Chemistry 58 Due Today Stoichiometry Worksheet Gas Laws











































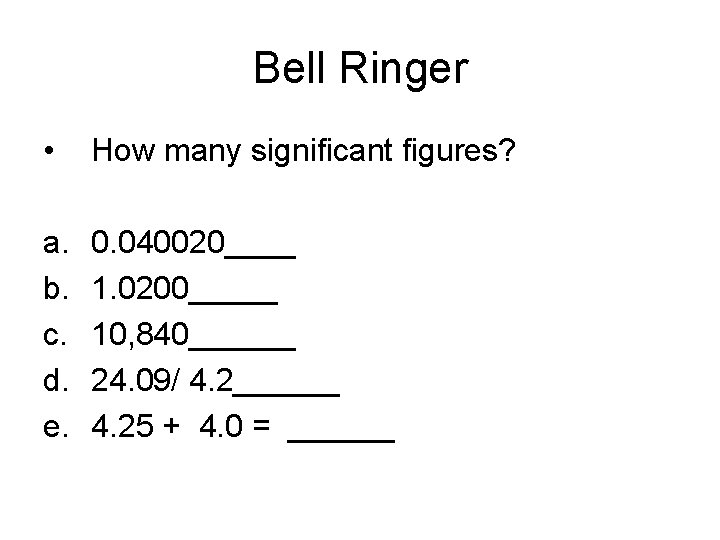






- Slides: 94

Chemistry (5/8) Due Today: • Stoichiometry Worksheet • Gas Laws Graphs Objectives: • Complete Stoichiometry Quiz • Interpret and discuss the Gas Laws graphs by answering the questions in your packet.

Chemistry (5/9) Due Today: • Stoichiometry Worksheet-late • Gas Laws Graphs Objectives: • Discuss the Gas Laws you read about from the text and applied to graphs. • Discuss Stoichiometry Quiz Homework: Study for Stoichiometry Re-Take Quiz

Gas Laws Assignment Chapter 14. 2 in Text • Graph each set of data using a line graph. • Interpret each graph by answering the questions in the packet.

Pre-AP Chemistry (5/13) Due Today: • Gas Laws Graphs Objectives: • Discuss and apply Gas Laws. Homework: Gas Law applications

Chemistry (5/13) Objectives: • Discuss and apply Gas Laws using your graphs. Homework: Gas Law applications and review for final.

Chemistry (5/15) Objectives: • Discuss and apply Gas Laws using your graphs. Due Today: • Gas Law Graphs Homework: Gas Law applications and review for final.

Chemistry II (5/18) Due Today: • Gas Law Assignments Objectives: • I an apply the gas laws to real-world applications.

Gas Laws Behavior of a gas when two of the following variables change: • temperature of a gas (o. C or K) • pressure of a gas (atm, mm. Hg, Pa) • volume of a gas ( m. L, cm 3) Illustrate in your notes how a sample of gas in a balloon would be affected by a change in each variable above.

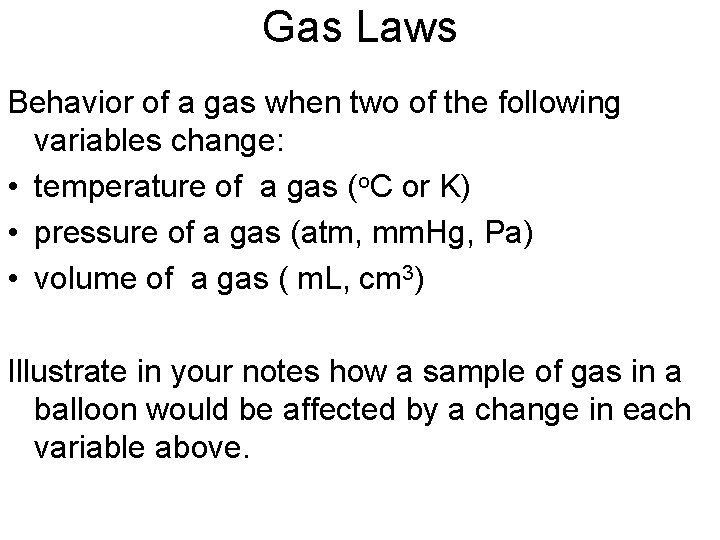
Boyle’s Law If temperature is held constant, what is the relationship between the volume and pressure of a gas? blog. cencophysics. com

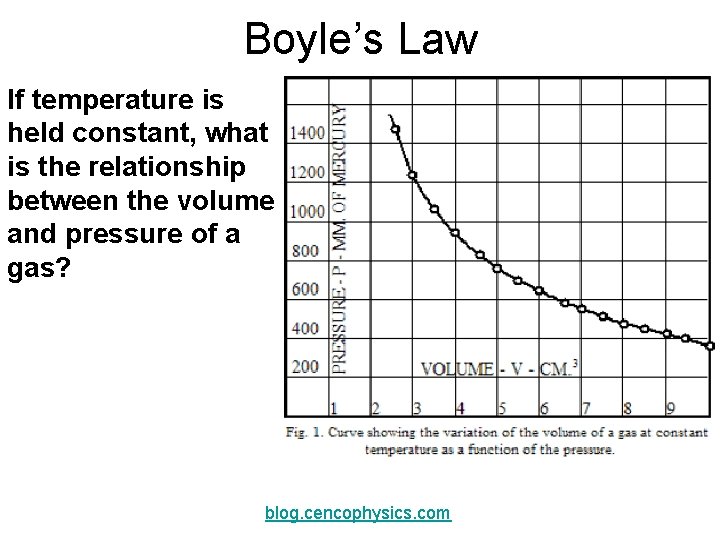
Charles’ Law If pressure is held constant, what is the relationship between temperature and volume of a gas? www. kchemistry. com

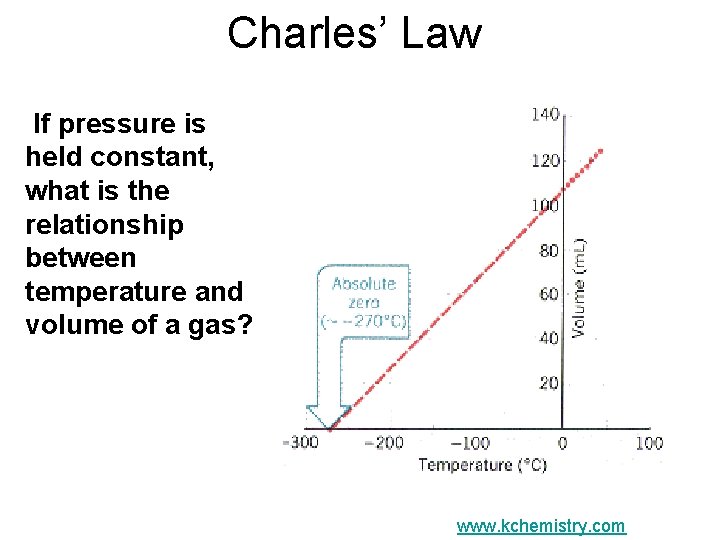
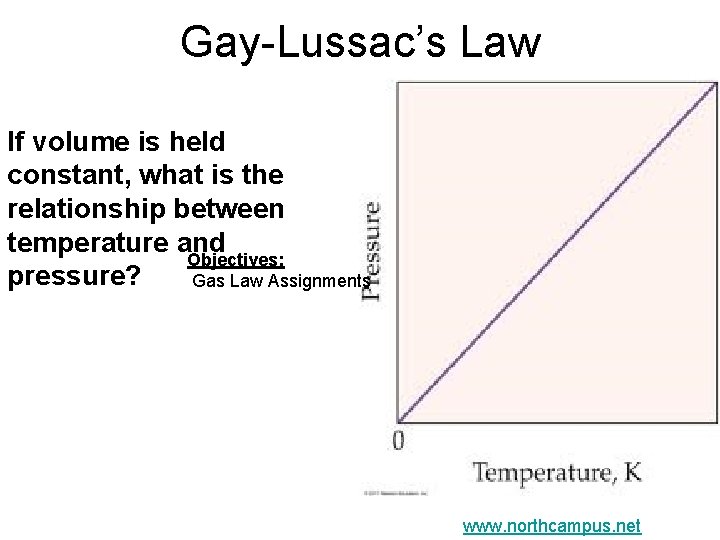
Gay-Lussac’s Law If volume is held constant, what is the relationship between temperature and Objectives: pressure? Gas Law Assignments www. northcampus. net

Chemistry II (5/19) Due Today: • Gas Law Assignments Objectives: • I can apply the gas laws to real-world applications qualitatively and quantitatively.

Gas Laws Applications 1. 2. 3. 4. 5. 6. 7. 8. 9.

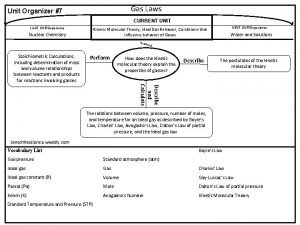
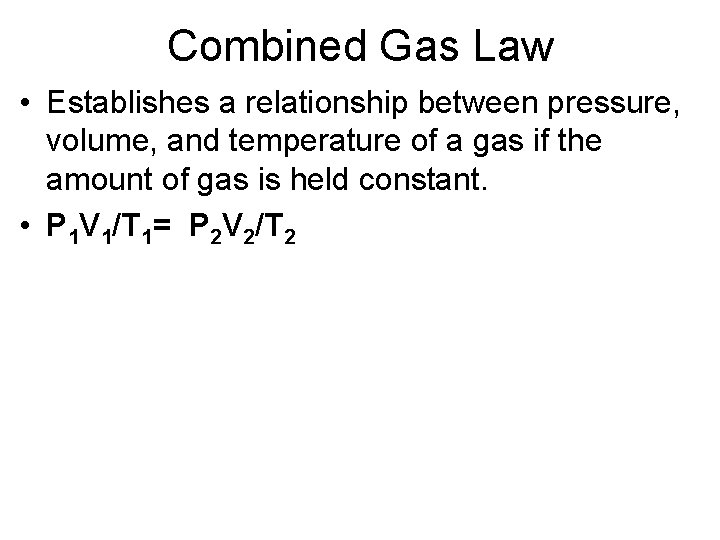
Combined Gas Law • Establishes a relationship between pressure, volume, and temperature of a gas if the amount of gas is held constant. • P 1 V 1/T 1= P 2 V 2/T 2

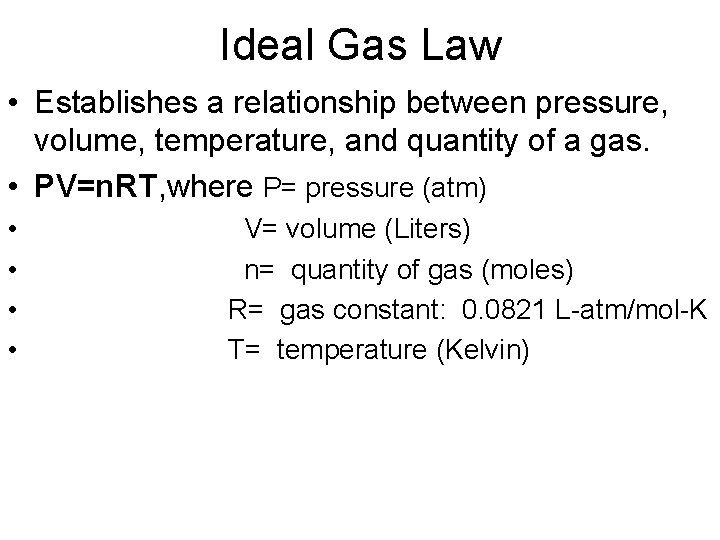
Ideal Gas Law • Establishes a relationship between pressure, volume, temperature, and quantity of a gas. • PV=n. RT, where P= pressure (atm) • • V= volume (Liters) n= quantity of gas (moles) R= gas constant: 0. 0821 L-atm/mol-K T= temperature (Kelvin)

Chemistry (5/16) Due Today: Gas Laws Graph Objectives: Review for Final

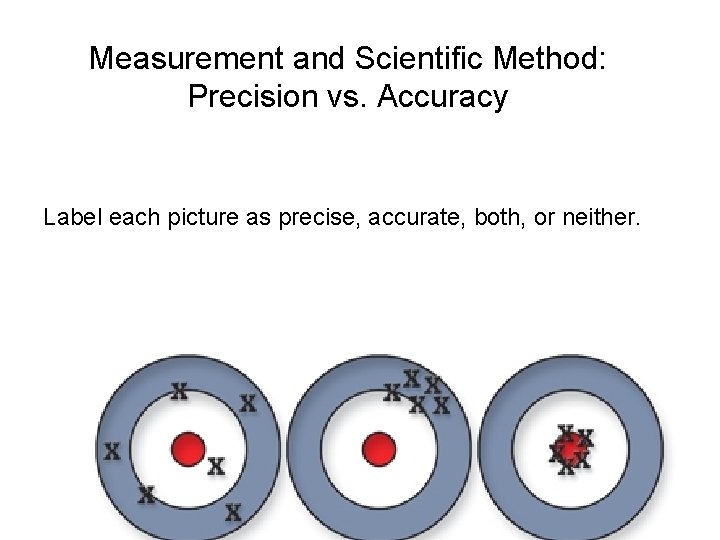
Measurement and Scientific Method: Precision vs. Accuracy Label each picture as precise, accurate, both, or neither.

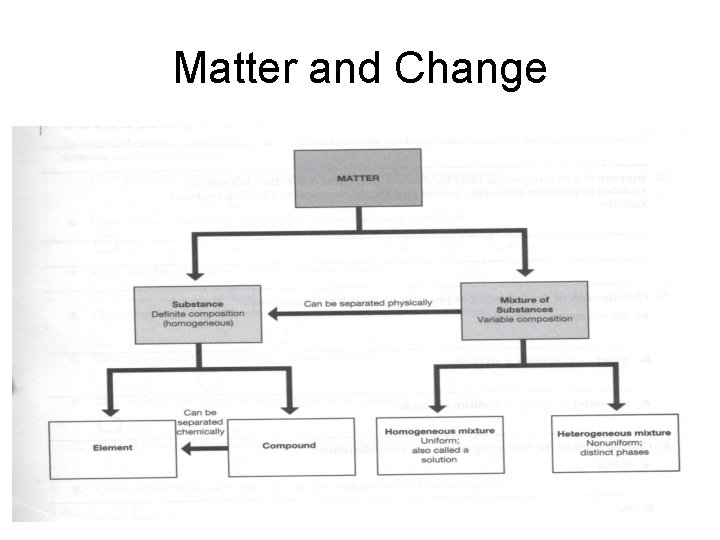
Matter and Change

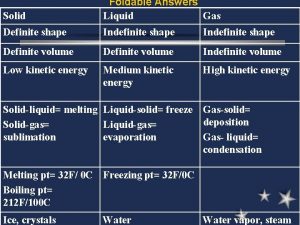
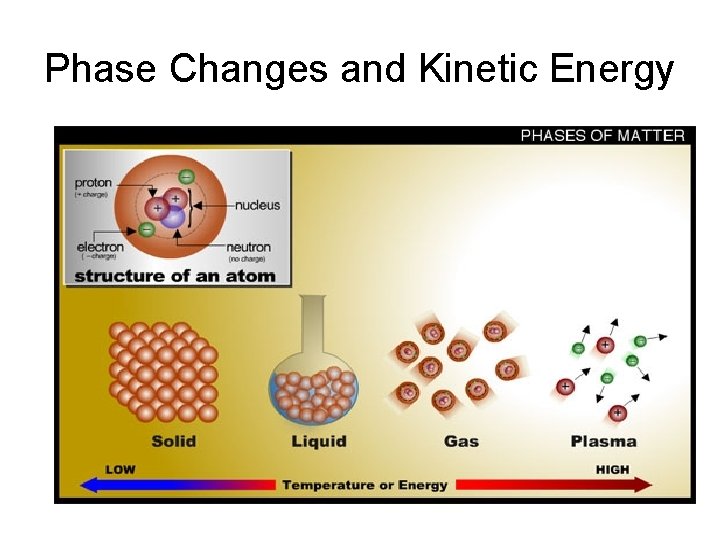
Phase Changes and Kinetic Energy

Atomic Structure

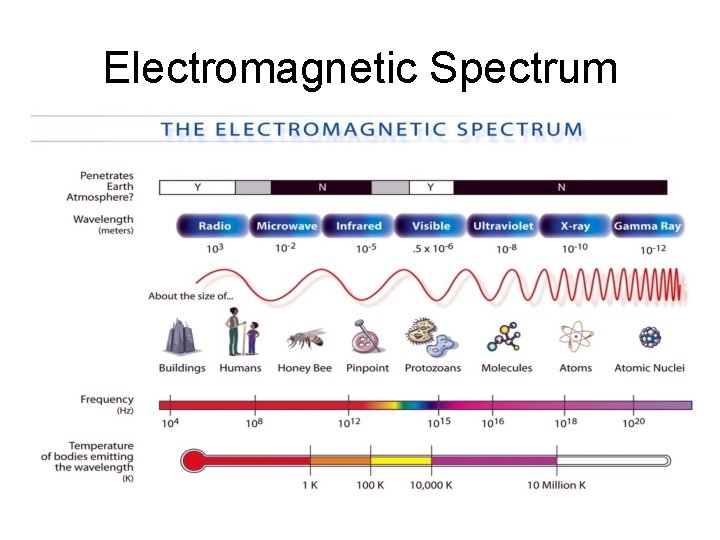
Electromagnetic Spectrum

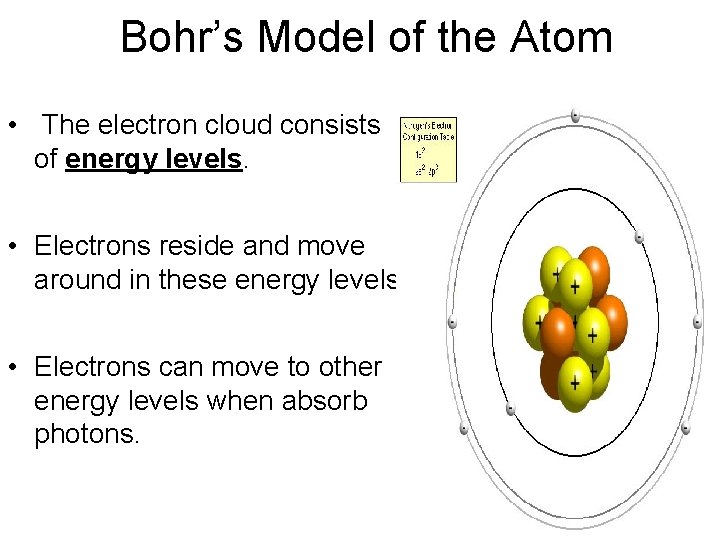
Bohr’s Model of the Atom • The electron cloud consists of energy levels. • Electrons reside and move around in these energy levels. • Electrons can move to other energy levels when absorb photons.

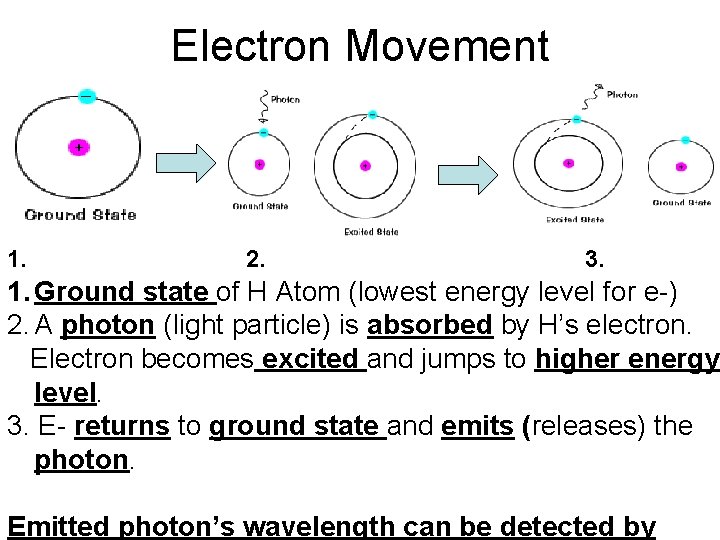
Electron Movement 1. 2. 3. 1. Ground state of H Atom (lowest energy level for e-) 2. A photon (light particle) is absorbed by H’s electron. Electron becomes excited and jumps to higher energy level. 3. E- returns to ground state and emits (releases) the photon. Emitted photon’s wavelength can be detected by

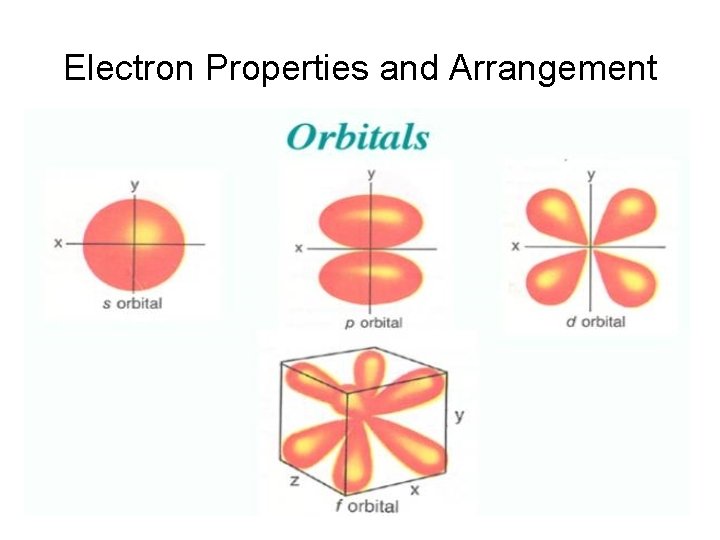
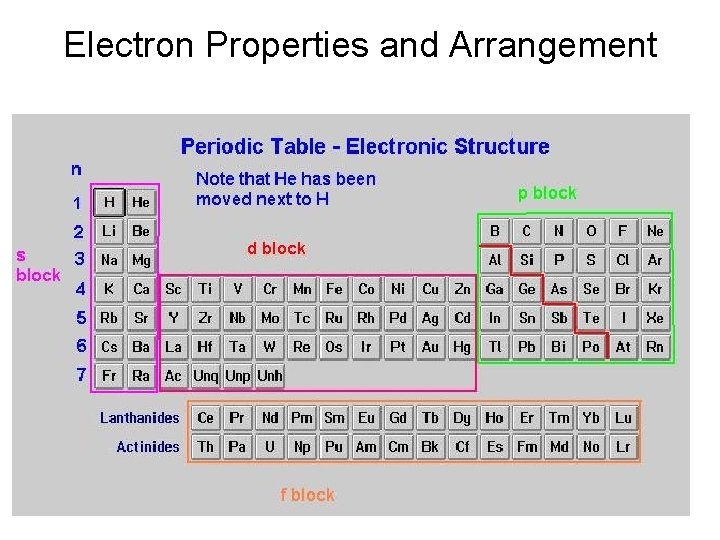
Electron Properties and Arrangement

Electron Properties and Arrangement

Electron Properties and Arrangement

Periodic Table

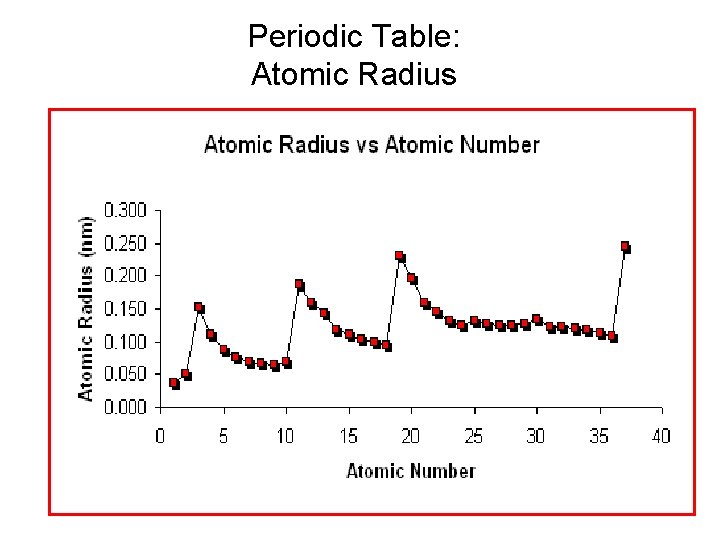
Periodic Table: Atomic Radius

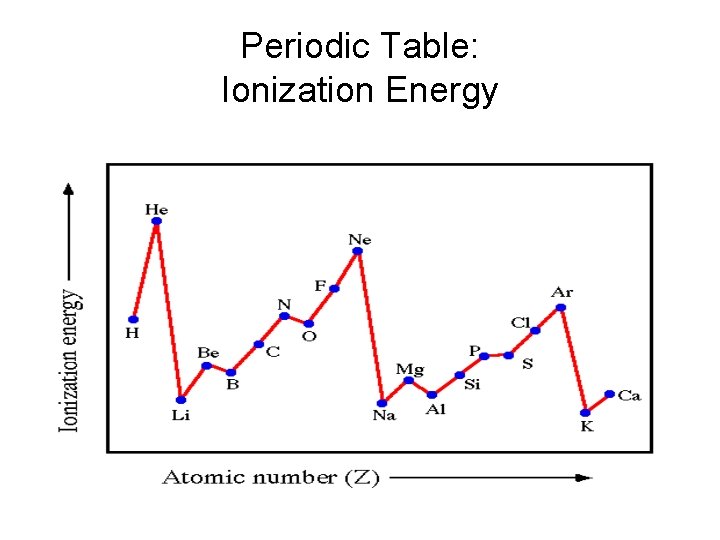
Periodic Table: Ionization Energy

Chemical Bonding

Chemical Reactions

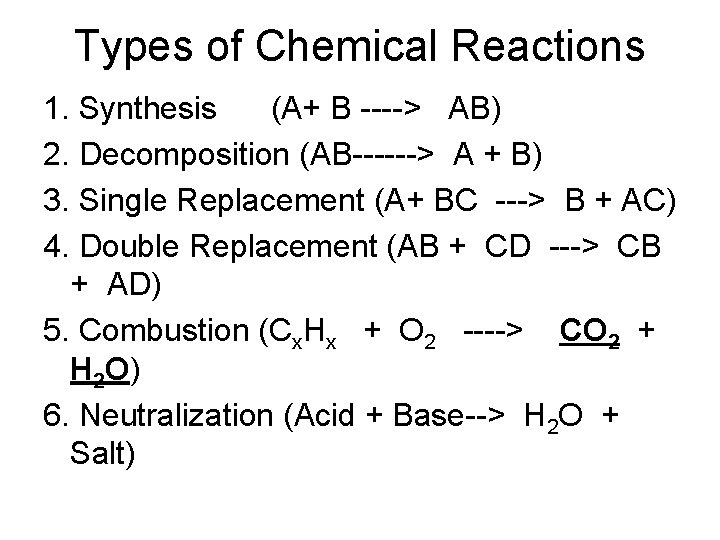
Types of Chemical Reactions 1. Synthesis (A+ B ----> AB) 2. Decomposition (AB------> A + B) 3. Single Replacement (A+ BC ---> B + AC) 4. Double Replacement (AB + CD ---> CB + AD) 5. Combustion (Cx. Hx + O 2 ----> CO 2 + H 2 O) 6. Neutralization (Acid + Base--> H 2 O + Salt)

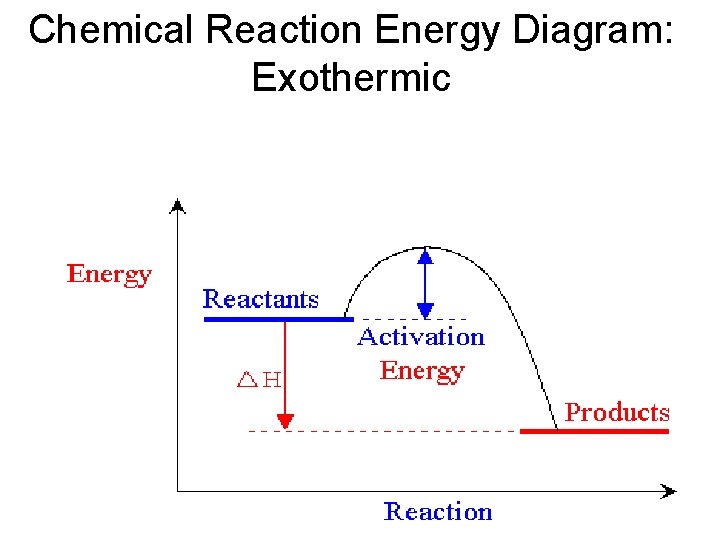
Chemical Reaction Energy Diagram: Exothermic

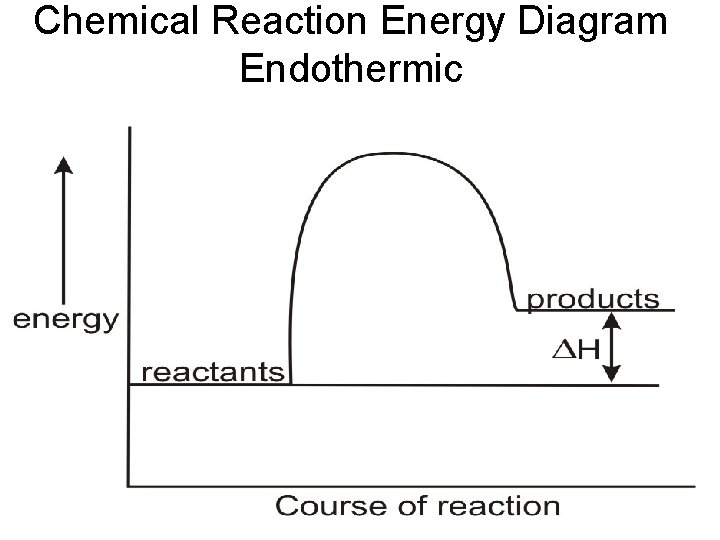
Chemical Reaction Energy Diagram Endothermic

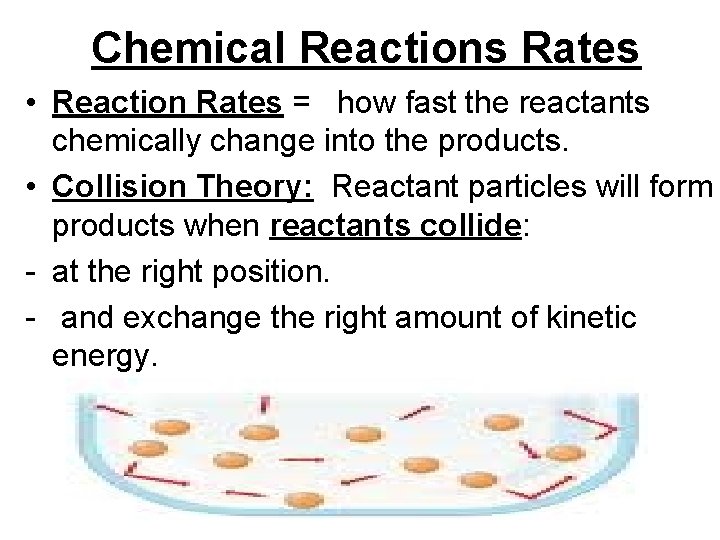
Chemical Reactions Rates • Reaction Rates = how fast the reactants chemically change into the products. • Collision Theory: Reactant particles will form products when reactants collide: - at the right position. - and exchange the right amount of kinetic energy.

Nuclear Chemistry

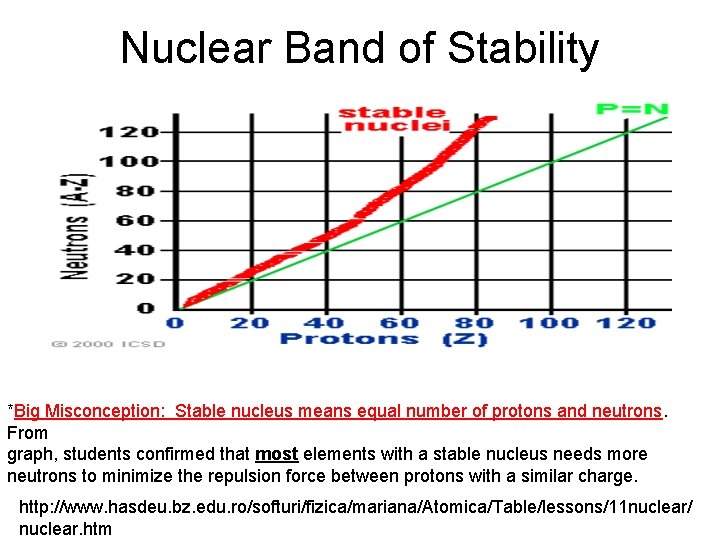
Nuclear Band of Stability *Big Misconception: Stable nucleus means equal number of protons and neutrons. From graph, students confirmed that most elements with a stable nucleus needs more neutrons to minimize the repulsion force between protons with a similar charge. http: //www. hasdeu. bz. edu. ro/softuri/fizica/mariana/Atomica/Table/lessons/11 nuclear/ nuclear. htm

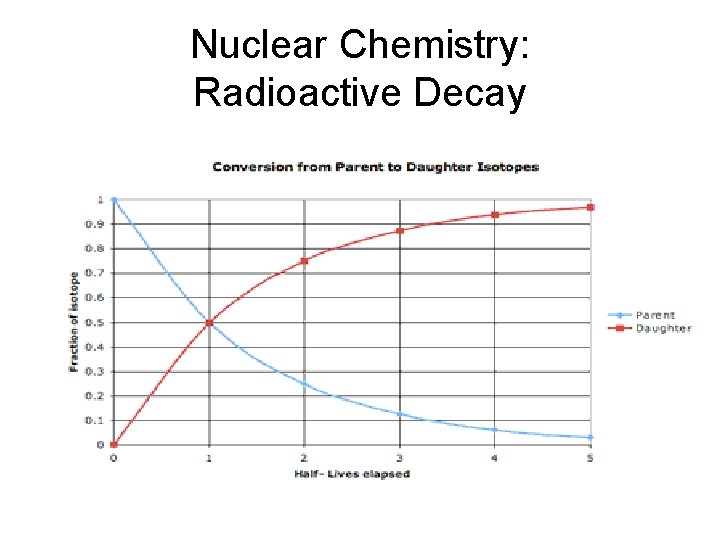
Nuclear Chemistry: Radioactive Decay

Chemical Quantities

Stoichiometry

Chemistry Final What does Chemistry study?

Scientific Notation 1. Convert to scientific notation: 2, 350, 921

Scientific Notation • Convert to scientific notation. 0. 0000258

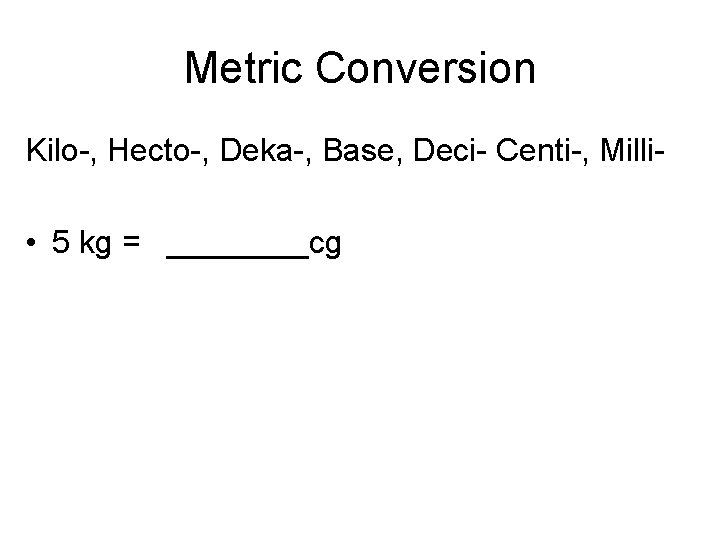
Metric Conversion Kilo-, Hecto-, Deka-, Base, Deci- Centi-, Milli- • 5 kg = ____cg

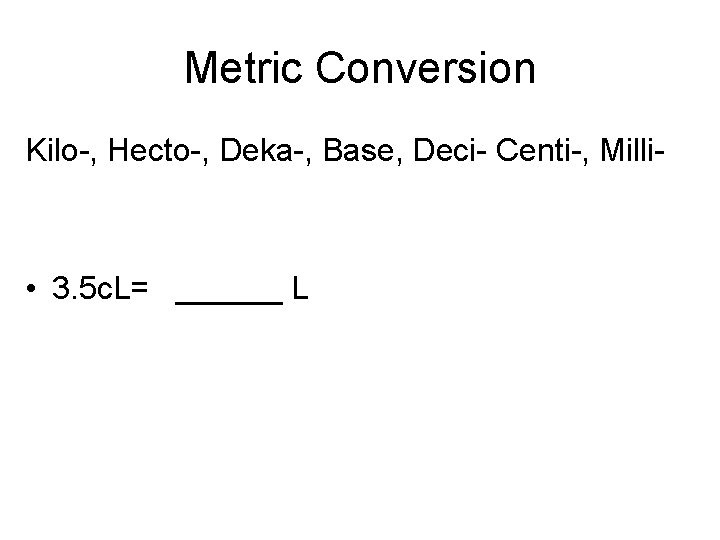
Metric Conversion Kilo-, Hecto-, Deka-, Base, Deci- Centi-, Milli- • 3. 5 c. L= ______ L

Metric and English Conversions How many seconds is in 10. 26 hrs?

Metric and English Conversions • Sara ran 2 miles in 16 minutes. How far did she run in kilometers? (1 mi= 1600 m)

Density What is the equation for density?

Density • A solution has a mass of 1200 g and a density of 1. 2 g/m. L. What is the volume?

Density • What is the density of a 50 m. L sample of water that has a mass of 49. 6 g?

Scientific Method • What are the 5 main steps to the scientific method?

Scientific Method What is the difference between qualitative and quantitative results?

Scientific Method • What is the difference between accuracy and precision?

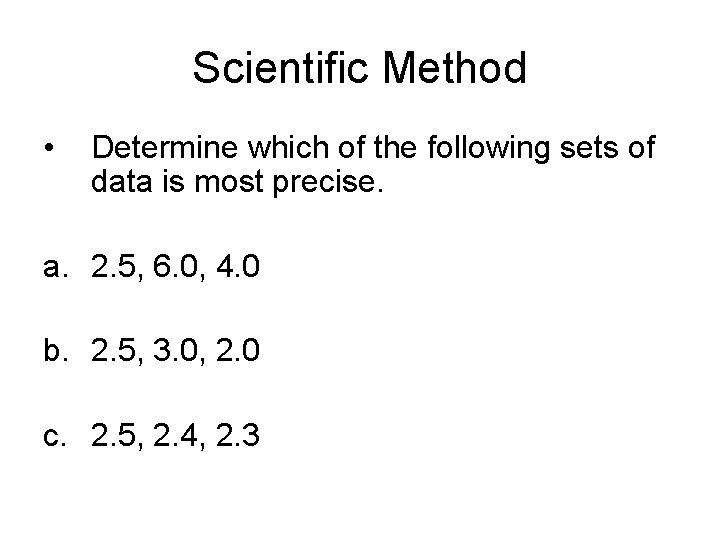
Scientific Method • Determine which of the following sets of data is most precise. a. 2. 5, 6. 0, 4. 0 b. 2. 5, 3. 0, 2. 0 c. 2. 5, 2. 4, 2. 3

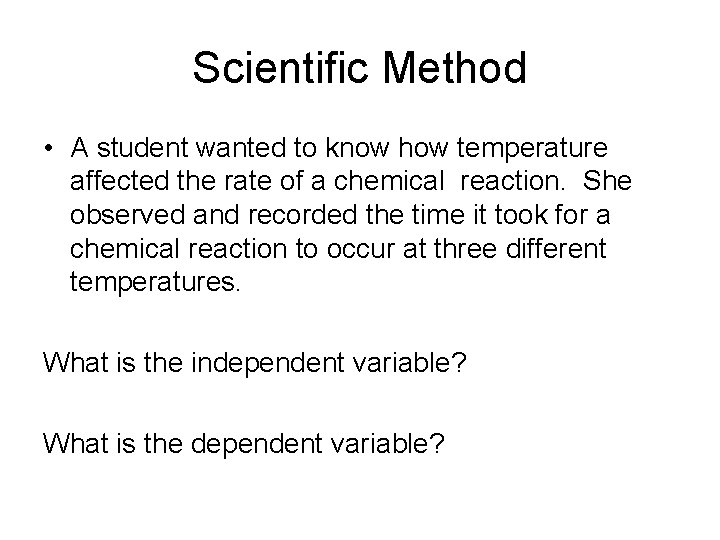
Scientific Method • A student wanted to know how temperature affected the rate of a chemical reaction. She observed and recorded the time it took for a chemical reaction to occur at three different temperatures. What is the independent variable? What is the dependent variable?

Significant Figures • How many significant figures? 0. 01020 g ____

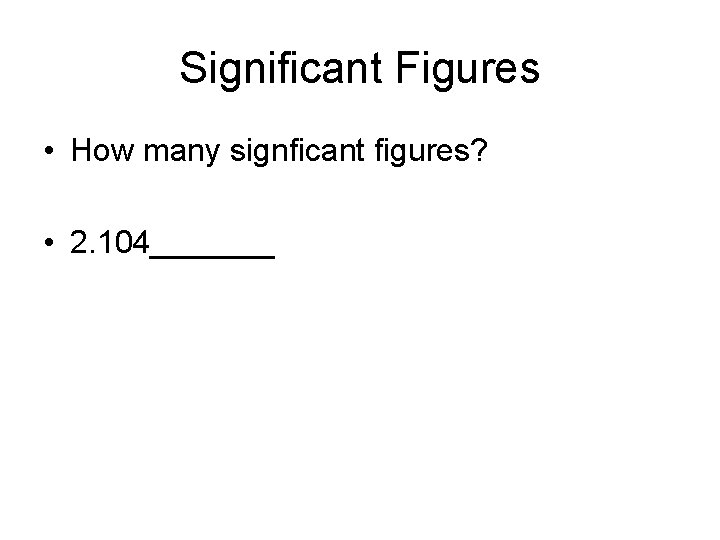
Significant Figures • How many signficant figures? • 2. 104_______

Calculating using Significant Figures • How many significant figures are in the answer? 1. 21 g x 0. 50 g =

Calculating using Signficant Figures • How many significant figures are in the answer? 0. 45 m + 2. 140 m + 1. 3 g+ 4 g =

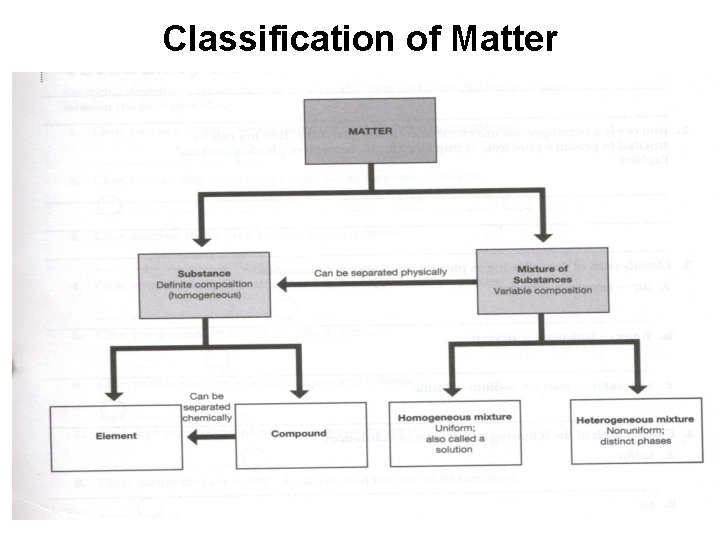
Matter • What are the two broad classes of matter?

Classification of Matter

Matter • What is the big difference between substances and mixtures?

Mixtures • Determine if the following mixtures are heterogenous or homogenous mixtures. a. Saltwater b. Vegetable soup

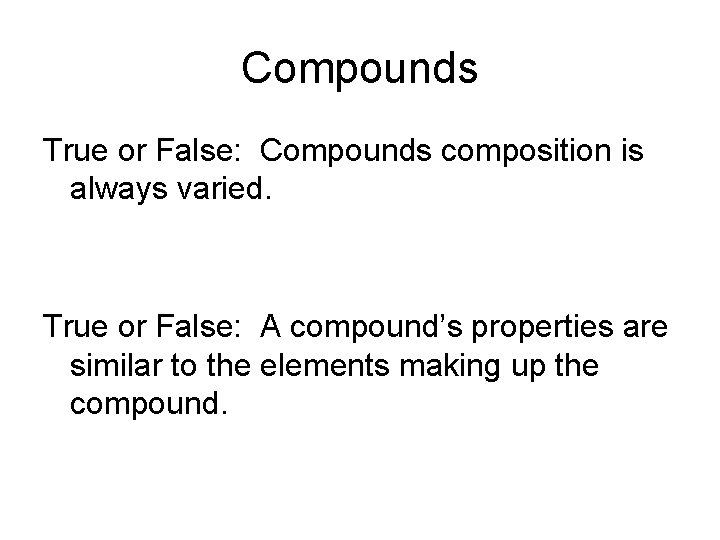
Compounds True or False: Compounds composition is always varied. True or False: A compound’s properties are similar to the elements making up the compound.

Physical Properties Give me example of physical properties.

Chemical properties Give me example of chemical properties.

Physical or Chemical Change • Determine if the following are chemical or physical changes a. Boiling water b. Digesting food c. Burning fire wood d. Dissolving sugar in water

Mixture or Compound? • air • Na(OH) • lead • Na(OH)solution

Atomic Theory • Who believed matter consisted of air, wind, fire, and earth? • Who was the first to propose that matter is composed of atoms?

Atomic Theory • Who was the first person to confirm using the scientific method that matter was composed of atoms?

Dalton’s Atomic Theory • Identify important concepts of Dalton’s Atomic Theory.

The Atom • What are the two subatomic particles that have charge?

The Atom • What are the two subatomic particles that contribute mass to an atom?

The Atom • What is the difference between mass number and atomic mass?

Atomic Subparticles • Nitrogen is a neutral atom. It has 7 neutrons and 7 electrons. • What is Nitrogen’s number of protons? • What is Nitrogen’s atomic number? • What is Nitrogen’s mass number?

Atomic Subparticles • Ca 2+ has a mass number of 40 and an atomic number of 20. • How may protons does Ca 2+ have? • How many electrons does Ca 2+ have? • How many neutrons does Ca 2+ have?

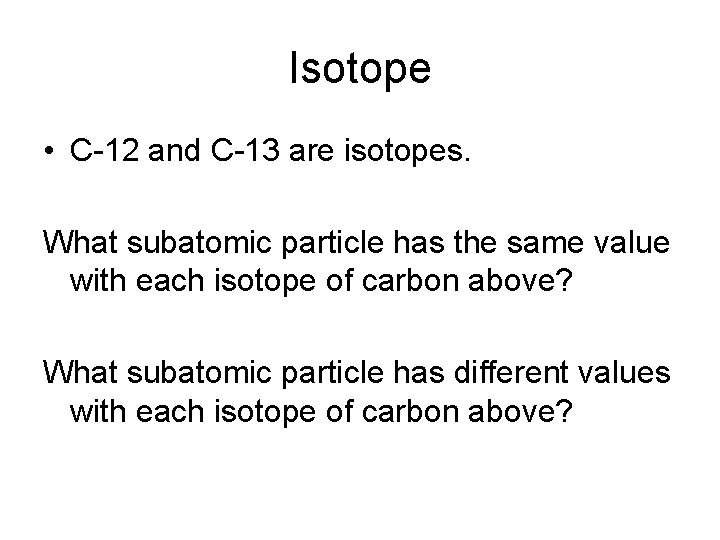
The Atom • What is an isotope?
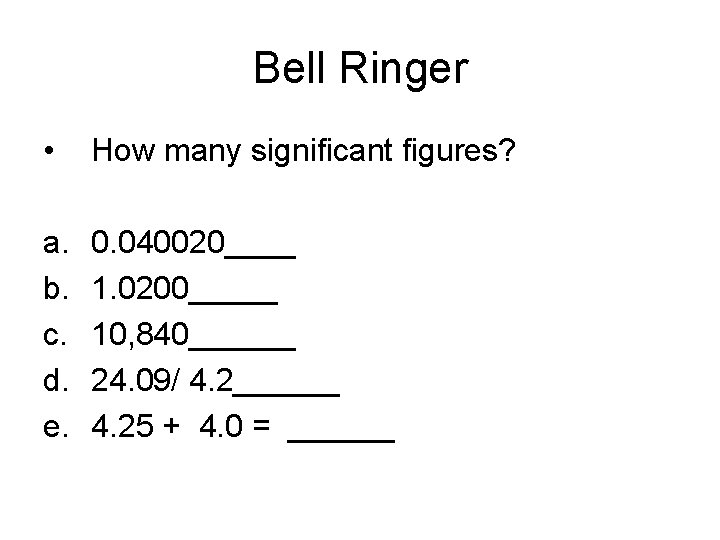
Bell Ringer • How many significant figures? a. b. c. d. e. 0. 040020____ 1. 0200_____ 10, 840______ 24. 09/ 4. 2______ 4. 25 + 4. 0 = ______

Isotope • C-12 and C-13 are isotopes. What subatomic particle has the same value with each isotope of carbon above? What subatomic particle has different values with each isotope of carbon above?

Radioisotope • What is a radioisotope? • What are three types of radioisotope particles we talked about?

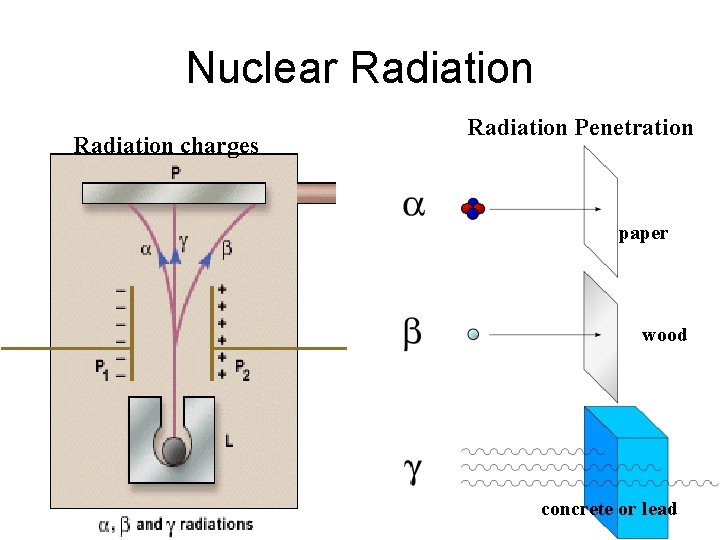
Nuclear Radiation charges Radiation Penetration paper wood concrete or lead

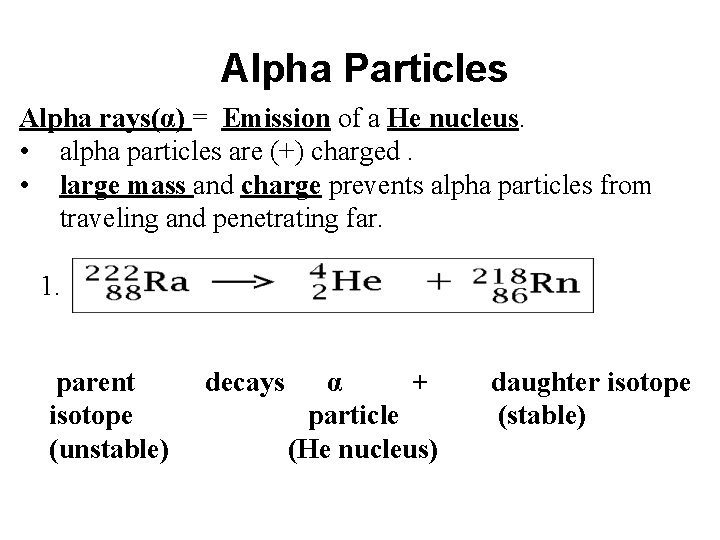
Alpha Particles Alpha rays(α) = Emission of a He nucleus. • alpha particles are (+) charged. • large mass and charge prevents alpha particles from traveling and penetrating far. 1. parent isotope (unstable) decays α + particle (He nucleus) daughter isotope (stable)

Nuclear Decay with α Particles • Mass number decreases by four • Atomic Number decreases by two (Daughter isotope that is more stable)

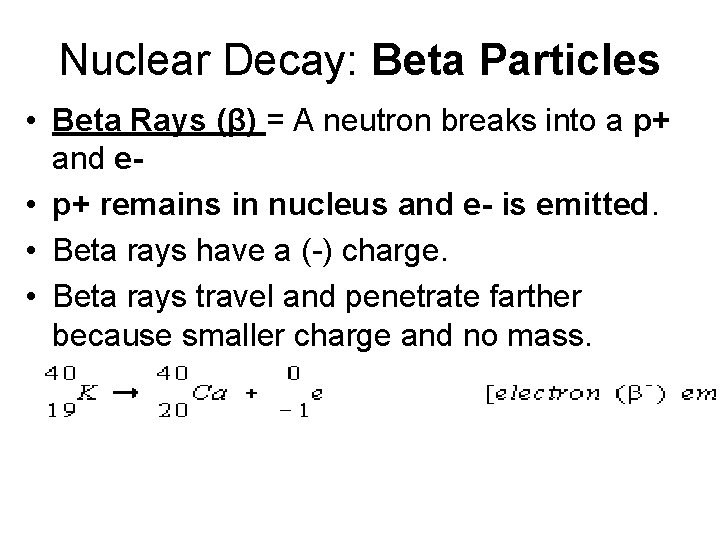
Nuclear Decay: Beta Particles • Beta Rays (β) = A neutron breaks into a p+ and e • p+ remains in nucleus and e- is emitted. • Beta rays have a (-) charge. • Beta rays travel and penetrate farther because smaller charge and no mass.

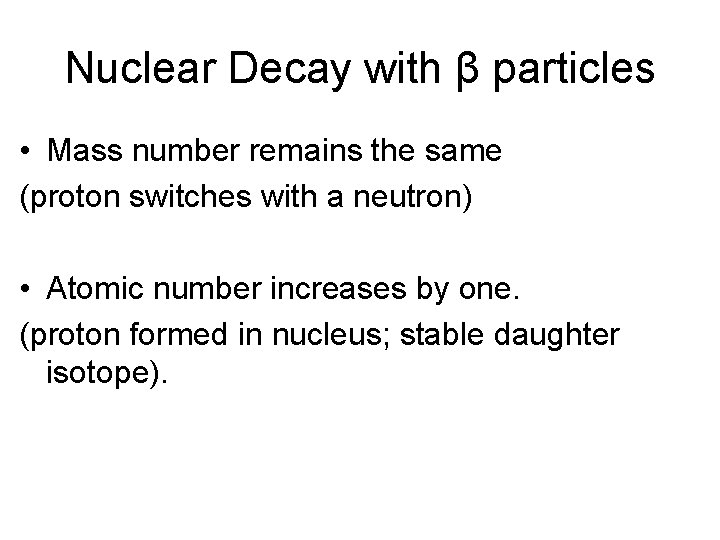
Nuclear Decay with β particles • Mass number remains the same (proton switches with a neutron) • Atomic number increases by one. (proton formed in nucleus; stable daughter isotope).

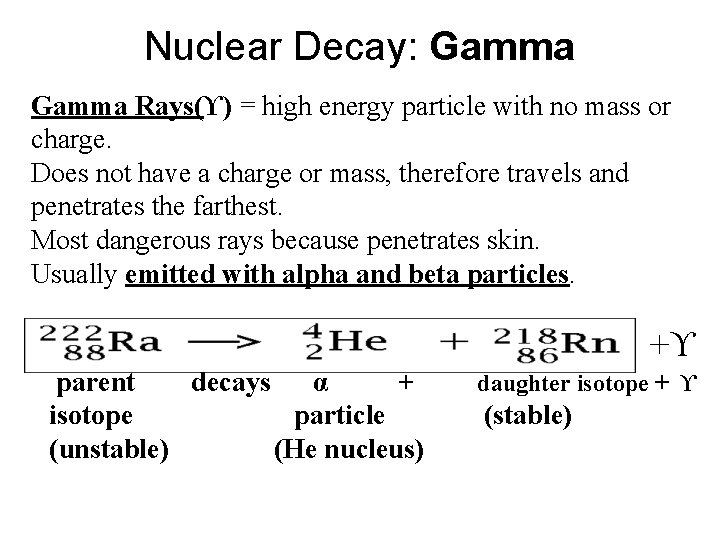
Nuclear Decay: Gamma Rays(ϒ) = high energy particle with no mass or charge. Does not have a charge or mass, therefore travels and penetrates the farthest. Most dangerous rays because penetrates skin. Usually emitted with alpha and beta particles. +ϒ parent decays α + isotope particle (unstable) (He nucleus) daughter isotope + ϒ (stable)

Nuclear Decay with ϒ rays • Mass number remains the same (ϒ rays has no mass) • Atomic number remains the same (ϒ rays has no mass or charge)

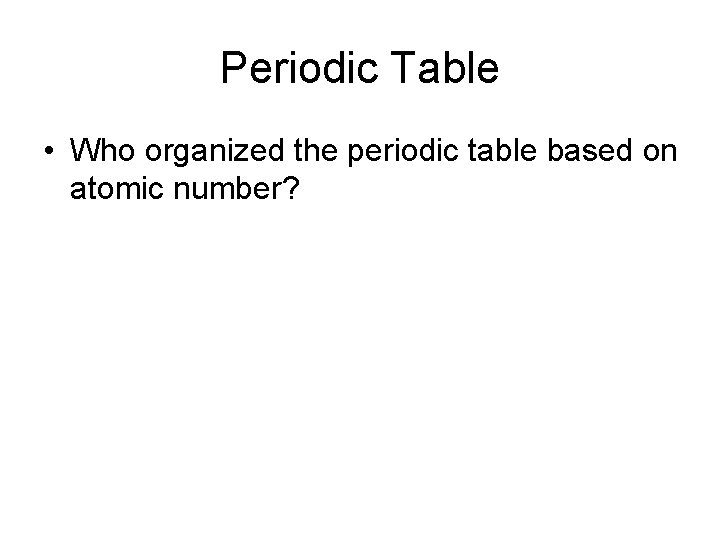
Periodic Table • Who organized the periodic table based on atomic mass?

Periodic Table • Who organized the periodic table based on atomic number?

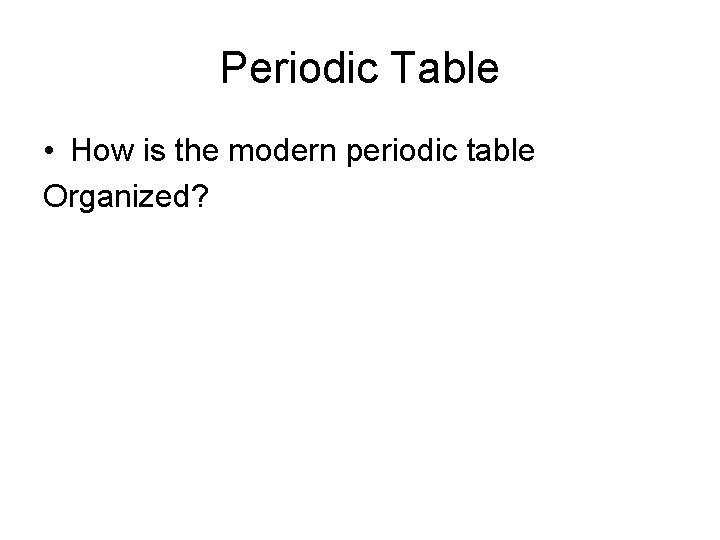
Periodic Table • How is the modern periodic table Organized?

Periodic Table • What is another name for rows on the periodic table?

Periodic Table • What is another name for the columns on the periodic table?

Periodic Table • Give me an example of a metal, nonmetal, and a metalloid

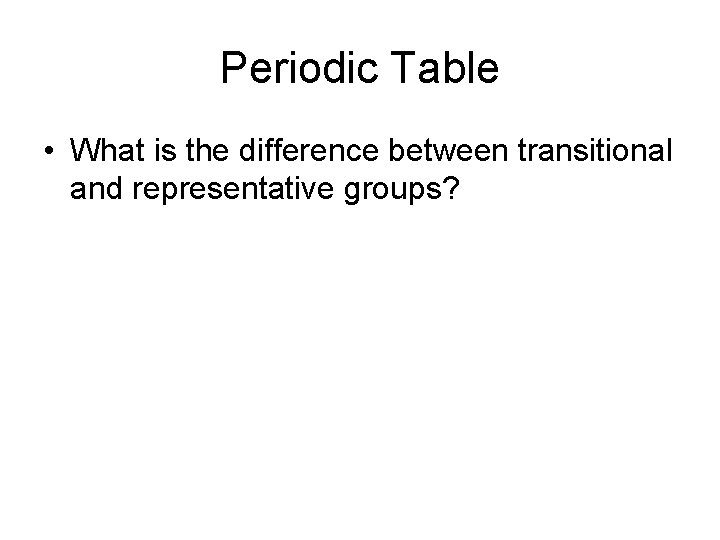
Periodic Table • What is the difference between transitional and representative groups?
 Rock me avogadro
Rock me avogadro Gas stoichiometry worksheet
Gas stoichiometry worksheet Ap chemistry gas laws
Ap chemistry gas laws Density gas law
Density gas law Charles' law worksheet with answers
Charles' law worksheet with answers Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Thermite reaction formula
Thermite reaction formula Stoichiometry is
Stoichiometry is Chapter 11 stoichiometry test
Chapter 11 stoichiometry test Stoichiometry test review
Stoichiometry test review General chemistry 1 stoichiometry
General chemistry 1 stoichiometry Ap chemistry stoichiometry
Ap chemistry stoichiometry 38/2
38/2 Gas stoichiometry
Gas stoichiometry Gas stoichiometry
Gas stoichiometry Explain dalton's law of partial pressure
Explain dalton's law of partial pressure Today meeting or today's meeting
Today meeting or today's meeting In todays class
In todays class Today meeting or today's meeting
Today meeting or today's meeting Galton details
Galton details Today's lesson or today lesson
Today's lesson or today lesson Example of repitition
Example of repitition Facts about montesquieu
Facts about montesquieu Assignment due today
Assignment due today Assignment due today
Assignment due today Assignment due today
Assignment due today Homework due today
Homework due today Black cat analogy
Black cat analogy Reports due today!
Reports due today! Homework due today
Homework due today Central vein liver
Central vein liver That was due today
That was due today Homework
Homework Regolith
Regolith Homework due today
Homework due today Homework due today
Homework due today Mole tunnel stoichiometry
Mole tunnel stoichiometry Defining stoichiometry worksheet answers
Defining stoichiometry worksheet answers Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Ha solo due lati paralleli e due lati congruenti
Ha solo due lati paralleli e due lati congruenti Liberty chapter 20
Liberty chapter 20 Slidetodoc
Slidetodoc Due piccole sfere identiche sono sospese a due punti
Due piccole sfere identiche sono sospese a due punti Gas laws crash course
Gas laws crash course Direct vs indirect relationship
Direct vs indirect relationship All the gas laws
All the gas laws Combined gas law
Combined gas law All the gas laws
All the gas laws Different gas laws
Different gas laws Combined gas law
Combined gas law Gas law conceptual questions
Gas law conceptual questions Sample problem of boyle's law
Sample problem of boyle's law Boyle's gas law formula
Boyle's gas law formula Different gas laws
Different gas laws Combined gas laws
Combined gas laws Which gas law relates pressure and temperature
Which gas law relates pressure and temperature State charle's law.
State charle's law. Charles law formula
Charles law formula Gas law graphic organizer
Gas law graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Graham's law in real life
Graham's law in real life Kmt gas laws
Kmt gas laws Gas law formulas
Gas law formulas Empirical gas law
Empirical gas law Avogadro's law relationship
Avogadro's law relationship Indefinite shape and indefinite volume
Indefinite shape and indefinite volume Due diligence letter of intent
Due diligence letter of intent Chapter 13 marketing in todays world
Chapter 13 marketing in todays world Chapter 13 marketing in today's world
Chapter 13 marketing in today's world Chapter 5 natural laws and car control worksheet answers
Chapter 5 natural laws and car control worksheet answers Chapter 9 natural laws and car control
Chapter 9 natural laws and car control Newtons law of motion
Newtons law of motion 21 irrefutable laws of leadership worksheet pdf
21 irrefutable laws of leadership worksheet pdf Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Pseudo reduced specific volume
Pseudo reduced specific volume An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Conclusion
Conclusion Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Volume molare
Volume molare Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Kinetika kimia
Kinetika kimia Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Chemistry dimensions 2 worksheet solutions
Chemistry dimensions 2 worksheet solutions Dimensional analysis worksheet #1
Dimensional analysis worksheet #1 Writing ionic formulas
Writing ionic formulas Chemistry separating mixtures worksheet
Chemistry separating mixtures worksheet Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Ape man for neon
Ape man for neon Nuclear chemistry review worksheet answer key
Nuclear chemistry review worksheet answer key