Liquids and Intermolecular Forces The forces holding solids






- Slides: 38

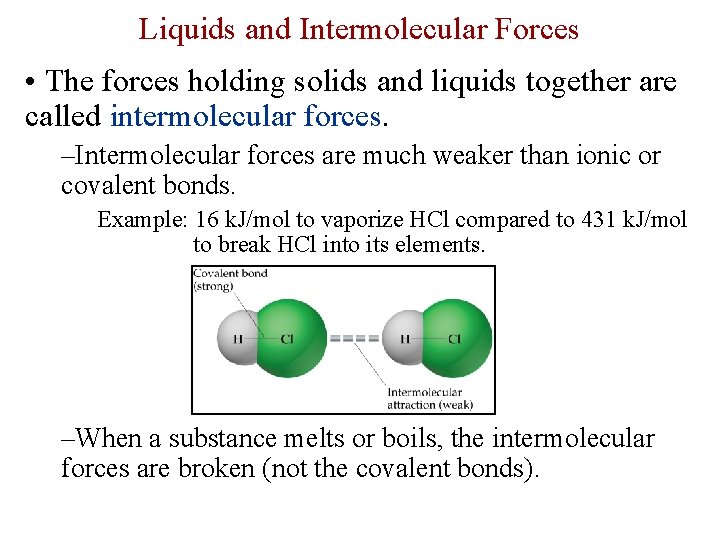
Liquids and Intermolecular Forces • The forces holding solids and liquids together are called intermolecular forces. –Intermolecular forces are much weaker than ionic or covalent bonds. Example: 16 k. J/mol to vaporize HCl compared to 431 k. J/mol to break HCl into its elements. –When a substance melts or boils, the intermolecular forces are broken (not the covalent bonds).

Intermolecular Forces • Boiling point reflects intermolecular force strength. - A high boiling point indicates strong attractive forces. - A high melting point also reflects strong attractive forces. • There are 4 basic types of intermolecular forces… 1) ion-dipole forces 2) dipole-dipole forces 3) London dispersion forces (induced dipole-induced dipole) 4) hydrogen bonding…(a special type of dipole-dipole force!) *Hydrogen bonds just wanna have FON

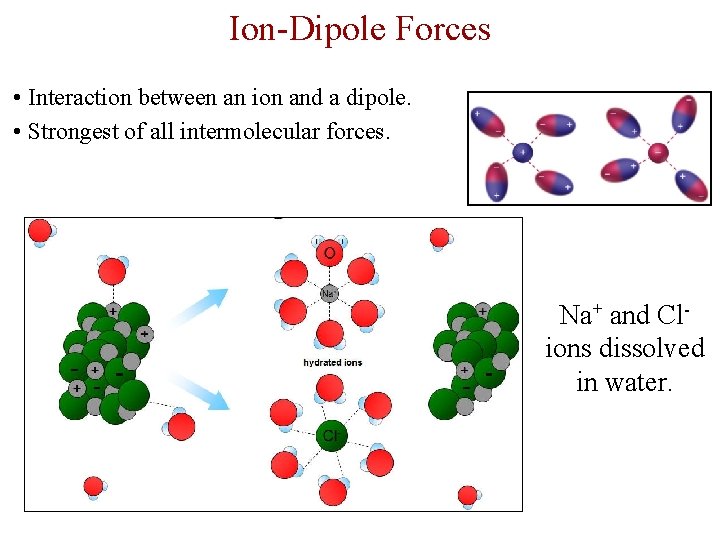
Ion-Dipole Forces • Interaction between an ion and a dipole. • Strongest of all intermolecular forces. Na+ and Clions dissolved in water.

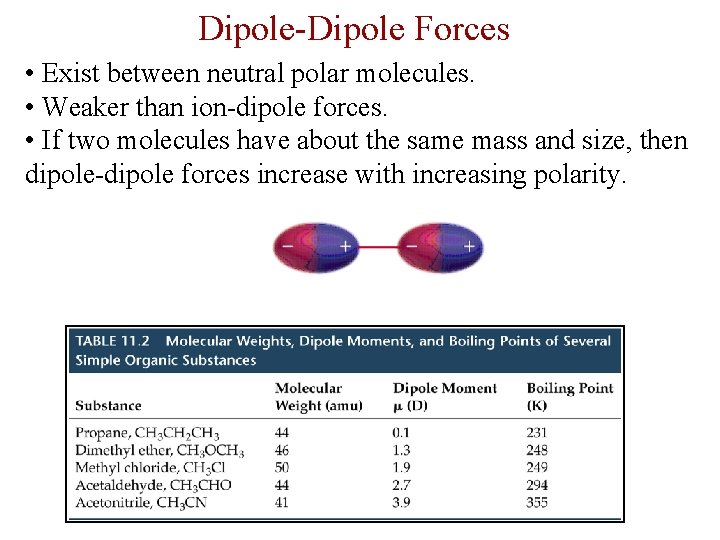
Dipole-Dipole Forces • Exist between neutral polar molecules. • Weaker than ion-dipole forces. • If two molecules have about the same mass and size, then dipole-dipole forces increase with increasing polarity.

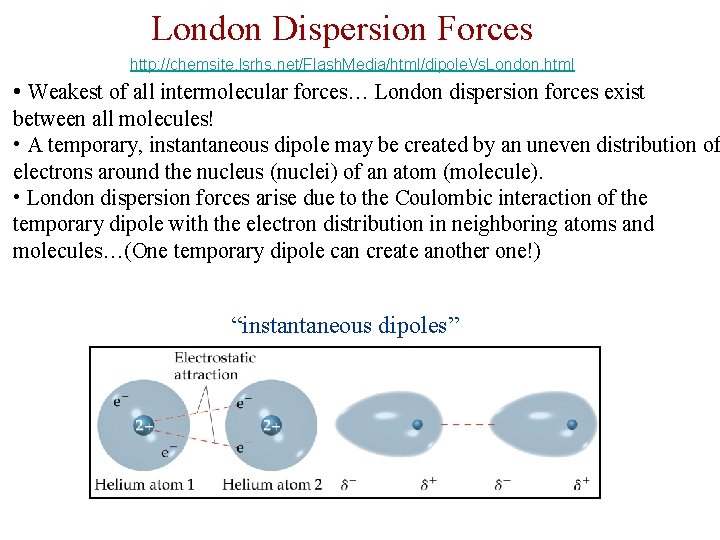
London Dispersion Forces http: //chemsite. lsrhs. net/Flash. Media/html/dipole. Vs. London. html • Weakest of all intermolecular forces… London dispersion forces exist between all molecules! • A temporary, instantaneous dipole may be created by an uneven distribution of electrons around the nucleus (nuclei) of an atom (molecule). • London dispersion forces arise due to the Coulombic interaction of the temporary dipole with the electron distribution in neighboring atoms and molecules…(One temporary dipole can create another one!) “instantaneous dipoles”

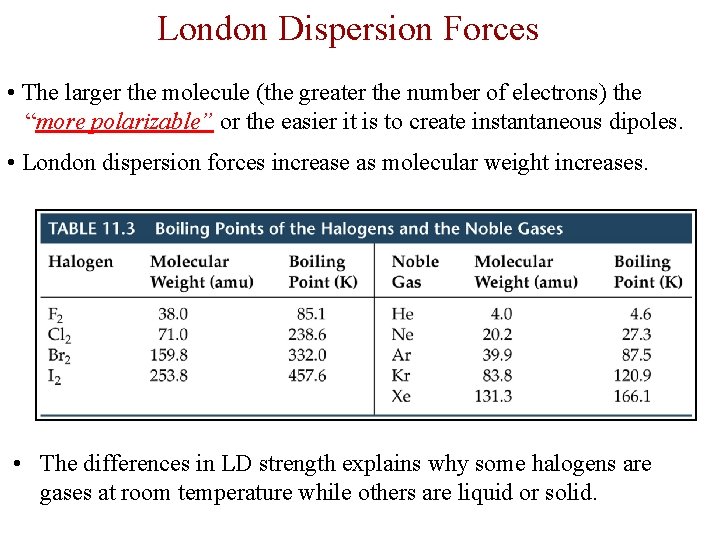
London Dispersion Forces • The larger the molecule (the greater the number of electrons) the “more polarizable” or the easier it is to create instantaneous dipoles. • London dispersion forces increase as molecular weight increases. • The differences in LD strength explains why some halogens are gases at room temperature while others are liquid or solid.

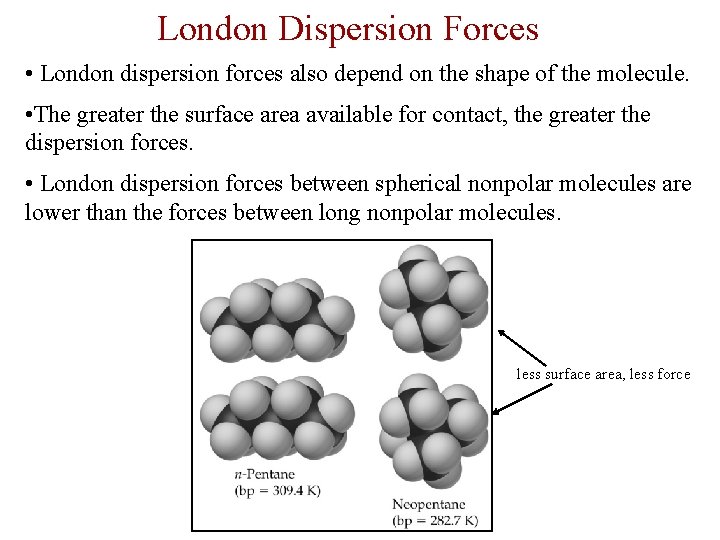
London Dispersion Forces • London dispersion forces also depend on the shape of the molecule. • The greater the surface area available for contact, the greater the dispersion forces. • London dispersion forces between spherical nonpolar molecules are lower than the forces between long nonpolar molecules. less surface area, less force

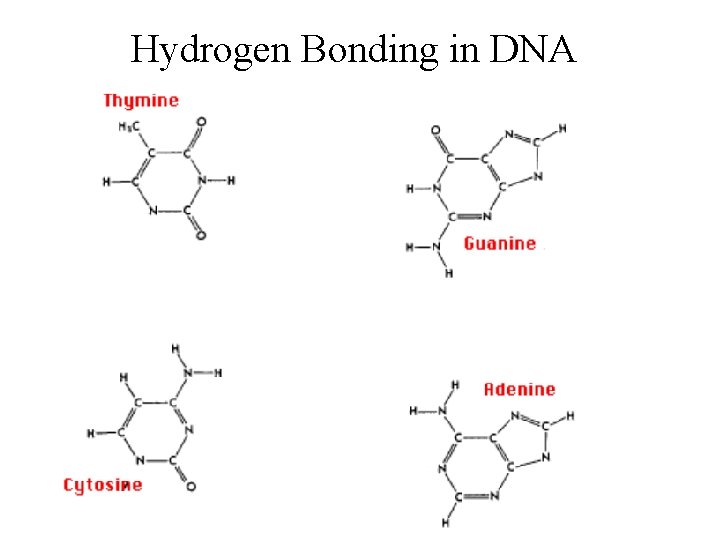
Hydrogen Bonding • A special case of dipole-dipole forces. • By experiments, the boiling pts. of compounds with H-F, H-O, and H-N bonds are abnormally high. The intermolecular forces are therefore abnormally strong. • H-bonding requires… 1) H bonded to a small, highly electronegative element (most important for compounds of F, O, and N) 2) an unshared electron pair on a nearby small highly electronegative ion or atom (usually F, O, or N on another molecule).

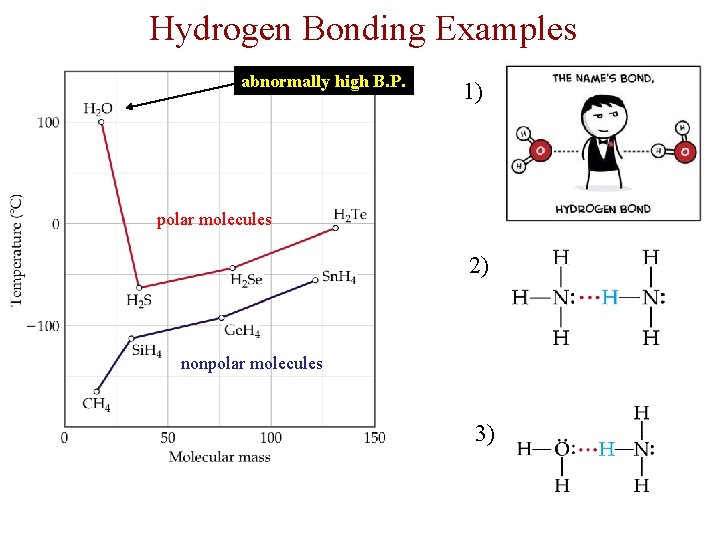
Hydrogen Bonding Examples abnormally high B. P. 1) polar molecules 2) nonpolar molecules 3)

Hydrogen Bonding in DNA

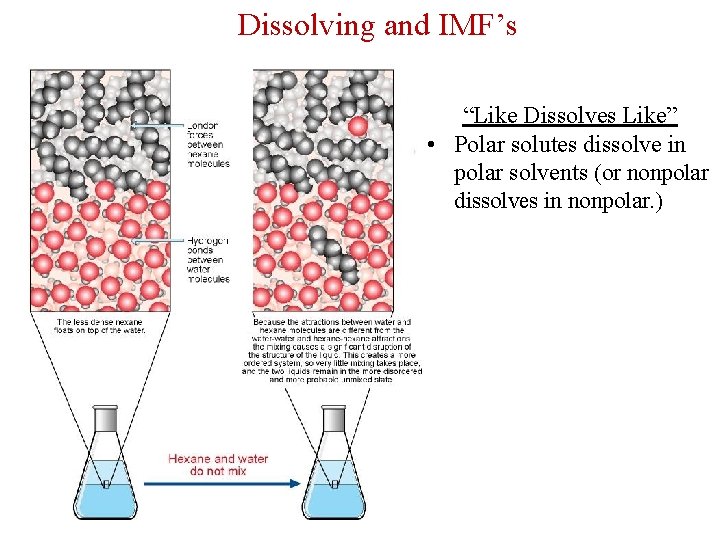
Dissolving and IMF’s “Like Dissolves Like” • Polar solutes dissolve in polar solvents (or nonpolar dissolves in nonpolar. )


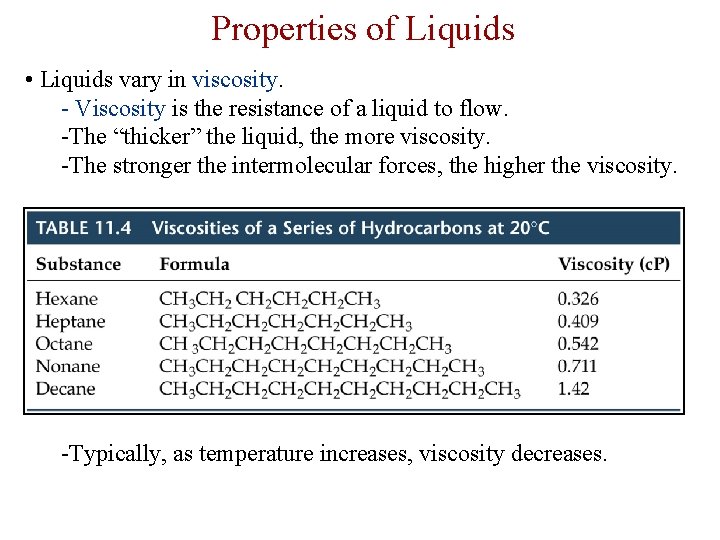
Properties of Liquids • Liquids vary in viscosity. - Viscosity is the resistance of a liquid to flow. -The “thicker” the liquid, the more viscosity. -The stronger the intermolecular forces, the higher the viscosity. -Typically, as temperature increases, viscosity decreases.

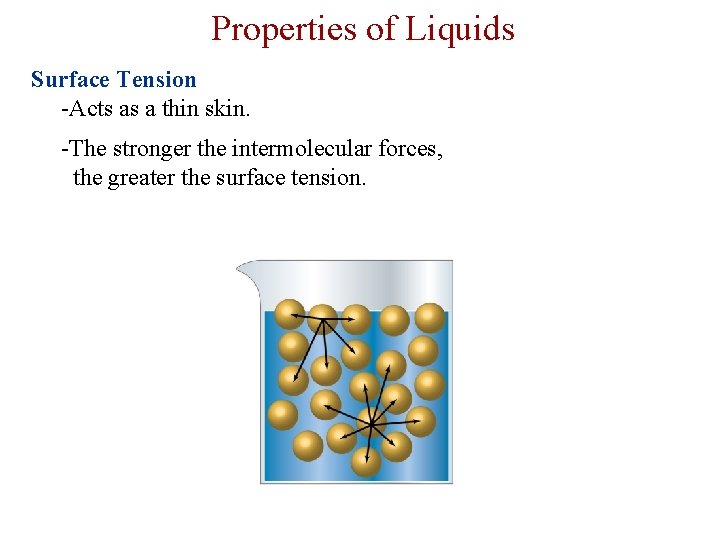
Properties of Liquids Surface Tension -Acts as a thin skin. -The stronger the intermolecular forces, the greater the surface tension.

Fun Facts: (1) “Soap makes water wetter” by reducing the surface tension. (2) Soap doesn’t kill germs. Instead, the germs are attracted to the soap suds and are washed down the drain. • Normally, oil and water don't mix, so they separate into two different layers. Soap breaks up the oil into smaller drops, which can mix with the water. • It works because soap is made up of molecules with two very different ends. One end of the soap molecules is polar – they are hydrophilic. The other end of the soap molecule is nonpolar - they are hydrophobic. • The hydrophobic ends of soap molecules attach to the oil. The hydrophilic ends stick out into the water. This causes a drop of oil to be suspended in the water. • This is how soap cleans your hands - it causes drops of grease and dirt to be pulled off your hands and suspended in water. These drops are washed away when you rinse your hands.

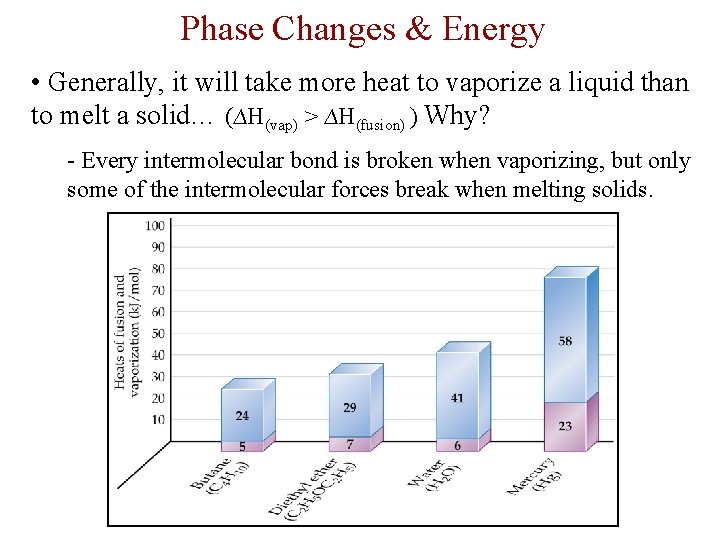
Phase Changes & Energy • Generally, it will take more heat to vaporize a liquid than to melt a solid… (∆H(vap) > ∆H(fusion) ) Why? - Every intermolecular bond is broken when vaporizing, but only some of the intermolecular forces break when melting solids.

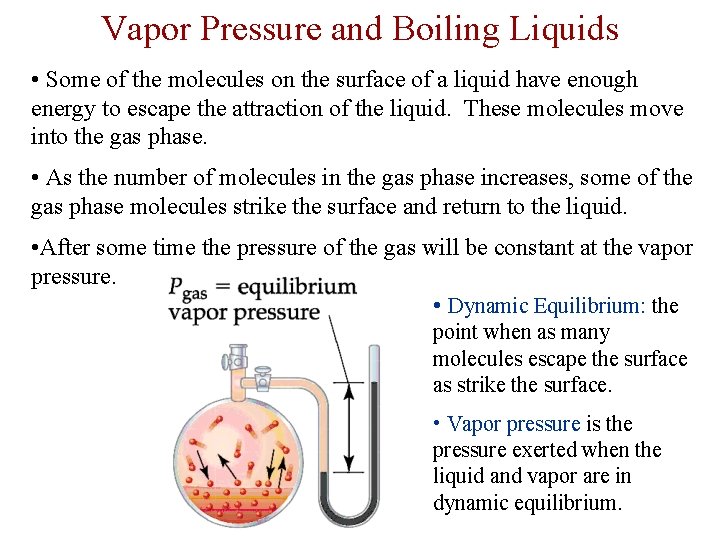
Vapor Pressure and Boiling Liquids • Some of the molecules on the surface of a liquid have enough energy to escape the attraction of the liquid. These molecules move into the gas phase. • As the number of molecules in the gas phase increases, some of the gas phase molecules strike the surface and return to the liquid. • After some time the pressure of the gas will be constant at the vapor pressure. • Dynamic Equilibrium: the point when as many molecules escape the surface as strike the surface. • Vapor pressure is the pressure exerted when the liquid and vapor are in dynamic equilibrium.

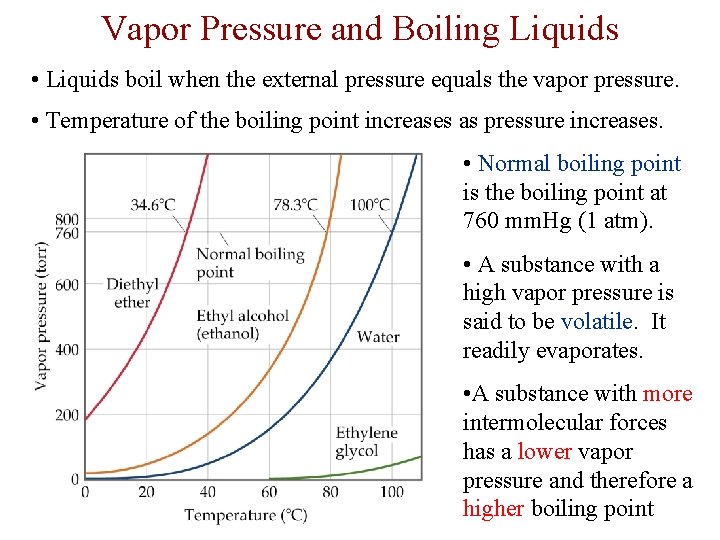
Vapor Pressure and Boiling Liquids • Liquids boil when the external pressure equals the vapor pressure. • Temperature of the boiling point increases as pressure increases. • Normal boiling point is the boiling point at 760 mm. Hg (1 atm). • A substance with a high vapor pressure is said to be volatile. It readily evaporates. • A substance with more intermolecular forces has a lower vapor pressure and therefore a higher boiling point

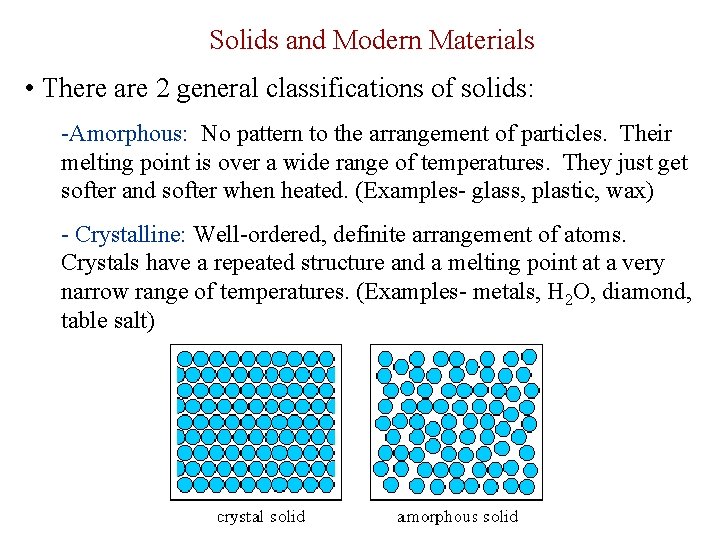
Solids and Modern Materials • There are 2 general classifications of solids: -Amorphous: No pattern to the arrangement of particles. Their melting point is over a wide range of temperatures. They just get softer and softer when heated. (Examples- glass, plastic, wax) - Crystalline: Well-ordered, definite arrangement of atoms. Crystals have a repeated structure and a melting point at a very narrow range of temperatures. (Examples- metals, H 2 O, diamond, table salt)

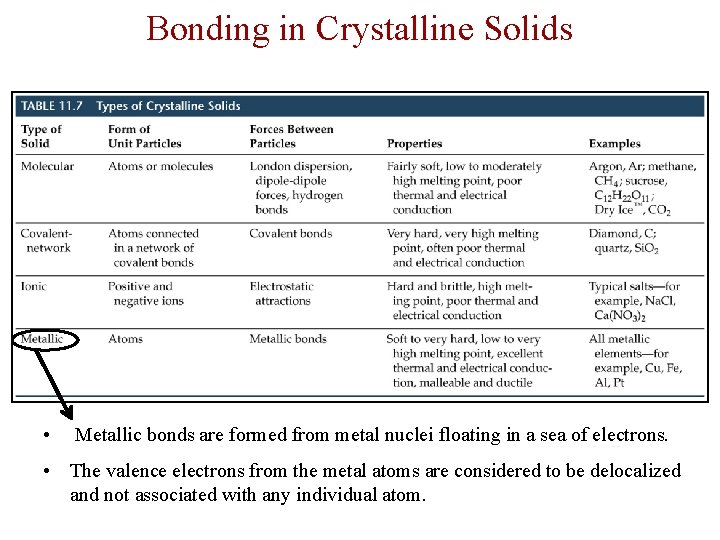
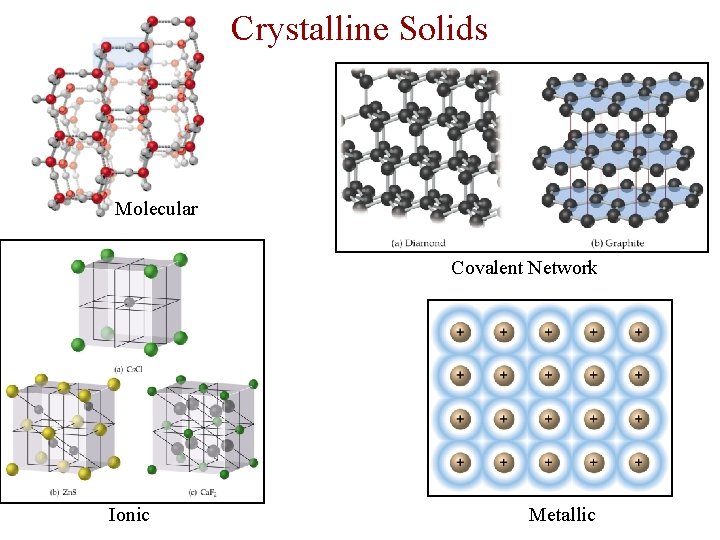
Crystalline Solids There are four types of crystalline solid: - Molecular (formed from molecules) - usually soft with low melting points and poor conductivity. - Covalent network (formed from atoms) - very hard with very high melting points and poor conductivity. - Ionic (formed form ions) - hard, brittle, high melting points and poor conductivity. - Metallic (formed from metal atoms) - soft or hard, high melting points, good conductivity, malleable and ductile.

Bonding in Crystalline Solids • Metallic bonds are formed from metal nuclei floating in a sea of electrons. • The valence electrons from the metal atoms are considered to be delocalized and not associated with any individual atom.

Crystalline Solids Molecular Covalent Network Ionic Metallic

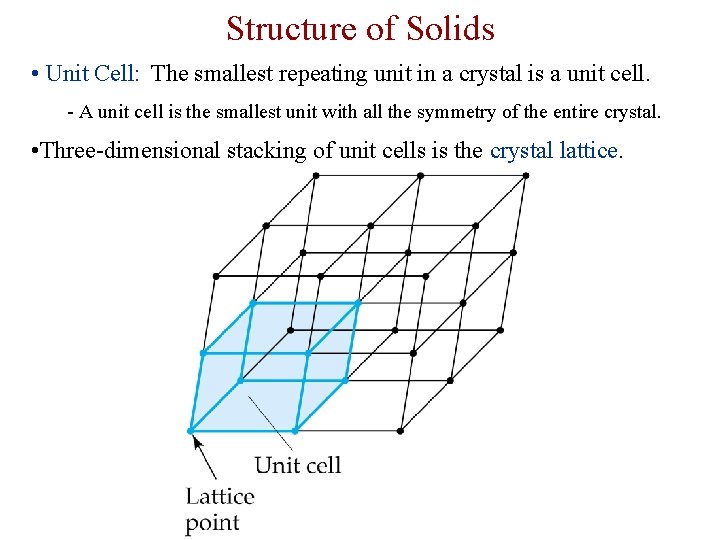
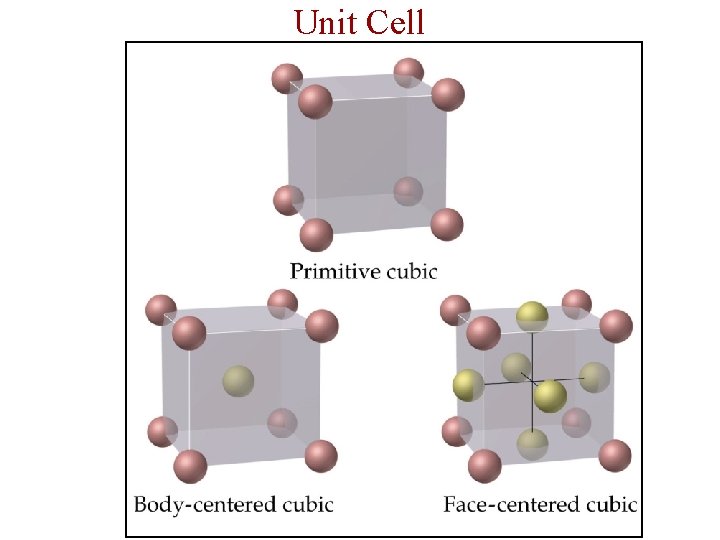
Structure of Solids • Unit Cell: The smallest repeating unit in a crystal is a unit cell. - A unit cell is the smallest unit with all the symmetry of the entire crystal. • Three-dimensional stacking of unit cells is the crystal lattice.

Unit Cell

Delocalized π- bonding in Molecular Solids Here’s a random fact… A long-standing assumption in chemistry is that molecular solids will always be insulators. But in 1970, some molecular solids that conduct electricity were discovered. Conduction was the result of delocalized pi bonding in the molecular solids. Molecular Solids: Polymers • Molecular solids are usually composed of very large molecules, or polymers, with important commercial and biological applications.

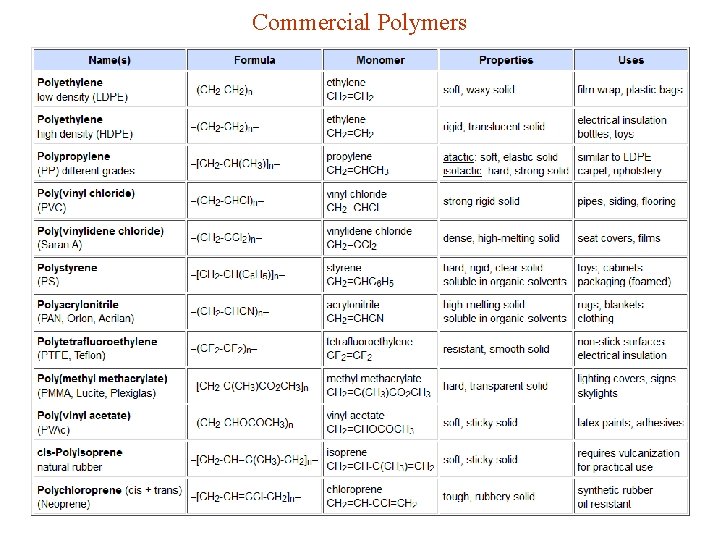
Commercial Polymers

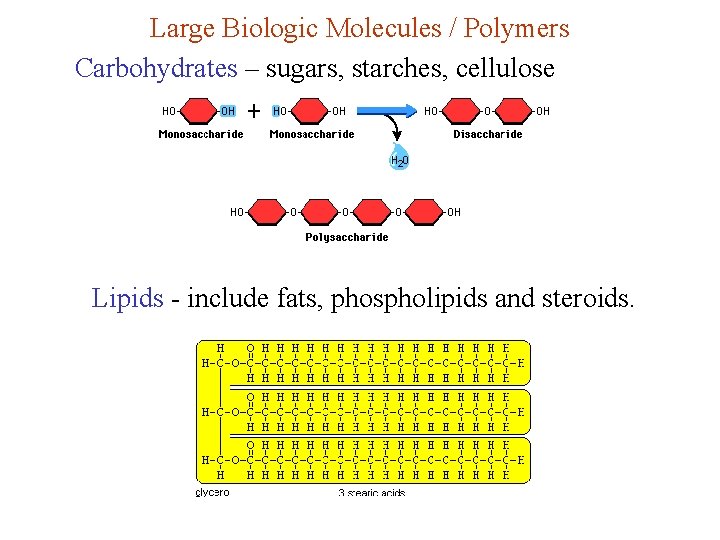
Large Biologic Molecules / Polymers Carbohydrates – sugars, starches, cellulose Lipids - include fats, phospholipids and steroids.

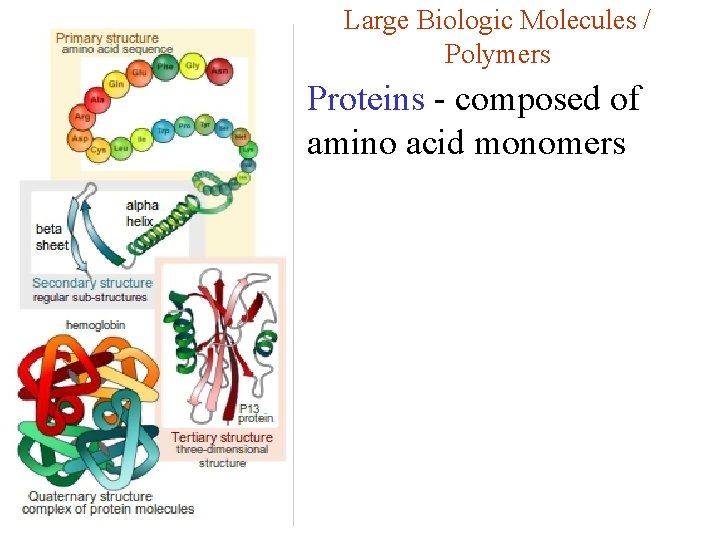
Large Biologic Molecules / Polymers Proteins - composed of amino acid monomers

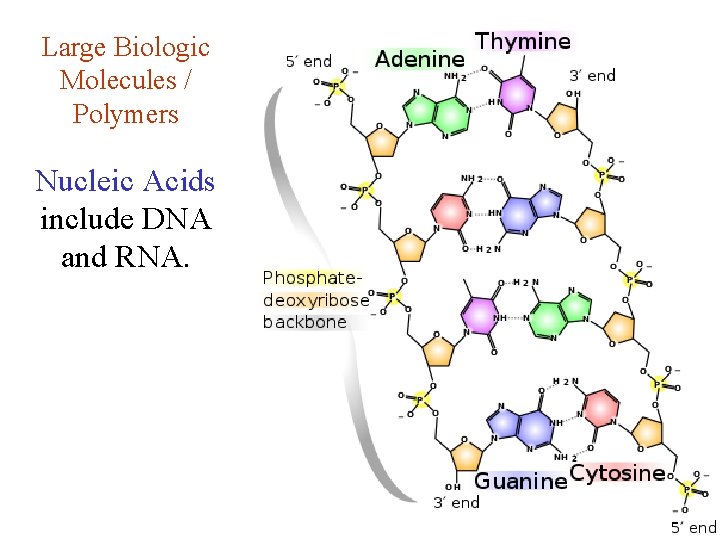
Large Biologic Molecules / Polymers Nucleic Acids include DNA and RNA.

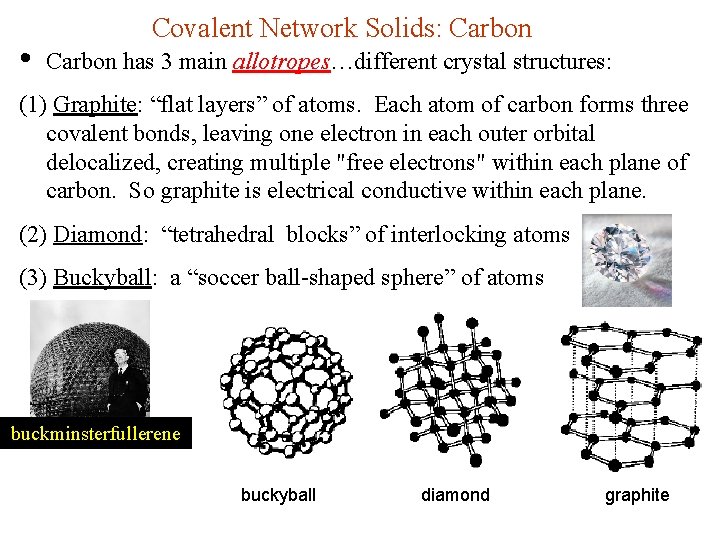
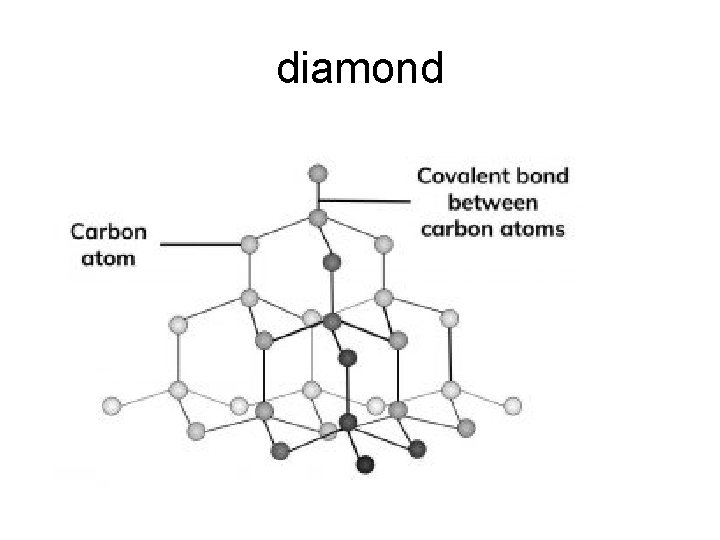
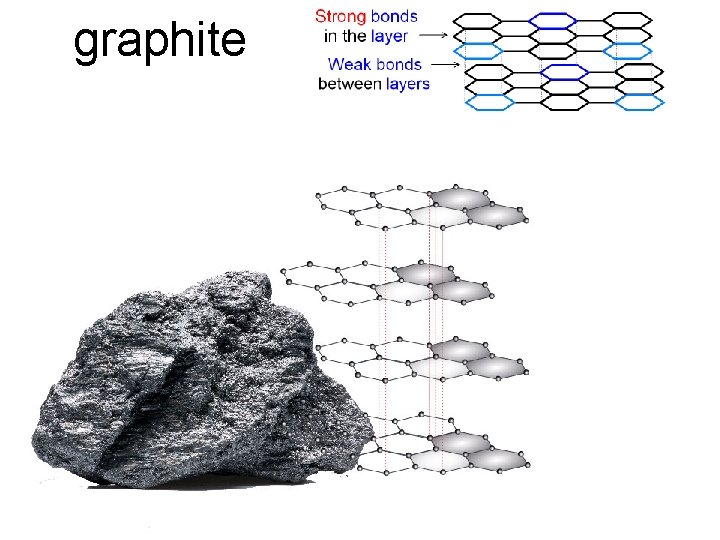
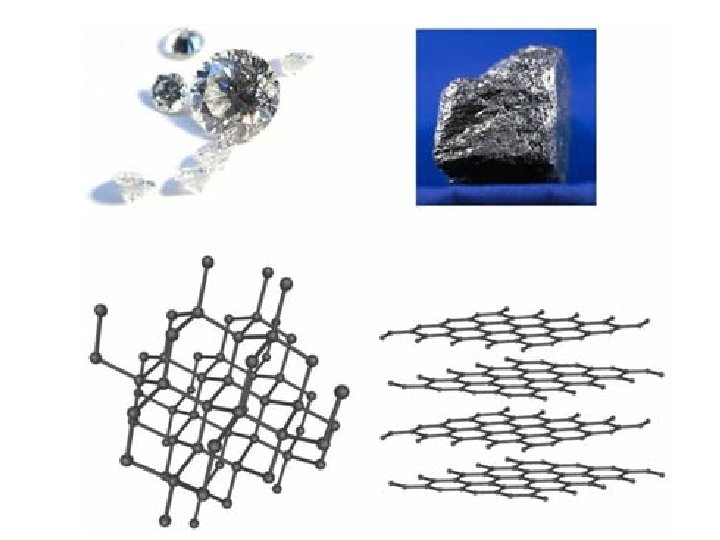
• Covalent Network Solids: Carbon has 3 main allotropes…different crystal structures: (1) Graphite: “flat layers” of atoms. Each atom of carbon forms three covalent bonds, leaving one electron in each outer orbital delocalized, creating multiple "free electrons" within each plane of carbon. So graphite is electrical conductive within each plane. (2) Diamond: “tetrahedral blocks” of interlocking atoms (3) Buckyball: a “soccer ball-shaped sphere” of atoms buckminsterfullerene buckyball diamond graphite

diamond

graphite


C 60 Buckyball

Covalent Network Solids: Silicon • Silicon is a covalent network solid and a semiconductor. • Silicon forms a three-dimensional network similar in geometry to a diamond. • Silicon’s conductivity increases as temperature increases.

Covalent Network Solids to memorize • • • Silicon Carbon (diamond, graphite) Silicon carbide Si. C Silicon dioxide Si. O 2 Boron nitride BN

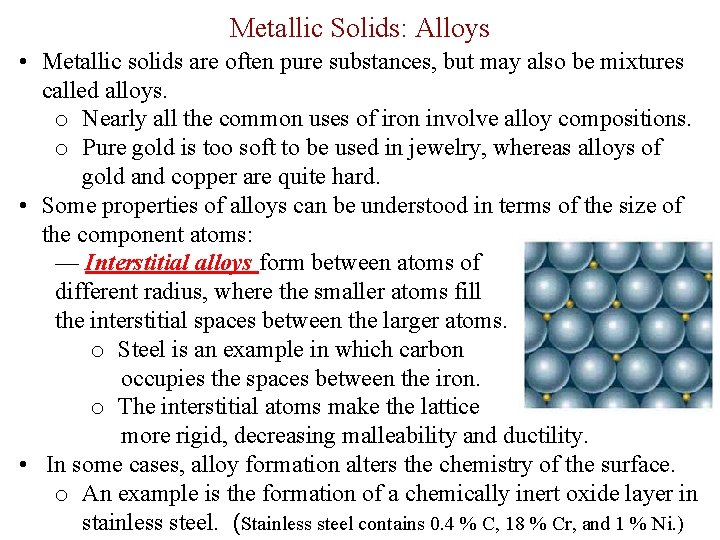
Metallic Solids: Alloys • Metallic solids are often pure substances, but may also be mixtures called alloys. o Nearly all the common uses of iron involve alloy compositions. o Pure gold is too soft to be used in jewelry, whereas alloys of gold and copper are quite hard. • Some properties of alloys can be understood in terms of the size of the component atoms: — Interstitial alloys form between atoms of different radius, where the smaller atoms fill the interstitial spaces between the larger atoms. o Steel is an example in which carbon occupies the spaces between the iron. o The interstitial atoms make the lattice more rigid, decreasing malleability and ductility. • In some cases, alloy formation alters the chemistry of the surface. o An example is the formation of a chemically inert oxide layer in stainless steel. (Stainless steel contains 0. 4 % C, 18 % Cr, and 1 % Ni. )

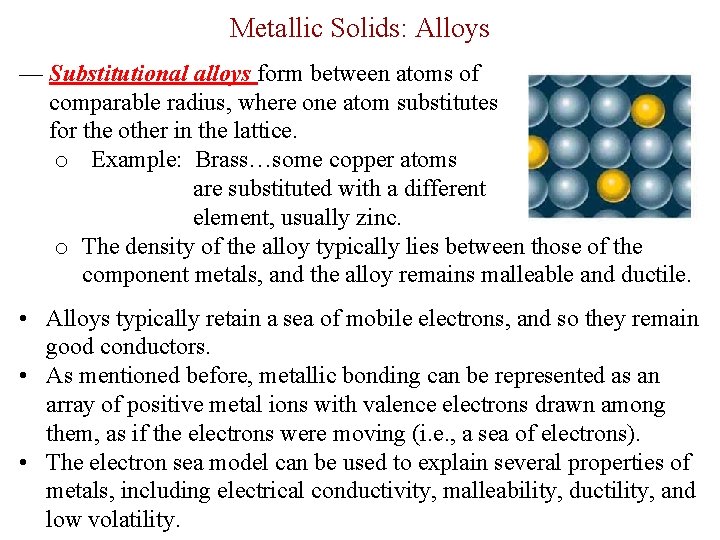
Metallic Solids: Alloys — Substitutional alloys form between atoms of comparable radius, where one atom substitutes for the other in the lattice. o Example: Brass…some copper atoms are substituted with a different element, usually zinc. o The density of the alloy typically lies between those of the component metals, and the alloy remains malleable and ductile. • Alloys typically retain a sea of mobile electrons, and so they remain good conductors. • As mentioned before, metallic bonding can be represented as an array of positive metal ions with valence electrons drawn among them, as if the electrons were moving (i. e. , a sea of electrons). • The electron sea model can be used to explain several properties of metals, including electrical conductivity, malleability, ductility, and low volatility.