AP Chemistry Intermolecular Forces and States of Matter


- Slides: 11

AP Chemistry Intermolecular Forces and States of Matter

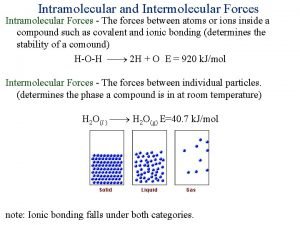
Chemical properties are related only to chemical composition; physical properties are related to chemical composition AND the physical state of the substance at the time. O H 2

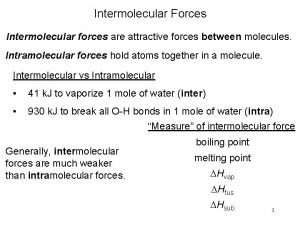
intermolecular forces (IMFs): forces between molecules -- largely determine the physical properties of molecular liquids and solids covalent STRENGTH OF IMFs… GASES < LIQUIDS < SOLIDS Solids w/highly-ordered structures are crystalline. ionic and metallic

Intermolecular Forces (IMFs) -- these are much weaker than ionic or covalent bonds (< 15% as strong) -- In vaporizing water, we overcome the IMFs between water molecules, but. . . NOT the bonds holding the molecule together. H Boiling (or freezing) doesn’t affect these O H

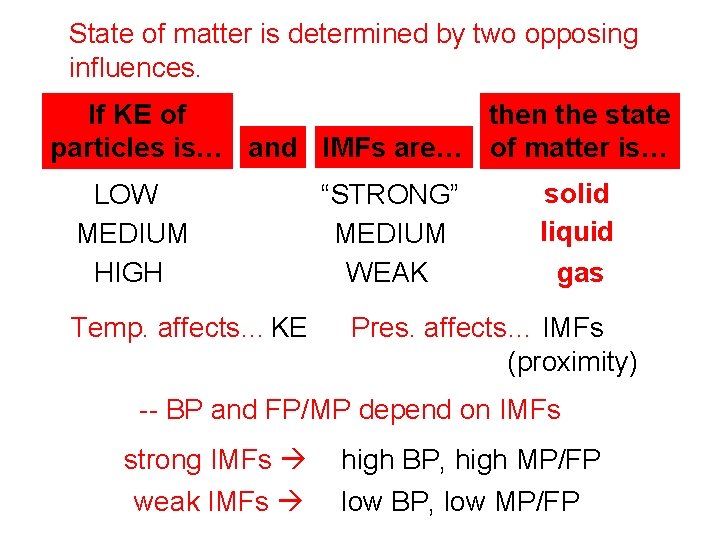
State of matter is determined by two opposing influences. If KE of then the state particles is… and IMFs are… of matter is… LOW MEDIUM HIGH Temp. affects… KE “STRONG” MEDIUM WEAK solid liquid gas Pres. affects… IMFs (proximity) -- BP and FP/MP depend on IMFs strong IMFs weak IMFs high BP, high MP/FP low BP, low MP/FP

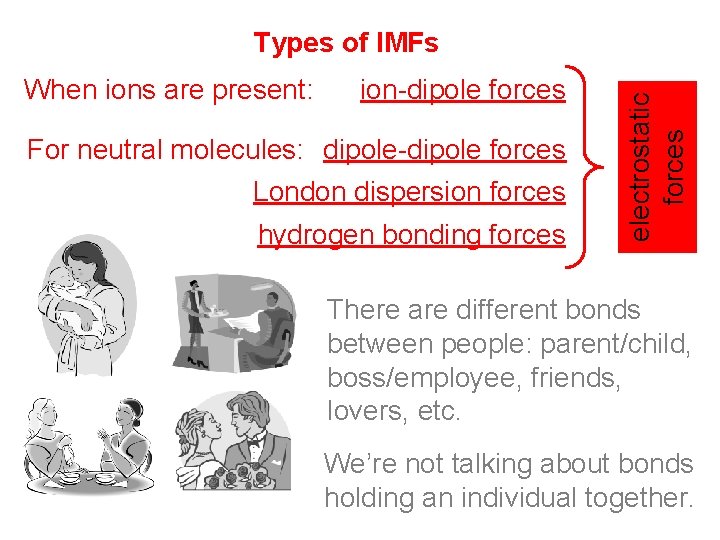
When ions are present: ion-dipole forces For neutral molecules: dipole-dipole forces London dispersion forces hydrogen bonding forces electrostatic forces Types of IMFs There are different bonds between people: parent/child, boss/employee, friends, lovers, etc. We’re not talking about bonds holding an individual together.

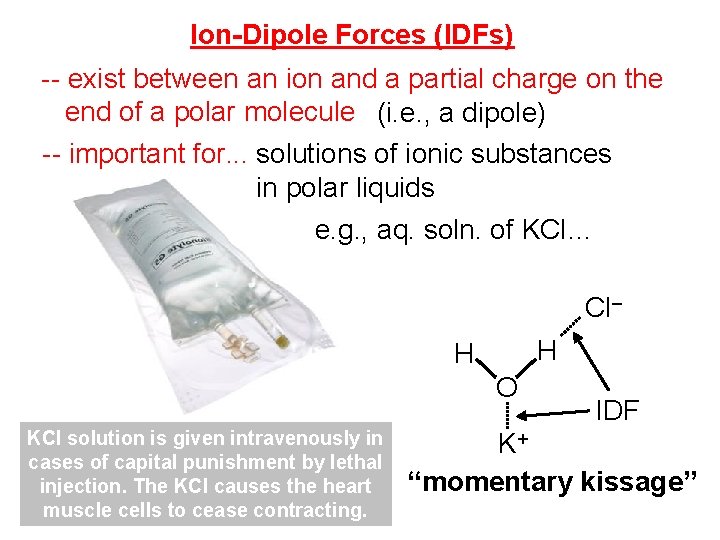
Ion-Dipole Forces (IDFs) -- exist between an ion and a partial charge on the end of a polar molecule (i. e. , a dipole) -- important for. . . solutions of ionic substances in polar liquids e. g. , aq. soln. of KCl… Cl– H H O KCl solution is given intravenously in cases of capital punishment by lethal injection. The KCl causes the heart muscle cells to cease contracting. IDF K+ “momentary kissage”

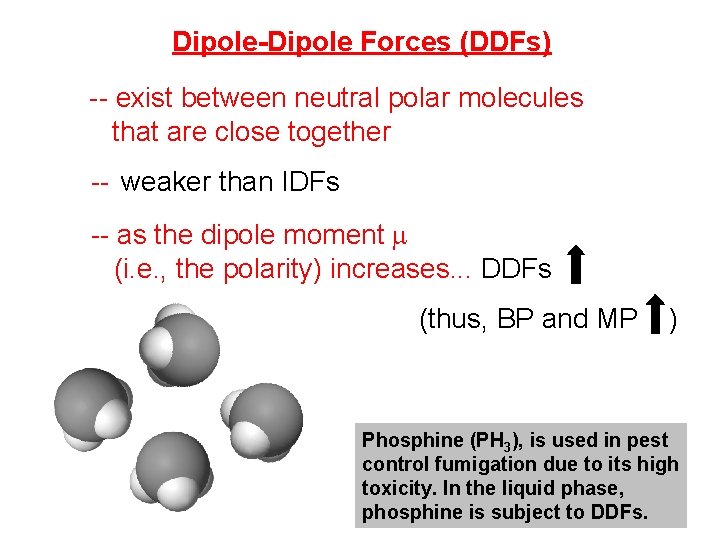
Dipole-Dipole Forces (DDFs) -- exist between neutral polar molecules that are close together -- weaker than IDFs -- as the dipole moment m (i. e. , the polarity) increases. . . DDFs (thus, BP and MP ) Phosphine (PH 3), is used in pest control fumigation due to its high toxicity. In the liquid phase, phosphine is subject to DDFs.

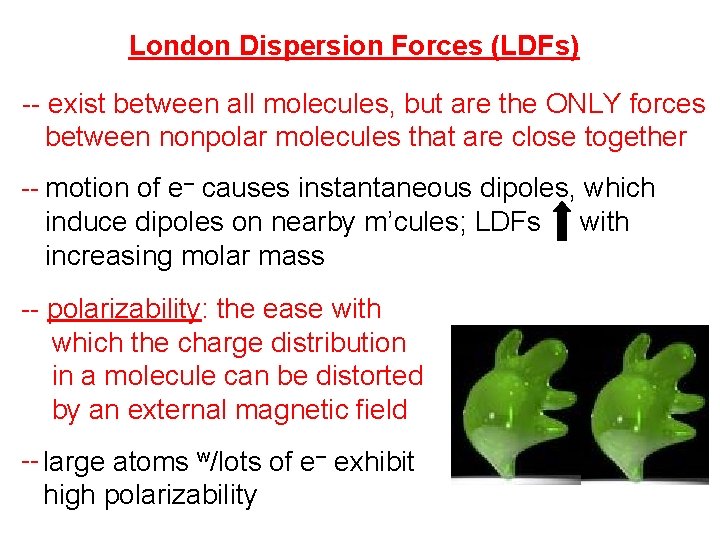
London Dispersion Forces (LDFs) -- exist between all molecules, but are the ONLY forces between nonpolar molecules that are close together -- motion of e– causes instantaneous dipoles, which induce dipoles on nearby m’cules; LDFs with increasing molar mass -- polarizability: the ease with which the charge distribution in a molecule can be distorted by an external magnetic field -- large atoms w/lots of e– exhibit high polarizability

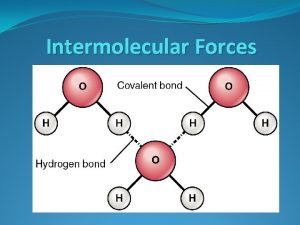
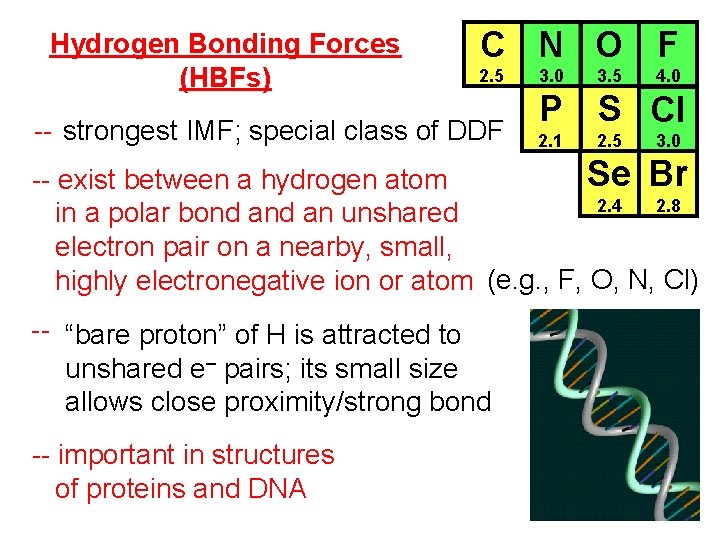
Hydrogen Bonding Forces (HBFs) C N O F 2. 5 -- strongest IMF; special class of DDF 3. 0 3. 5 4. 0 P S Cl 2. 1 2. 5 3. 0 Se Br -- exist between a hydrogen atom 2. 4 2. 8 in a polar bond an unshared electron pair on a nearby, small, highly electronegative ion or atom (e. g. , F, O, N, Cl) -- “bare proton” of H is attracted to unshared e– pairs; its small size allows close proximity/strong bond -- important in structures of proteins and DNA

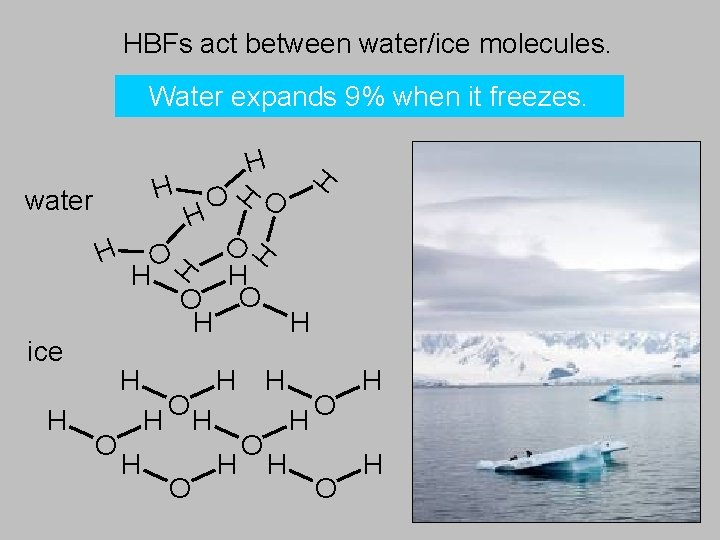
HBFs act between water/ice molecules. Water expands 9% when it freezes. H O H H O O H ice H H O H H H O O H water H H O O H H
 Intramolecular forces vs intermolecular forces
Intramolecular forces vs intermolecular forces Inter vs intramolecular
Inter vs intramolecular Intermolecular force of attraction
Intermolecular force of attraction Intermolecular forces of matter
Intermolecular forces of matter Cnof chemistry
Cnof chemistry Intermolecular forces in water
Intermolecular forces in water Geckos and intermolecular forces
Geckos and intermolecular forces Similarities of intermolecular and intramolecular forces
Similarities of intermolecular and intramolecular forces Viscosity and intermolecular forces
Viscosity and intermolecular forces Intermolecular forces capillary action
Intermolecular forces capillary action What are the weakest attractions between molecules
What are the weakest attractions between molecules Pg 147
Pg 147