Introduction to Gas Laws Boyles Law Charles Law

Introduction to Gas Laws Boyle’s Law Charles’ Law and Gay-Lussac’s Laws
Kelvin Temperature Scale A temperature scale called the Kelvin scale is used when dealing with gases. This scale uses – 273 o. C as the zero point. On this scale there is a point called absolute zero that is theoretical point when the gas would have no volume. (This does not happen!) To convert from degrees Celsius to Kelvins: TK = To. C + 273
Boyle’s Law Pressure and Volume This law states that at a constant temperature, as the pressure on a gas increase, the volume of the gas decreases proportionally, the volume and pressure of a gas are inversely proportional.

This law can be written as: P 1 V 1 = P 2 V 2 Where: P 1 is the initial pressure V 1 is the initial volume P 2 is the final pressure V 2 is the final volume Robert Boyle (1627 -1691)

Example #1: A 2. 0 L party balloon at 98 k. Pa is taken to the top of a mountain where the pressure is 75 k. Pa. Assume the temperature is the same. What is the new volume of the balloon? P 1 V 1= P 2 V 2 (98 k. Pa)(2. 0 L) = (75 k. Pa) V 2 2. 6 L = V 2

Charles’ Law Volume and Temperature This law states that the volume of a gas varies directly with its temperature in Kelvin, if the pressure and amount of gas are constant.

This law can be written as: Where: V 1 is the initial volume T 1 is the initial temperature V 2 is the final volume T 2 is the final temperature Temperature must be in Kelvin
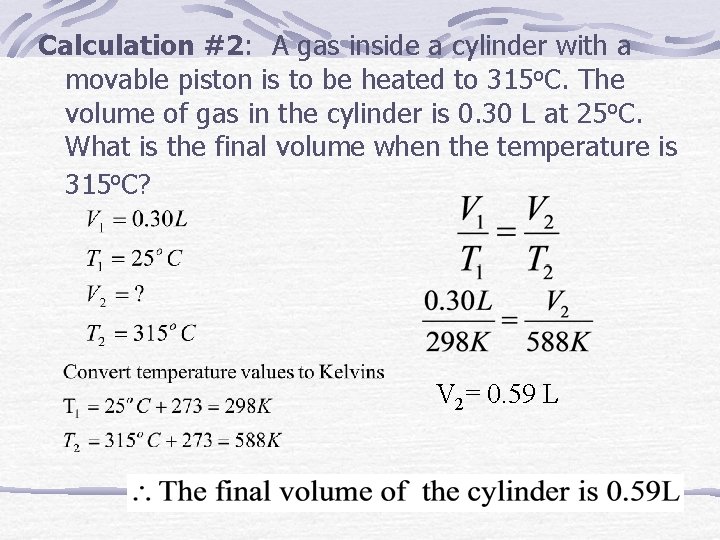
Calculation #2: A gas inside a cylinder with a movable piston is to be heated to 315 o. C. The volume of gas in the cylinder is 0. 30 L at 25 o. C. What is the final volume when the temperature is 315 o. C? V 2= 0. 59 L
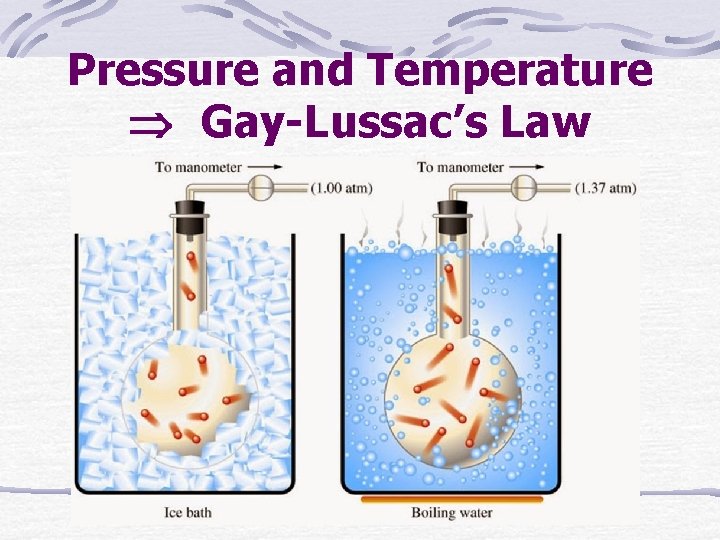
Pressure and Temperature Gay-Lussac’s Law

This law states that the pressure of any gas is directly proportional to its Kelvin temperature. For this law, there must be a constant volume and a fixed mass of gas.

As the temperature decreases, gas particles move more slowly and collide less frequently with each other, causing pressure to decrease. When temperature increases, the gas particles move faster, causing more collisions and increasing the pressure.

Joseph Louis Gay. Lussac (1778 – 1850)
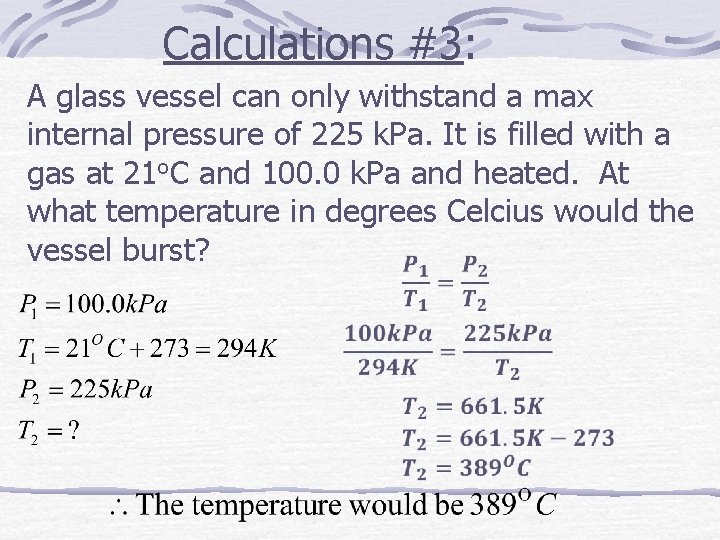
Calculations #3: A glass vessel can only withstand a max internal pressure of 225 k. Pa. It is filled with a gas at 21 o. C and 100. 0 k. Pa and heated. At what temperature in degrees Celcius would the vessel burst?

- Slides: 14