General Chemistry Gas Laws More Gas Laws Even























































- Slides: 60



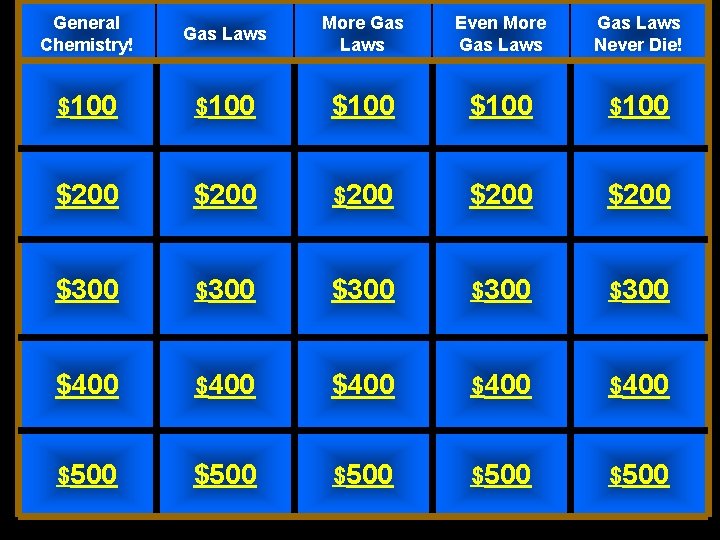
General Chemistry! Gas Laws More Gas Laws Even More Gas Laws Never Die! $100 $100 $200 $200 $300 $300 $400 $400 $500 $500

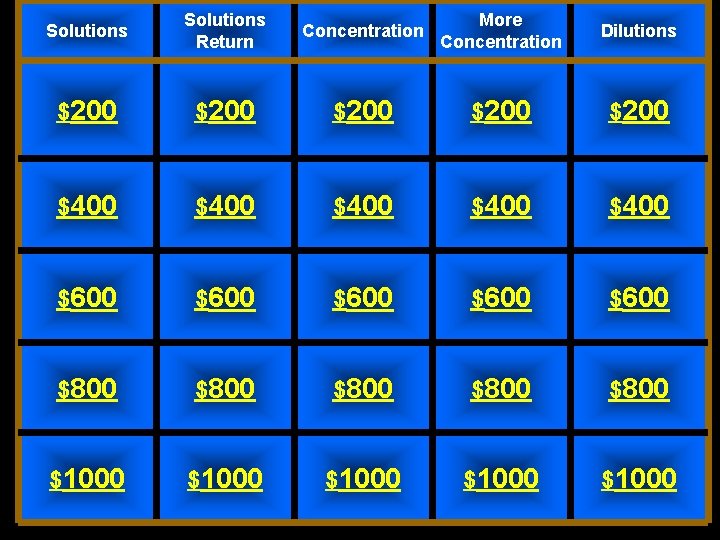
Solutions Return Concentration More Concentration Dilutions $200 $200 $400 $400 $600 $600 $800 $800 $1000 $1000

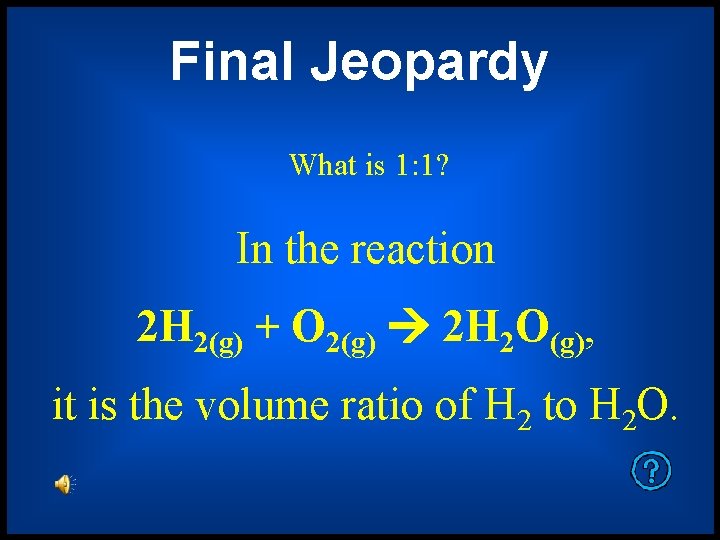
Final Jeopardy Gas Laws

Final Jeopardy What is 1: 1? In the reaction 2 H 2(g) + O 2(g) 2 H 2 O(g), it is the volume ratio of H 2 to H 2 O.

$100 What is the mole ratio? It is the ratio obtained from the coefficients in a balanced chemical equation. General Chemistry!

$200 What is 0. 0555 mol H 2 O? The number of moles in 1. 000 g of water vapor. General Chemistry!

$300 What are 0 C (or 273 K) and 1 atm (or 101. 3 k. Pa)? The values for temperature and pressure at STP (Standard Temperature and Pressure). General Chemistry!

$400 What is a synthesis reaction? A type of reaction in which two simple substances combine to form a new, more complex substance. General Chemistry!

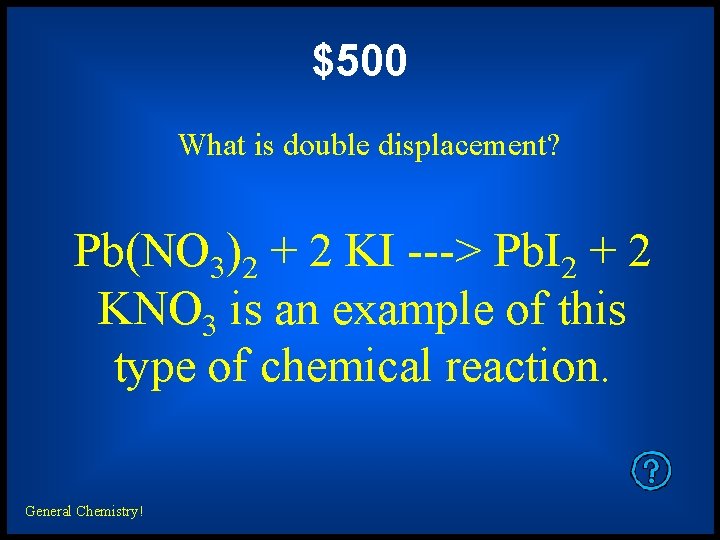
$500 What is double displacement? Pb(NO 3)2 + 2 KI ---> Pb. I 2 + 2 KNO 3 is an example of this type of chemical reaction. General Chemistry!

$100 What is Charles’s Law? This gas law relates temperature and volume at a constant pressure. Gas Laws

$200 What are temperature and pressure? Gay-Lussac’s Law relates these two variables. Gas Laws

$300 What is Boyle’s Law? This gas law holds temperature constant. Gas Laws

$400 What is the Combined Gas Law? This gas law allows for temperature, pressure, and volume to vary simultaneously. Gas Laws

$500 What is P 1 V 1 = P 2 V 2? This is the equation for Boyle’s Law. Gas Laws

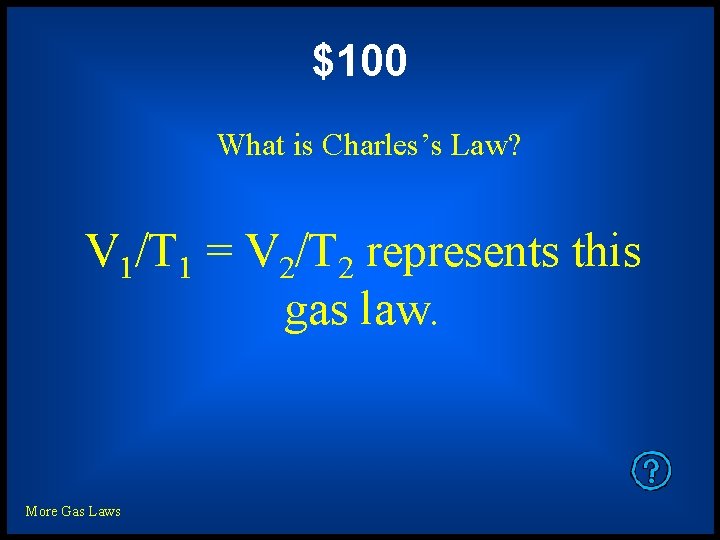
$100 What is Charles’s Law? V 1/T 1 = V 2/T 2 represents this gas law. More Gas Laws

$200 What is increase? In Gay-Lussac’s Law, the temperature will do this as the pressure increases. More Gas Laws

$300 What is halve? At a constant temperature, doubling the volume of a gas will cause the pressure to do this. More Gas Laws

$400 What is double V 1? The value of T 2 if the initial volume is doubled. More Gas Laws

$500 What are Charles’s Law and Gay-Lussac’s Law? These two gas laws exhibit direct relationships. More Gas Laws

$100 What is inverse? A relationship in which one factor increases as the other decreases is called this. Even More Gas Laws

$200 What is Dalton’s Law of Partial Pressures? This law states that the total pressure is equal to the sum of the pressures of each component in a mixture of gases. Even More Gas Laws

$300 What are moles and volume? Avogadro’s Law relates these two variables. Even More Gas Laws

$400 What is 47. 04 L? At STP, 2. 1 moles of CH 4 will occupy this much space. Even More Gas Laws

$500 What is 84. 3 k. Pa? The pressure of nitrogen in a mixture with oxygen when the total pressure is 98. 4 k. Pa and the pressure of oxygen is 14. 1 k. Pa. Even More Gas Laws

$100 What are moles? It is the quantity represented by “n” in the Ideal Gas Law equation. Gas Laws Never Die!

$200 What is 0. 082 L atm/K mol? The Ideal Gas Constant (R) has this value when working with pressures in atmospheres. Gas Laws Never Die!

$300 What is collisions of particles with the walls of the container? According to KMT, pressure is the result of this. Gas Laws Never Die!

$400 What is the average kinetic energy (speed) of the particles? According to KMT, temperature is the result of this. Gas Laws Never Die!

$500 What are extremely low temperatures or extremely high pressures? Real gases behave like ideal gases except under these two extreme conditions. Gas Laws Never Die!

$200 What is homogeneous? A mixture in which all component are evenly mixed throughout Solutions

$400 What is heterogeneous? A mixture that is not evenly mixed throughout. Solutions

$600 What is solute? That which is dissolved in a solution. Solutions

$800 What is solvent? The part of the solution that the other part dissolves into. Solutions

$1000 What is aqueous? A solution in which water is the solvent. Solutions

$200 What is immiscible? Liquids that do not dissolve in one another. Solutions Return

$400 What is solubility? The degree to which a substance will dissolve in a given solvent. Solutions Return

$600 What is solubility curve? A graph that shows the amount of solute that will dissolve in a solvent across a wide range of temperatures. Solutions Return

$800 What is an electrolyte? An aqueous solution that conducts electricity. Solutions Return

$1000 What is a serial dilution? The step-wise process of making a very dilute solution from a very concentrated one. Solutions Return

$200 What is saturated? A solution that holds all the solute it can under the given conditions. Concentration

$400 What is unsaturated? A solution that holds less solute than it can under the given conditions. Concentration

$600 What is supersaturated? A solution that holds more solute than it normally would under the given conditions. Concentration

$800 What is concentrated? A solution in which is large amount of solute is held relative to the amount of solvent. Concentration

$1000 What is dilute? A solution in which a very small amount of solute is held relative to the amount of solvent. Concentration

$200 What is molarity? A measure of the moles of solute per liter of solution. More Concentration

$400 What is molality? A measure of the moles of solute per kilogram of solvent. More Concentration

$600 What is 1. 1? The molarity if 4. 4 moles of solute are dissolved in 4 liters of solution. More Concentration

$800 What is 6? The number of moles of solvent used to make a 1. 5 molal solution in 4 kg of solvent. More Concentration

$1000 What is 1? The number of moles of solvent used to make a 0. 5 molar solution of 2 liters. More Concentration

$200 What are non-polar? These types of solvents will dissolve non-polar solutes. Dilutions

$400 What is 1. 500 M? The new molarity if I add 350. 0 m. L of distilled water to 50. 00 m. L of 12 M HCl. Dilutions

$600 What is 3. 500 M? The molarity of a solution if I dissolve 42. 00 g of sodium hydroxide into distilled to produce a total volume of 300. 0 m. L. Dilutions

$800 What is 0. 72 M? The new concentration (M) when I add 30. 0 m. L of a 6. 00 M KCl solution to 220. 0 m. L of distilled water. Dilutions

$1000 What is 3. 42 M? The concentration of a solution if I dissolve 100. 0 g of Na. Cl into distilled water to produce a total volume of 500 m. L. Dilutions

Daily Double f f

Daily Double c v

Daily Double f f

The Jeopardy champion!