The Cardio MEMS HF System Introduction 39019 MAT2003654
































- Slides: 101

The Cardio. MEMS™ HF System

Introduction 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 2

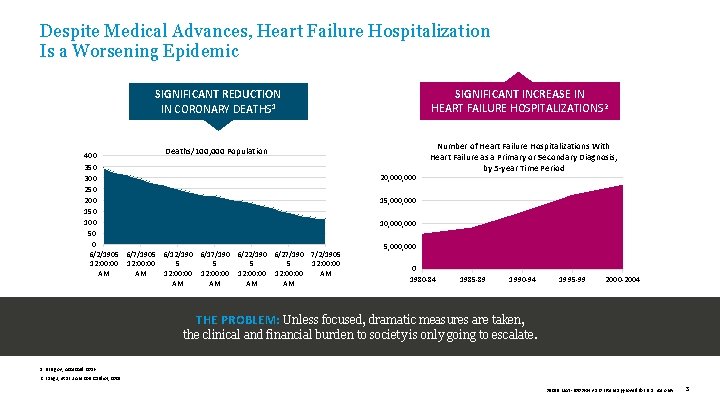
Despite Medical Advances, Heart Failure Hospitalization Is a Worsening Epidemic SIGNIFICANT INCREASE IN HEART FAILURE HOSPITALIZATIONS 2 SIGNIFICANT REDUCTION IN CORONARY DEATHS 1 Deaths/100, 000 Population 400 350 300 250 200 150 100 50 0 6/2/1905 6/7/1905 6/12/190 6/17/190 6/22/190 6/27/190 7/2/1905 12: 00: 00 5 5 12: 00 AM AM 12: 00: 00 AM AM AM 20, 000 Number of Heart Failure Hospitalizations With Heart Failure as a Primary or Secondary Diagnosis, by 5 -year Time Period 15, 000 10, 000 5, 000 0 1980 -84 1985 -89 1990 -94 1995 -99 2000 -2004 THE PROBLEM: Unless focused, dramatic measures are taken, the clinical and financial burden to society is only going to escalate. 1. NIH. gov, Accessed 2016. 2. Fang J, et al. J Am Coll Cardiol, 2008. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 3

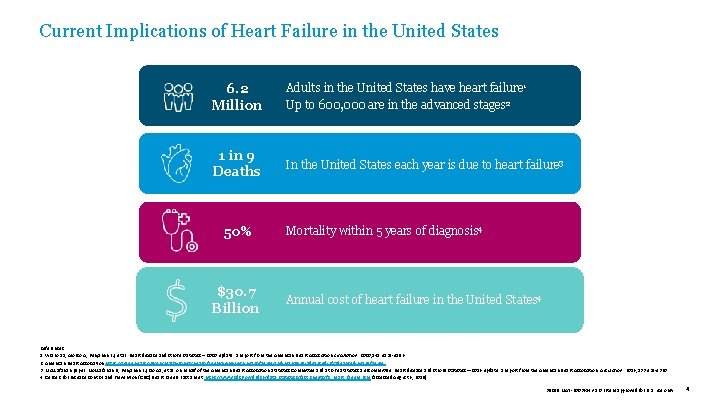
Current Implications of Heart Failure in the United States 6. 2 Million Adults in the United States have heart failure 1 Up to 600, 000 are in the advanced stages 2 1 in 9 Deaths In the United States each year is due to heart failure 3 50% $30. 7 Billion Mortality within 5 years of diagnosis 4 Annual cost of heart failure in the United States 4 References: 1. Virano SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics – 2020 update: a report from the American Heart Association. Circulation. 2020; 141: e 139 -e 596. 2. American Heart Association https: //www. heart. org/en/health-topics/heart-failure/living-with-heart-failure-and-managing-advanced-hf/advanced-heart-failure. 3. Mozzafarian paper: Mozzafarian D, Benjamin EJ, Go AS, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics— 2016 update: a report from the American Heart Association. Circulation. 2016; 133: e 38 -e 360. 4. Centers for Disease Control and Prevention (CDC) Heart Failure Fact Sheet. https: //www. cdc. gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure. htm (accessed August 6, 2018). 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 4

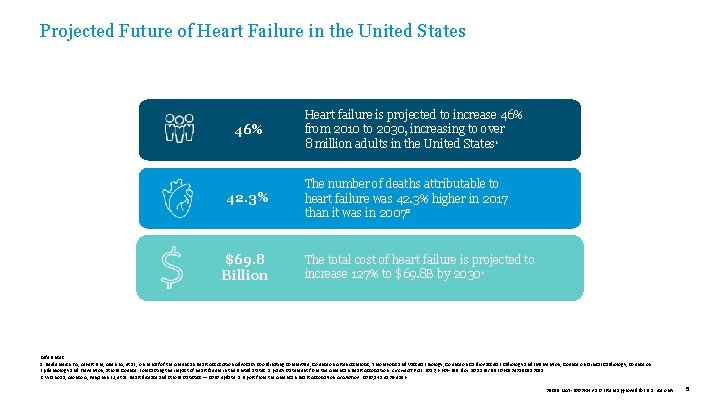
Projected Future of Heart Failure in the United States 46% 42. 3% $69. 8 Billion Heart failure is projected to increase 46% from 2010 to 2030, increasing to over 8 million adults in the United States 1 The number of deaths attributable to heart failure was 42. 3% higher in 2017 than it was in 20072 The total cost of heart failure is projected to increase 127% to $69. 8 B by 20301 References: 1. Heidenreich PA, Albert NM, Allen LA, et al. ; on behalf of the American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013; 6: 606 -619. doi: 10. 1161/HHF. 0 b 013 e 318291329 a. 2. Virano SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics — 2020 update: a report from the American Heart Association. Circulation. 2020; 141: e 139 -e 596. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 5

THE CHALLENGE Current Tools Are Ineffective in Reducing the Clinical and Economic Burdens of Heart Failure 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 6

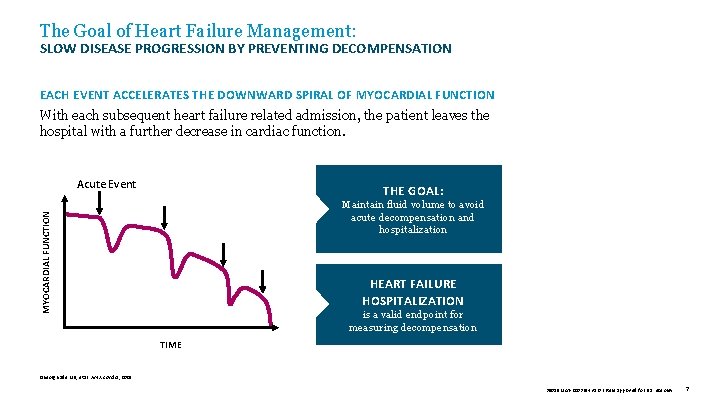
The Goal of Heart Failure Management: SLOW DISEASE PROGRESSION BY PREVENTING DECOMPENSATION EACH EVENT ACCELERATES THE DOWNWARD SPIRAL OF MYOCARDIAL FUNCTION With each subsequent heart failure related admission, the patient leaves the hospital with a further decrease in cardiac function. Acute Event THE GOAL: MYOCARDIAL FUNCTION Maintain fluid volume to avoid acute decompensation and hospitalization HEART FAILURE HOSPITALIZATION is a valid endpoint for measuring decompensation TIME Gheorghiade MD, et al. Am J. Cardiol , 2005. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 7

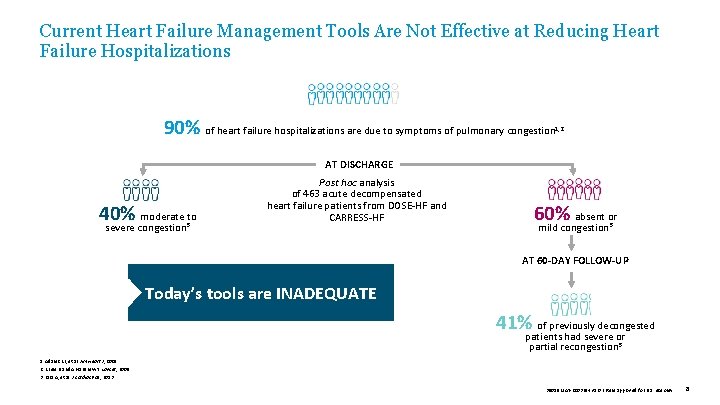
Current Heart Failure Management Tools Are Not Effective at Reducing Heart Failure Hospitalizations 90% of heart failure hospitalizations are due to symptoms of pulmonary congestion 1, 2 AT DISCHARGE 40% moderate to severe congestion 3 Post hoc analysis of 463 acute decompensated heart failure patients from DOSE-HF and CARRESS-HF 60% absent or mild congestion 3 AT 60 -DAY FOLLOW-UP Today’s tools are INADEQUATE 41% of previously decongested patients had severe or partial recongestion 3 1. Adams KF, et al. Am Heart J , 2005. 2. Krum H and Abraham WT. Lancet, 2009. 3. Lala A, et al. J Cardiac Fail, 2013. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 8

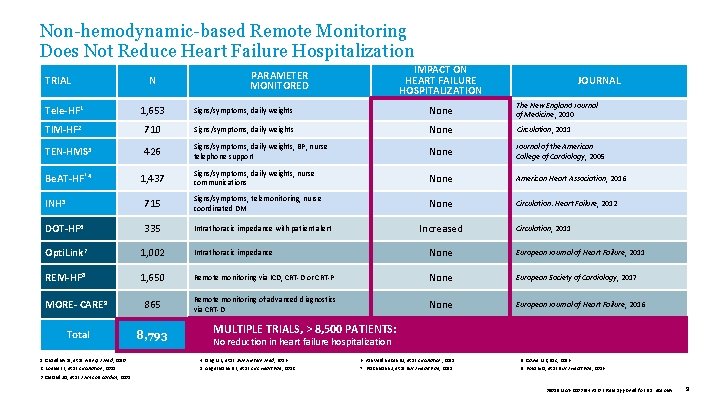
Non-hemodynamic-based Remote Monitoring Does Not Reduce Heart Failure Hospitalization TRIAL N IMPACT ON HEART FAILURE HOSPITALIZATION PARAMETER MONITORED JOURNAL Tele-HF 1 1, 653 Signs/symptoms, daily weights None The New England Journal of Medicine, 2010 TIM-HF 2 710 Signs/symptoms, daily weights None Circulation, 2011 TEN-HMS 3 426 Signs/symptoms, daily weights, BP, nurse telephone support None Journal of the American College of Cardiology, 2005 Be. AT-HF‡ 4 1, 437 Signs/symptoms, daily weights, nurse communications None American Heart Association, 2016 None Circulation: Heart Failure, 2012 INH 5 715 Signs/symptoms, telemonitoring, nurse coordinated DM DOT-HF 6 335 Intrathoracic impedance with patient alert Opti. Link 7 1, 002 Intrathoracic impedance None European Journal of Heart Failure, 2011 REM-HF 8 1, 650 Remote monitoring via ICD, CRT-D or CRT-P None European Society of Cardiology, 2017 MORE- CARE 9 865 Remote monitoring of advanced diagnostics via CRT-D None European Journal of Heart Failure, 2016 Total 8, 793 Increased Circulation, 2011 MULTIPLE TRIALS, > 8, 500 PATIENTS: No reduction in heart failure hospitalization 1. Chaudhry SI, et al. N Engl J Med , 2010. 4. Ong MK, et al. JAMA Intern Med , 2016. 6. van Veldhuisen DJ, et al. Circulation , 2011. 8. Cowie MR, ESC, 2016. 2. Koehler F, et al. Circulation , 2011. 5. Angermann DE, et al. Circ Heart Fail , 2012. 7. Brachmann J, et al. Eur J Heart Fail , 2011. 9. Boriani G, et al. Eur J Heart Fail , 2016. 3. Cleland JG, et al. J Am Coll Cardiol , 2005. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 9

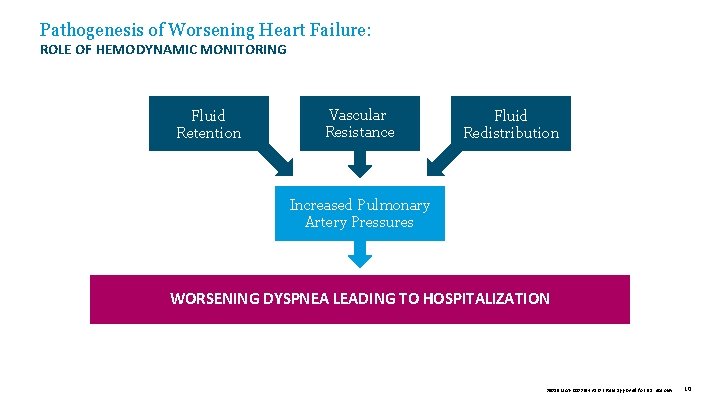
Pathogenesis of Worsening Heart Failure: ROLE OF HEMODYNAMIC MONITORING Fluid Retention Vascular Resistance Fluid Redistribution Increased Pulmonary Artery Pressures WORSENING DYSPNEA LEADING TO HOSPITALIZATION 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 10

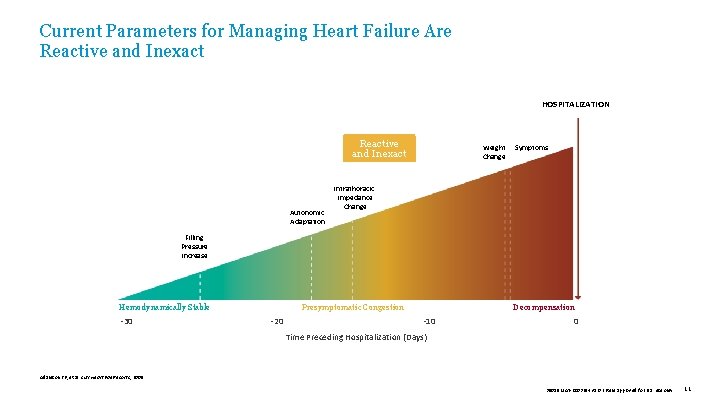
Current Parameters for Managing Heart Failure Are Reactive and Inexact HOSPITALIZATION Reactive and Inexact Autonomic Adaptation Weight Change Symptoms Intrathoracic Impedance Change Filling Pressure Increase Hemodynamically Stable -30 Presymptomatic Congestion -20 Decompensation -10 0 Time Preceding Hospitalization (Days) Adamson PB, et al. Curr Heart Fail Reports , 2009. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 11

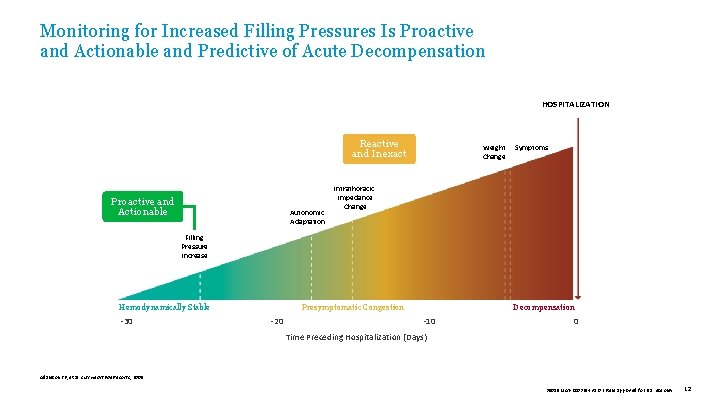
Monitoring for Increased Filling Pressures Is Proactive and Actionable and Predictive of Acute Decompensation HOSPITALIZATION Reactive and Inexact Proactive and Actionable Autonomic Adaptation Weight Change Symptoms Intrathoracic Impedance Change Filling Pressure Increase Hemodynamically Stable -30 Presymptomatic Congestion -20 Decompensation -10 0 Time Preceding Hospitalization (Days) Adamson PB, et al. Curr Heart Fail Reports , 2009. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 12

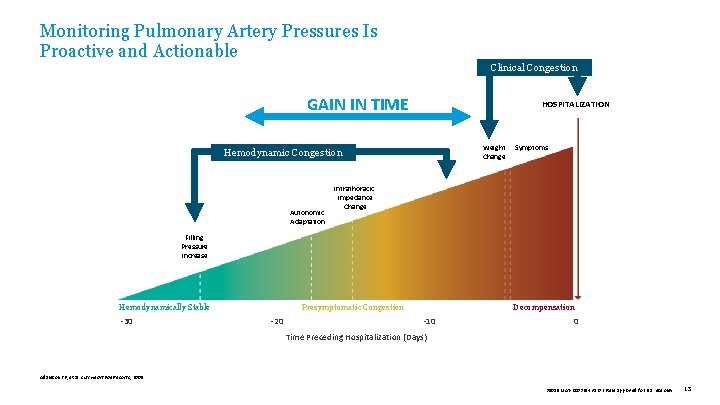
Monitoring Pulmonary Artery Pressures Is Proactive and Actionable Clinical Congestion GAIN IN TIME HOSPITALIZATION Reactive Hemodynamic Congestion and Inexact Proactive and Actionable Autonomic Adaptation Weight Change Symptoms Intrathoracic Impedance Change Filling Pressure Increase Hemodynamically Stable -30 Presymptomatic Congestion -20 Decompensation -10 0 Time Preceding Hospitalization (Days) Adamson PB, et al. Curr Heart Fail Reports , 2009. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 13

INTRODUCTION TO THE CARDIOMEMS™ HF SYSTEM A Personalized, Proactive Approach to Managing Heart Failure by Monitoring PA Pressure 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 14

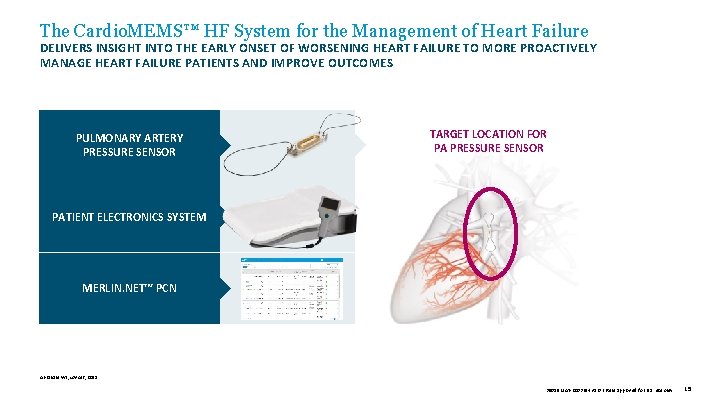
The Cardio. MEMS™ HF System for the Management of Heart Failure DELIVERS INSIGHT INTO THE EARLY ONSET OF WORSENING HEART FAILURE TO MORE PROACTIVELY MANAGE HEART FAILURE PATIENTS AND IMPROVE OUTCOMES PULMONARY ARTERY PRESSURE SENSOR TARGET LOCATION FOR PA PRESSURE SENSOR PATIENT ELECTRONICS SYSTEM MERLIN. NET™ PCN Abraham WT, Lancet, 2011. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 15

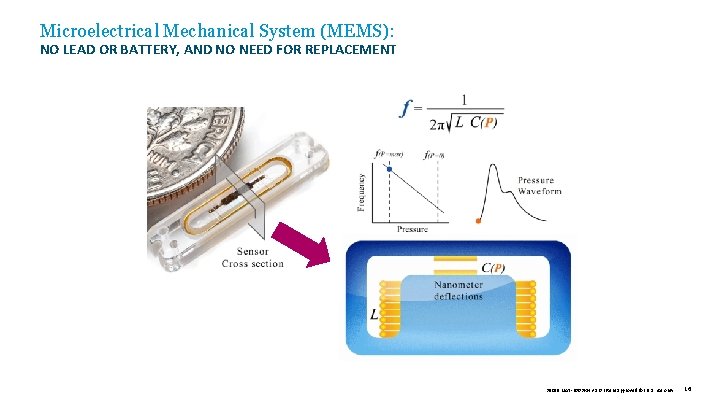
Microelectrical Mechanical System (MEMS): NO LEAD OR BATTERY, AND NO NEED FOR REPLACEMENT 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 16

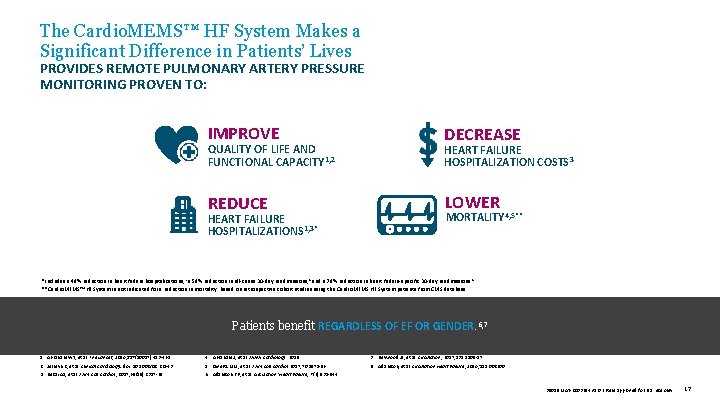
The Cardio. MEMS™ HF System Makes a Significant Difference in Patients’ Lives PROVIDES REMOTE PULMONARY ARTERY PRESSURE MONITORING PROVEN TO: IMPROVE H DECREASE QUALITY OF LIFE AND FUNCTIONAL CAPACITY 1, 2 HEART FAILURE HOSPITALIZATION COSTS 3 REDUCE LOWER HEART FAILURE HOSPITALIZATIONS 1, 3* MORTALITY 4, 5** *Includes a 48% reduction in heart failure hospitalizations, 1 a 58% reduction in all-cause 30 -day readmissions, 8 and a 78% reduction in heart failure-specific 30 -day readmissions. 8 **Cardio. MEMS™ HF System is not indicated for a reduction in mortality. Based on retrospective cohort studies using the Cardio. MEMS HF System patients from CMS database. Patients benefit REGARDLESS OF EF OR GENDER. 6, 7 1. Abraham WT, et al. The Lancet, 2016; 387(10017): 453 -461. 2. Jermyn R, et al. Clinical Cardiology. doi: 10. 1002/clc. 22643. 3. Desai AS, et al. J Am Coll Cardiol , 2017; 69(19): 2357 -65. 4. Abraham J, et al. JAMA Cardiology. 2019. 5. Givertz MM, et al. J Am Coll Cardiol 2017; 70: 1875 -86. 6. Adamson PB, et al. Circulation: Heart Failure, 7 (6): 935 -944. 7. Heywood JT, et al. Circulation, 2017; 135: 1509 -17. 8. Adamson, et al. Circulation Heart Failure, 2016; 115. 002600. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 17

The Cardio. MEMS™ HF System Implant Procedure THE PA PRESSURE SENSOR IS INSERTED DURING A RIGHT HEART CATHETERIZATION PROCEDURE VIA FEMORAL VEIN APPROACH. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 18

THE CHAMPION TRIAL DESIGN Evaluating the Cardio. MEMS™ HF System Through Clinical Studies and Real-world Experience 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 19

Summary of the CHAMPION Randomized Clinical Trial: 550 PREVIOUSLY HOSPITALIZED NYHA CLASS III PATIENTS 1 -3 Pulmonary Artery Pressure Medication Changes Based on Pulmonary Artery Pressure (p < 0. 0001) Pulmonary Artery Pressure Reduction (p = 0. 008) Reduction in Heart Failure Hospitalizations (p < 0. 0001) MANAGING PRESSURES TO TARGET GOAL RANGES • PA pressure systolic: 15– 35 mm. Hg • PA pressure diastolic: 8– 20 mm. Hg • PA pressure mean: 10– 25 mm. Hg Using diuretics and vasodilators, in addition to GDMTs Quality of Life Improvement (p = 0. 024) 1. Abraham WT, et al. Lancet, 2011. 2. Abraham WT, et al. Lancet, 2016. 3. Adamson PB, et al. J Card Fail , 2010. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 20

THE CHAMPION TRIAL RESULTS Evidence That PA Pressure-guided Therapy Reduces Heart Failure Hospitalizations 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 21

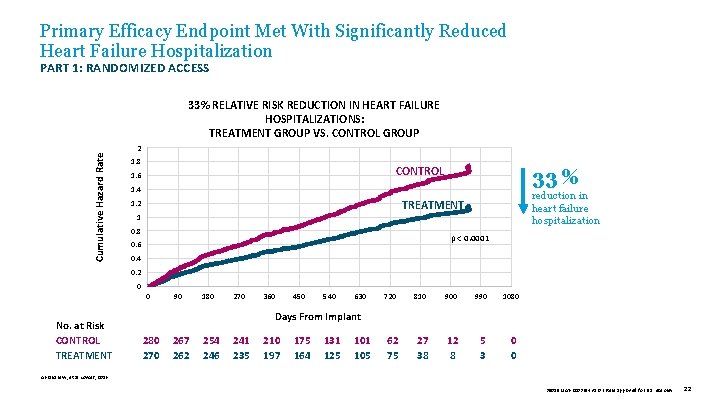
Primary Efficacy Endpoint Met With Significantly Reduced Heart Failure Hospitalization PART 1: RANDOMIZED ACCESS Cumulative Hazard Rate 33% RELATIVE RISK REDUCTION IN HEART FAILURE HOSPITALIZATIONS: TREATMENT GROUP VS. CONTROL GROUP 2 1. 8 CONTROL 1. 6 33 % 1. 4 reduction in heart failure hospitalization TREATMENT 1. 2 1 0. 8 p < 0. 0001 0. 6 0. 4 0. 2 0 0 No. at Risk CONTROL TREATMENT 90 180 270 360 450 540 630 720 810 900 990 1080 62 75 27 38 12 8 5 3 0 0 Days From Implant 280 270 267 262 254 246 241 235 210 197 175 164 131 125 101 105 Abraham W, et al. Lancet, 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 22

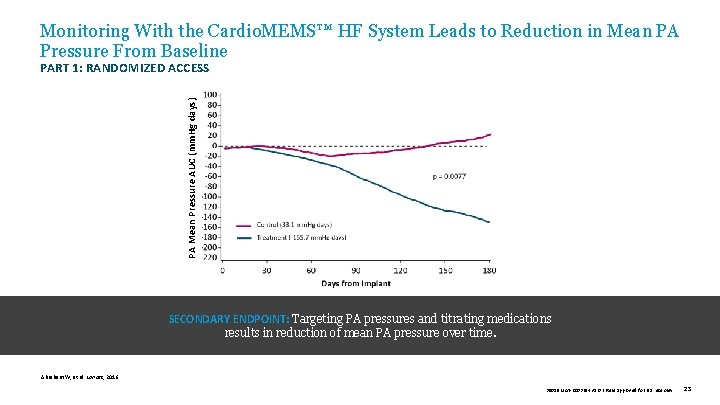
Monitoring With the Cardio. MEMS™ HF System Leads to Reduction in Mean PA Pressure From Baseline PA Mean Pressure AUC (mm. Hg days) PART 1: RANDOMIZED ACCESS SECONDARY ENDPOINT: Targeting PA pressures and titrating medications results in reduction of mean PA pressure over time. Abraham W, et al. Lancet, 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 23

PROSPECTIVE ANALYSIS: HFp. EF Subgroup The CHAMPION Trial Subgroup Analyses 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 24

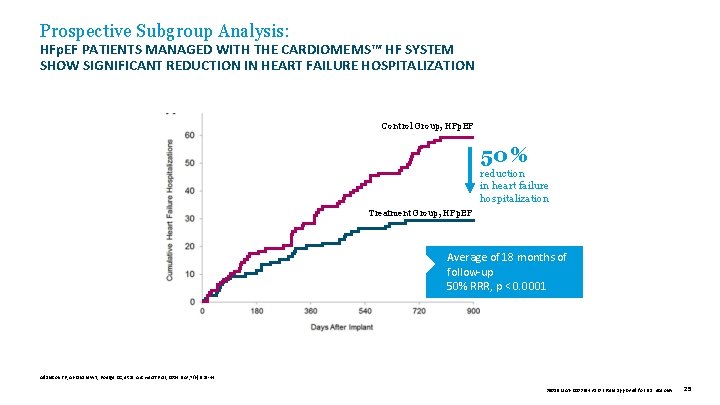
Prospective Subgroup Analysis: HFp. EF PATIENTS MANAGED WITH THE CARDIOMEMS™ HF SYSTEM SHOW SIGNIFICANT REDUCTION IN HEART FAILURE HOSPITALIZATION Control Group, HFp. EF 50 % reduction in heart failure hospitalization Treatment Group, HFp. EF Average of 18 months of follow-up 50% RRR, p < 0. 0001 Adamson PB, Abraham WT, Bourge RC, et al. Circ Heart Fail , 2014 Nov; 7(6): 935 -44. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 25

PROSPECTIVE ANALYSIS: HFr. EF Subgroup — Givertz The CHAMPION Trial Subgroup Analyses 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 26

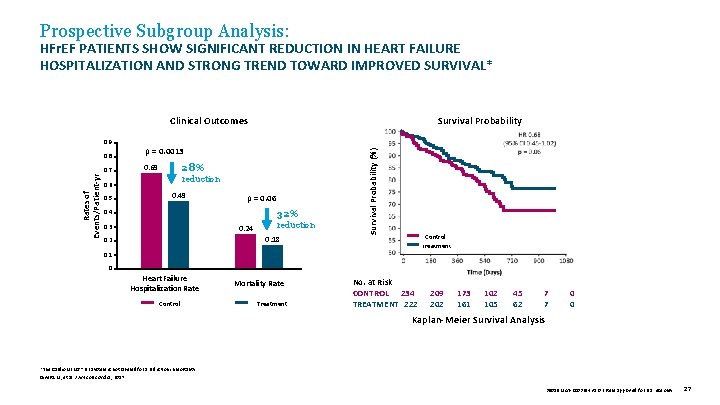
Prospective Subgroup Analysis: HFr. EF PATIENTS SHOW SIGNIFICANT REDUCTION IN HEART FAILURE HOSPITALIZATION AND STRONG TREND TOWARD IMPROVED SURVIVAL* Clinical Outcomes Rates of Events/Patient-yr 0. 8 0. 7 p = 0. 0013 28% 0. 69 reduction 0. 6 0. 5 0. 49 p = 0. 06 32% 0. 4 0. 3 0. 24 reduction 0. 18 0. 2 Survival Probability (%) 0. 9 Survival Probability Control Treatment 0. 1 0 Heart Failure Hospitalization Rate Control Mortality Rate Treatment No. at Risk CONTROL 234 TREATMENT 222 209 202 173 161 102 105 45 62 7 7 0 0 Kaplan-Meier Survival Analysis *The Cardio. MEMS™ HF System is not labeled for a reduction in mortality. Givertz M, et al. J Am Coll Cardiol , 2017. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 27

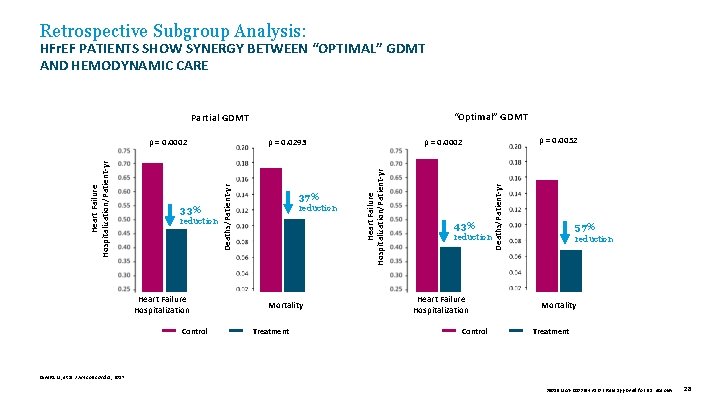
Retrospective Subgroup Analysis: HFr. EF PATIENTS SHOW SYNERGY BETWEEN “OPTIMAL” GDMT AND HEMODYNAMIC CARE “Optimal” GDMT Partial GDMT Heart Failure Hospitalization Control reduction Mortality Treatment 43% reduction Heart Failure Hospitalization Control Deaths/Patient-yr reduction 37% Heart Failure Hospitalization/Patient-yr 33% p = 0. 0052 p = 0. 0002 p = 0. 0293 Deaths/Patient-yr Heart Failure Hospitalization/Patient-yr p = 0. 0002 57% reduction Mortality Treatment Givertz M, et al. J Am Coll Cardiol, 2017. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 28

RETROSPECTIVE ANALYSIS: THERAPY GUIDED BY PAP ALONE VS. SIGNS AND SYMPTOMS — GOLDBERG The CHAMPION Trial Subgroup Analyses 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 29

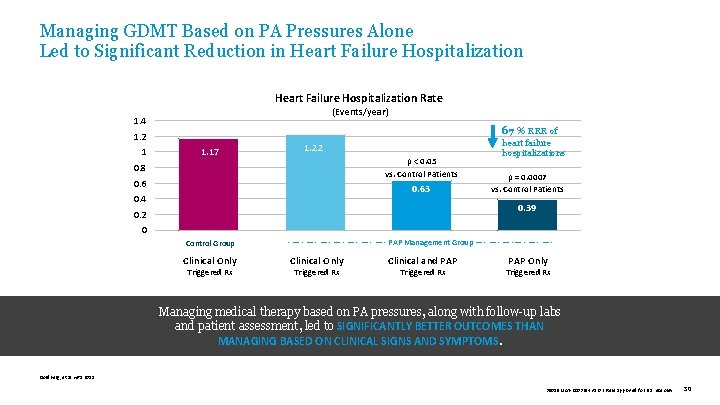
Managing GDMT Based on PA Pressures Alone Led to Significant Reduction in Heart Failure Hospitalization Rate (Events/year) 1. 4 67 % RRR of 1. 2 1 1. 17 1. 22 p < 0. 05 vs. Control Patients 0. 8 0. 63 0. 4 heart failure hospitalizations p = 0. 0007 vs. Control Patients 0. 39 0. 2 0 Control Group Clinical Only Triggered Rx - — - — - PAP Management Group — - — -— - — - Clinical Only Triggered Rx Clinical and PAP Triggered Rx PAP Only Triggered Rx Managing medical therapy based on PA pressures, along with follow-up labs and patient assessment, led to SIGNIFICANTLY BETTER OUTCOMES THAN MANAGING BASED ON CLINICAL SIGNS AND SYMPTOMS. Goldberg, et al. HRS 2015. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 30

COMMERCIAL EXPERIENCE Analyzing the Cardio. MEMS™ HF System in a Commercial Setting 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 31

COMMERCIAL EXPERIENCE First 2, 000 Commercial Patients Heywood JT, et al. Circulation. 2017. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 32

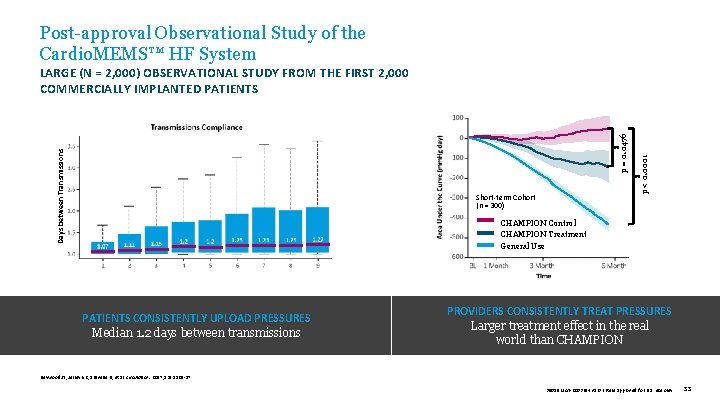
Post-approval Observational Study of the Cardio. MEMS™ HF System Short-term Cohort (n = 300) p < 0. 0001 Days between Transmissions p = 0. 0476 LARGE (N = 2, 000) OBSERVATIONAL STUDY FROM THE FIRST 2, 000 COMMERCIALLY IMPLANTED PATIENTS CHAMPION Control CHAMPION Treatment General Use PATIENTS CONSISTENTLY UPLOAD PRESSURES Median 1. 2 days between transmissions PROVIDERS CONSISTENTLY TREAT PRESSURES Larger treatment effect in the real world than CHAMPION Heywood JT, Jermyn R, Shavelle D, et al. Circulation. 2017; 135: 1509 -17. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 33

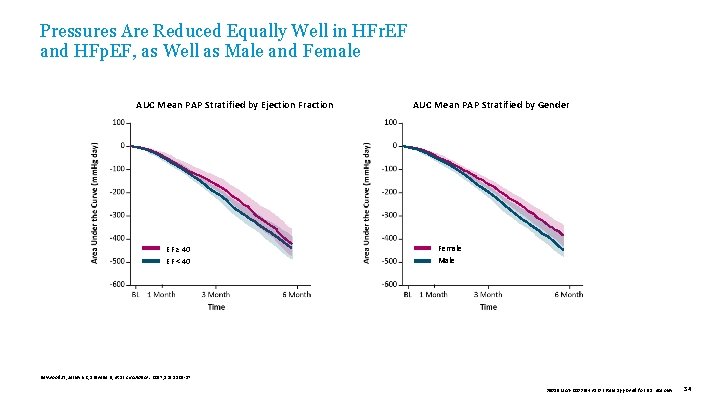
Pressures Are Reduced Equally Well in HFr. EF and HFp. EF, as Well as Male and Female AUC Mean PAP Stratified by Ejection Fraction EF ≥ 40 EF < 40 AUC Mean PAP Stratified by Gender Female Male Heywood JT, Jermyn R, Shavelle D, et al. Circulation. 2017; 135: 1509 -17. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 34

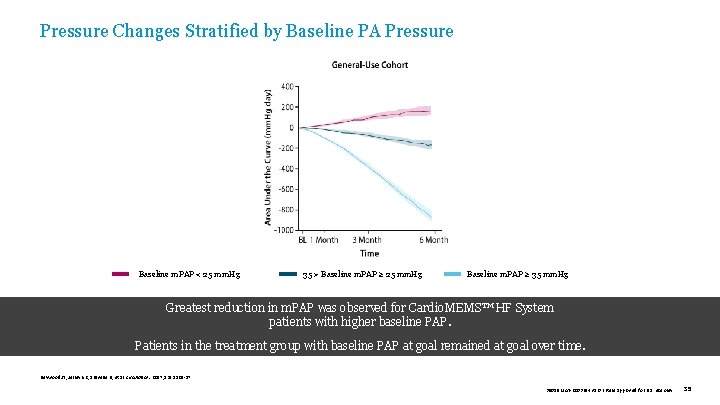
Pressure Changes Stratified by Baseline PA Pressure Baseline m. PAP < 25 mm. Hg 35 > Baseline m. PAP ≥ 25 mm. Hg Baseline m. PAP ≥ 35 mm. Hg Greatest reduction in m. PAP was observed for Cardio. MEMS™ HF System . patients with higher baseline PAP. Patients in the treatment group with baseline PAP at goal remained at goal over time. Heywood JT, Jermyn R, Shavelle D, et al. Circulation. 2017; 135: 1509 -17. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 35

COMMERCIAL EXPERIENCE Reduced Heart Failure Hospitalizations and Economic Impact Desai AS, et al. JACC. 2017. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 36

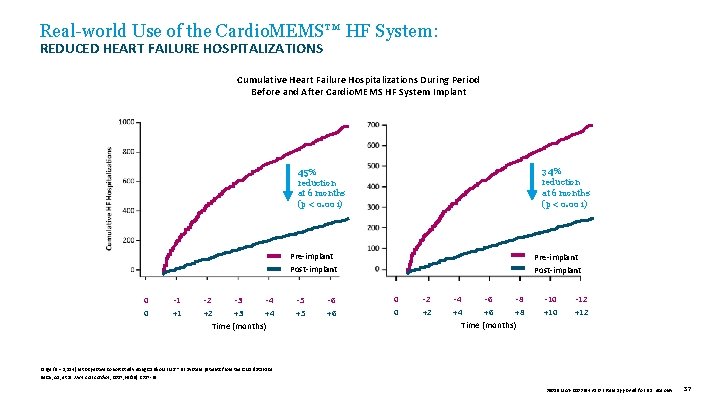
Real-world Use of the Cardio. MEMS™ HF System: REDUCED HEART FAILURE HOSPITALIZATIONS Cumulative Heart Failure Hospitalizations During Period Before and After Cardio. MEMS HF System Implant 34% reduction at 6 months (p < 0. 001) 45% reduction at 6 months (p < 0. 001) Pre-implant Post-implant 0 0 -1 +1 -2 -3 -4 +2 +3 +4 Time (months) -5 +5 -6 +6 Pre-implant Post-implant 0 0 -2 +2 -4 -6 -8 +4 +6 +8 Time (months) -10 +10 -12 +12 Large (N = 1, 114) retrospective cohort study using Cardio. MEMS™ HF System patients from the CMS database. Desai, AS, et al. J Am Coll Cardiol , 2017; 69(19): 2357 -65. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 37

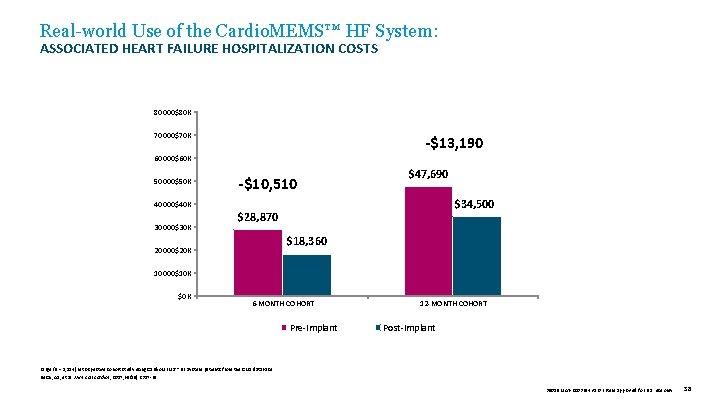
Real-world Use of the Cardio. MEMS™ HF System: ASSOCIATED HEART FAILURE HOSPITALIZATION COSTS 80000$80 K 70000$70 K -$13, 190 60000$60 K 50000$50 K -$10, 510 $47, 690 $34, 500 40000$40 K 30000$30 K $28, 870 $18, 360 20000$20 K 10000$10 K $0 K 6 -MONTH COHORT Pre-Implant 12 -MONTH COHORT Post-Implant Large (N = 1, 114) retrospective cohort study using Cardio. MEMS™ HF System patients from the CMS database. Desai, AS, et al. J Am Coll Cardiol , 2017; 69(19): 2357 -65. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 38

COMMERCIAL EXPERIENCE Lower Mortality and Heart Failure Hospitalization Following Cardio. MEMS™ PA Sensor Implant Abraham J, et al. JAMA Cardiology. 2019. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 39

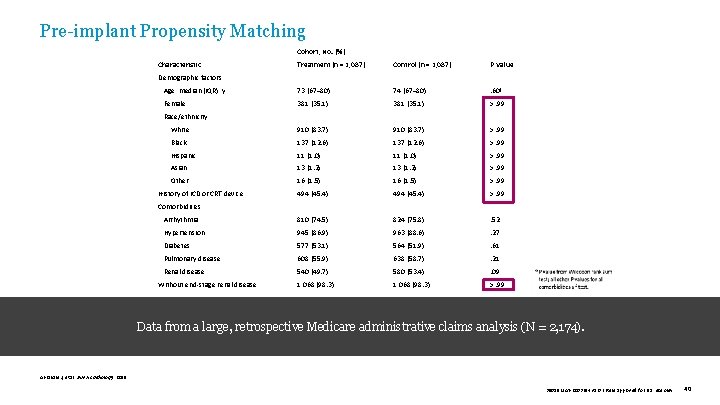
Pre-implant Propensity Matching Cohort, No. (%) Characteristic Treatment (n = 1, 087) Control (n = 1, 087) P Value Age, median (IQR), y 73 (67– 80) 74 (67– 80) . 60ª Female 381 (35. 1) >. 99 White 910 (83. 7) >. 99 Black 137 (12. 6) >. 99 Hispanic 11 (1. 0) >. 99 Asian 13 (1. 2) >. 99 Other 16 (1. 5) >. 99 494 (45. 4) >. 99 Arrhythmia 810 (74. 5) 824 (75. 8) . 52 Hypertension 945 (86. 9) 963 (88. 6) . 27 Diabetes 577 (53. 1) 564 (51. 9) . 61 Pulmonary disease 608 (55. 9) 638 (58. 7) . 21 Renal disease 540 (49. 7) 580 (53. 4) . 09 Without end-stage renal disease 1, 068 (98. 3) >. 99 Demographic factors Race/ethnicity History of ICD or CRT device Comorbidities Data from a large, retrospective Medicare administrative claims analysis (N = 2, 174). Abraham J, et al. JAMA Cardiology. 2019. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 40

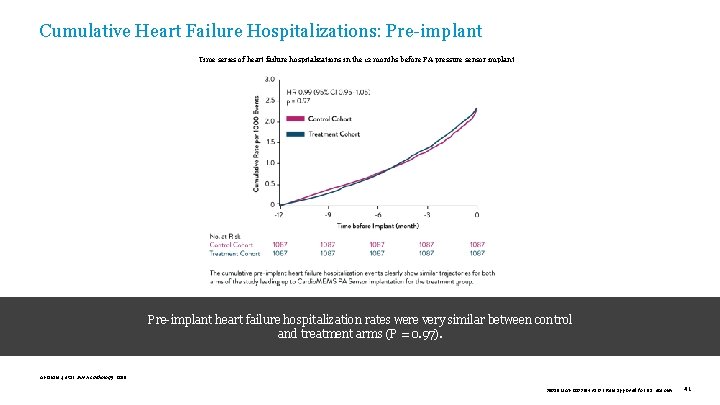
Cumulative Heart Failure Hospitalizations: Pre-implant Time series of heart failure hospitalizations in the 12 months before PA pressure sensor implant Pre-implant heart failure hospitalization rates were very similar between control and treatment arms (P = 0. 97). Abraham J, et al. JAMA Cardiology. 2019. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 41

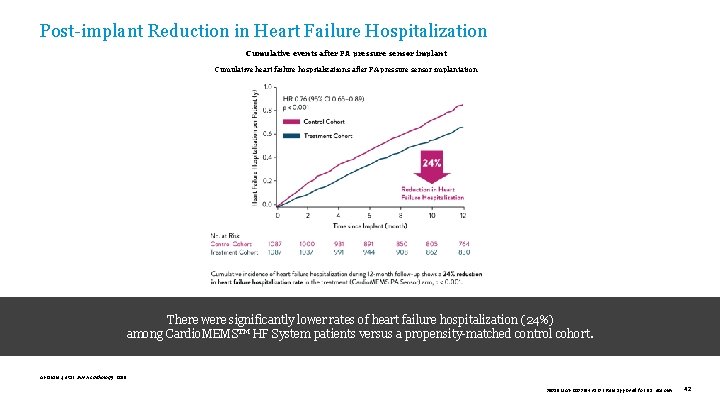
Post-implant Reduction in Heart Failure Hospitalization Cumulative events after PA pressure sensor implant Cumulative heart failure hospitalizations after PA pressure sensor implantation There were significantly lower rates of heart failure hospitalization (24%) among Cardio. MEMS™ HF System patients versus a propensity-matched control cohort. Abraham J, et al. JAMA Cardiology. 2019. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 42

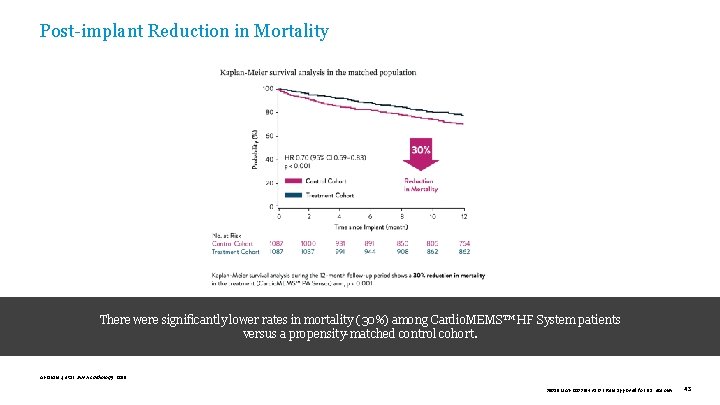
Post-implant Reduction in Mortality There were significantly lower rates in mortality (30%) among Cardio. MEMS™ HF System patients versus a propensity-matched control cohort. Abraham J, et al. JAMA Cardiology. 2019. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 43

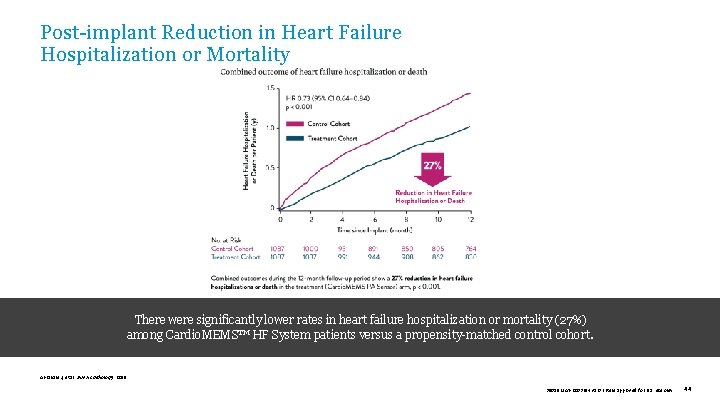
Post-implant Reduction in Heart Failure Hospitalization or Mortality There were significantly lower rates in heart failure hospitalization or mortality (27%) among Cardio. MEMS™ HF System patients versus a propensity-matched control cohort. Abraham J, et al. JAMA Cardiology. 2019. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 44

COMMERCIAL EXPERIENCE U. S. Post-approval Study Shavelle D, et al. Circulation: Heart Failure. 2020. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 45

Cardio. MEMS Post-approval Study: Study Design A PROSPECTIVE, MULTICENTER, OPEN-LABEL TRIAL OF 1, 200 PATIENTS WITH NYHA CLASS III HEART FAILURE AND A HEART FAILURE HOSPITALIZATION WITHIN THE PRIOR 12 MONTHS Primary Efficacy Endpoint: Reduction in the rate of heart failure hospitalization at 1 -year post-implant compared with the year prior to enrollment Primary Safety Endpoints: Freedom from DSRC > 80% at 2 years Freedom from sensor failure > 90% at 2 years Supplemental Analysis: Heart failure hospitalization or death at 1 year Death at 1 year Patient compliance Outcomes in subgroups Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 46

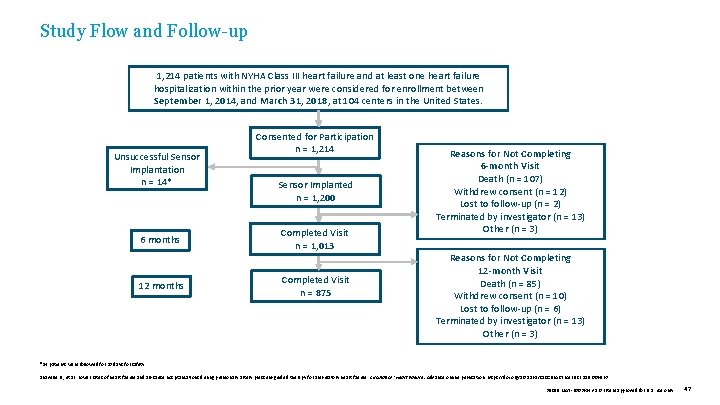
Study Flow and Follow-up 1, 214 patients with NYHA Class III heart failure and at least one heart failure hospitalization within the prior year were considered for enrollment between September 1, 2014, and March 31, 2018, at 104 centers in the United States. Unsuccessful Sensor Implantation n = 14* 6 months 12 months Consented for Participation n = 1, 214 Sensor Implanted n = 1, 200 Completed Visit n = 1, 013 Completed Visit n = 875 Reasons for Not Completing 6 -month Visit Death (n = 107) Withdrew consent (n = 12) Lost to follow-up (n = 2) Terminated by investigator (n = 13) Other (n = 3) Reasons for Not Completing 12 -month Visit Death (n = 85) Withdrew consent (n = 10) Lost to follow-up (n = 6) Terminated by investigator (n = 13) Other (n = 3) *14 patients were followed for 30 days for safety. Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 47

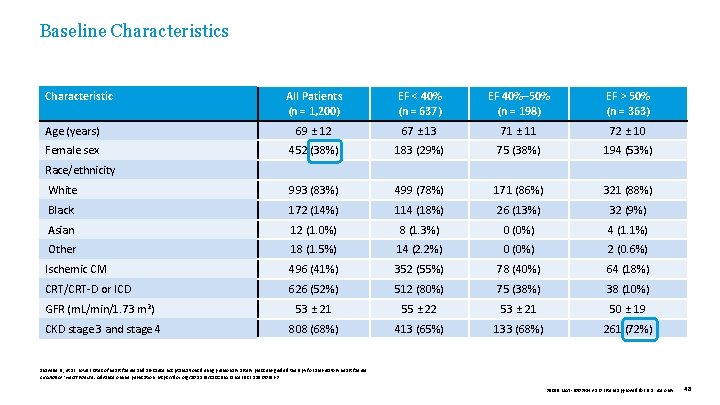
Baseline Characteristics Characteristic All Patients (n = 1, 200) EF < 40% (n = 637) EF 40%– 50% (n = 198) EF > 50% (n = 363) Age (years) 69 ± 12 67 ± 13 71 ± 11 72 ± 10 Female sex 452 (38%) 183 (29%) 75 (38%) 194 (53%) White 993 (83%) 499 (78%) 171 (86%) 321 (88%) Black 172 (14%) 114 (18%) 26 (13%) 32 (9%) Asian 12 (1. 0%) 8 (1. 3%) 0 (0%) 4 (1. 1%) Other 18 (1. 5%) 14 (2. 2%) 0 (0%) 2 (0. 6%) Ischemic CM 496 (41%) 352 (55%) 78 (40%) 64 (18%) CRT/CRT-D or ICD 626 (52%) 512 (80%) 75 (38%) 38 (10%) GFR (m. L/min/1. 73 m 2) 53 ± 21 55 ± 22 53 ± 21 50 ± 19 CKD stage 3 and stage 4 808 (68%) 413 (65%) 133 (68%) 261 (72%) Race/ethnicity Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 48

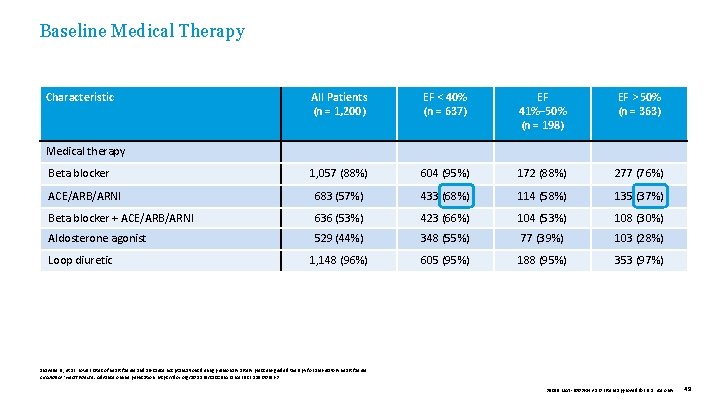
Baseline Medical Therapy Characteristic EF < 40% (n = 637) EF 41%– 50% (n = 198) EF > 50% (n = 363) 1, 057 (88%) 604 (95%) 172 (88%) 277 (76%) ACE/ARB/ARNI 683 (57%) 433 (68%) 114 (58%) 135 (37%) Beta blocker + ACE/ARB/ARNI 636 (53%) 423 (66%) 104 (53%) 108 (30%) Aldosterone agonist 529 (44%) 348 (55%) 77 (39%) 103 (28%) 1, 148 (96%) 605 (95%) 188 (95%) 353 (97%) Medical therapy Beta blocker Loop diuretic All Patients (n = 1, 200) Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 49

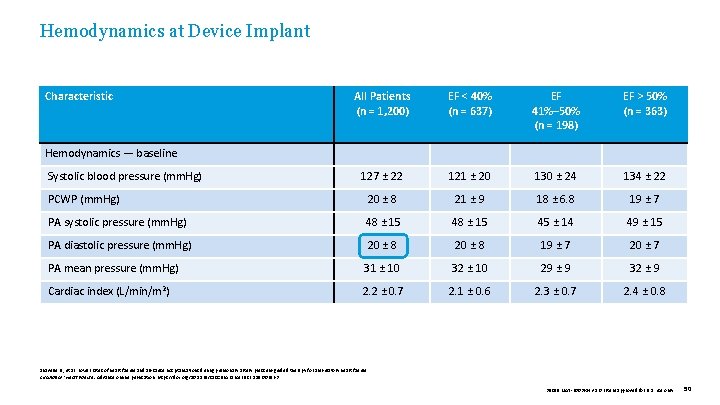
Hemodynamics at Device Implant Characteristic All Patients (n = 1, 200) EF < 40% (n = 637) EF 41%– 50% (n = 198) EF > 50% (n = 363) 127 ± 22 121 ± 20 130 ± 24 134 ± 22 20 ± 8 21 ± 9 18 ± 6. 8 19 ± 7 PA systolic pressure (mm. Hg) 48 ± 15 45 ± 14 49 ± 15 PA diastolic pressure (mm. Hg) 20 ± 8 19 ± 7 20 ± 7 PA mean pressure (mm. Hg) 31 ± 10 32 ± 10 29 ± 9 32 ± 9 2. 2 ± 0. 7 2. 1 ± 0. 6 2. 3 ± 0. 7 2. 4 ± 0. 8 Hemodynamics — baseline Systolic blood pressure (mm. Hg) PCWP (mm. Hg) Cardiac index (L/min/m 2) Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 50

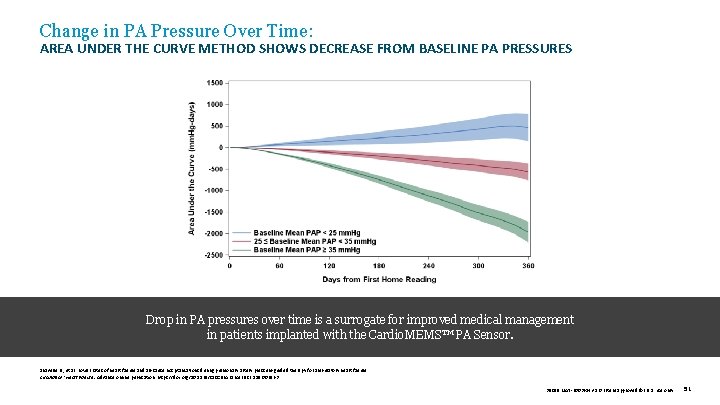
Change in PA Pressure Over Time: AREA UNDER THE CURVE METHOD SHOWS DECREASE FROM BASELINE PA PRESSURES Drop in PA pressures over time is a surrogate for improved medical management in patients implanted with the Cardio. MEMS™ PA Sensor. Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 51

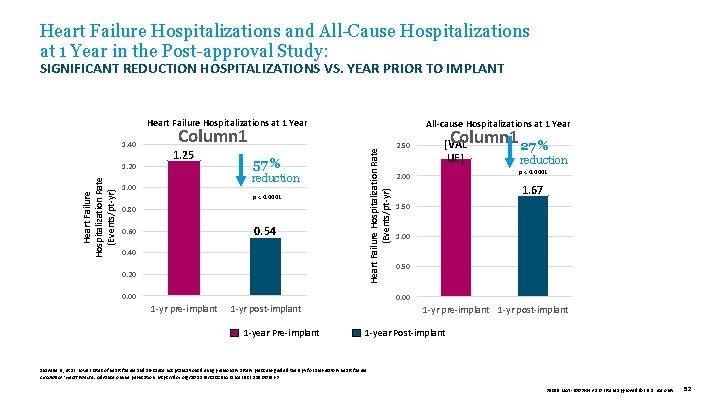
Heart Failure Hospitalizations and All-Cause Hospitalizations at 1 Year in the Post-approval Study: SIGNIFICANT REDUCTION HOSPITALIZATIONS VS. YEAR PRIOR TO IMPLANT Heart Failure Hospitalizations at 1 Year Heart Failure Hospitalization Rate (Events/pt-yr) 1. 20 Column 1 1. 25 57 % reduction 1. 00 p < 0. 0001 0. 80 0. 54 0. 60 0. 40 0. 20 Heart Failure Hospitalization Rate (Events/pt-yr) 1. 40 All-cause Hospitalizations at 1 Year 0. 00 2. 50 Column 1 27 % [VAL UE] reduction p < 0. 0001 2. 00 1. 67 1. 50 1. 00 0. 50 0. 00 1 -yr pre-implant 1 -yr post-implant 1 -year Pre-implant 1 -yr post-implant 1 -year Post-implant Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 52

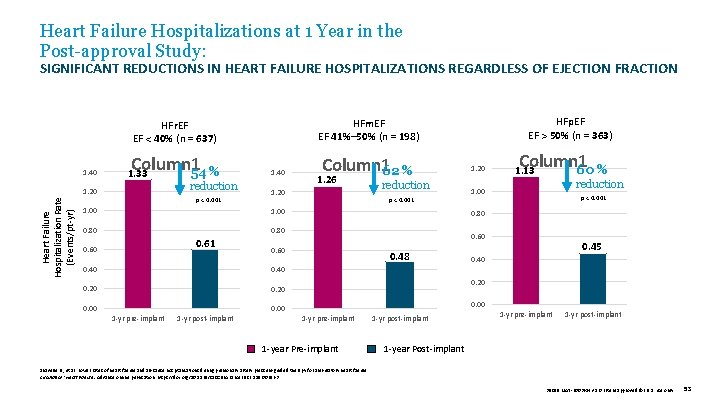
Heart Failure Hospitalizations at 1 Year in the Post-approval Study: SIGNIFICANT REDUCTIONS IN HEART FAILURE HOSPITALIZATIONS REGARDLESS OF EJECTION FRACTION Heart Failure Hospitalization Rate (Events/pt-yr) 1. 40 Column 1 54 % 1. 33 1. 20 reduction p < 0. 001 1. 00 1. 40 Column 162 % 1. 26 1. 20 reduction p < 0. 001 1. 00 0. 80 0. 60 0. 40 0. 20 0. 00 1 -yr pre-implant 1 -yr post-implant Column 1 60 % 1. 13 reduction 1. 00 p < 0. 001 0. 60 0. 40 1. 20 0. 80 0. 61 HFp. EF EF > 50% (n = 363) HFm. EF EF 41%– 50% (n = 198) HFr. EF EF < 40% (n = 637) 0. 48 0. 45 0. 40 0. 20 0. 00 1 -yr pre-implant 1 -year Pre-implant 1 -yr post-implant 1 -yr pre-implant 1 -yr post-implant 1 -year Post-implant Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 53

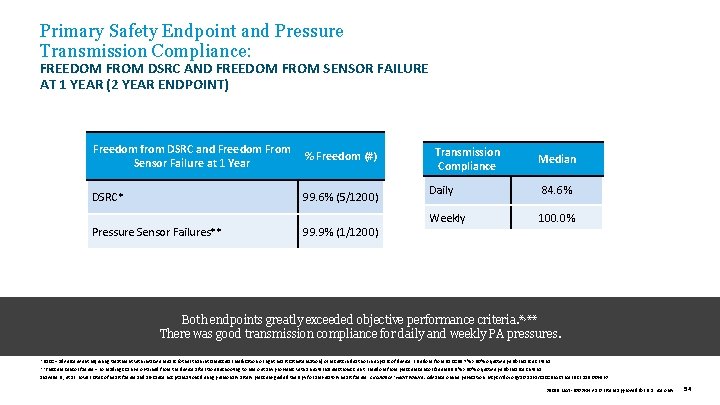
Primary Safety Endpoint and Pressure Transmission Compliance: FREEDOM FROM DSRC AND FREEDOM FROM SENSOR FAILURE AT 1 YEAR (2 YEAR ENDPOINT) Freedom from DSRC and Freedom From % Freedom (#) Sensor Failure at 1 Year DSRC* 99. 6% (5/1200) Pressure Sensor Failures** 99. 9% (1/1200) Transmission Compliance Median Daily 84. 6% Weekly 100. 0% Both endpoints greatly exceeded objective performance criteria. *, ** There was good transmission compliance for daily and weekly PA pressures. *DSRC = adverse event requiring treatment with invasive means (other than intramuscular medication or right heart catheterization) or results in death or in explant of device. Freedom from DSRC 99. 7% > 80% objective performance criteria. **Pressure sensor failure = no readings can be obtained from the device after troubleshooting to rule out any problems with an external electronics unit. Freedom from pressure sensor failure 99. 9% > 90% objective performance criteria. Shavelle D. , et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure. Circulation: Heart Failure. Advance online publication. https: //doi. org/10. 1161/CIRCHEARTFAILURE. 119. 006863 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 54

COMMERCIAL EXPERIENCE Commercial Experience in a European Healthcare System: MEMS-HF Angermann C, et al. European Journal of Heart Failure. 2020. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 55

MEMS-HF Prospective Study MEMS-HF Summary Prospective single-arm, multicenter, open-label trial (N = 234) to confirm the safety and effectiveness of the Cardio. MEMS™ HF System in a real-world setting outside of the United States. • The first prospective multicenter Cardio. MEMS HF System study to show improved quality of life scores • Results from Germany, the Netherlands and Ireland are consistent with the positive results of the U. S. Post-approval Study of 1, 200 patients • PA pressure-guided therapy using the Cardio. MEMS HF System was safe with few device-/system-related complications and few pressure sensor failures Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 56

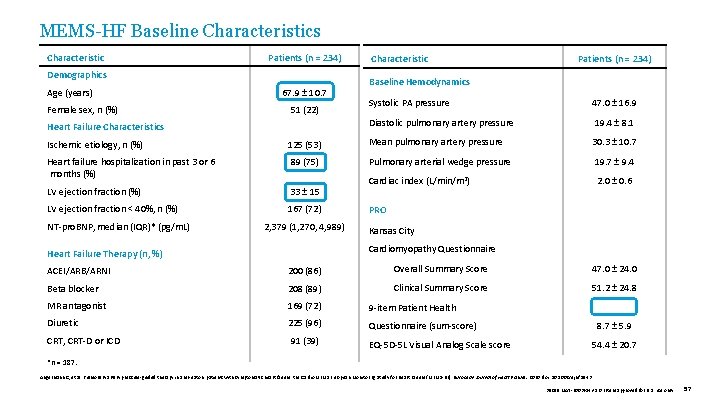
MEMS-HF Baseline Characteristics Characteristic Patients (n = 234) Demographics Age (years) Female sex, n (%) 67. 9 ± 10. 7 51 (22) Heart Failure Characteristics Characteristic Patients (n = 234) Baseline Hemodynamics Systolic PA pressure 47. 0 ± 16. 9 Diastolic pulmonary artery pressure 19. 4 ± 8. 1 Ischemic etiology, n (%) 125 (53) Mean pulmonary artery pressure 30. 3 ± 10. 7 Heart failure hospitalization in past 3 or 6 months (%) 89 (75) Pulmonary arterial wedge pressure 19. 7 ± 9. 4 LV ejection fraction (%) 33 ± 15 Cardiac index (L/min/m 2) 2. 0 ± 0. 6 LV ejection fraction < 40%, n (%) 167 (72) NT-pro. BNP, median (IQR)* (pg/m. L) 2, 379 (1, 270, 4, 989) PRO Kansas City Cardiomyopathy Questionnaire Heart Failure Therapy (n, %) ACEI/ARB/ARNI 200 (86) Overall Summary Score 47. 0 ± 24. 0 Beta blocker 208 (89) Clinical Summary Score 51. 2 ± 24. 8 MR antagonist 169 (72) 9 -item Patient Health Diuretic 225 (96) Questionnaire (sum-score) CRT, CRT-D or ICD 91 (39) EQ-5 D-5 L Visual Analog Scale score 8. 7 ± 5. 9 54. 4 ± 20. 7 *n = 187. Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 57

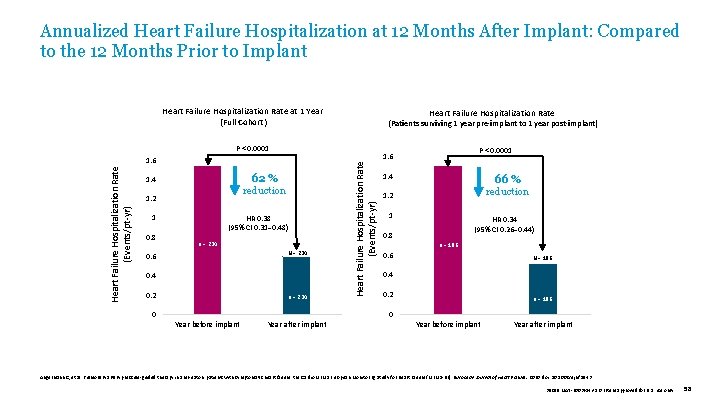
Annualized Heart Failure Hospitalization at 12 Months After Implant: Compared to the 12 Months Prior to Implant Heart Failure Hospitalization Rate at 1 Year (Full Cohort) Heart Failure Hospitalization Rate (Patients surviving 1 year pre-implant to 1 year post-implant) 62 % 1. 4 reduction 1. 2 1 0. 8 HR 0. 38 (95% CI 0. 31– 0. 48) n = 234 N = 234 0. 6 0. 4 0. 2 n = 234 Heart Failure Hospitalization Rate (Events/pt-yr) P < 0. 0001 1. 6 1. 4 66 % 1. 2 reduction 1 HR 0. 34 (95% CI 0. 26– 0. 44) 0. 8 n = 186 0. 6 N = 186 0. 4 0. 2 n = 186 0 0 Year before implant Year after implant Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 58

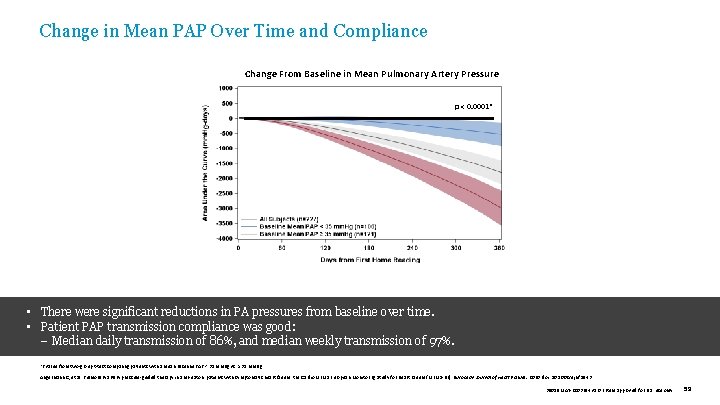
Change in Mean PAP Over Time and Compliance Change From Baseline in Mean Pulmonary Artery Pressure p < 0. 0001* • There were significant reductions in PA pressures from baseline over time. • Patient PAP transmission compliance was good: – Median daily transmission of 86%, and median weekly transmission of 97%. *P value from two-group t-test comparing patients with a mean baseline PAP < 35 mm. Hg vs. ≥ 35 mm. Hg. Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 59

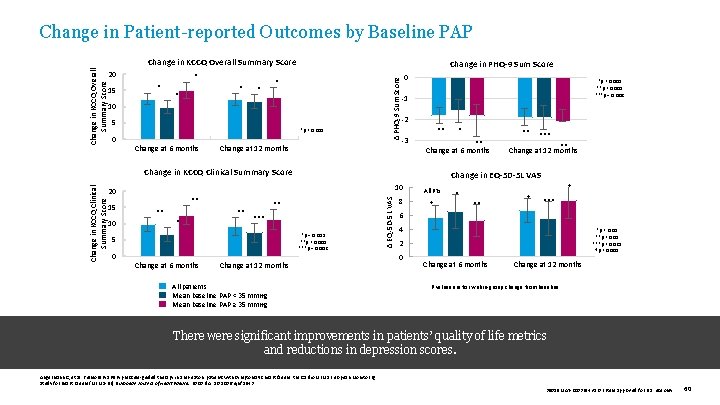
Change in KCCQ Overall Summary Score 20 15 * * * Change in PHQ-9 Sum Score * 10 5 *p < 0. 001 0 Change at 6 months Δ PHQ-9 Sum Score Change in KCCQ Overall Summary Score Change in Patient-reported Outcomes by Baseline PAP Change at 12 months 0 *p < 0. 005 **p < 0. 001 ***p = 0. 002 -1 -2 ** -3 10 ** *** *p = 0. 003 **p < 0. 001 ***p = 0. 002 5 0 Change at 6 months *** ** Change at 12 months Change in EQ-5 D-5 L VAS 10 Change at 12 months All patients Mean baseline PAP < 35 mm. Hg Mean baseline PAP ≥ 35 mm. Hg Δ EQ-5 D-5 L VAS Change in KCCQ Clinical Summary Score 15 ** ** Change at 6 months Change in KCCQ Clinical Summary Score 20 * 8 All Pts † † * ** † *** 6 4 *p < 0. 01 **p < 0. 05 ***p < 0. 025 †p < 0. 005 2 0 Change at 6 months Change at 12 months P values are for within-group change from baseline. There were significant improvements in patients’ quality of life metrics and reductions in depression scores. Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 60

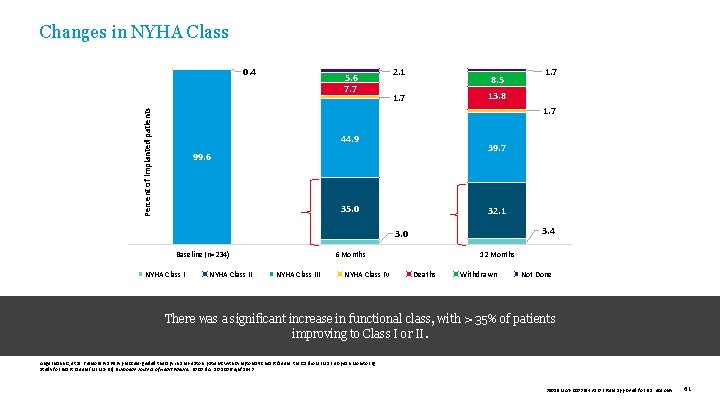
Changes in NYHA Class 5. 6 7. 7 2. 1 8. 5 1. 7 13. 8 1. 7 Percent of implanted patients Percent of implanted subjects 0. 4 44. 9 39. 7 99. 6 35. 0 32. 1 3. 4 3. 0 Baseline (n=234) NYHA Class II 6 Months NYHA Class III NYHA Class IV 12 Months Deaths Withdrawn Not Done There was a significant increase in functional class, with > 35% of patients improving to Class I or II. Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 61

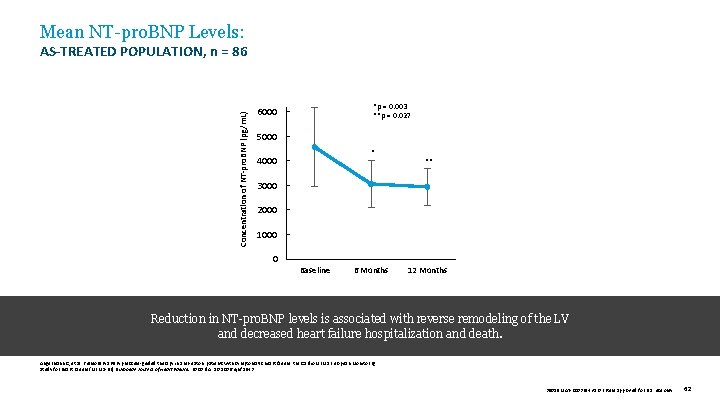
Mean NT-pro. BNP Levels: Concentration of NT-pro. BNP (pg/m. L) AS-TREATED POPULATION, n = 86 *p = 0. 003 **p = 0. 027 6000 5000 * 4000 ** 3000 2000 1000 0 Baseline 6 Months 12 Months Reduction in NT-pro. BNP levels is associated with reverse remodeling of the LV and decreased heart failure hospitalization and death. Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 62

Cardio. MEMS™ HF System Summary Results from all current clinical studies continue to strengthen the clinical evidence supporting the Cardio. MEMS HF System as the best way to proactively monitor and manage patients with heart failure, and reinforce our messaging that: The Cardio. MEMS HF System is the only technology proven through clinical trial data for managing heart failure patients using PA pressure monitoring Patient-transmitted PA pressure readings are proactive, reliable and actionable The Cardio. MEMS HF System is a heart failure disease management system, thereby preventing heart failure hospitalizations and resulting in improved patient QOL and functional status The Cardio. MEMS HF System allows physicians to optimize medical therapies for each patient, making it a personalized approach to heart failure management Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 63

Questions 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 64

Appendix I. ADDITIONAL SLIDES FOR THE CHALLENGE II. ADDITIONAL SLIDES FOR THE CHAMPION TRIAL DESIGN III. ADDITIONAL SLIDES FOR THE CHAMPION TRIAL RESULTS: SUBANALYSES IV. ADDITIONAL SLIDES FOR MEMS-HF MEDICATION CHANGES V. ADDITIONAL SUPPORTING SLIDES VI. REFERENCES 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 65

APPENDIX I The Challenge Additional Slides WEIGHT CHANGE SENSITIVITY LONG TERM MORTALITY RISK 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 66

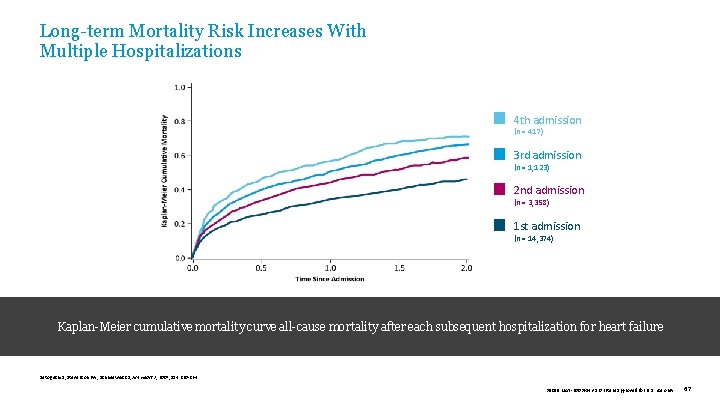
Long-term Mortality Risk Increases With Multiple Hospitalizations 4 th admission (n = 417) 3 rd admission (n = 1, 123) 2 nd admission (n = 3, 358) 1 st admission (n = 14, 374) Kaplan-Meier cumulative mortality curve all-cause mortality after each subsequent hospitalization for heart failure Setoguchi S, Stevenson LW, Schneeweiss S, Am Heart J, 2007; 154: 260 -264. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 67

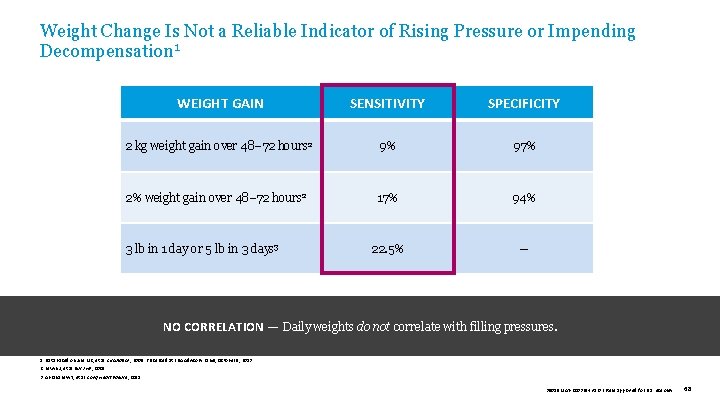
Weight Change Is Not a Reliable Indicator of Rising Pressure or Impending Decompensation 1 WEIGHT GAIN SENSITIVITY SPECIFICITY 2 kg weight gain over 48– 72 hours 2 9% 97% 2% weight gain over 48– 72 hours 2 17% 94% 22. 5% — 3 lb in 1 day or 5 lb in 3 days 3 NO CORRELATION — Daily weights do not correlate with filling pressures. 1. Data based on Zile MR, et al. Circulation , 2008. Presented at FDA Advisory Panel, October 9, 2013. 2. Lewin J, et al. Eur J HF, 2005. 3. Abraham WT, et al. Cong Heart Failure , 2011. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 68

APPENDIX II CHAMPION Trial Design Additional slides TRIAL DESIGN DEVICE/SYSTEM NNT Data 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 69

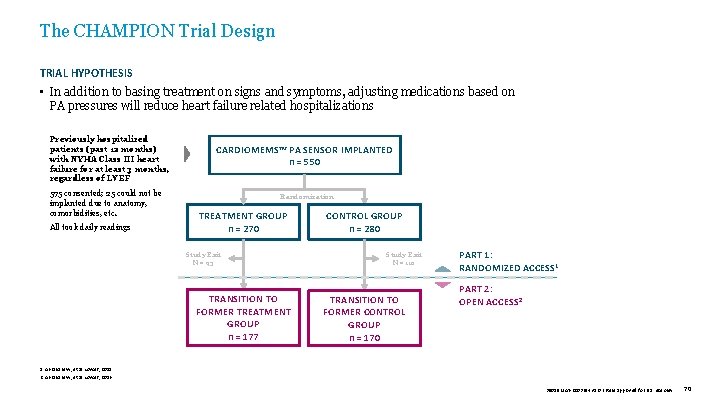
The CHAMPION Trial Design TRIAL HYPOTHESIS • In addition to basing treatment on signs and symptoms, adjusting medications based on PA pressures will reduce heart failure related hospitalizations Previously hospitalized patients (past 12 months) with NYHA Class III heart failure for at least 3 months, regardless of LVEF 575 consented; 25 could not be implanted due to anatomy, comorbidities, etc. All took daily readings CARDIOMEMS™ PA SENSOR IMPLANTED n = 550 Randomization TREATMENT GROUP n = 270 Study Exit N = 93 TRANSITION TO FORMER TREATMENT GROUP n = 177 CONTROL GROUP n = 280 Study Exit N = 110 TRANSITION TO FORMER CONTROL GROUP n = 170 PART 1: RANDOMIZED ACCESS 1 PART 2: OPEN ACCESS 2 1. Abraham W, et al. Lancet, 2011. 2. Abraham W, et al. Lancet, 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 70

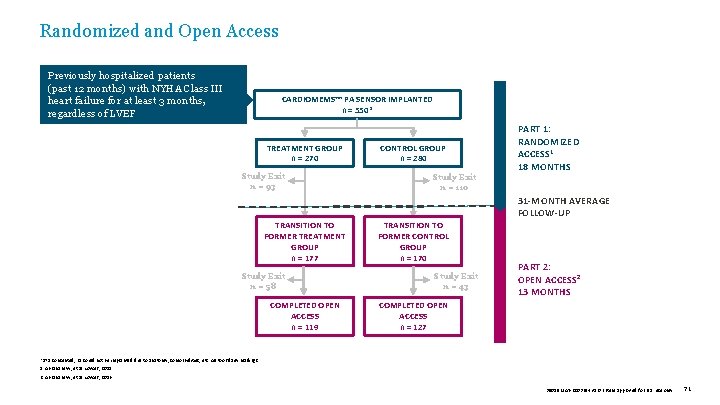
Randomized and Open Access Previously hospitalized patients (past 12 months) with NYHA Class III heart failure for at least 3 months, regardless of LVEF CARDIOMEMS™ PA SENSOR IMPLANTED n = 550* TREATMENT GROUP n = 270 Study Exit n = 93 CONTROL GROUP n = 280 Study Exit n = 110 PART 1: RANDOMIZED ACCESS 1 18 MONTHS 31 -MONTH AVERAGE FOLLOW-UP TRANSITION TO FORMER TREATMENT GROUP n = 177 Study Exit n = 58 COMPLETED OPEN ACCESS n = 119 TRANSITION TO FORMER CONTROL GROUP n = 170 Study Exit n = 43 PART 2: OPEN ACCESS 2 13 MONTHS COMPLETED OPEN ACCESS n = 127 *575 consented; 25 could not be implanted due to anatomy, comorbidities, etc. All took daily readings. 1. Abraham W, et al. Lancet, 2011. 2. Abraham W, et al. Lancet, 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 71

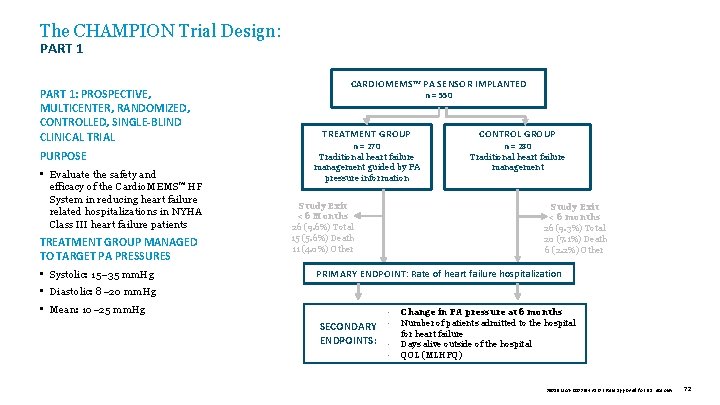
The CHAMPION Trial Design: PART 1: PROSPECTIVE, MULTICENTER, RANDOMIZED, CONTROLLED, SINGLE-BLIND CLINICAL TRIAL PURPOSE • Evaluate the safety and efficacy of the Cardio. MEMS™ HF System in reducing heart failure related hospitalizations in NYHA Class III heart failure patients TREATMENT GROUP MANAGED TO TARGET PA PRESSURES • Systolic: 15– 35 mm. Hg CARDIOMEMS™ PA SENSOR IMPLANTED n = 550 TREATMENT GROUP n = 270 Traditional heart failure management guided by PA pressure information Study Exit < 6 Months 26 (9. 6%) Total 15 (5. 6%) Death 11 (4. 0%) Other CONTROL GROUP n = 280 Traditional heart failure management Study Exit < 6 months 26 (9. 3%) Total 20 (7. 1%) Death 6 (2. 2%) Other PRIMARY ENDPOINT: Rate of heart failure hospitalization • Diastolic: 8– 20 mm. Hg • Mean: 10– 25 mm. Hg SECONDARY ENDPOINTS: • • Change in PA pressure at 6 months Number of patients admitted to the hospital for heart failure Days alive outside of the hospital QOL (MLHFQ) 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 72

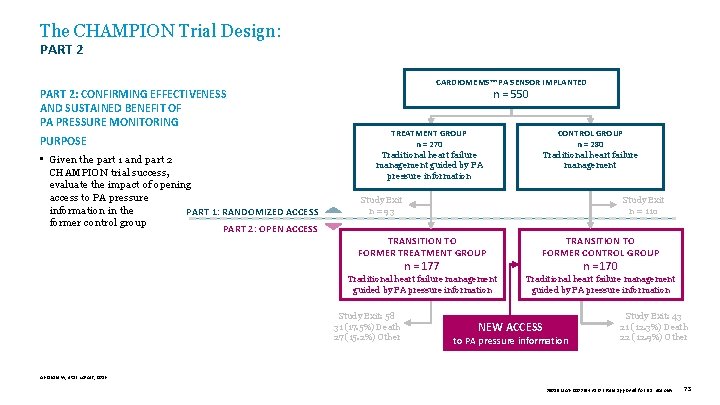
The CHAMPION Trial Design: PART 2: CONFIRMING EFFECTIVENESS AND SUSTAINED BENEFIT OF PA PRESSURE MONITORING PURPOSE • Given the part 1 and part 2 CHAMPION trial success, evaluate the impact of opening access to PA pressure information in the PART 1: RANDOMIZED ACCESS former control group PART 2: OPEN ACCESS CARDIOMEMS™ PA SENSOR IMPLANTED n = 550 TREATMENT GROUP n = 270 Traditional heart failure management guided by PA pressure information CONTROL GROUP n = 280 Traditional heart failure management Study Exit n = 93 Study Exit n = 110 TRANSITION TO FORMER TREATMENT GROUP TRANSITION TO FORMER CONTROL GROUP Traditional heart failure management guided by PA pressure information n = 177 Study Exit: 58 31 (17. 5%) Death 27 (15. 2%) Other n = 170 NEW ACCESS to PA pressure information Study Exit: 43 21 (12. 3%) Death 22 (12. 9%) Other Abraham W, et al. Lancet, 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 73

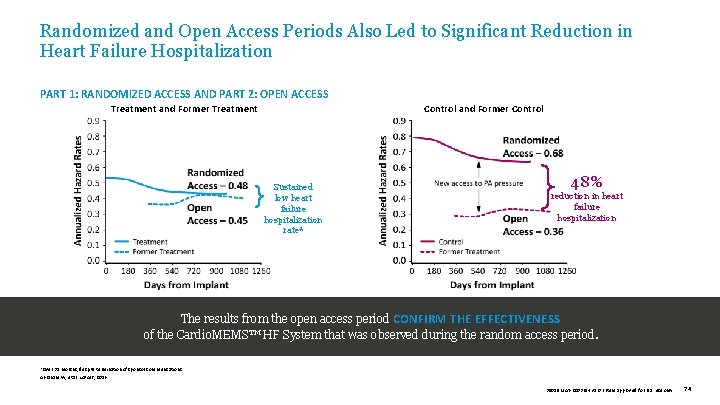
Randomized and Open Access Periods Also Led to Significant Reduction in Heart Failure Hospitalization PART 1: RANDOMIZED ACCESS AND PART 2: OPEN ACCESS Treatment and Former Treatment Control and Former Control Sustained low heart failure hospitalization rate* 48% reduction in heart failure hospitalization The results from the open access period CONFIRM THE EFFECTIVENESS of the Cardio. MEMS™ HF System that was observed during the random access period. *Over 31 months, despite termination of sponsor communications. Abraham W, et al. Lancet, 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 74

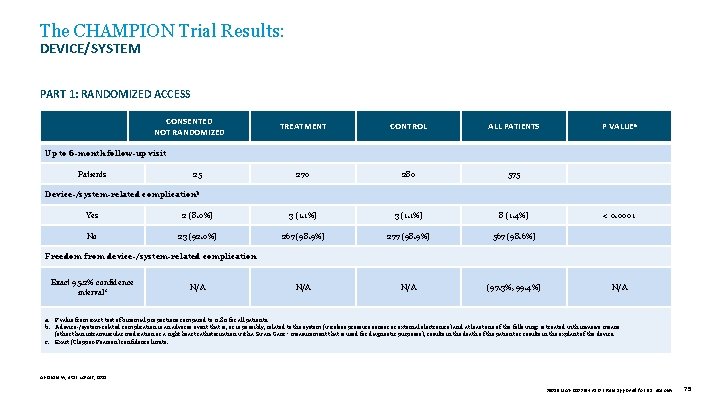
The CHAMPION Trial Results: DEVICE/SYSTEM PART 1: RANDOMIZED ACCESS CONSENTED NOT RANDOMIZED TREATMENT CONTROL ALL PATIENTS P VALUEa 270 280 575 Up to 6 -month follow-up visit Patients 25 Device-/system-related complicationb Yes 2 (8. 0%) 3 (1. 1%) 8 (1. 4%) < 0. 0001 No 23 (92. 0%) 267 (98. 9%) 277 (98. 9%) 567 (98. 6%) N/A (97. 3%, 99. 4%) N/A Freedom from device-/system-related complication Exact 95. 2% confidence intervalc N/A a. P value from exact test of binomial proportions compared to 0. 80 for all patients. b. A device-/system-related complication is an adverse event that is, or is possibly, related to the system (wireless pressure sensor or external electronics) and at least one of the following: is treated with invasive means (other than intramuscular medication or a right heart catheterization with a Swan-Ganz ‡ measurement that is used for diagnostic purposes), results in the death of the patient or results in the explant of the device. c. Exact (Clopper-Pearson) confidence limits. Abraham W, et al. Lancet, 2011. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 75

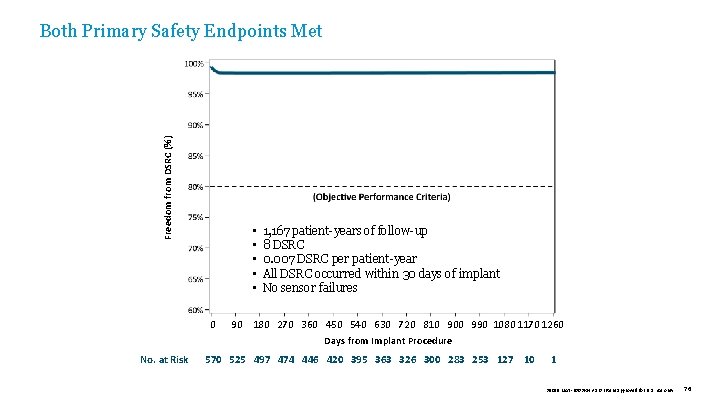
Freedom from DSRC (%) Both Primary Safety Endpoints Met • • • 0 No. at Risk 1, 167 patient-years of follow-up 8 DSRC 0. 007 DSRC per patient-year All DSRC occurred within 30 days of implant No sensor failures 90 180 270 360 450 540 630 720 810 900 990 1080 1170 1260 Days from Implant Procedure 570 525 497 474 446 420 395 363 326 300 283 253 127 10 1 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 76

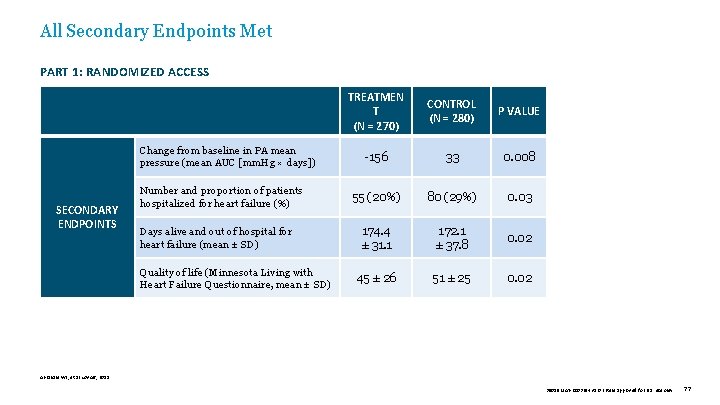
All Secondary Endpoints Met PART 1: RANDOMIZED ACCESS PART 2: OPEN ACCESS Change from baseline in PA mean pressure (mean AUC [mm. Hg × days]) SECONDARY ENDPOINTS Number and proportion of patients hospitalized for heart failure (%) Days alive and out of hospital for heart failure (mean ± SD) Quality of life (Minnesota Living with Heart Failure Questionnaire, mean ± SD) TREATMEN T (N = 270) CONTROL (N = 280) P VALUE -156 33 0. 008 55 (20%) 80 (29%) 0. 03 174. 4 ± 31. 1 172. 1 ± 37. 8 0. 02 45 ± 26 51 ± 25 0. 02 Abraham WT, et al. Lancet, 2011. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 77

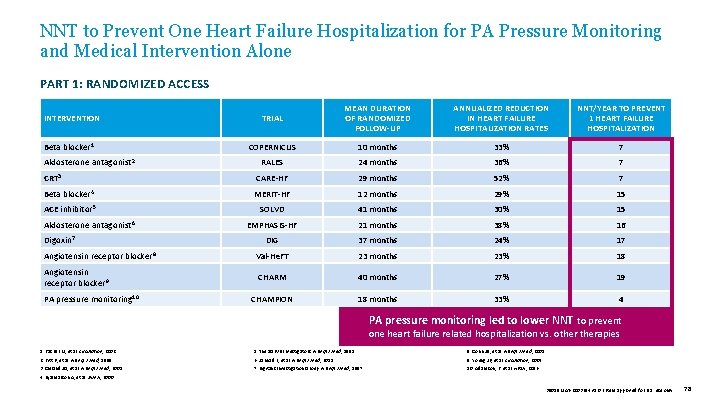
NNT to Prevent One Heart Failure Hospitalization for PA Pressure Monitoring and Medical Intervention Alone PART 1: RANDOMIZED ACCESS INTERVENTION Beta blocker 1 Aldosterone antagonist 2 TRIAL MEAN DURATION OF RANDOMIZED FOLLOW-UP ANNUALIZED REDUCTION IN HEART FAILURE HOSPITALIZATION RATES NNT/YEAR TO PREVENT 1 HEART FAILURE HOSPITALIZATION COPERNICUS 10 months 33% 7 RALES 24 months 36% 7 CRT 3 CARE-HF 29 months 52% 7 Beta blocker 4 MERIT-HF 12 months 29% 15 ACE inhibitor 5 Aldosterone antagonist 6 Digoxin 7 SOLVD 41 months 30% 15 EMPHASIS-HF 21 months 38% 16 DIG 37 months 24% 17 Angiotensin receptor blocker 8 Val-He. FT 23 months 23% 18 Angiotensin receptor blocker 9 CHARM 40 months 27% 19 CHAMPION 18 months 33% 4 PA pressure monitoring 10 PA pressure monitoring led to lower NNT to prevent one heart failure related hospitalization vs. otherapies 1. Packer M, et al. Circulation, 2002. 5. The SOLVD Investigators. N Engl J Med , 1991. 8. Cohn JN, et al. N Engl J Med , 2001. 2. Pitt B, et al. N Engl J Med, 1999. 6. Zannad F, et al. N Engl J Med , 2011. 9. Young JB, et al. Circulation, 2004. 3. Cleland JG, et al. N Engl J Med, 2005. 7. Digitalis Investigation Group. N Engl J Med , 1997. 10. Adamson, P. et al. HFSA, 2016. 4. Hjalmarson A, et al. JAMA , 2000. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 78

APPENDIX III The CHAMPION Trial Subgroup Analyses • HFr. EF with CRT on GDMT • Medicare-eligible populations • PA-guided medical management • Medication changes • Common comorbidities • Patients with CKD • Patients with PHTN • Patients with COPD 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 79

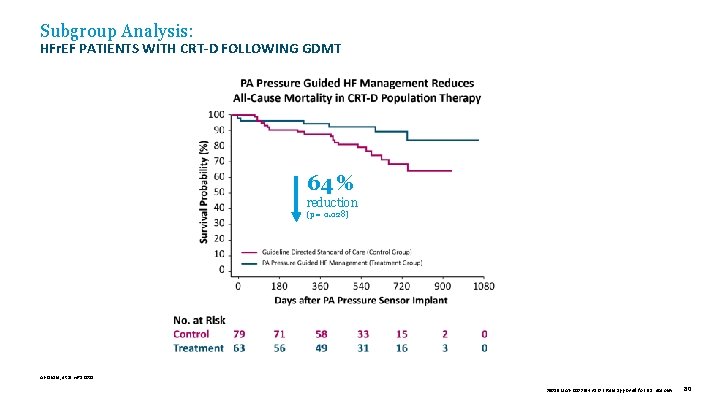
Subgroup Analysis: HFr. EF PATIENTS WITH CRT-D FOLLOWING GDMT 64 % reduction (p = 0. 028) Abraham, et al. HRS 2015. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 80

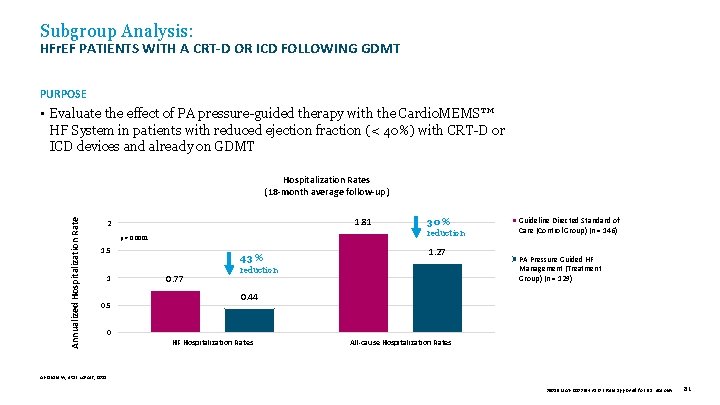
Subgroup Analysis: HFr. EF PATIENTS WITH A CRT-D OR ICD FOLLOWING GDMT PURPOSE • Evaluate the effect of PA pressure-guided therapy with the Cardio. MEMS™ HF System in patients with reduced ejection fraction (< 40%) with CRT-D or ICD devices and already on GDMT Annualized Hospitalization Rates (18 -month average follow-up) 1. 81 2 p = 0. 0001 1. 5 1 0. 5 43 % 0. 77 30 % reduction 1. 27 reduction Guideline Directed Standard of Care (Control Group) (n = 146) PA Pressure Guided HF Management (Treatment Group) (n = 129) 0. 44 0 HF Hospitalization Rates All-cause Hospitalization Rates Abraham W, et al. Lancet, 2011. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 81

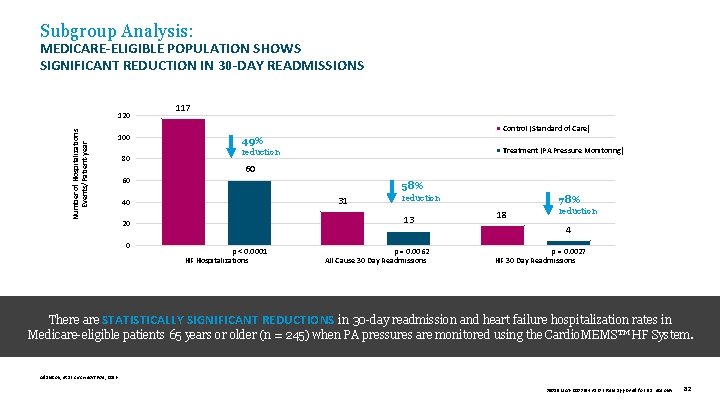
Subgroup Analysis: MEDICARE-ELIGIBLE POPULATION SHOWS SIGNIFICANT REDUCTION IN 30 -DAY READMISSIONS Number of Hospitalizations Events/Patient-year 120 100 80 117 Control (Standard of Care) 49% Treatment (PA Pressure Monitoring) reduction 60 60 58% 31 40 13 20 0 reduction p < 0. 0001 HF Hospitalizations p = 0. 0062 All Cause 30 Day Readmissions 78% 18 reduction 4 p = 0. 0027 HF 30 Day Readmissions There are STATISTICALLY SIGNIFICANT REDUCTIONS in 30 -day readmission and heart failure hospitalization rates in Medicare-eligible patients 65 years or older (n = 245) when PA pressures are monitored using the Cardio. MEMS™ HF System. Adamson, et al. Circ Heart Fail , 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 82

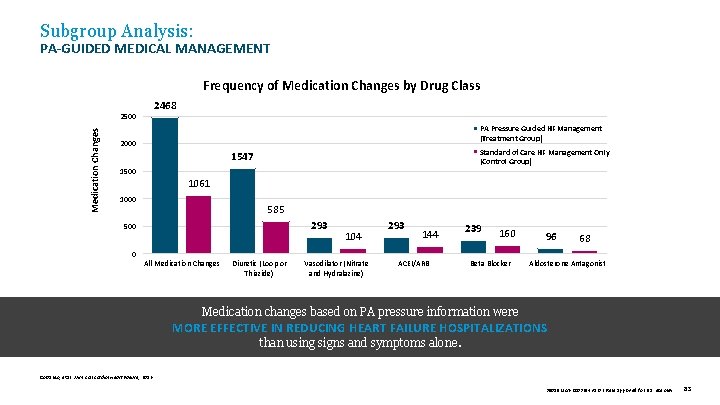
Subgroup Analysis: PA-GUIDED MEDICAL MANAGEMENT Frequency of Medication Changes by Drug Class 2468 Medication Changes 2500 PA Pressure Guided HF Management (Treatment Group) 2000 Standard of Care HF Management Only (Control Group) 1547 1500 1061 1000 585 293 500 104 293 144 239 160 96 68 0 All Medication Changes Diuretic (Loop or Thiazide) Vasodilator (Nitrate and Hydralazine) ACEI/ARB Beta Blocker Aldosterone Antagonist Medication changes based on PA pressure information were MORE EFFECTIVE IN REDUCING HEART FAILURE HOSPITALIZATIONS than using signs and symptoms alone. Costanzo, et al. J Am Coll Cardiol Heart Failure, 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 83

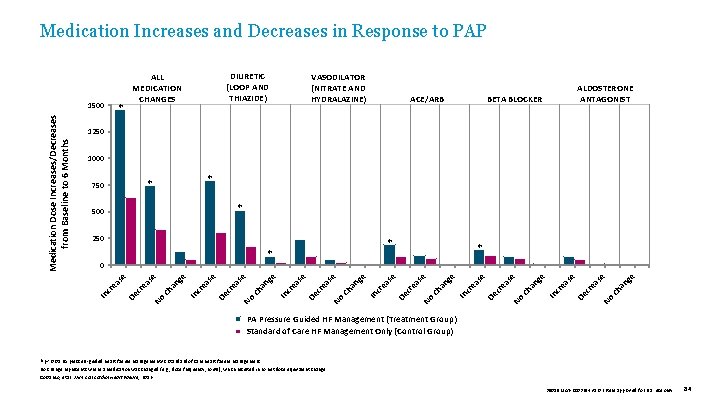
Medication Increases and Decreases in Response to PAP ✽ VASODILATOR (NITRATE AND HYDRALAZINE) ACE/ARB ALDOSTERONE ANTAGONIST BETA BLOCKER 1250 1000 ✽ ✽ 750 ✽ 500 250 ✽ ✽ ✽ e C ha ng se ea No cr se De ea cr In ha ng e No C ea se cr De In cr ea se ha ng e se C ea No cr De cr ea se e In No C ha ng se ea cr De cr ea se e In ha ng se C ea No cr De cr ea se e In ha ng C ea No cr De ea cr In se 0 se Medication Dose Increases/Decreases from Baseline to 6 Months 1500 DIURETIC (LOOP AND THIAZIDE) ALL MEDICATION CHANGES PA Pressure Guided HF Management (Treatment Group) Standard of Care HF Management Only (Control Group) ✽p < 0. 05 PA pressure-guided heart failure management vs. standard of care heart failure management. No change represents where a medication was changed (e. g. , dose frequency, route), which resulted in no net dose equivalent change. Costanzo, et al. J Am Coll Cardiol Heart Failure, 2016. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 84

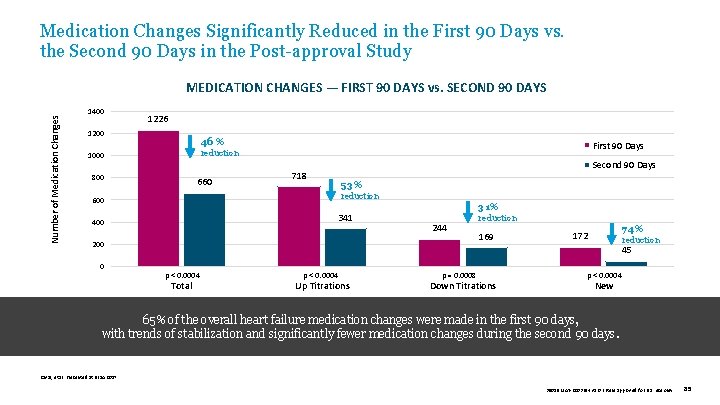
Medication Changes Significantly Reduced in the First 90 Days vs. the Second 90 Days in the Post-approval Study Number of Medication Changes MEDICATION CHANGES — FIRST 90 DAYS vs. SECOND 90 DAYS 1400 1226 1200 46 % First 90 Days reduction 1000 800 660 Second 90 Days 718 53% reduction 600 341 400 31% 244 200 0 reduction 169 74% 172 reduction 45 p < 0. 0004 Total p < 0. 0004 Up Titrations p = 0. 0008 Down Titrations p < 0. 0004 New 65% of the overall heart failure medication changes were made in the first 90 days, with trends of stabilization and significantly fewer medication changes during the second 90 days. Raval, et al. Presented at HFSA 2017. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 85

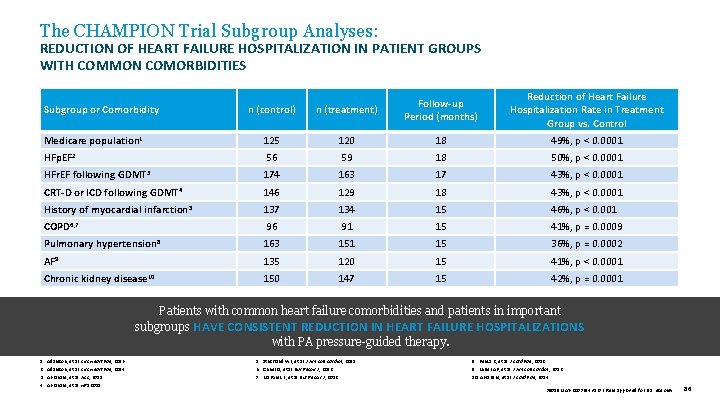
The CHAMPION Trial Subgroup Analyses: REDUCTION OF HEART FAILURE HOSPITALIZATION IN PATIENT GROUPS WITH COMMON COMORBIDITIES n (control) n (treatment) Follow-up Period (months) Reduction of Heart Failure Hospitalization Rate in Treatment Group vs. Control Medicare population 1 125 120 18 49%, p < 0. 0001 HFp. EF 2 56 59 18 50%, p < 0. 0001 HFr. EF following GDMT 3 174 163 17 43%, p < 0. 0001 CRT-D or ICD following GDMT 4 146 129 18 43%, p < 0. 0001 History of myocardial infarction 5 137 134 15 46%, p < 0. 0010 COPD 6, 7 96 91 15 41%, p = 0. 0009 Pulmonary hypertension 8 163 151 15 36%, p = 0. 0002 AF 9 135 120 15 41%, p < 0. 0001 Chronic kidney disease 10 150 147 15 42%, p = 0. 0001 Subgroup or Comorbidity Patients with common heart failure comorbidities and patients in important subgroups HAVE CONSISTENT REDUCTION IN HEART FAILURE HOSPITALIZATIONS with PA pressure-guided therapy. 1. 2. 3. 4. Adamson, et al. Circ Heart Fail , 2016. Adamson, et al. Circ Heart Fail , 2014. Abraham, et al. ACC, 2015. Abraham, et al. HRS 2015. 5. Strickland WL, et al. J Am Coll Cardiol , 2011. 6. Criner G, et al. Eur Respir J , 2012. 7. Martinez F, et al. Eur Respir J , 2012. 8. Benza R, et al. J Card Fail , 2012. 9. Miller AB, et al. J Am Coll Cardiol , 2012. 10. Abraham, et al. J Card Fail , 2014. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 86

The CHAMPION Trial Subanalysis: PATIENTS WITH CKD PURPOSE • Compared Class III heart failure patients with CKD managed with PA pressure (n = 150) to those managed with SOC (n = 147) 42 % 42% reduction REDUCTION in heart failure hospitalizations for heart failure patients with CKD who were managed with PA pressure compared to SOC Changes in CKD indicators (creatinine and GFR) were not adversely affected in the PA pressure-monitored group. Intensified heart failure medical therapy due to PA pressure monitoring was safe and did not adversely affect renal function. 0. 48 vs. 83, HR 0. 58, p < 0. 001 There was a REDUCTION IN HEART FAILURE HOSPITALIZATIONS in heart failure patients with comorbid pulmonary hypertension. Abraham W, et al. Lancet, 2011. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 87

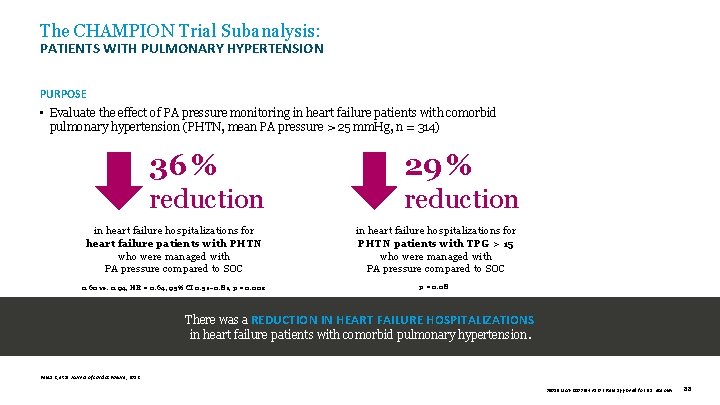
The CHAMPION Trial Subanalysis: PATIENTS WITH PULMONARY HYPERTENSION PURPOSE • Evaluate the effect of PA pressure monitoring in heart failure patients with comorbid pulmonary hypertension (PHTN, mean PA pressure > 25 mm. Hg, n = 314) 36 % 29 % reduction in heart failure hospitalizations for heart failure patients with PHTN who were managed with PA pressure compared to SOC in heart failure hospitalizations for PHTN patients with TPG > 15 who were managed with PA pressure compared to SOC 0. 60 vs. 0. 94, HR = 0. 64, 95% CI 0. 51– 0. 81, p = 0. 002 p = 0. 08 There was a REDUCTION IN HEART FAILURE HOSPITALIZATIONS in heart failure patients with comorbid pulmonary hypertension. Benza R, et al. Journal of Cardiac Failure , 2012. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 88

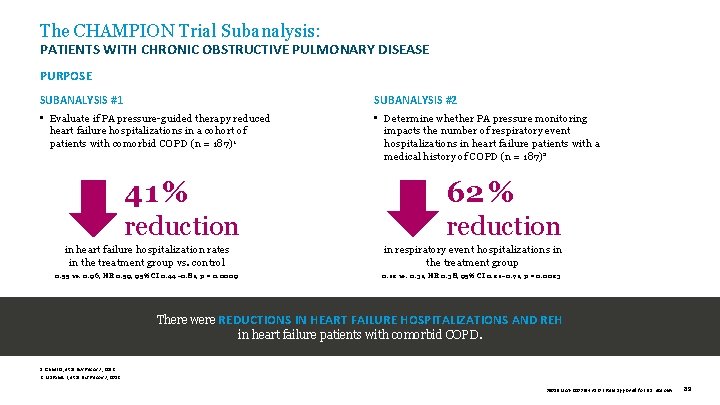
The CHAMPION Trial Subanalysis: PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE PURPOSE SUBANALYSIS #1 SUBANALYSIS #2 • Evaluate if PA pressure-guided therapy reduced heart failure hospitalizations in a cohort of patients with comorbid COPD (n = 187)1 • Determine whether PA pressure monitoring impacts the number of respiratory event hospitalizations in heart failure patients with a medical history of COPD (n = 187)2 41 % 62 % reduction in heart failure hospitalization rates in the treatment group vs. control 0. 55 vs. 0. 96, HR 0. 59, 95% CI 0. 44– 0. 81, p = 0. 0009 in respiratory event hospitalizations in the treatment group 0. 12 vs. 0. 31, HR 0. 38, 95% CI 0. 21– 0. 71, p = 0. 0023 There were REDUCTIONS IN HEART FAILURE HOSPITALIZATIONS AND REH in heart failure patients with comorbid COPD. 1. Criner G, et al. Eur Respir J , 2012. 2. Martinez F, et al. Eur Respir J , 2012. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 89

MEDICATION CHANGES APPENDIX IV Additional Supporting Slides MEMS-HF 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 90

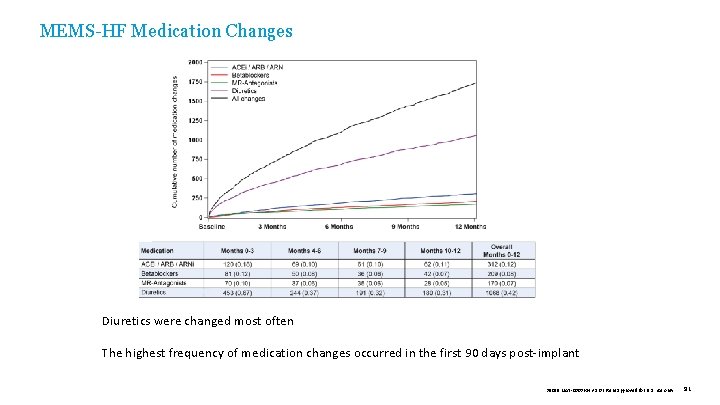
MEMS-HF Medication Changes Diuretics were changed most often The highest frequency of medication changes occurred in the first 90 days post-implant 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 91

HEART FAILURE CLASSIFICATION MEDICARE READMISSION REDUCTION PROGRAM — COMPARISON APPENDIX V Additional Supporting Slides 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 92

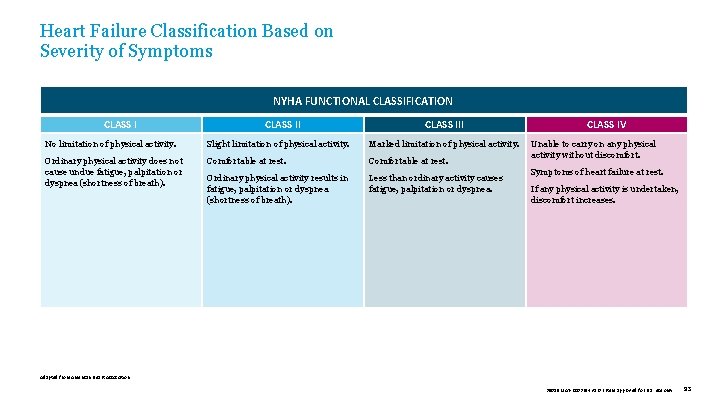
Heart Failure Classification Based on Severity of Symptoms NYHA FUNCTIONAL CLASSIFICATION CLASS III No limitation of physical activity. Slight limitation of physical activity. Marked limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation or dyspnea (shortness of breath). Comfortable at rest. Ordinary physical activity results in fatigue, palpitation or dyspnea (shortness of breath). Less than ordinary activity causes fatigue, palpitation or dyspnea. CLASS IV Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases. Adapted from American Heart Association. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 93

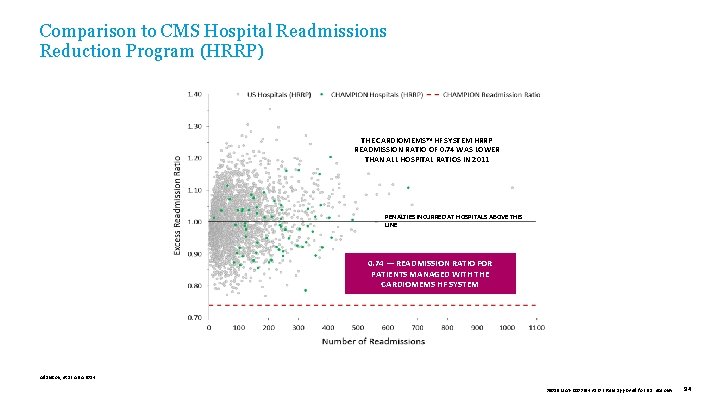
Comparison to CMS Hospital Readmissions Reduction Program (HRRP) THE CARDIOMEMS™ HF SYSTEM HRRP READMISSION RATIO OF 0. 74 WAS LOWER THAN ALL HOSPITAL RATIOS IN 2011 PENALTIES INCURRED AT HOSPITALS ABOVE THIS LINE 0. 74 — READMISSION RATIO FOR PATIENTS MANAGED WITH THE CARDIOMEMS HF SYSTEM Adamson, et al. AHA 2014. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 94

APPENDIX VI References 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 95

References Abraham J, et al. Association of Ambulatory Hemodynamic Monitoring with Clinical Outcomes in a Concurrent Matched Cohort Analysis. JAMA Cardiology. 2019; 4(6): 556 -563. Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014 Nov; 7(6): 935 -44. Abraham WT, et al. (2014) Benefits of pulmonary artery pressure monitoring in patients with NYHA class III heart failure and chronic kidney disease: results from the CHAMPION Trial. Journal of Cardiac Failure, 20(Suppl S 93). Adamson PB, et al. (2016) Pulmonary artery pressure-guided heart failure management reduces 30 -day readmissions. Circ Heart Fail. 115. 002600. Abraham WT, Compton S, Haas G et al. Intrathoracic Impedance vs. Daily Weight Monitoring for Predicting Worsening Heart Failure Events: Results of the Fluid Accumulation Status Trial (FAST). Congest Heart Fail 2011 March; 17(2): 51 -5. Adamson PB, et al. (2016, September). Economic impact of hemodynamic monitoring in heart failure patients: estimating the number needed to treat and the break even point using a claims database. Presented at HFSA in Orlando, FL. Abraham WT, et al. (2016, September) Pulmonary artery pressures decrease over time during heart failure management guided by ambulatory hemodynamic monitoring in real world setting. Paper presented at HFSA in Orlando, FL. Adamson PB. Cardio. MEMS HF System Clinical Protocol Example. St. Jude Medical Website Resource. 2015. Abraham WT, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011; 377(9766): 658 -66. Abraham WT, et al. Pulmonary artery pressure management in heart failure patients with cardiac resynchronization therapy or implantable cardioverter defibrillator devices significantly reduces heart failure hospitalizations and mortality above and beyond background guide-directed medical therapy. Abstract AB 37 -03 presented at HRS 2015, Boston. Abraham WT, et al. Pulmonary Artery Pressure Management in Heart Failure Patients with Reduced Ejection Fraction Significantly Reduces Heart Failure Hospitalizations and Mortality Above and Beyond Background Guide. Directed Medical Therapy ACC 2015, San Diego Abstract 902 -04. Abraham WT, Stevenson LW, Bourge RC, et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet. 2016; 387(10017): 453 -461. Adams KF Jr, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100, 000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005 Feb; 149(2): 209 -16. Adamson PB. (graph adapted from) Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep. 2009; 6(4): 28792. Adamson PB, et al. (2014). Impact of wireless pulmonary artery pressure monitoring on heart failure hospitalizations and 30 -day readmissions in Medicare-eligible patients with NYHA class III heart failure: results from the CHAMPION trial. Circulation, 133, A 16744. Adamson PB, Gold MR, Bourge RC, et al. (2010). Reducing Decompensation Events Utilizing Intra. Cardiac Pressures in Patients With Chronic HF (REDUCEhf). Journal of Cardiac Failure, 16(11), 913. Alam A. (2016, March). Improved Quality of Life Scores and Exercise Capacity with Remote Pulmonary Artery Pressure Monitoring in Patients with Chronic Heart Failure. Abstract presented at ACC in 2016. Angermann C, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the Cardio. MEMS European Monitoring Study for Heart Failure (MEMS-HF). European Journal of Heart Failure. 2020. doi: 10. 1002/ejhf. 1943 Angermann CE, Stork S, Gelbrich G, et al. (2012). Mode of Action and Effects of Standardized Collaborative Disease Management on Mortality and Morbidity in Patients with Systolic Heart Failure: The Interdisciplinary Network for Heart Failure (INH) Study. Circ Heart Failure 2012; 5; 25 -35. Araujo-Gutierrez R, Ibarra-Cortez SH, Park MH, et al. (2016). Reduction in Healthcare Utilization in Patients with Remote Hemodynamic Pulmonary Artery Pressure Monitoring Device: A Real-World, Post Clinical Trial Single Center Experience. Journal of Cardiac Failure, 22(8). Benza R, Bourge R, Adamson P, et al. Heart failure hospitalizations are reduced in heart failure patients with comorbid pulmonary hypertension using a wireless implanted pulmonary artery pressure monitoring system. J Cardiac Fail. 2012; 18(Suppl 8): S 99. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 96

References Blecker S, Paul M, Taksler G, et al. (2013). Heart Failure–Associated Hospitalizations in the United States. J Am Coll Cardiol, 61(12), 1259 -1267. Boriani G, Da Costa A, Guesada A, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators; results of the MORE CARE multicentre randomized controlled trial. Eur J Heart Fail. 2017; DOI: 10. 1002/ejhf. 626. Bourge RC, Abraham WT, Adamson PB, et al. (2008). Randomized Controlled Trial of an Implantable Continuous Hemodynamic Monitor in Patients With Advanced Heart Failure. J Am Coll Cardiol, 51(11), 1073 -1079. Brachmann J, et al. Fluid status monitoring with a wireless network to reduce cardiovascular-related hospitalizations and mortality in heart failure: rationale and design of the Opti. Link HF Study (Optimization of Heart Failure Management using Opti. Vol Fluid Status Monitoring and Care. Link). Eur J Heart Fail. 2011; 13: 796– 804. Capomolla S, et al. Echo-Doppler and clinical evaluations to define hemodynamic profile in patients with chronic heart failure: accuracy and influence on therapeutic management. Eur J Heart Fail. 2005 Jun; 7(4): 624 -30. Carey SA, Bass K, Dormer A, et al. (2016, September). Our Experience with Cardio. MEMS™ in a Large Advanced Heart Failure Program. Abstract presented at HFSA in Orlando, FL. CDC NCHS National Hospital Discharge Survey, 20002010. Chaudhry SI, Mattera JA, Curtis JP, et al. (2010). Telemonitoring in patients with heart failure. N Engl J Med, 363, 2301 -2309. Cleland JG, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005; 352: 1539 -49. Cleland JG, Louis AA, Rigby AS, et al. TENS-HMS Investigators. (2005) Noninvasive Home Telemonitoring for Patients With Heart Failure at High Risk of Recurrent Admission and Death: The Trans-European Network-Home. Care Management System (TEN-HMS) study. J Am Coll Cardiol, 45, 1654 -1664. Cohn JN, Tognoni G for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001; 345: 1667 -75. During Ambulatory Pulmonary Artery Pressure Monitoring. J Am Coll Cardiol Heart Fail. 2016. Cowie MR, et al. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur Heart J. 2013 Aug; 34(31): 2472 -80 Cowie MR. REM-HF: Remote monitoring: an evaluation of implantable devices for management of heart failure patients. European Society of Cardiology 2016 Congress; August 28, 2016; Rome, Italy. Abstract 1223. Criner G, et al. Impact of wireless implanted pulmonary artery pressure monitoring system in heart failure patients with comorbid chronic obstructive pulmonary disease. Eur Resp J. 2012: 40(Suppl 56): P 9761. Curtis LH, et al. Incidence and prevalence of heart failure in elderly persons, 1994 -2003. Arch Intern Med. 2008 Feb 25; 168(4): 418 -24. Desai AS, et al. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in “Real-World” Clinical Practice J Am Coll Cardiol. 2017; 69(19): 2357 -65. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336: 525 -533. Fang J, et al. Heart failure related hospitalization in the U. S. , 1979 -2004. J Am Coll Cardiol, Vol 52, Issue 6, 5 Aug 2008, p 428 -434. Gheorghiade MD, et al. (graph adapted from) Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol. 2005; 96[suppl]: 11 G-17 G. Givertz MM, Stevenson LW, Costanzo MR, et al. , on behalf of the CHAMPION Trial Investigators. Pulmonary artery pressure–guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017; 70: 1875 -86. Goldberg L, et al. (2015, May). Pressure for action: Implantable pulmonary artery pressure sensor measurements alone beat clinical signs to guide prevention of heart failure hospitalizations. Abstract AB 36 -02 presented at HRS in Boston, MA. Costanzo MR, Stevenson LW, Adamson PB, et al. Interventions Linked to Decreased Heart Failure Hospitalizations 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 97

References Heidenreich PA, Albert NM, Allen LA, , et al. ; on behalf of the American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013; 6: 606 -619. doi: 10. 1161/HHF. 0 b 013 e 318291329 a. Heidenreich PA, et al. Forecasting the impact of heart failure in the United States. A policy statement from the American Heart Association. Circ Heart Fail. 2013; 6: 606 -619. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of PA pressures in 2000 patients implanted with the Cardio. MEMS sensor. Circulation. 2017; 135: 1509 -17. obstructive pulmonary disease using a wireless implanted pulmonary artery pressure monitoring system. Eur Resp J. 2012; 40(Suppl 56): 2832. Miller AB, et al. Impact of remote, wireless pulmonary artery hemodynamic monitoring in patients with atrial fibrillation and chronic heart failure: Insights from the CHAMPION clinical trial. J Am Coll Cardiol. 2012; 59(13 Supplement): E 868. Mozaffarian D, et al. Heart Disease and Stroke Statistics — 2016 Update: A report from the American Heart Association. Circulation. 2016. National Institute of Health. NHLBI Face Book, Fiscal Year 2012. http: //www. nhlbi. nih. gov/about/documents/factbook/2012/chapter 4. Accessed September 2016. Hjalmarson A, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERITHF). JAMA. 2000; 283: 1295 -302. O‘Brien DP, Wolfson AM, Fong MW, et al. (2016). Hemodynamic Guided Therapy for Heart Failure: Initial Clinical Experience with the Cardio. MEMS™ HF System. Journal of Cardiac Failure, 22(8). Jermyn R, et al. (2016, September). Real World transmission compliance in patients managed with hemodynamic guided medical care using an implantable sensor. Paper presented at HFSA in Orlando, FL. Okumura N, et al. Importance of Clinical Worsening of Heart Failure Treated in the Outpatient Setting: Evidence From the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF). Circulation. 2016; 133: 2254 -2262. Koehler F, Winkler S, Schieber M, et al. Telemedical Interventional Monitoring in Heart Failure Investigators. (2011). Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The Telemedical Interventional Monitoring in Heart Failure study. Circulation, 123, 1873 -1880. 5 Dec 15. Krum H, Abraham WT. Heart failure. Lancet. 2009; 373(9667): 941 -55. Krumholz HM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30 -day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009; 2: 407 -13. Lala A, et al. The Tides of Congestion during and after Hospitalization for Acute Decompensated Heart Failure. J Cardiac Fail. 2013; 19(8): S 81. Lewin J, et al. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur J Heart Fail. 2005 Oct; 7(6): 953 -7. Lindenfeld J, et al. Hemodynamic-GUIDEd management of Heart Failure (GUIDE-HF). Am Heart J. 2019; 214: 18 -27. Ong, MK, Romano, PS, Edgington, S, et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition–Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016; 176(3): 310 -318. Packer M, et al; Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. Circulation 2002; 106: 2194 -9. Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709 -717. Ponikowski P, Voors AA, Anker SD, et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J, 37(27), 2129 -2200. Rathman LD, Unruh LN, Nissley CD, et al. (2016, June). Cardio. MEMS: proving that failure is not the only option for HF patients. Poster and paper presented at AAHFN in Scottsdale, AZ. Martinez F, et al. Respiratory event hospitalizations are reduced in heart failure patients with comorbid chronic 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 98

References Rathman L, et al. (2016, September). Pulmonary Artery Pressure Monitoring. Is it feasible in the community setting? Presented at HFSA in Orlando, FL. Wexler DJ, et al. Predictors of costs of caring for elderly patients discharged with heart failure. Am Heart J 2001; 142: 350 -7. Rathman L, et al. (2016, September). Holding up both ends of the bargain: ambulatory hemodynamic monitoring using Cardio. MEMS. Presented at HFSA in Orlando, FL. Whellan DJ, PARTNERS Study Investigators, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010 Apr 27; 55(17): 1803 -10. a. Raval, Nirav Y. , et al. Significant Reductions in Heart Failure Hospitalizations with the Pulmonary Artery Pressure Guided HF System: Preliminary Observations From the Cardio. MEMS Post Approval Study. Abstracts From the HFSA 2017 21 st Annual Meeting, Journal of Cardiac Failure August 2017 23(8) Supplement: S 27 Roger VL, et al. Trends in heart failure incidence and survival in a community-based population. JAMA 2004; 292: 34450. Setoguchi S, Stevenson LW, Schneeweiss S. Am Heart J 2007; 154: 260 -266. Shavelle D, et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: One year outcomes from the Cardio. MEMS Post-Approval Study. Circulation: Heart Failure. 2020; e 006836 Strickland WL, et al. The utility of remote wireless pulmonary artery pressure monitoring in patients with or without a history of myocardial infarction: Experience from the CHAMPION clinical trial. J Am Coll Cardiol. 2011; 58(20 Supplement): B 130. Yamokoski L, (AAHFN 2015). From clinical research to clinical practice: development of a comprehensive workflow for the Cardio. MEMS™ Heart Failure System. Ohio State University. Yancy CW, ADHERE Scientific Advisory Committee and Investigators, et al. Clinical presentation, management, and in -hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006 Jan 3; 47(1): 76 -84. Epub 2005 Dec 15. Yancy CW, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A report of the ACC/AHA Task Force on Practice Guidelines. Circulation. 2013; 128: e 240 -e 327. Young JB, et al. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction. Results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004 Oct 26; 110(17): 2618 -26. Epub 2004 Oct 18. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991; 325: 293 -302. Zannad F, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011; 364: 11 -21. van Veldhuisen DJ, et al. for DOT-HF Investigators. Intrathoracic Impedance Monitoring, Audible Patient Alerts, and Outcome in Patients With Heart Failure. Circulation. 2011 Oct 18; 124(16): 1719 -1726. Zile MR, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008 Sep 30; 118(14): 1433 -41. Epub 2008 Sep 15. Verdejo HE, et al. Comparison of a radiofrequency-based wireless pressure sensor to Swan-Ganz ‡ catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J Am Coll Cardiol. 2007; 50(25): 2375 -82. Virano SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics — 2020 update: a report from the American Heart Association. Circulation. 2020; 141: e 139 -e 596. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only. 99

Abbott One St. Jude Medical Dr. , St. Paul, MN 55117 USA, Tel: 1 651 756 2000 Cardiovascular. Abbott/Cardio. MEMS Rx Only Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use. Cardio. MEMS™ HF System Indications and Usage: The Cardio. MEMS™ HF System is indicated for wirelessly measuring and monitoring pulmonary artery (PA) pressure and heart rate in New York Heart Association (NYHA) Class III heart failure patients who have been hospitalized for heart failure in the previous year. The hemodynamic data are used by physicians for heart failure management and with the goal of reducing heart failure hospitalizations. Cardio. MEMS™ HF System Contraindications: The Cardio. MEMS HF System is contraindicated for patients with an inability to take dual antiplatelet or anticoagulants for one month post implant. Cardio. MEMS™ HF System Potential Adverse Events: Potential adverse events associated with the implantation procedure include, but are not limited to, the following: Infection, Arrhythmias, Bleeding, Hematoma, Thrombus, Myocardial infarction, Transient ischemic attack, Stroke, Death, and Device embolization. ™ Indicates a trademark of the Abbott group of companies. ‡ Indicates a third party trademark, which is property of its respective owner. © 2020 Abbott. All Rights Reserved. 39019 MAT-2003654 v 1. 0 | Item approved for U. S. use only.

 Mems inertial navigation system
Mems inertial navigation system Nintendo mems
Nintendo mems Mems stiction
Mems stiction Mems magnetic actuator
Mems magnetic actuator Hagen poiseuille law
Hagen poiseuille law Sugar mems
Sugar mems Mems gyroscope
Mems gyroscope Thomas mems
Thomas mems Mems accelerometer
Mems accelerometer Mems cantilever beam
Mems cantilever beam Mems
Mems Is mems a female
Is mems a female Mems
Mems Mems mirror
Mems mirror Mems
Mems Mems
Mems Ariwatch
Ariwatch Max mems
Max mems Mems speaker
Mems speaker Mems cleanroom
Mems cleanroom Mems
Mems Memsag
Memsag Vddi cardio
Vddi cardio Semnele stopului cardiorespirator
Semnele stopului cardiorespirator Cristina skrypnyk
Cristina skrypnyk Index cardio thoracique
Index cardio thoracique Angle de raccordement radio thorax
Angle de raccordement radio thorax Aortic knob
Aortic knob Cardio vision
Cardio vision Site:slidetodoc.com
Site:slidetodoc.com Cauze reversibile de stop cardio-respirator
Cauze reversibile de stop cardio-respirator Rr2tss que significa
Rr2tss que significa Cardio clearance meaning
Cardio clearance meaning Cardio sense
Cardio sense Index cardiothoracique calcul
Index cardiothoracique calcul Prefijo cardio ejemplos
Prefijo cardio ejemplos Tête de méduse cirrhose
Tête de méduse cirrhose Pa cardio
Pa cardio Barorécepteur et chémorécepteur
Barorécepteur et chémorécepteur Cardio soft
Cardio soft Cardio map
Cardio map Velo cardio facial syndrome
Velo cardio facial syndrome Trusight cardio
Trusight cardio 4tech cardio
4tech cardio Discovery cardio care programme
Discovery cardio care programme Digeorge syndrome
Digeorge syndrome Electro cardio gram
Electro cardio gram Atl cardio
Atl cardio Cardio alex
Cardio alex Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Closed open and isolated systems
Closed open and isolated systems Digestive system respiratory system and circulatory system
Digestive system respiratory system and circulatory system Body paragraph
Body paragraph Urinary system introduction
Urinary system introduction The digestive system introduction
The digestive system introduction Introduction to systems analysis and design
Introduction to systems analysis and design Single entry system introduction
Single entry system introduction Introduction to restaurant management system
Introduction to restaurant management system Hierarchical structure of operating system
Hierarchical structure of operating system Operating system definition
Operating system definition Mis meaning
Mis meaning