Homework 8 due Today Homework 9 posted and







- Slides: 16

Homework 8: due Today Homework 9 posted and due Monday 4/2

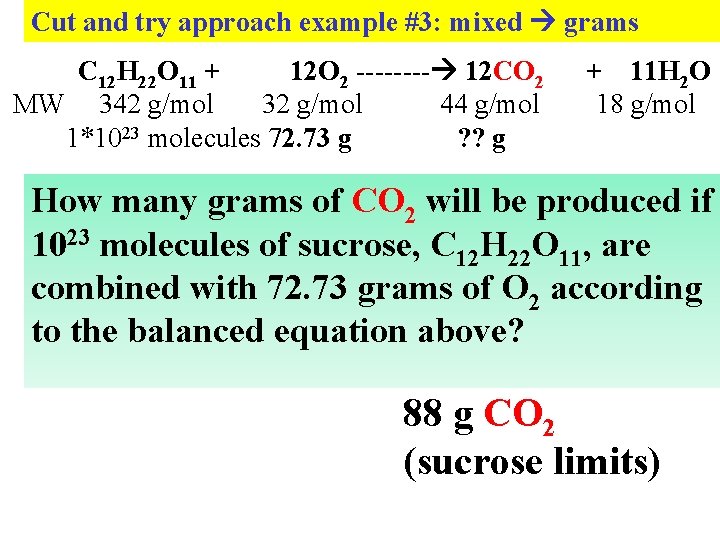
Cut and try approach example #3: mixed grams C 12 H 22 O 11 + 12 O 2 ---- 12 CO 2 MW 342 g/mol 32 g/mol 44 g/mol 1*1023 molecules 72. 73 g ? ? g + 11 H 2 O 18 g/mol How many grams of CO 2 will be produced if 1023 molecules of sucrose, C 12 H 22 O 11, are combined with 72. 73 grams of O 2 according to the balanced equation above? 88 g CO 2 (sucrose limits)

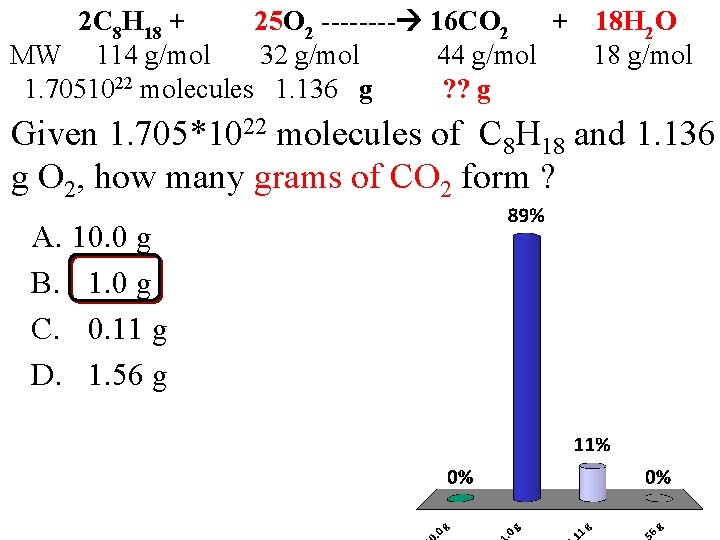
2 C 8 H 18 + 25 O 2 ---- 16 CO 2 + 18 H 2 O MW 114 g/mol 32 g/mol 44 g/mol 18 g/mol 1. 7051022 molecules 1. 136 g ? ? g Given 1. 705*1022 molecules of C 8 H 18 and 1. 136 g O 2, how many grams of CO 2 form ? A. 10. 0 g B. 1. 0 g C. 0. 11 g D. 1. 56 g

Need more practice ? ? ?

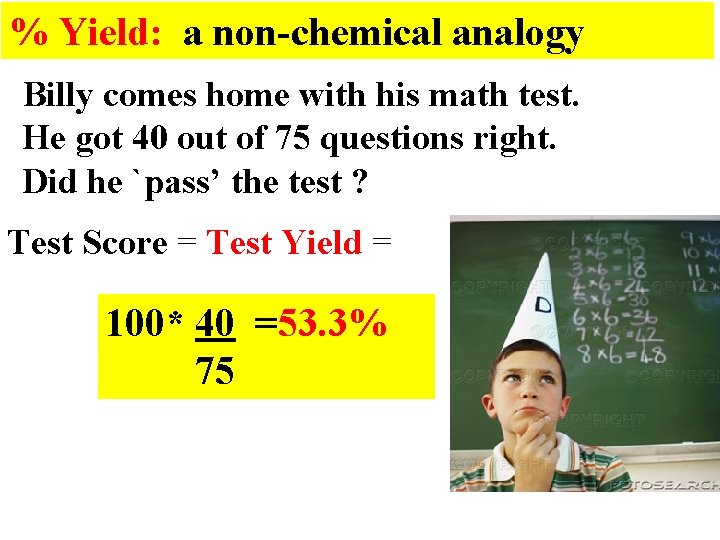
% Yield: a non-chemical analogy Billy comes home with his math test. He got 40 out of 75 questions right. Did he `pass’ the test ? Test Score = Test Yield = 100* 40 =53. 3% 75

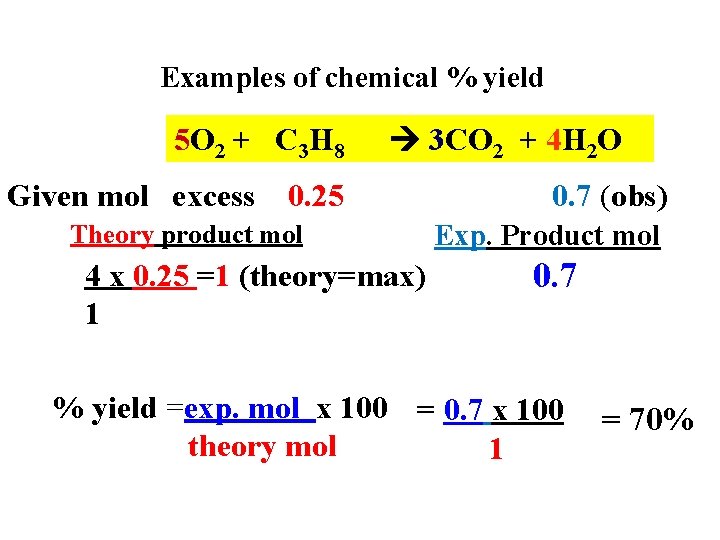
Examples of chemical % yield 5 O 2 + C 3 H 8 Given mol excess 3 CO 2 + 4 H 2 O 0. 25 Theory product mol 4 x 0. 25 =1 (theory=max) 1 0. 7 (obs) Exp. Product mol 0. 7 % yield =exp. mol x 100 = 0. 7 x 100 theory mol 1 = 70%

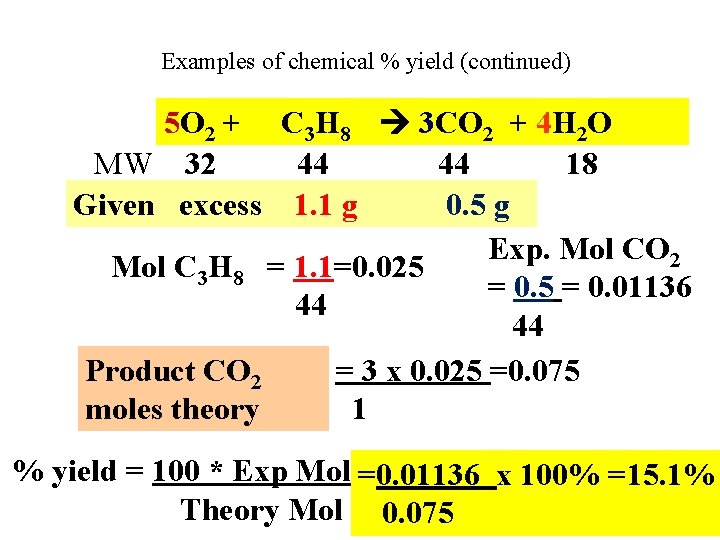
Examples of chemical % yield (continued) 5 O 2 + C 3 H 8 3 CO 2 + 4 H 2 O MW 32 44 44 18 Given excess 1. 1 g 0. 5 g Exp. Mol CO 2 Mol C 3 H 8 = 1. 1=0. 025 = 0. 01136 44 44 Product CO 2 = 3 x 0. 025 =0. 075 moles theory 1 % yield = 100 * Exp Mol =0. 01136 x 100% =15. 1% Theory Mol 0. 075

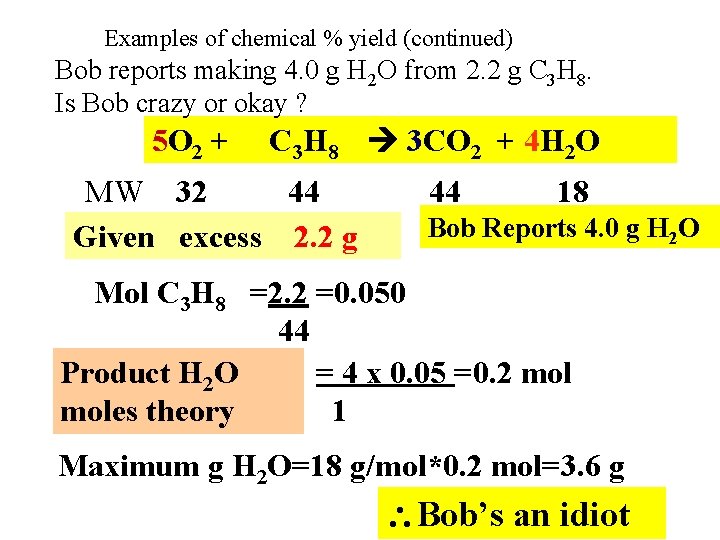
Examples of chemical % yield (continued) Bob reports making 4. 0 g H 2 O from 2. 2 g C 3 H 8. Is Bob crazy or okay ? 5 O 2 + C 3 H 8 3 CO 2 + 4 H 2 O MW 32 44 Given excess 2. 2 g 44 18 Bob Reports 4. 0 g H 2 O Mol C 3 H 8 =2. 2 =0. 050 44 Product H 2 O = 4 x 0. 05 =0. 2 moles theory 1 Maximum g H 2 O=18 g/mol*0. 2 mol=3. 6 g Bob’s an idiot

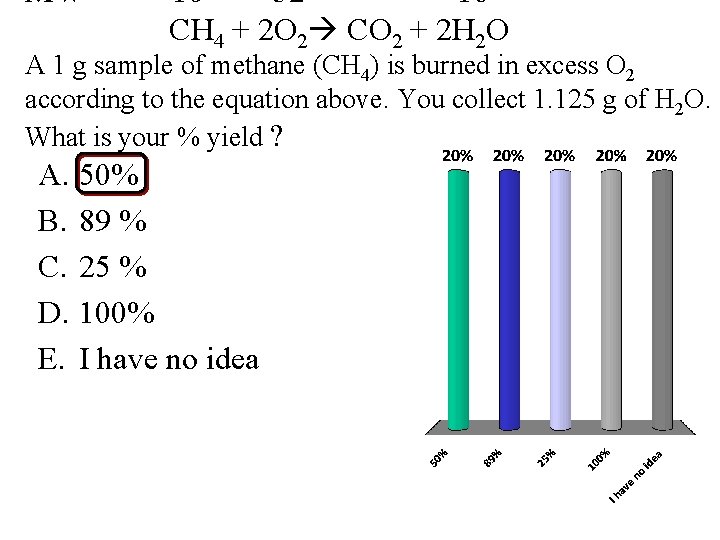
MW 16 32 18 CH 4 + 2 O 2 CO 2 + 2 H 2 O A 1 g sample of methane (CH 4) is burned in excess O 2 according to the equation above. You collect 1. 125 g of H 2 O. What is your % yield ? A. 50% B. 89 % C. 25 % D. 100% E. I have no idea

Need more practice ? ?

Where we are on the mole road trip…. We is done with moles !!!

And now, after many weeks of &^%!! Moles …. for something completely different…. CLASSICAL REACTIONs

Today’s Student Learning Objective (SLO): SLO #6 Students will be able to: write, balance, identify and predict common reaction classes (metatheses, acidbase, redox). Translation: What happens when I add this to that ?


CLASSICAL REACTIONs (continued) What to read… chapter 4 ( + a bit of Ch. 14) üMetatheses 131 -140 üAcid-base 140 -147; 549 -552 üOxidation-reduction 147 -159

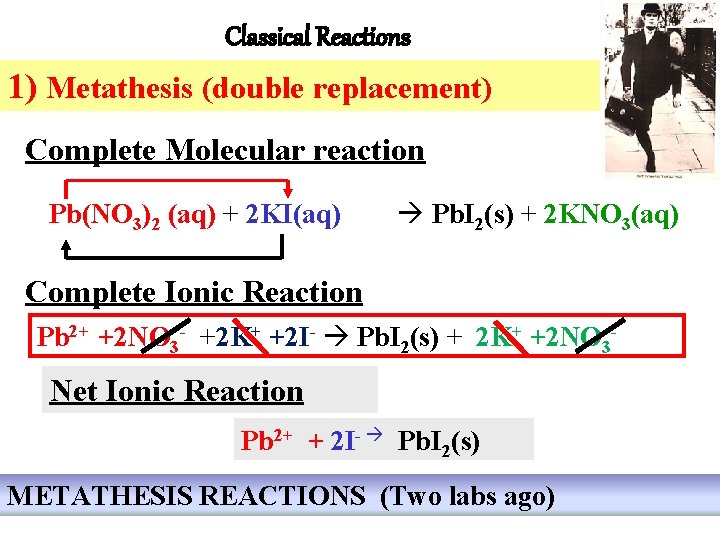
Classical Reactions 1) Metathesis (double replacement) Complete Molecular reaction Pb(NO 3)2 (aq) + 2 KI(aq) Pb. I 2(s) + 2 KNO 3(aq) Complete Ionic Reaction Pb 2+ +2 NO 3 - +2 K+ +2 I- Pb. I 2(s) + 2 K+ +2 NO 3 - Net Ionic Reaction Pb 2+ + 2 I- Pb. I 2(s) METATHESIS REACTIONS (Two labs ago)