INTERMOLECULAR FORCES Liquids and Solids Chapter 11 Liquids




















- Slides: 34

INTERMOLECULAR FORCES Liquids and Solids Chapter 11

Liquids vs. Solids �Physical properties are due to intermolecular forces �Understood in terms of kinetic-molecular theory �Gases are highly compressible and assume the shape and volume of their container �Liquids are almost incompressible, assume the shape but not the volume of the container �Solids are incompressible and have a definite shape and volume

Liquids vs. Solids �Solids and liquids are condensed phases �Converting a gas into a liquid or solid requires the molecules to get closer to each other �Forces holding solids and liquids together are called intermolecular forces

INTERMOLECULAR FORCES

Intermolecular Forces �Attraction between molecules �Weaker than ionic or covalent bonds (16 k. J/mol vs. 431 k. J/mol for HCl) �Melting or boiling breaks intermolecular forces �Condensing forms intermolecular forces �Melting points / Boiling points reflect strength of intermolecular forces �High melting/boiling points indicates strong attractive forces

Intermolecular Forces �Van der Waals forces exist between neutral molecules �Includes London-dispersion forces, dipole-dipole forces, and hydrogen-bonding forces �Ion-dipole interactions are important in solutions �ALL are WEAK electrostatic interactions �(~15% as strong as a covalent or ionic bond)

Van der Waals Forces �Ion-Dipole �Interaction between an ion and the partial charge on the end of a polar molecule (dipole) �Important in formation of solution between ionic substances in polar liquids (ex. Na. Cl in water) �Dipole-Dipole �Exist between neutral polar molecules �Polar molecules attract each other �Need to be close together to form strong attractions �Weaker than ion-dipole forces

Van der Waals Forces London Dispersion Forces �Weakest of all intermolecular forces �Possible for two adjacent neutral molecules to affect each other �Nucleus of one molecule (atom) attracts the electrons in an adjacent molecule (atom) �Electron “clouds” become distorted – temporary �Temporary distortion creates an instantaneous dipole �One instantaneous dipole can create an instantaneous dipole in a nearby molecule (atom) �Temporary dipoles attract each other

Van der Waals Forces London Dispersion Forces �Molecules must be very close together for these attractive forces to occur �Polarizability is the ease with which an electron cloud can be deformed �The larger the molecule- the more polarizable it is �Forces increase as molecular weight increases �Forces depend on the shape of the molecule

Van der Waals Forces Hydrogen Bonds �Boiling points of compounds with hydrogen bonded to an electronegative atom are abnormally high �Special case of dipole-dipole interactions �Requires: �H bonded to a small electronegative element �An unshared pair of electrons on a nearby small electronegative atom/ion �Hydrogen only has one electron, so in an electronegative bond it is “electron bare”

PROPERTIES IN LIQUIDS

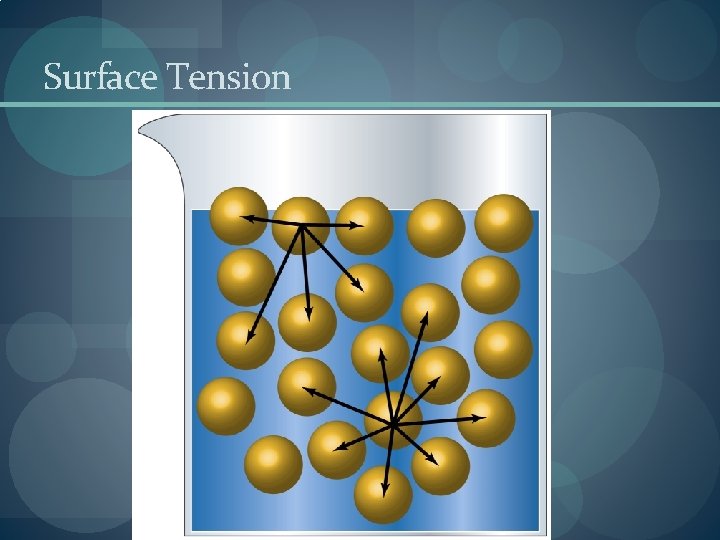
Properties in Liquids � Viscosity is the resistance of a liquid to flow. � A liquid flows by sliding molecules over each other. � The stronger the intermolecular forces, the higher the viscosity. �Surface Tension � Bulk molecules (those in the liquid) are equally attracted to their neighbors. � Surface molecules are only attracted inwards towards the bulk molecules

Surface Tension

Surface Tension � Surface tension is the amount of energy required to increase the surface area of a liquid. � Cohesive forces bind molecules to each other. � Adhesive forces bind molecules to a surface � Meniscus is the shape of the liquid surface. � Adhesive > Cohesive : U-shaped meniscus (water) � Capillary Action: When a narrow glass tube is placed in water, the meniscus pulls the water up the tube

PHASE CHANGES

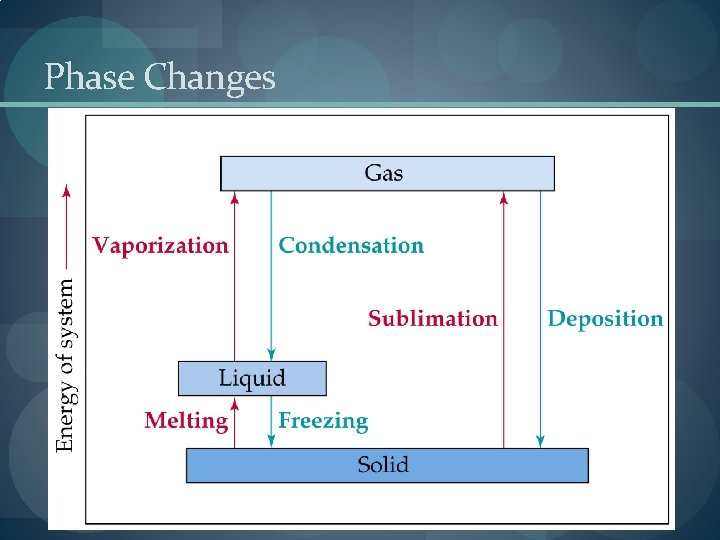
Phase Changes

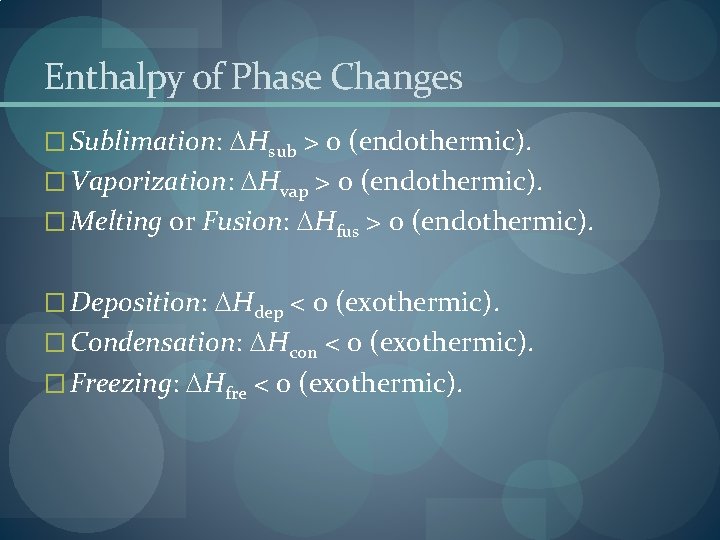
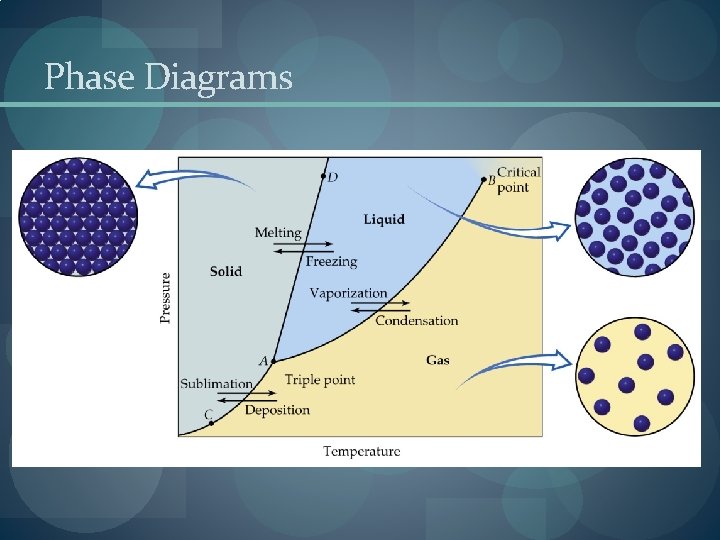
Enthalpy of Phase Changes � Sublimation: Hsub > 0 (endothermic). � Vaporization: Hvap > 0 (endothermic). � Melting or Fusion: Hfus > 0 (endothermic). � Deposition: Hdep < 0 (exothermic). � Condensation: Hcon < 0 (exothermic). � Freezing: Hfre < 0 (exothermic).

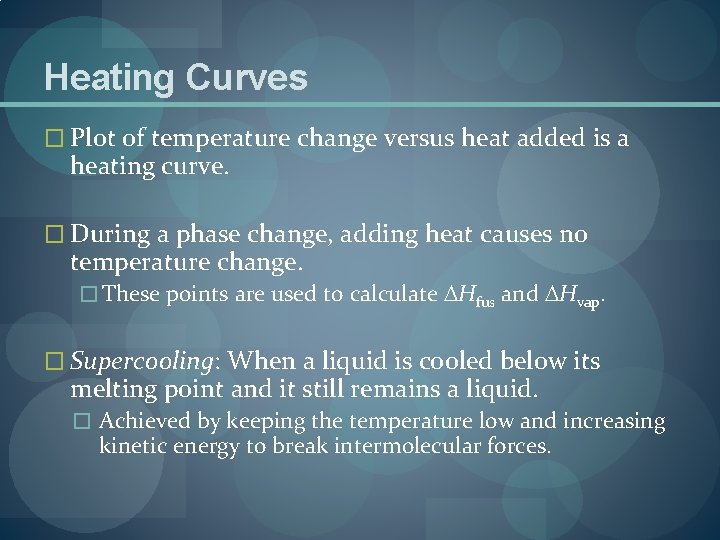
Heating Curves � Plot of temperature change versus heat added is a heating curve. � During a phase change, adding heat causes no temperature change. � These points are used to calculate Hfus and Hvap. � Supercooling: When a liquid is cooled below its melting point and it still remains a liquid. � Achieved by keeping the temperature low and increasing kinetic energy to break intermolecular forces.


Critical Temperature and Pressure � Gases liquefied by increasing pressure at some temperature. � Critical temperature: the minimum temperature for liquefaction of a gas using pressure. � Critical pressure: pressure required for liquefaction.

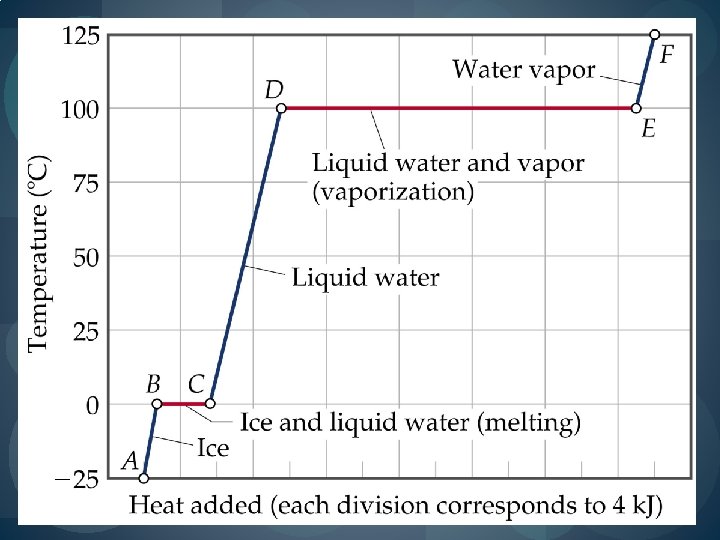
Vapor Pressure on a Molecular Level

Vapor Pressure � Dynamic Equilibrium: the point when as many molecules escape the surface as strike the surface. � Vapor pressure is the pressure exerted when the liquid and vapor are in dynamic equilibrium. � Volatility, Vapor Pressure, and Temperature � If equilibrium is never established then the liquid evaporates. � Volatile substances evaporate rapidly. � The higher the temperature, the higher the average kinetic energy, the faster the liquid evaporates.

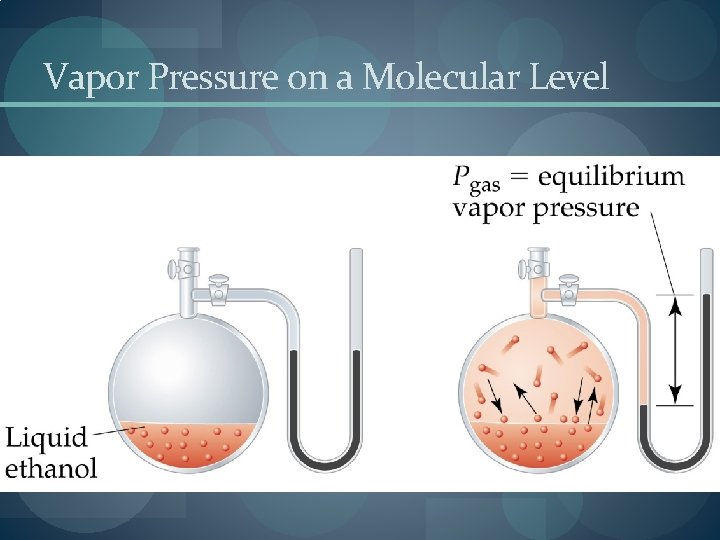
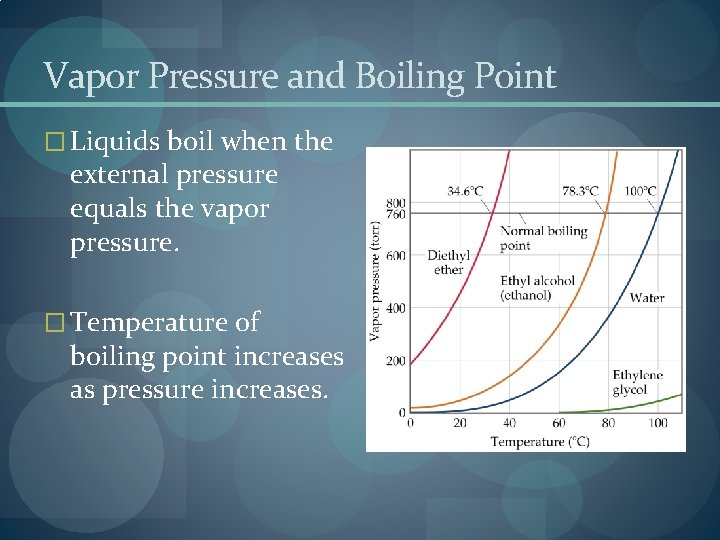
Vapor Pressure and Boiling Point � Liquids boil when the external pressure equals the vapor pressure. � Temperature of boiling point increases as pressure increases.

PHASE DIAGRAMS

Phase Diagrams

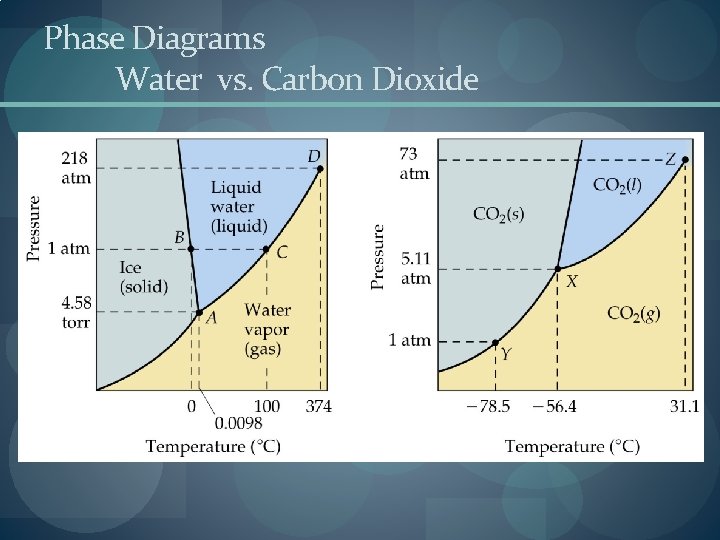
Phase Diagrams Water vs. Carbon Dioxide

SOLIDS

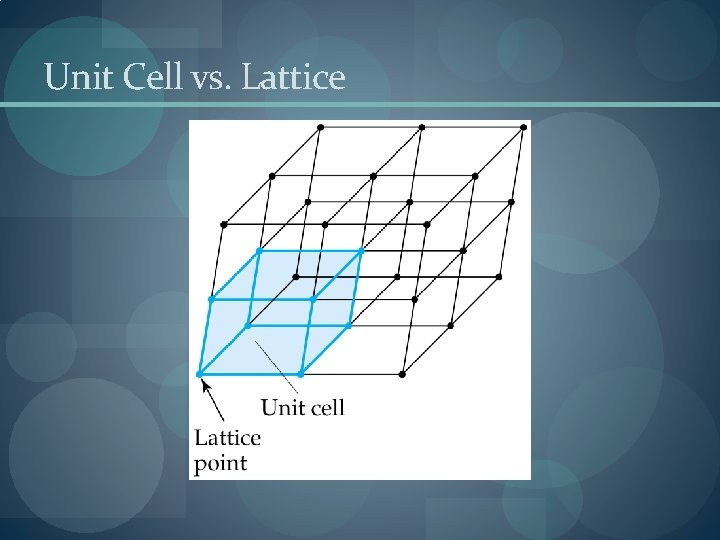
Unit Cells � Crystalline solid: well-ordered, definite arrangements of molecules, atoms or ions. � Crystals have an ordered, repeated structure. � The smallest repeating unit in a crystal is a unit cell. � Unit cell is the smallest unit with all the symmetry of the entire crystal. � Three-dimensional stacking of unit cells is the crystal lattice.

Unit Cell vs. Lattice

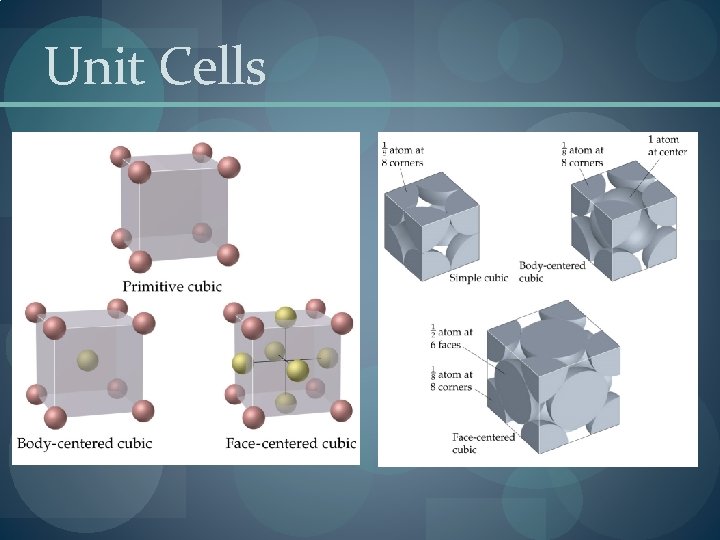
Three common types of unit cell. �Primitive cubic, atoms at the corners of a simple cube, � each atom shared by 8 unit cells; �Body-centered cubic (bcc), atoms at the corners of a cube plus one in the center of the body of the cube, � corner atoms shared by 8 unit cells, center atom completely enclosed in one unit cell; �Face-centered cubic (fcc), atoms at the corners of a cube plus one atom in the center of each face of the cube, � corner atoms shared by 8 unit cells, face atoms shared by 2 unit cells.

Unit Cells

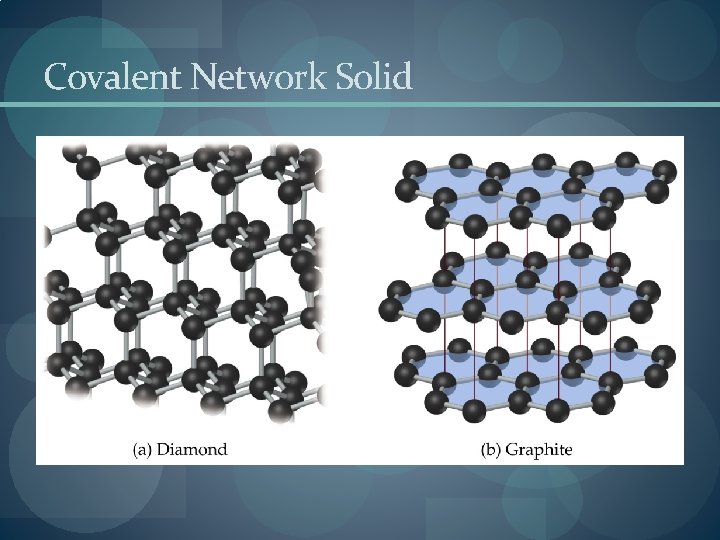
Solids: Four Types � Molecular (formed from molecules) - usually soft with low melting points and poor conductivity. �Covalent network (formed from atoms) - very hard with very high melting points and poor conductivity. �Ions (formed from ions) - hard, brittle, high melting points and poor conductivity. �Metallic (formed from metal atoms) - soft or hard, high melting points, good conductivity, malleable and ductile.

Covalent Network Solid

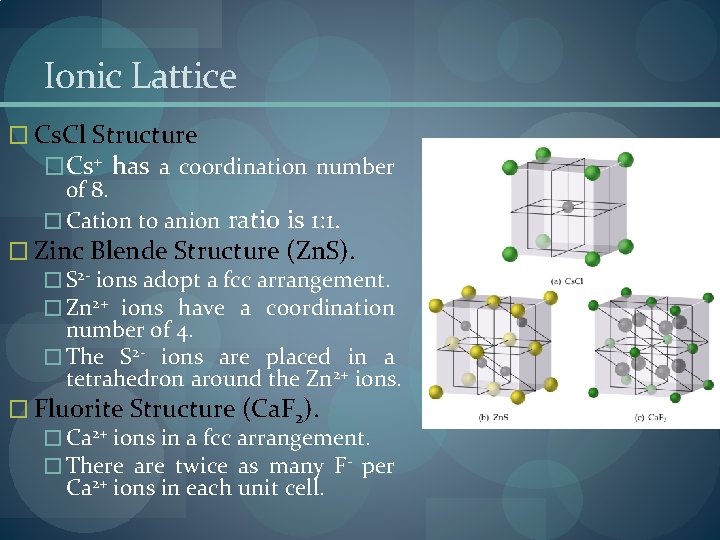
Ionic Lattice � Cs. Cl Structure �Cs+ has a coordination number of 8. � Cation to anion ratio is 1: 1. � Zinc Blende Structure (Zn. S). � S 2 - ions adopt a fcc arrangement. � Zn 2+ ions have a coordination number of 4. � The S 2 - ions are placed in a tetrahedron around the Zn 2+ ions. � Fluorite Structure (Ca. F 2). � Ca 2+ ions in a fcc arrangement. � There are twice as many F- per Ca 2+ ions in each unit cell.