Gas Laws The Gas Laws are mathematical n















- Slides: 24

Gas Laws

The Gas Laws are mathematical n The gas laws will describe HOW gases behave. n Gas behavior can be predicted by theory. n The amount of change can be calculated with mathematical equations. n You need to know both of these: theory, and the math

Variables for Gas Laws P=pressure n V=volume n T=temperature n n=number of moles n R=gas constant n

Units for Gas Laws atm, torr, k. Pa) n V=volume (Liters or L) n T=temperature (Kelvin or K) n n=number of moles (mol) n P=pressure (mm. Hg, n R=gas constant (atm x L/mol x K)

Robert Boyle (1627 -1691)

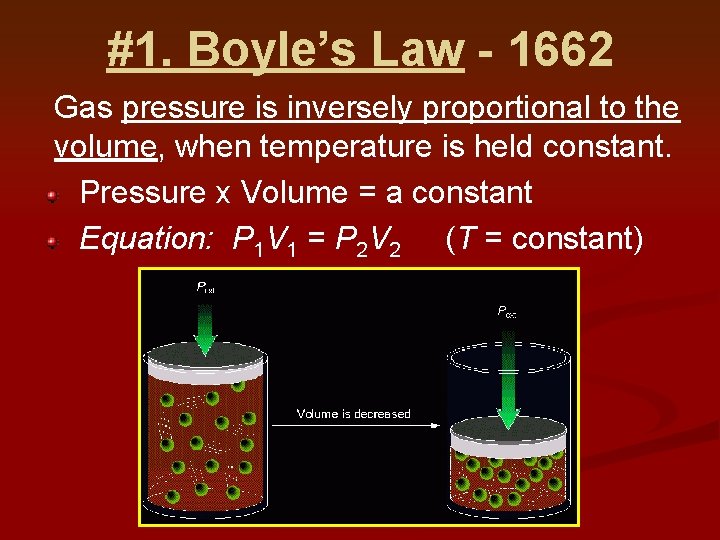
#1. Boyle’s Law - 1662 Gas pressure is inversely proportional to the volume, when temperature is held constant. Pressure x Volume = a constant Equation: P 1 V 1 = P 2 V 2 (T = constant)

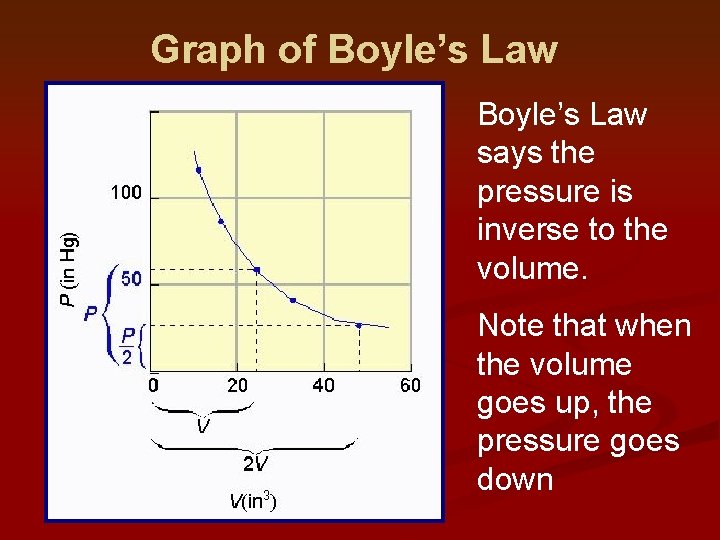
Graph of Boyle’s Law says the pressure is inverse to the volume. Note that when the volume goes up, the pressure goes down

Jacques Charles (1746 -1823)

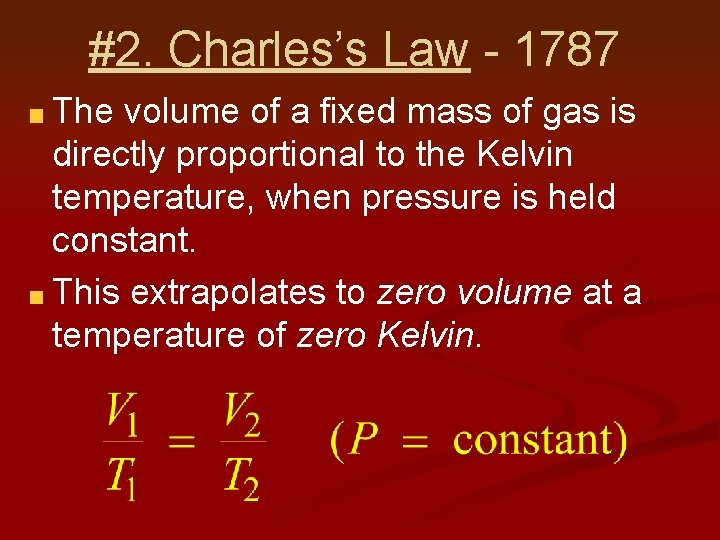
#2. Charles’s Law - 1787 The volume of a fixed mass of gas is directly proportional to the Kelvin temperature, when pressure is held constant. This extrapolates to zero volume at a temperature of zero Kelvin.

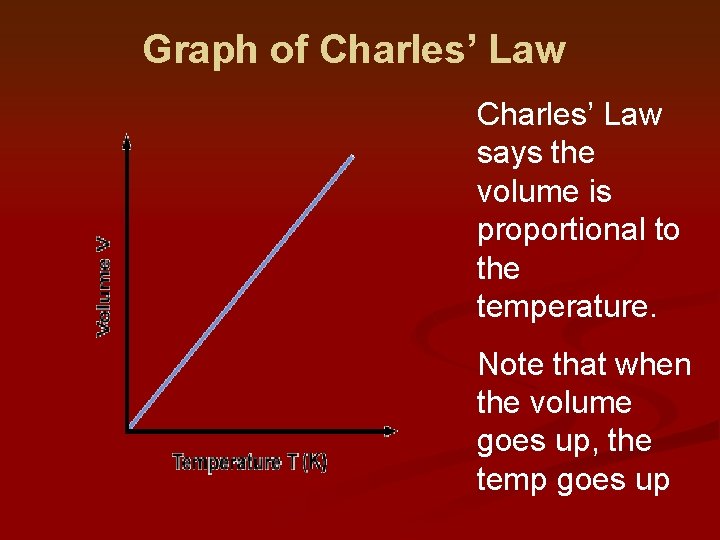
Graph of Charles’ Law says the volume is proportional to the temperature. Note that when the volume goes up, the temp goes up

Converting Celsius to Kelvin • Gas law problems involving temperature will always require that the temperature be in Kelvin. (Remember that no degree sign is shown with the kelvin scale. ) Kelvin = C + 273 and °C = Kelvin - 273

Converting pressure • 1 atm = 760 mm. Hg • 1 atm = 760 torr • 1 atm = 101. 3 k. Pa

Converting grams to moles • 1 mole = molar mass in grams

Joseph Louis Gay-Lussac (1778 – 1850)

#3. Gay-Lussac’s Law - 1802 • The pressure and Kelvin temperature of a gas are directly proportional, provided that the volume remains constant. • How does a pressure cooker affect the time needed to cook food?

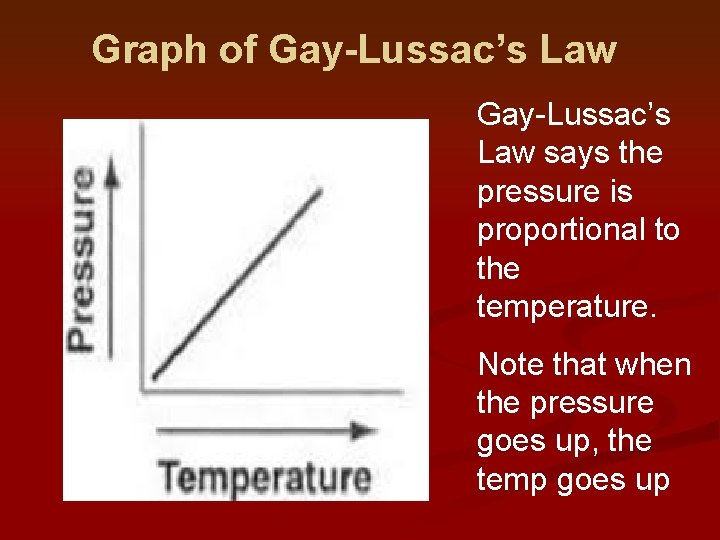
Graph of Gay-Lussac’s Law says the pressure is proportional to the temperature. Note that when the pressure goes up, the temp goes up

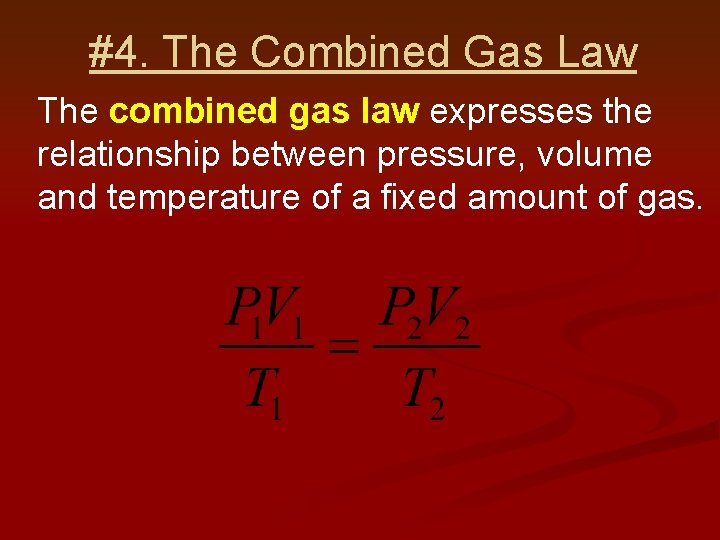
#4. The Combined Gas Law The combined gas law expresses the relationship between pressure, volume and temperature of a fixed amount of gas.

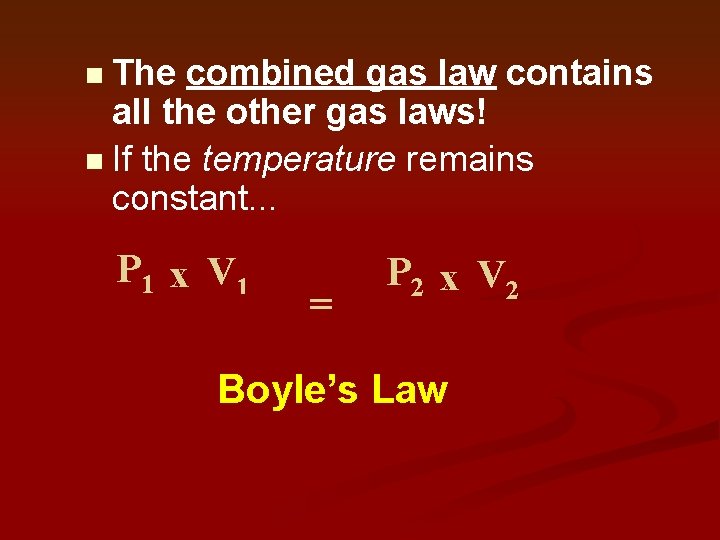
The combined gas law contains all the other gas laws! n If the temperature remains constant. . . n P 1 x V 1 T 1 = P 2 x V 2 T 2 Boyle’s Law

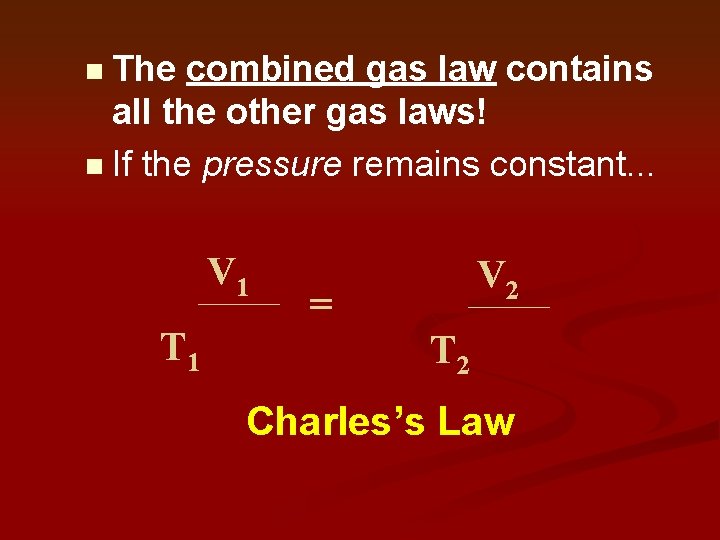
The combined gas law contains all the other gas laws! n If the pressure remains constant. . . n P 1 x V 1 T 1 = P 2 x V 2 T 2 Charles’s Law

u. The combined gas law contains all the other gas laws! u. If the volume remains constant. . . P 1 x V 1 T 1 = P 2 x V 2 T 2 Gay-Lussac’s Law

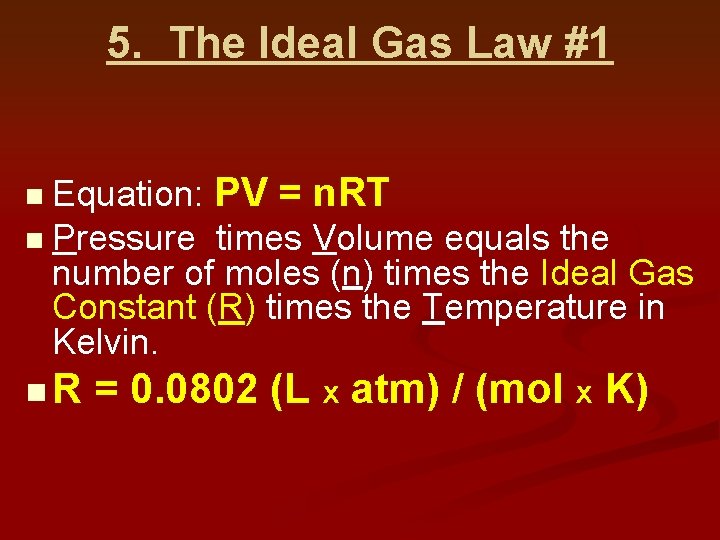
5. The Ideal Gas Law #1 Equation: PV = n. RT n Pressure times Volume equals the number of moles (n) times the Ideal Gas Constant (R) times the Temperature in Kelvin. n n. R = 0. 0802 (L x atm) / (mol x K)

The Ideal Gas Law n We now have a new way to count moles (the amount of matter), by measuring T, P, and V. P x V n= Rx. T

Ideal Gases We are going to assume the gases behave “ideally”- in other words, they obey the Gas Laws under all conditions of temperature and pressure n An ideal gas does not really exist, but it makes the math easier and is a close approximation. n Particles have no volume? Wrong! n No attractive forces? Wrong! n

Ideal Gases n There are no gases for which this is true (acting “ideal”); however, n Real gases behave this way at a) high temperature, and b) low pressure. n. Because at these conditions, a gas will stay a gas!
 Insidan region jh
Insidan region jh Mathematical vs non mathematical economics
Mathematical vs non mathematical economics Laws of mathematical logic
Laws of mathematical logic Useless laws weaken the necessary laws
Useless laws weaken the necessary laws Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Gấu đi như thế nào
Gấu đi như thế nào Thẻ vin
Thẻ vin Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Thể thơ truyền thống
Thể thơ truyền thống Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Slidetodoc
Slidetodoc Phép trừ bù
Phép trừ bù Lời thề hippocrates
Lời thề hippocrates Glasgow thang điểm
Glasgow thang điểm đại từ thay thế
đại từ thay thế Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể