Gas Laws Introduction to Gas Laws Key Terms







- Slides: 11

Gas Laws! Introduction to Gas Laws.

Key Terms Pressure: the amount of force per unit area of surface Newton: the SI unit force Pascal: the SI unit of pressure

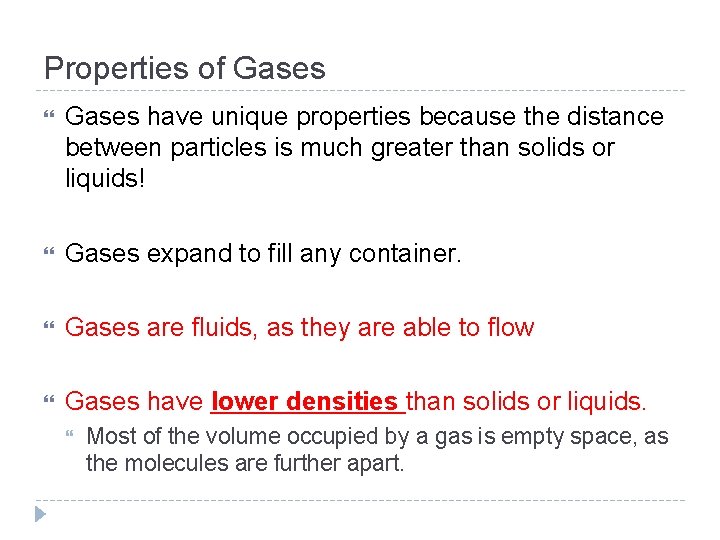
Properties of Gases have unique properties because the distance between particles is much greater than solids or liquids! Gases expand to fill any container. Gases are fluids, as they are able to flow Gases have lower densities than solids or liquids. Most of the volume occupied by a gas is empty space, as the molecules are further apart.

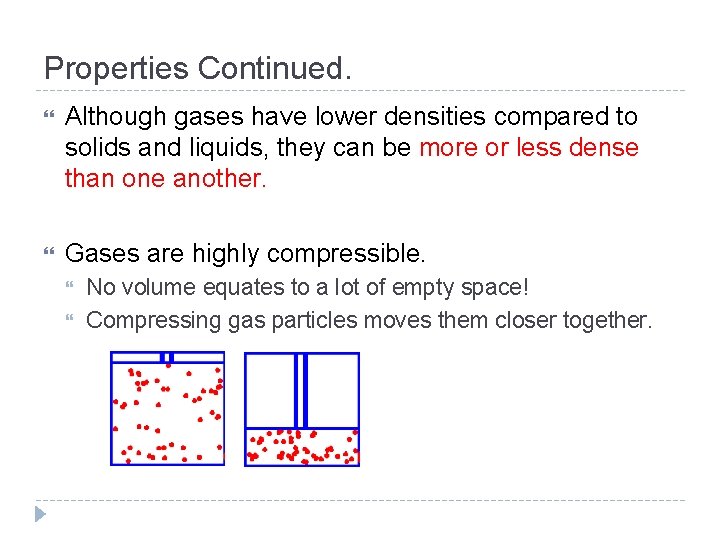
Properties Continued. Although gases have lower densities compared to solids and liquids, they can be more or less dense than one another. Gases are highly compressible. No volume equates to a lot of empty space! Compressing gas particles moves them closer together.

Air Pressure Collisions with gas molecules and with the surface of the earth cause air pressure. Less dense gases are at higher altitudes. Less dense air exerts less pressure, as their molecules collide less often. Your ears pop when you are in an airplane, as the air inside your ears changes to match the same pressure as the outside air.

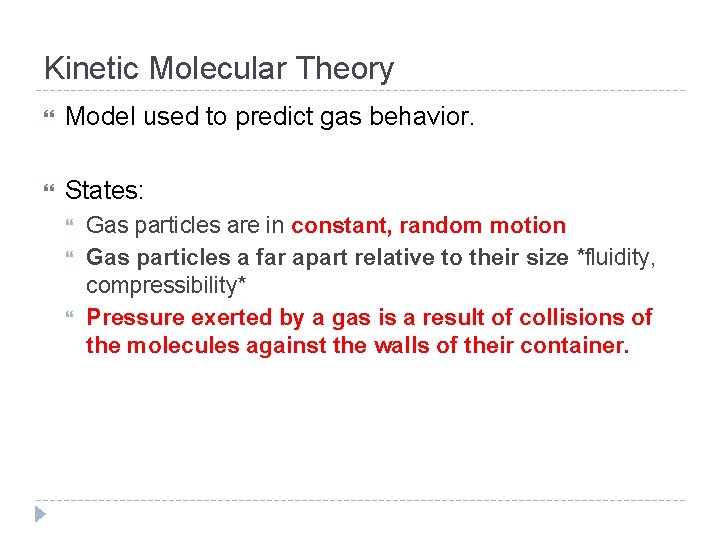
Kinetic Molecular Theory Model used to predict gas behavior. States: Gas particles are in constant, random motion Gas particles a far apart relative to their size *fluidity, compressibility* Pressure exerted by a gas is a result of collisions of the molecules against the walls of their container.

Pressure n. Which shoes create the most pressure?

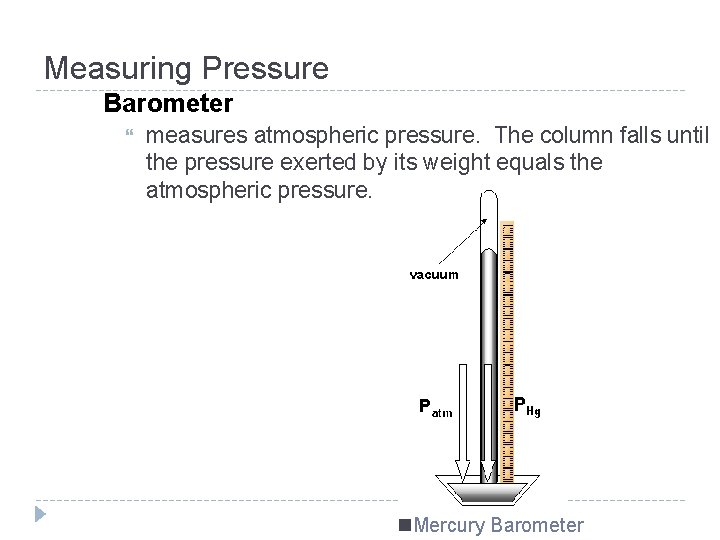
Measuring Pressure Barometer measures atmospheric pressure. The column falls until the pressure exerted by its weight equals the atmospheric pressure. n. Mercury Barometer

Pressure Units KEY UNITS AT SEA LEVEL 101. 325 k. Pa (kilopascal) 1 atm 760 mm Hg 760 torr 14. 7 psi

Converting Pressure Units - Practice Carbon dioxide has a pressure of 72. 7 atm. What is this value in pascals? The vapor pressure of water at 50ºC is 12. 33 k. Pa. What is this value in mm Hg? Convert 100 k. Pa to atmospheres. A tire is inflated to a 44. 7 psi. Calculate the total pressure in pascals.

Standard Temperature and Pressure At sea level, the atmosphere keeps mercury in a barometer at 760 mm. Hg or 1 atmosphere. Standard Temperature & Pressure : 0ºC and 1 atmosphere (atm)
 Ideal gas law examples
Ideal gas law examples Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Like terms and unlike terms in polynomials
Like terms and unlike terms in polynomials Combine like terms
Combine like terms Key partner business model canvas
Key partner business model canvas Key partners
Key partners Useless laws weaken the necessary laws
Useless laws weaken the necessary laws Introduction to construction drawings trade terms
Introduction to construction drawings trade terms Whats your name bob
Whats your name bob An introduction to cost terms and purposes
An introduction to cost terms and purposes An introduction to cost terms and purposes
An introduction to cost terms and purposes Match the following terms with their definitions:
Match the following terms with their definitions: