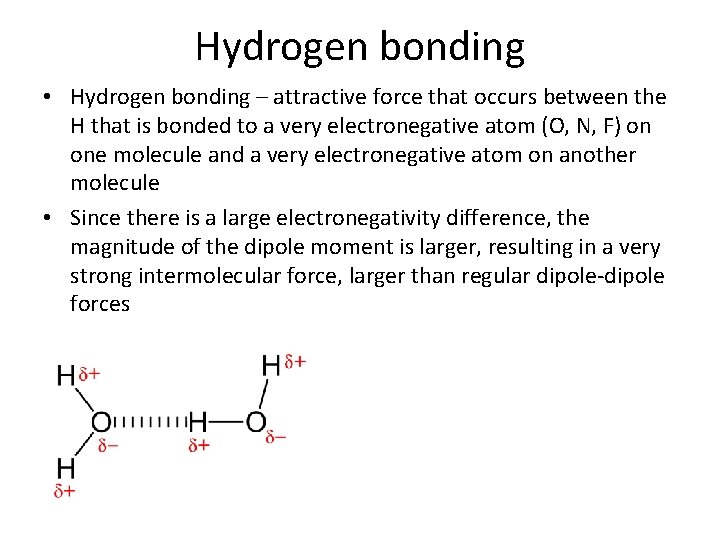
Hydrogen bonding • Hydrogen bonding – attractive force that occurs between the H that is bonded to a very electronegative atom (O, N, F) on one molecule and a very electronegative atom on another molecule • Since there is a large electronegativity difference, the magnitude of the dipole moment is larger, resulting in a very strong intermolecular force, larger than regular dipole-dipole forces


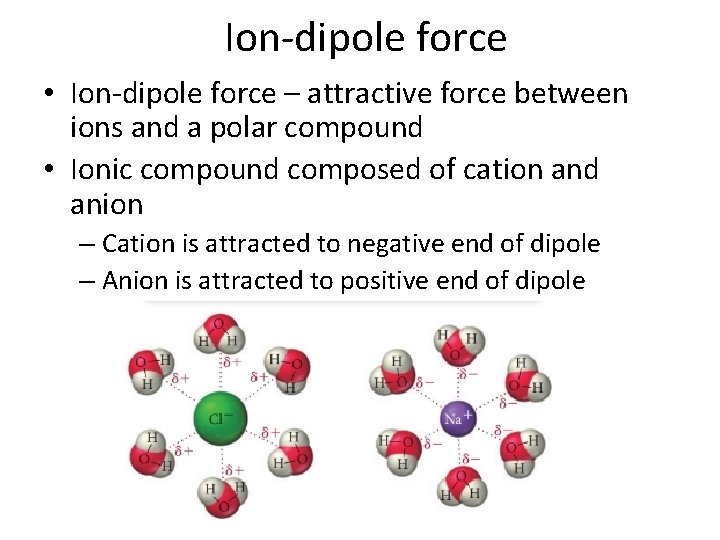
Ion-dipole force • Ion-dipole force – attractive force between ions and a polar compound • Ionic compound composed of cation and anion – Cation is attracted to negative end of dipole – Anion is attracted to positive end of dipole

Summary of intermolecular forces

Practice Identify the major interaction expected for the following molecules • Cl 2 • NH 3 • C 10 H 22 • KI • HBr Identify the major interaction expected between the following molecules • Water and ammonia (NH 3) • Formaldehyde (CH 2 O) and methane (CH 4)

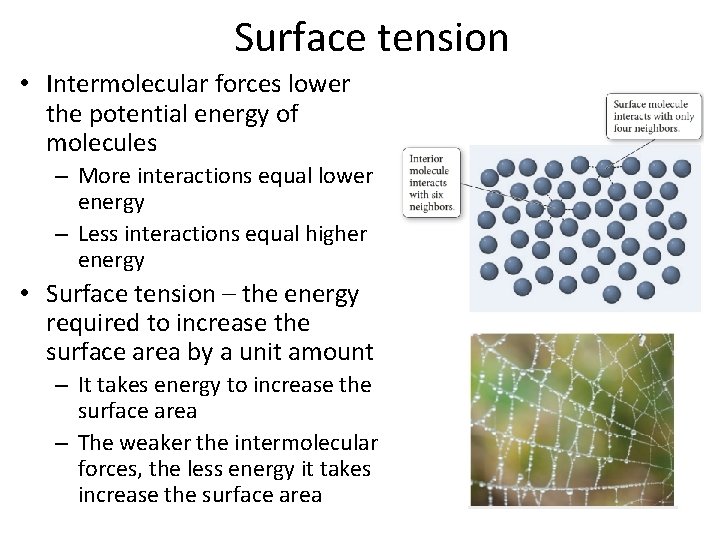
Surface tension • Intermolecular forces lower the potential energy of molecules – More interactions equal lower energy – Less interactions equal higher energy • Surface tension – the energy required to increase the surface area by a unit amount – It takes energy to increase the surface area – The weaker the intermolecular forces, the less energy it takes increase the surface area

Surface tension continued • Placing an object on the surface will increase the surface area of the liquid • Even if the object is more dense, it needs to overcome the surface tension of the liquid before it can sink, it needs to overcome the energy to move interior molecules to the surface

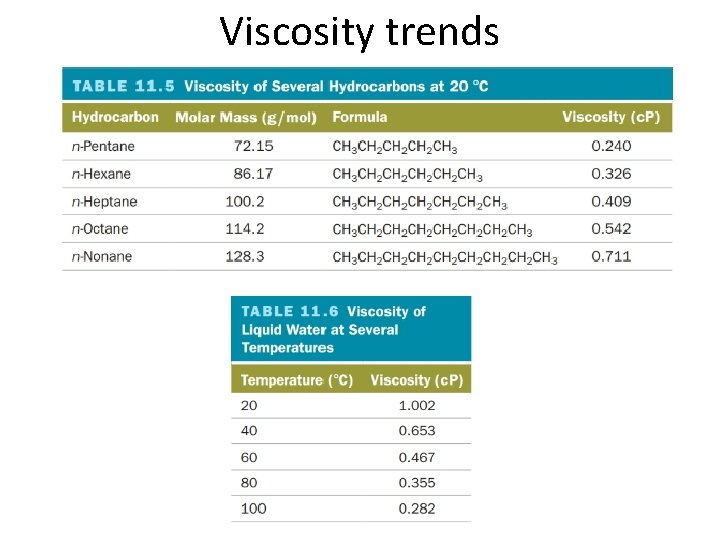
Viscosity • Intermolecular forces attract molecules to each other, making them less likely to flow • Viscosity – the resistance of a liquid to flow – Lower viscosity, faster flow • Water – Higher viscosity, slower flow • Maple syrup • All factors that enhance intermolecular forces (force type, shape and size) will affect viscosity • Temperature also affects viscosity – Higher temperature, lower viscosity – Lower temperature, higher viscosity

Viscosity trends

Capillary action • Capillary action – the ability of a liquid to flow against gravity up a narrow tube • Cohesive force – the attraction of the molecules in the liquid to each other • Adhesive force – the attraction of the molecules with the surface of the tube • Adhesive forces pull the liquid up a tube until gravity balances out the capillary action

Tube width and meniscus shape • Liquid will travel higher up more narrower tubes – Narrower tube increases adhesive forces per volume of liquid • Stronger adhesive forces cause liquid to creep up the sides to produce a concave meniscus shape • If cohesive forces are stronger then the meniscus will have a convex shape

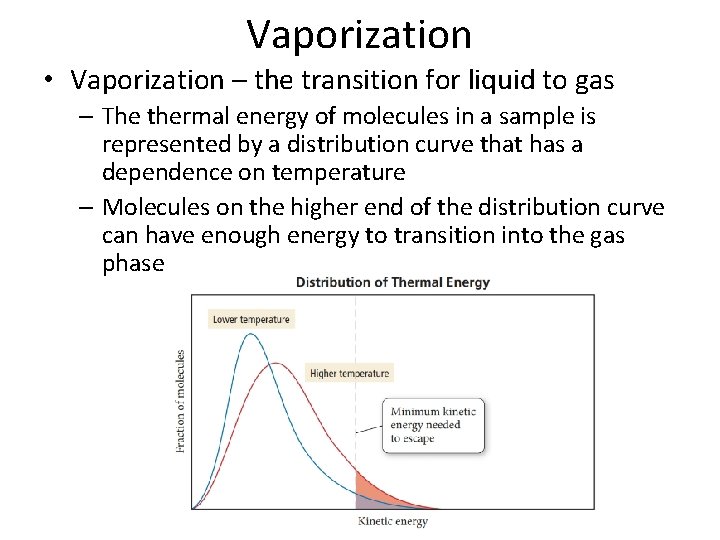
Vaporization • Vaporization – the transition for liquid to gas – The thermal energy of molecules in a sample is represented by a distribution curve that has a dependence on temperature – Molecules on the higher end of the distribution curve can have enough energy to transition into the gas phase

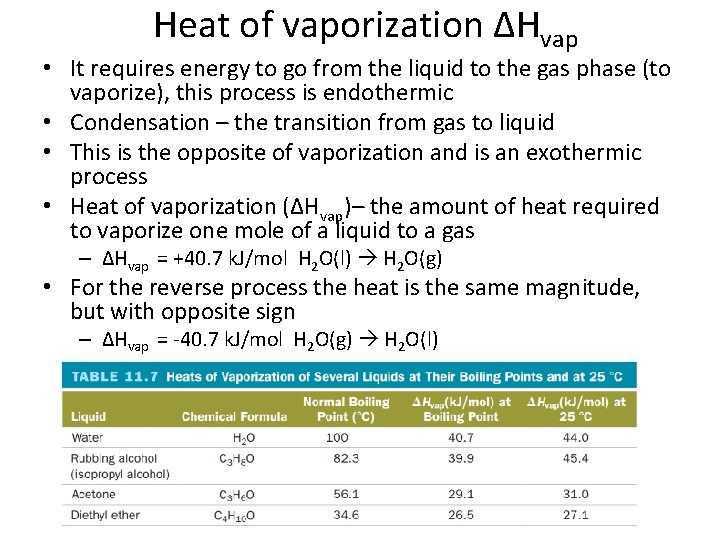
Heat of vaporization ΔHvap • It requires energy to go from the liquid to the gas phase (to vaporize), this process is endothermic • Condensation – the transition from gas to liquid • This is the opposite of vaporization and is an exothermic process • Heat of vaporization (ΔHvap)– the amount of heat required to vaporize one mole of a liquid to a gas – ΔHvap = +40. 7 k. J/mol H 2 O(l) H 2 O(g) • For the reverse process the heat is the same magnitude, but with opposite sign – ΔHvap = -40. 7 k. J/mol H 2 O(g) H 2 O(l)

Practice • How much heat does it take to boil 1 mol of water? • How much heat does it take to boil 45. 8 g of water? • Calculate the mass of water (in grams) that can be vaporized at its boiling point with 155 k. J of heat.

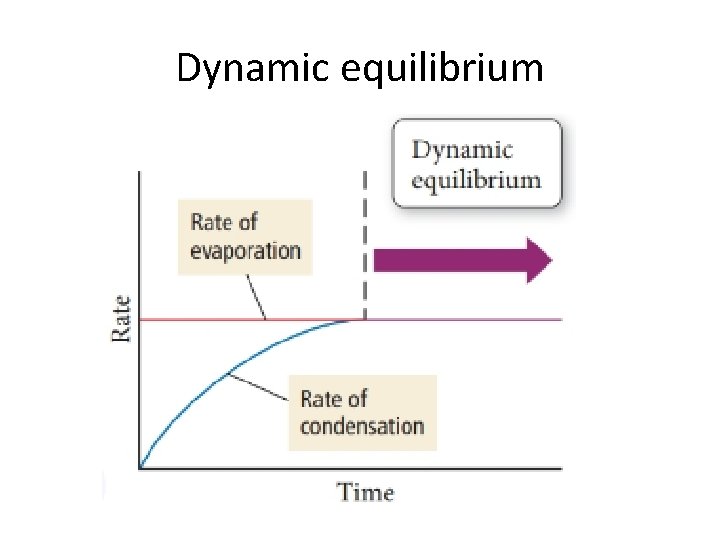
Vapor pressure • In open air, all liquid molecules in a sample can evaporate into the atmosphere and never come back!!! • In a sealed container liquid molecules can enter the gas phase, but they can also condense back down into the liquid phase • The rate of vaporization stays constant, while the rate of condensation increases until both rates are equal. At this point, a dynamic equilibrium has been reached • The pressure of a gas in a dynamic equilibrium with its liquid is its vapor pressure

Dynamic equilibrium

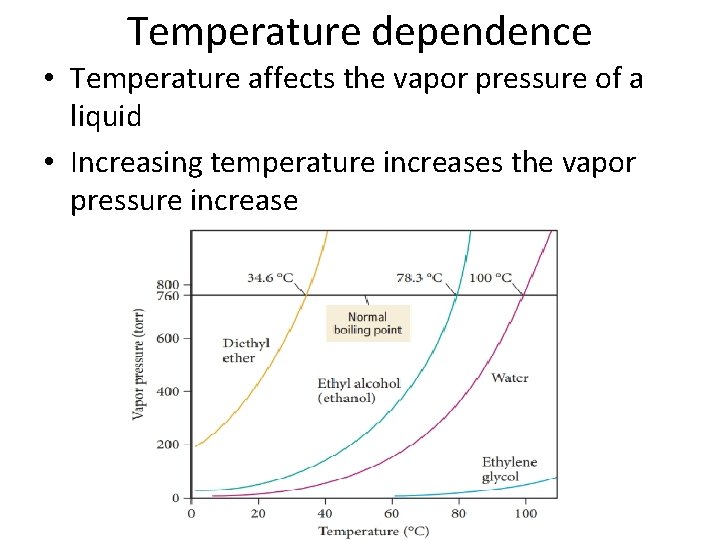
Temperature dependence • Temperature affects the vapor pressure of a liquid • Increasing temperature increases the vapor pressure increase

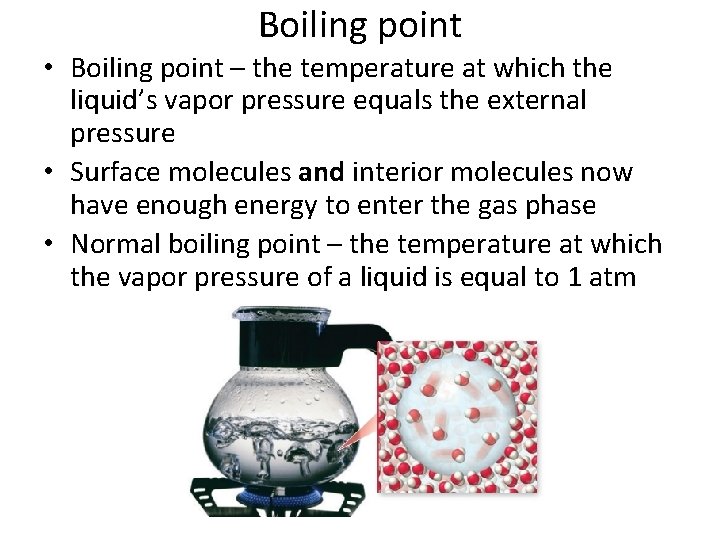
Boiling point • Boiling point – the temperature at which the liquid’s vapor pressure equals the external pressure • Surface molecules and interior molecules now have enough energy to enter the gas phase • Normal boiling point – the temperature at which the vapor pressure of a liquid is equal to 1 atm

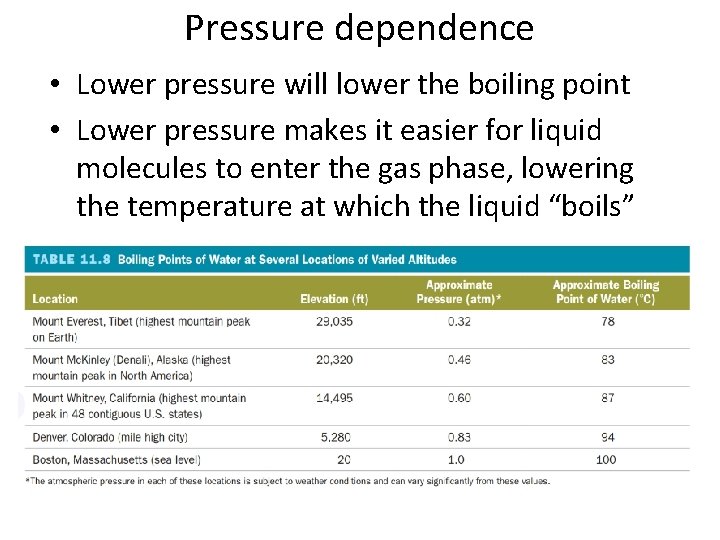
Pressure dependence • Lower pressure will lower the boiling point • Lower pressure makes it easier for liquid molecules to enter the gas phase, lowering the temperature at which the liquid “boils”

Critical point • Heating a liquid in a sealed container will increase the vapor pressure, producing more gas molecules, increasing the pressure of the container • Since the container is sealed and more gas molecules are being forced into the same space, the density of the gas increases • At the same time the density of the liquid is decreasing with the temperature increase • Eventually this will cause the liquid and gas phases to combine to make a single phase, at this point it is considered a supercritical fluid • The temperature at this point is the critical temperature (Tc) • The pressure at this point is the critical pressure (Pc)

• https: //www. youtube. com/watch? v=Rma. JVxafes. U

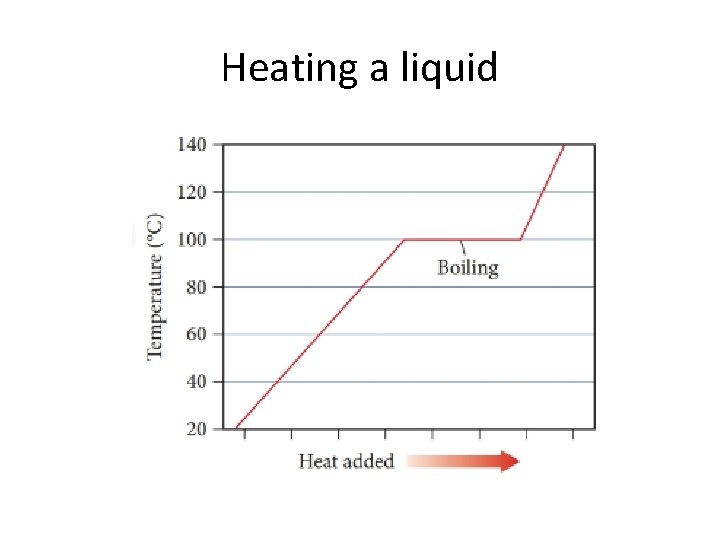
Heating a liquid

Practice • Will boiling macoroni in a pot occur faster in Norco, California (195 meters) or in Denver, Colorado (1609 m)? • Why does adding salt to water increase the boiling point?

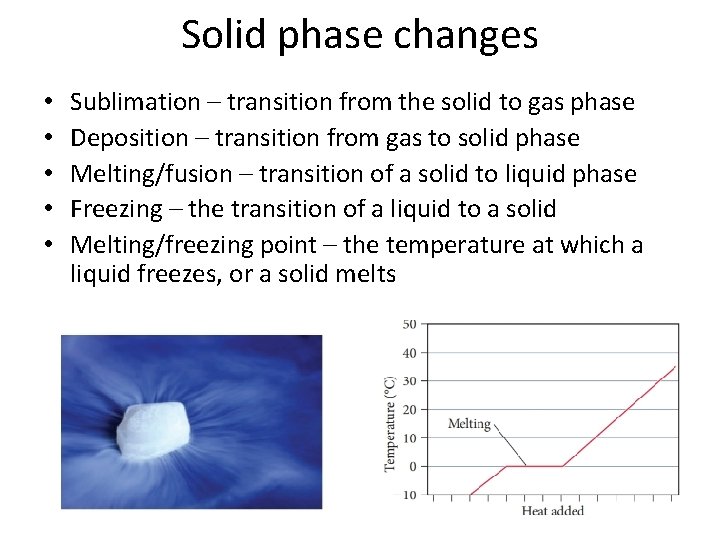
Solid phase changes • • • Sublimation – transition from the solid to gas phase Deposition – transition from gas to solid phase Melting/fusion – transition of a solid to liquid phase Freezing – the transition of a liquid to a solid Melting/freezing point – the temperature at which a liquid freezes, or a solid melts

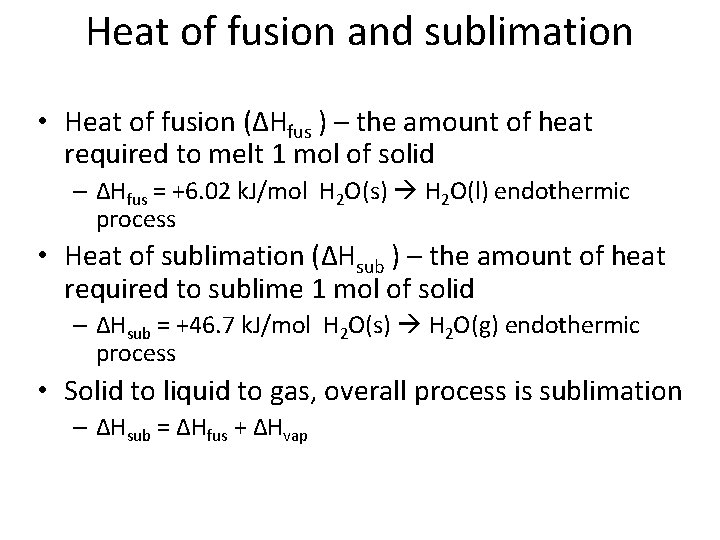
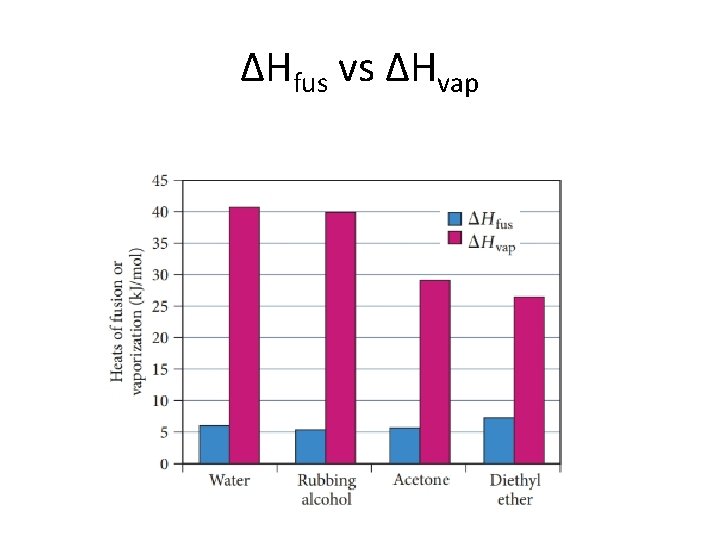
Heat of fusion and sublimation • Heat of fusion (ΔHfus ) – the amount of heat required to melt 1 mol of solid – ΔHfus = +6. 02 k. J/mol H 2 O(s) H 2 O(l) endothermic process • Heat of sublimation (ΔHsub ) – the amount of heat required to sublime 1 mol of solid – ΔHsub = +46. 7 k. J/mol H 2 O(s) H 2 O(g) endothermic process • Solid to liquid to gas, overall process is sublimation – ΔHsub = ΔHfus + ΔHvap

ΔHfus vs ΔHvap

Build a phase diagram (reminder)

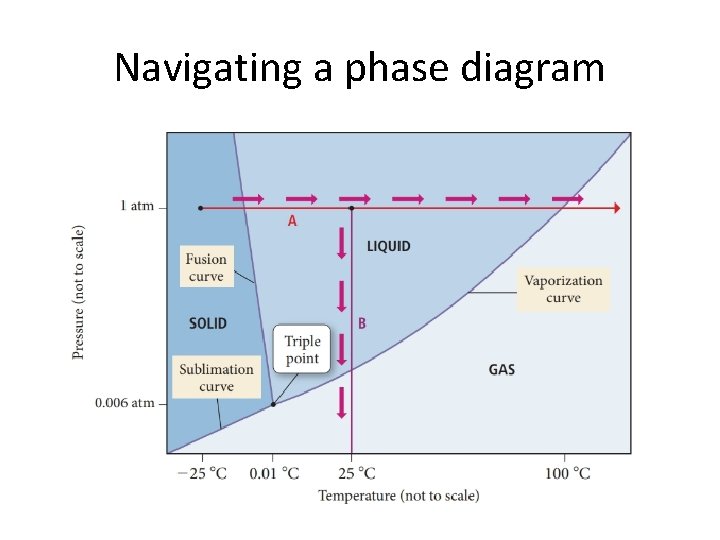
Phase diagrams • Temperature and pressure affect the phase of a substance • Phase diagrams show at a given temperature and pressure what a phase will be

Navigating a phase diagram

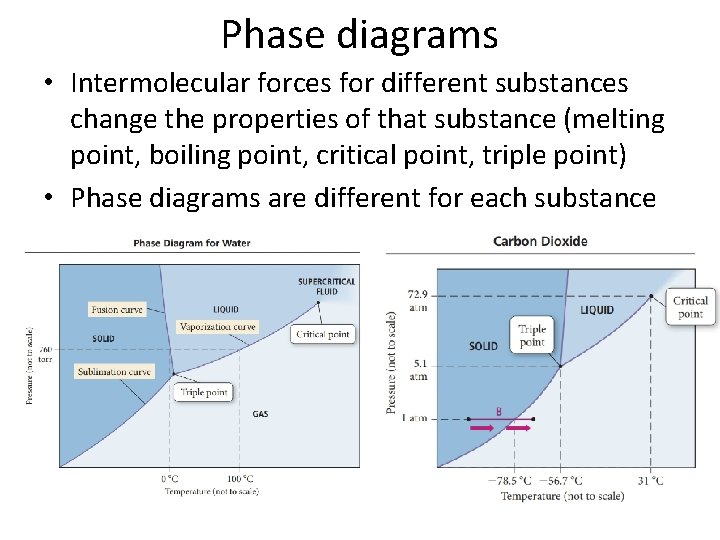
Phase diagrams • Intermolecular forces for different substances change the properties of that substance (melting point, boiling point, critical point, triple point) • Phase diagrams are different for each substance

Chapter 11 done Intermolecular S