Gas Laws Boyles and Charles Gas Laws Describes

Gas Laws: Boyle’s and Charles’
Gas Laws Describes the relationship between variables associated with gases Volume (V) Temperature (T) Pressure (P) Concentration/amount of gas (n) **Two variables change in relation to each other while the remaining variables are held constant.

Gas Laws 1) Boyle’s Law 2) Charle’s Law 3) Gay-Lussac Law 4) Avagadro’s Law 5) Combined Gas Law 6) Ideal Gas Law
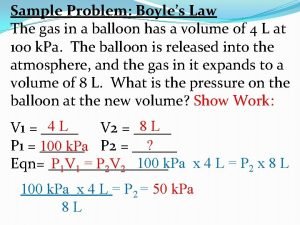
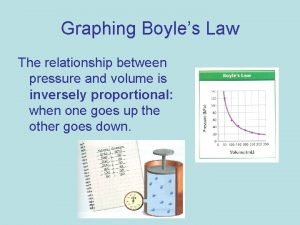
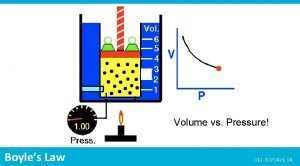
Boyle’s Law Pressure is INVERSELY proportional to the volume of the gas. Temperature and particle amount constant P , V Pressure and volume relationship P 1 V 1 = P 2 V 2 Remember to keep units the same.

Example 1 What would happen to the volume of a balloon filled with 0. 357 L of H 2 gas collected at 741. 3 mm. Hg if the atmospheric pressure increased to 758. 1 mm. Hg? (temperature is constant)

Example 2: What is the volume of a scuba tank if it takes 2000 L of air collected at 1 atm to fill the tank to a pressure of 150 atm? Temperature is constant

Example 3: Calculate the volume of a balloon that could be filled at 1. 00 atm with helium in a 2. 50 L compressed gas cylinder in which the pressure is 200 atm at 25°C.
Charle’s Law Temperature and volume relationship The volume of a gas is DIRECTLY proportional to the temperature T , V V 1 = V 2 SOOO T 1 = T 2 T 1 V 2 = T 2 V 1 Remember to keep units the same. Temperature MUST be in Kelvin

Example 1: A sample of O 2 gas with a volume of 0. 357 L was collected at 21°C. Calculate the volume of the gas when it is cooled to 0°C if the pressure remains constant.

Example 2: How hot will a 2. 3 L balloon have to get to expand to a volume of 400 L? Assume the initial balloon temperature is 25°C.

Homework Study for Kinetics Quiz.
- Slides: 11