Gas Laws Honors Chemistry The Gas Laws u
















- Slides: 31

Gas Laws Honors Chemistry

The Gas Laws u u u Describe HOW gases behave. Can be predicted by theory. • The Kinetic Theory Amount of change can be calculated with mathematical equations.

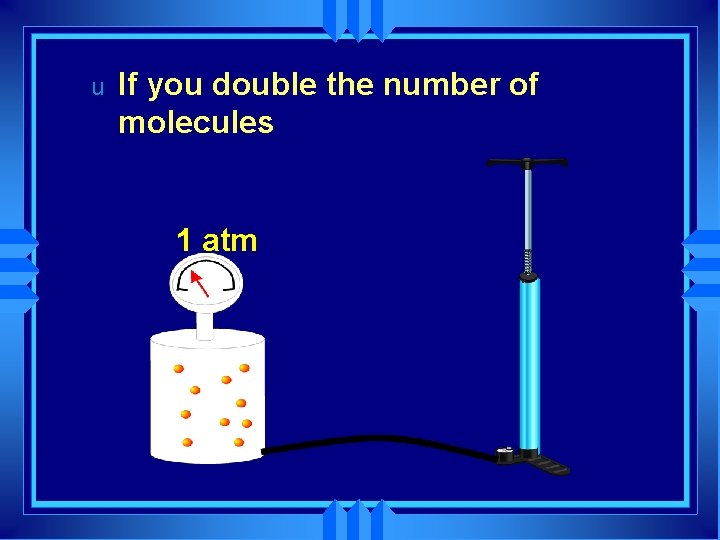
The effect of adding gas. When we blow up a balloon we are adding gas molecules. u Doubling the number of gas particles doubles the pressure (of the same volume at the same temperature). u

4 things u In order to completely describe a gas you need to measure 4 things 1. Pressure 2. Temperature 3. Volume 4. Number of particles 4

Pressure and the number of molecules are directly related u u More molecules means more collisions Fewer molecules means fewer collisions.

u If you double the number of molecules 1 atm

u u If you double the number of molecules You double the pressure. 2 atm

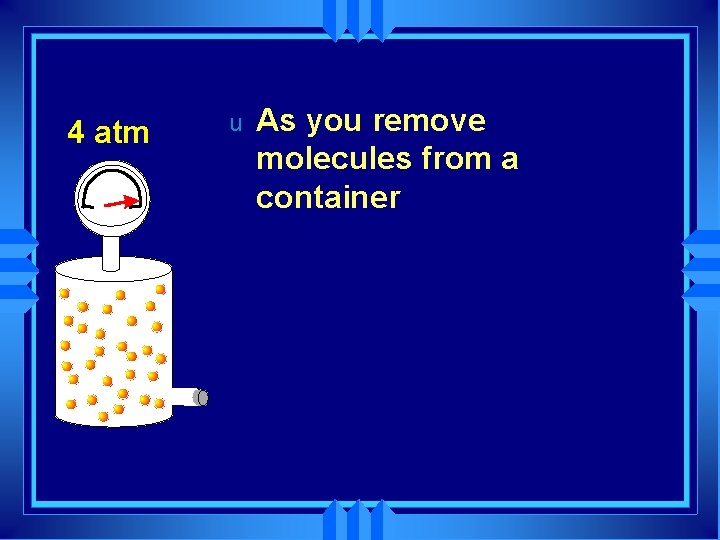
4 atm u As you remove molecules from a container

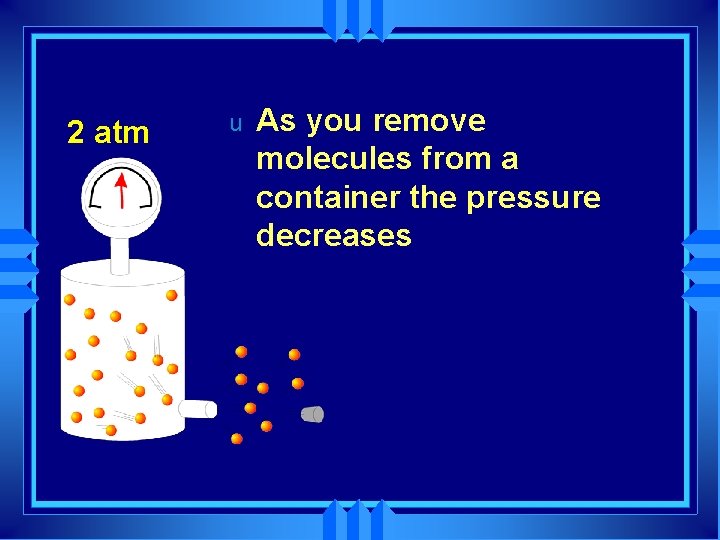
2 atm u As you remove molecules from a container the pressure decreases

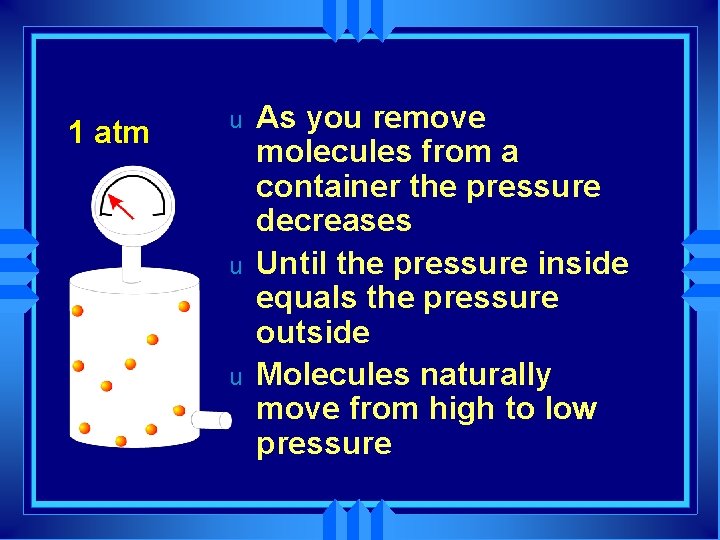
1 atm u u u As you remove molecules from a container the pressure decreases Until the pressure inside equals the pressure outside Molecules naturally move from high to low pressure

Changing the size of the container u u u In a smaller container molecules have less room to move Hit the sides of the container more often As volume decreases pressure increases.

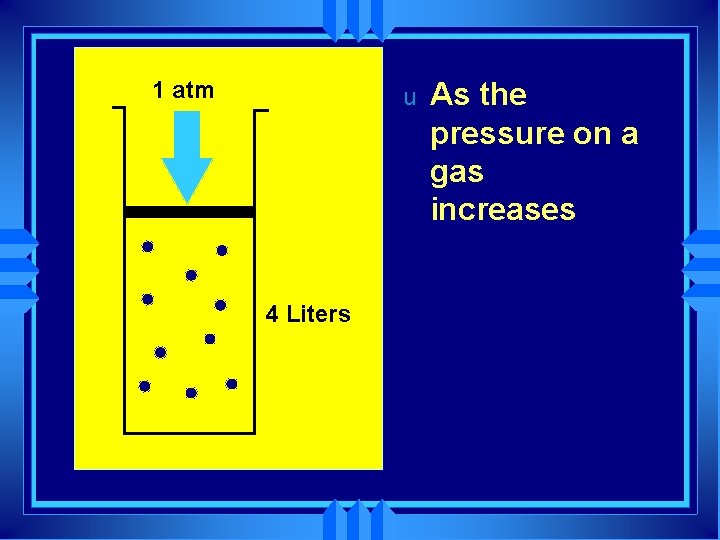
1 atm u 4 Liters As the pressure on a gas increases

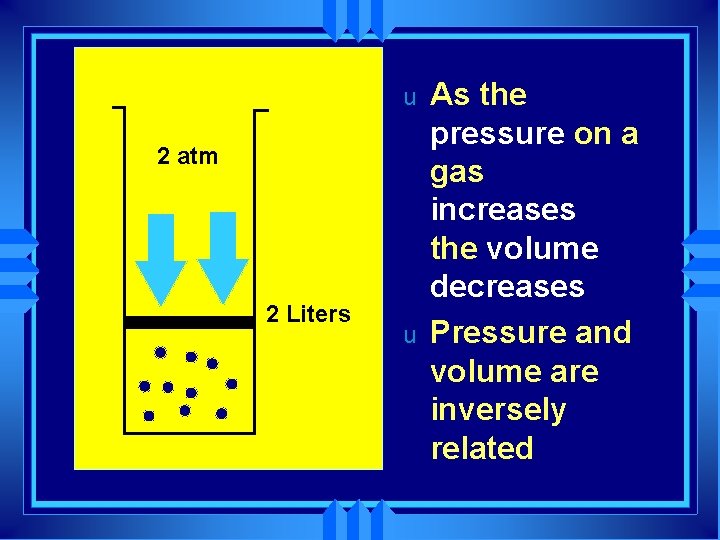
u 2 atm 2 Liters u As the pressure on a gas increases the volume decreases Pressure and volume are inversely related

Temperature u u u Raising the temperature of a gas increases the pressure if the volume is held constant. The molecules hit the walls harder. The only way to increase the temperature at constant pressure is to increase the volume.

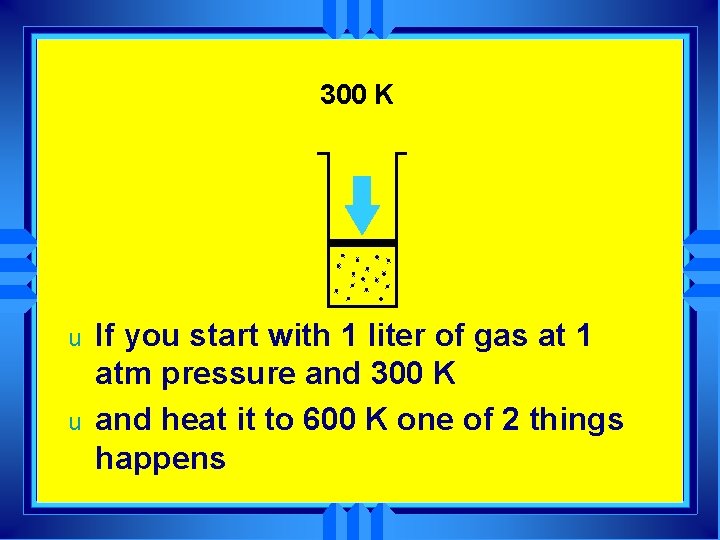
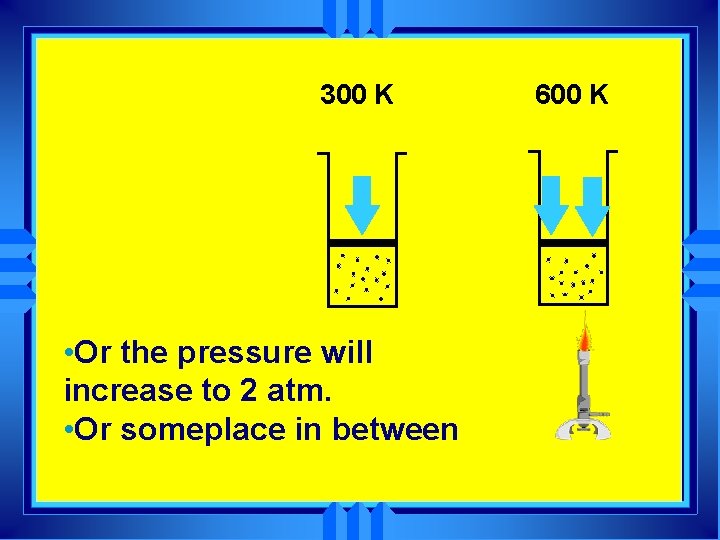
300 K u u If you start with 1 liter of gas at 1 atm pressure and 300 K and heat it to 600 K one of 2 things happens

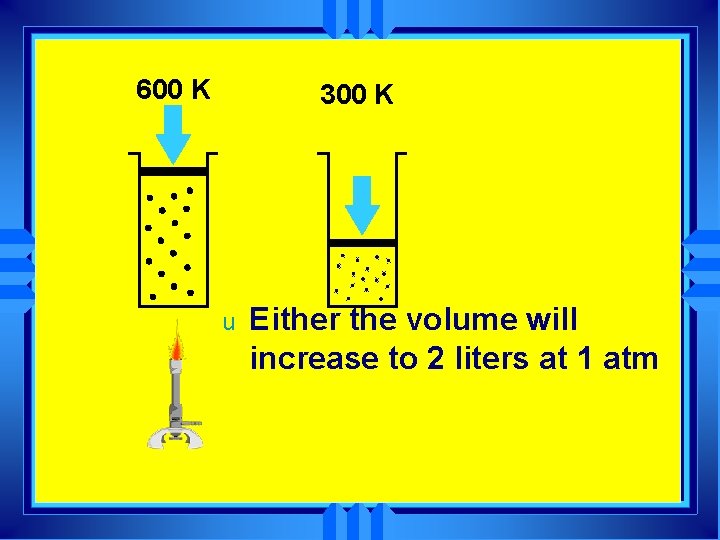
600 K 300 K u Either the volume will increase to 2 liters at 1 atm

300 K • Or the pressure will increase to 2 atm. • Or someplace in between 600 K

Ideal Gases u u In this section, we are going to assume the gases behave ideally Does not really exist • makes the math easier • close approximation. Assume particles have no volume Assume no attractive forces between molecules

Ideal Gases u u There are no gases for which this is true. Real gases behave this way at high temperature and low pressure.

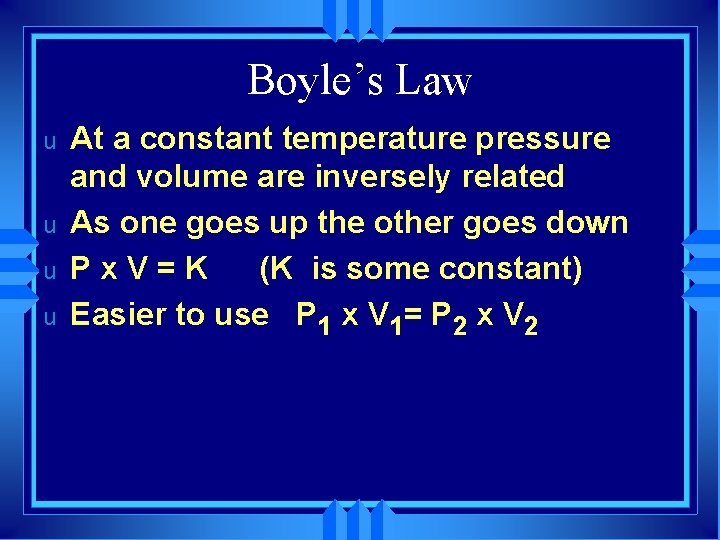
Boyle’s Law u u At a constant temperature pressure and volume are inversely related As one goes up the other goes down Px. V=K (K is some constant) Easier to use P 1 x V 1= P 2 x V 2

P V

Example 1 u A balloon is filled with 25 L of air at 1. 0 atm pressure. If the pressure is changed to 1. 5 atm what is the new volume?

Example 2 u A balloon is filled with 73 L of air at 1. 3 atm pressure. What pressure is needed to change to volume to 43 L?

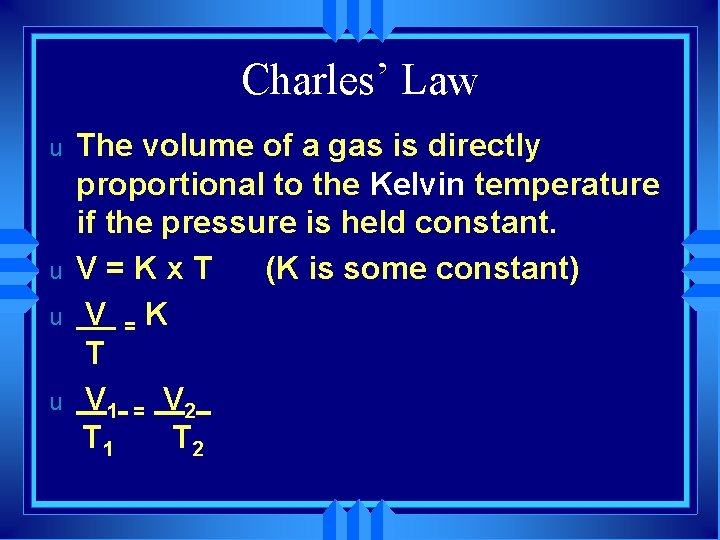
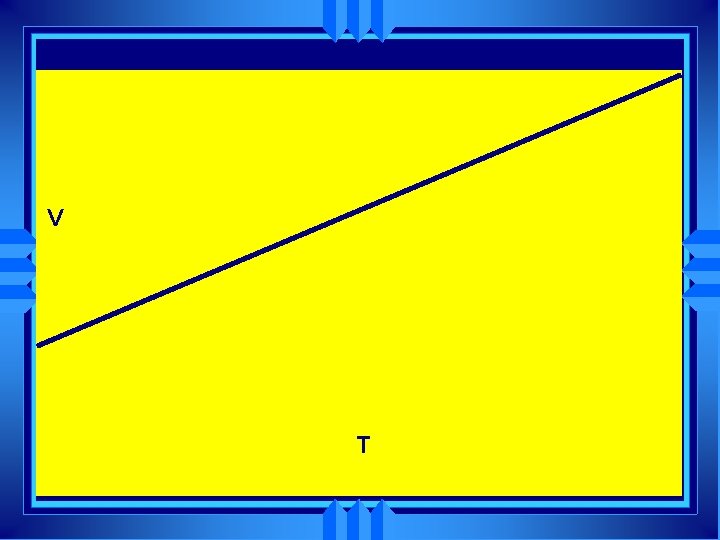
Charles’ Law u u The volume of a gas is directly proportional to the Kelvin temperature if the pressure is held constant. V=Kx. T (K is some constant) V =K T V 1 = V 2 T 1 T 2

V T

Example 3 u What is the temperature of a gas that is expanded from 2. 5 L at 25ºC to 4. 1 L at constant pressure.

Example 4 u What is the final volume of a gas that starts at 8. 3 L and 17ºC and is heated to 96ºC?

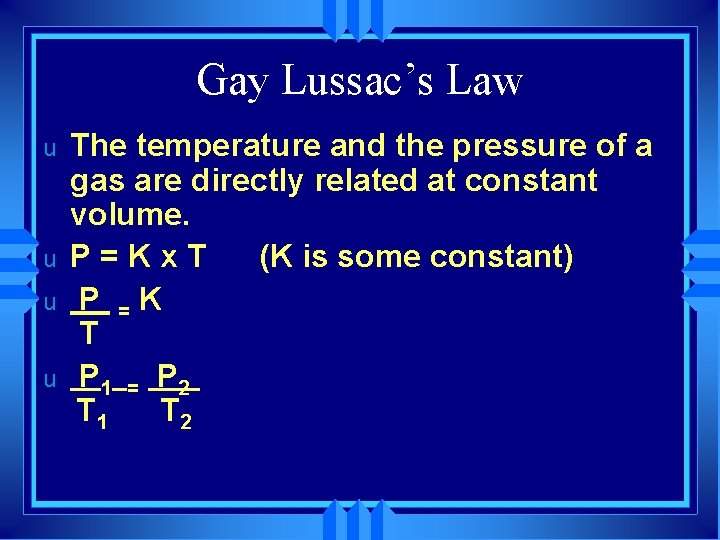
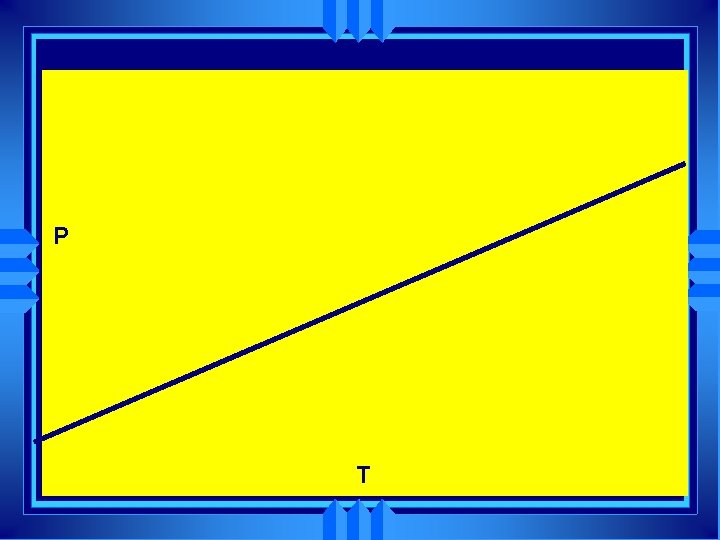
Gay Lussac’s Law u u The temperature and the pressure of a gas are directly related at constant volume. P=Kx. T (K is some constant) P =K T P 1 = P 2 T 1 T 2

P T

Example 5 u u What is the pressure inside a 0. 250 L can of deodorant that starts at 25ºC and 1. 2 atm if the temperature is raised to 100ºC? Example 6 At what temperature will the can above have a pressure of 2. 2 atm?

Answers 1. 2. 3. 4. 5. 6. 17 L 2. 2 atm 489 K or 215ºC 11 L 0. 31 L 546 K or 273ºC (270ºC )
 Kuei honors chemistry
Kuei honors chemistry Chemistry unit 4 review answers
Chemistry unit 4 review answers Honors chemistry summer assignment
Honors chemistry summer assignment Honors chemistry
Honors chemistry Ap chemistry gas laws
Ap chemistry gas laws Ideal gas equation
Ideal gas equation Charles de secondat
Charles de secondat Creighton university honors program
Creighton university honors program James scholar uiuc aces
James scholar uiuc aces Do no. 36, s. 2016
Do no. 36, s. 2016 Ucsb frap
Ucsb frap Honors physics semester 2 review
Honors physics semester 2 review Honors biology ecology test
Honors biology ecology test Uncle sam's toolbox honors
Uncle sam's toolbox honors Honors geometry quadrilaterals test
Honors geometry quadrilaterals test Honors earth science
Honors earth science Mrs blueprint
Mrs blueprint Hilton honors military program
Hilton honors military program Honors project
Honors project Honors biology properties of water lab
Honors biology properties of water lab Honors geometry parallel lines and transversals worksheet
Honors geometry parallel lines and transversals worksheet Tulane honors program
Tulane honors program Honors geometry chapter 3
Honors geometry chapter 3 Precalculus honors chapter 1 test
Precalculus honors chapter 1 test Honors physics projectile motion test
Honors physics projectile motion test Honors biology unit 4 test
Honors biology unit 4 test Smc scholars classes
Smc scholars classes Used so often as to lack freshness or originality
Used so often as to lack freshness or originality Math 3 honors
Math 3 honors Honors its atomic
Honors its atomic Ft pierce central
Ft pierce central Mahurin honors college
Mahurin honors college