Lesson 5 3 Gas Laws Chemistry 1 Honors





- Slides: 9

Lesson 5. 3 – Gas Laws Chemistry 1 Honors Dr. J. Venables Northwestern High School

Gas Laws • • • Boyle’s Law: P and V, at constant T Charles’ Law: V and T at constant P Gay-Lussac’s Law: P and T at constant V Combined Gas Law: P, V and T Ideal Gas Law: Moles join the Party (Thanks Avogadro).

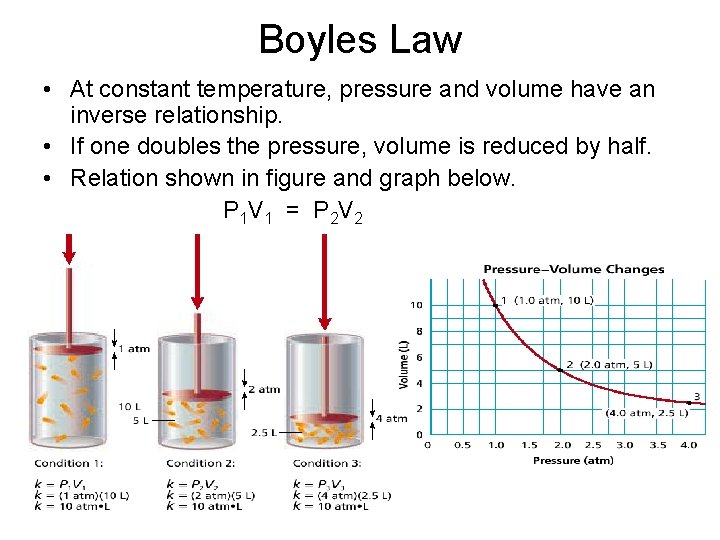
Boyles Law • At constant temperature, pressure and volume have an inverse relationship. • If one doubles the pressure, volume is reduced by half. • Relation shown in figure and graph below. P 1 V 1 = P 2 V 2

Practice 1. A sample of Ar gas occupies a volume of 1. 05 L at a pressure of 128 k. Pa. What is the pressure of the same gas sample if the volume is reduced to 0. 79 L? 2. A sample of O 2 gas occupies a volume of 225 m. L at a pressure of 0. 87 atm. What volume will the sample occupy at a pressure of 0. 37 atm?

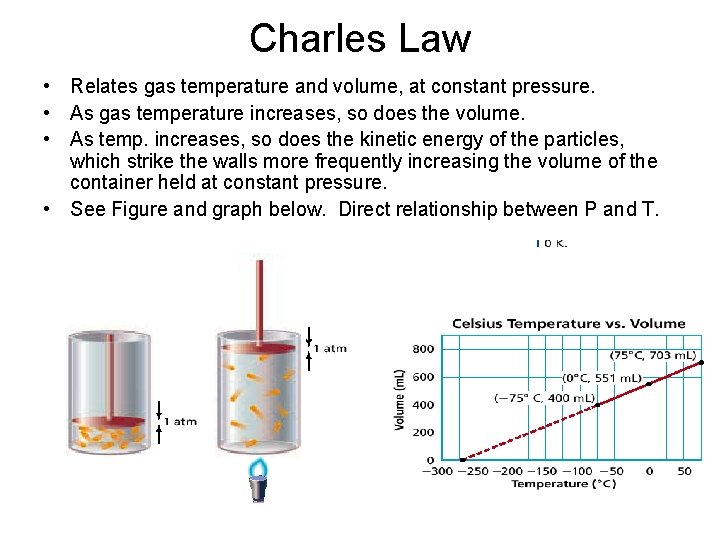
Charles Law • Relates gas temperature and volume, at constant pressure. • As gas temperature increases, so does the volume. • As temp. increases, so does the kinetic energy of the particles, which strike the walls more frequently increasing the volume of the container held at constant pressure. • See Figure and graph below. Direct relationship between P and T.

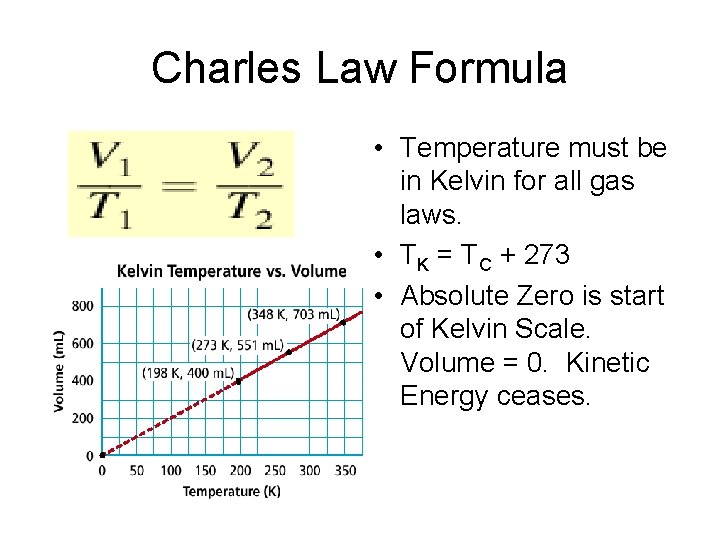
Charles Law Formula • Temperature must be in Kelvin for all gas laws. • TK = TC + 273 • Absolute Zero is start of Kelvin Scale. Volume = 0. Kinetic Energy ceases.

Practice 1. A gas sample occupies 1. 66 L at 257 K. What volume will it occupy at 395 K? 2. A gas sample occupies 75. 8 m. L at 25°C. At what temperature will the volume of the sample be 100. 0 m. L?

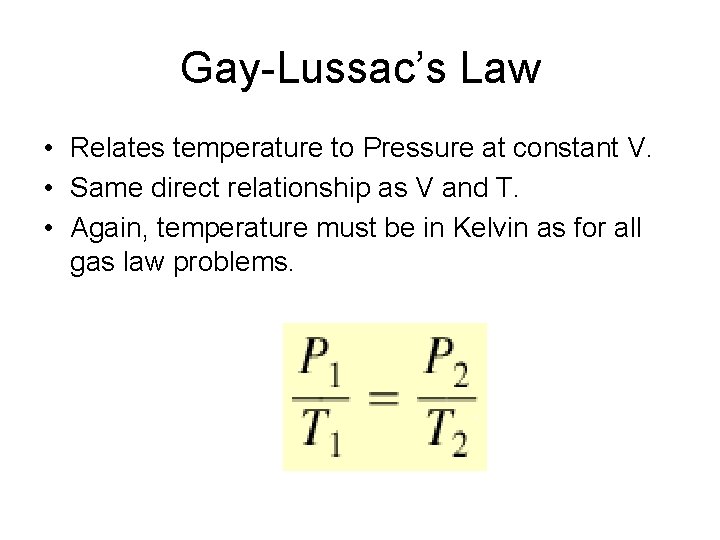
Gay-Lussac’s Law • Relates temperature to Pressure at constant V. • Same direct relationship as V and T. • Again, temperature must be in Kelvin as for all gas law problems.

Practice • A confined gas in a rigid container has a pressure of 855 k. Pa at 30°C. At what temperature will the pressure in the container reach 1500 k. Pa?