Announcements and Outline Sequential scanning reports due today









- Slides: 35

Announcements and Outline • Sequential scanning reports due today. Midterm exam is next Monday, Feb. 26. • – – • • Group members (up to 3) Organism Probe Question Checkouts during TBA time next week (see schedule) – Review using grading rubric Paper Discussion Fluorescent Probes 1. 2. 3. 4. 5. After paper discussion Mostly multiple choice test on the lecture content. Project proposals are also due next Monday. Provide: – – A. B. C. D. Nuclear stains Ion indicators Organelle probes Membrane probes Tracers Lab: Onion epithelium as a source of live cells. TBA Time: review for checkout

A. Paper Discussion • • • Today (Rachel): Tan et al. 2005 Feb. 26 (Ellen): March 12 (Emily) March 19 (Amy) March 26 (Amanda) April 2 (Andrea) April 9 (Brittaney) April 16 (Lauren) April 23 (Joe and Molly):

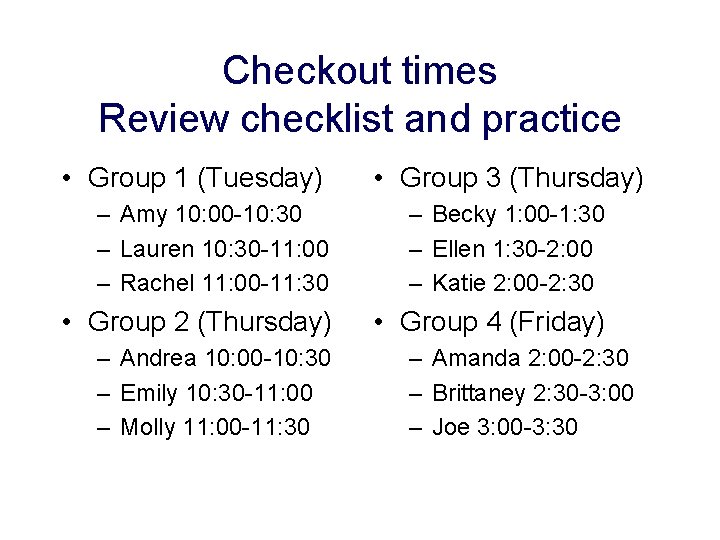
Checkout times Review checklist and practice • Group 1 (Tuesday) • Group 3 (Thursday) – Amy 10: 00 -10: 30 – Lauren 10: 30 -11: 00 – Rachel 11: 00 -11: 30 – Becky 1: 00 -1: 30 – Ellen 1: 30 -2: 00 – Katie 2: 00 -2: 30 • Group 2 (Thursday) • Group 4 (Friday) – Andrea 10: 00 -10: 30 – Emily 10: 30 -11: 00 – Molly 11: 00 -11: 30 – Amanda 2: 00 -2: 30 – Brittaney 2: 30 -3: 00 – Joe 3: 00 -3: 30

Another project idea • You might consider a “teaching” project instead of a “research” project. • For example, prepare your own cryostat section of mouse kidney or intestine and do a demonstration of correct controls for double or triple labeling. – – Background control: no label Bleed-through control: single labeled Double-labeled sample Simultaneous versus sequential scanning • Make a poster for prominent display in the Microscope facility, described how to do controls for double-labeling.

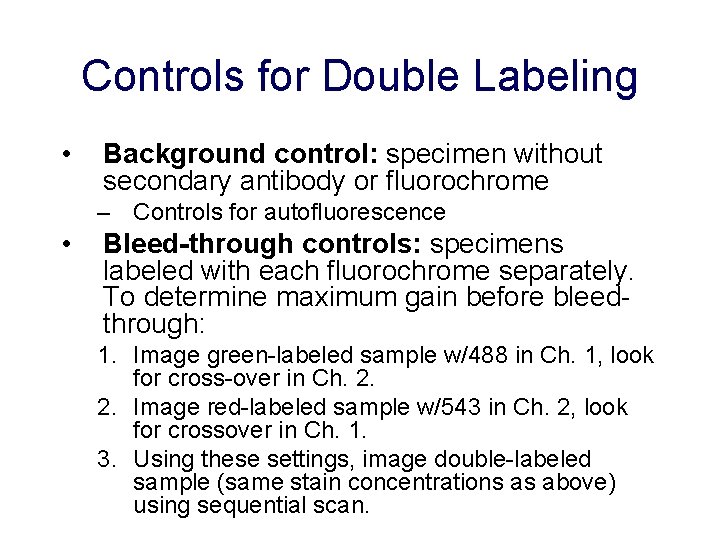
Controls for Double Labeling • Background control: specimen without secondary antibody or fluorochrome – Controls for autofluorescence • Bleed-through controls: specimens labeled with each fluorochrome separately. To determine maximum gain before bleedthrough: 1. Image green-labeled sample w/488 in Ch. 1, look for cross-over in Ch. 2. 2. Image red-labeled sample w/543 in Ch. 2, look for crossover in Ch. 1. 3. Using these settings, image double-labeled sample (same stain concentrations as above) using sequential scan.

B. Fluorescent Probes • Molecular Probes (Invitrogen) – www. probes. com – Catalog contains thousands of fluorescent probes, with valuable technical information. • Confocal Microscopy Listserver – To subscribe, send message “subscribe confocal” to listserv@ubvm. cc. buffalo. edu with nothing in the subject line, without the quotation marks. – Archive: http: //listserv/ascu. buffalo. edu/archives/confocal. ht ml

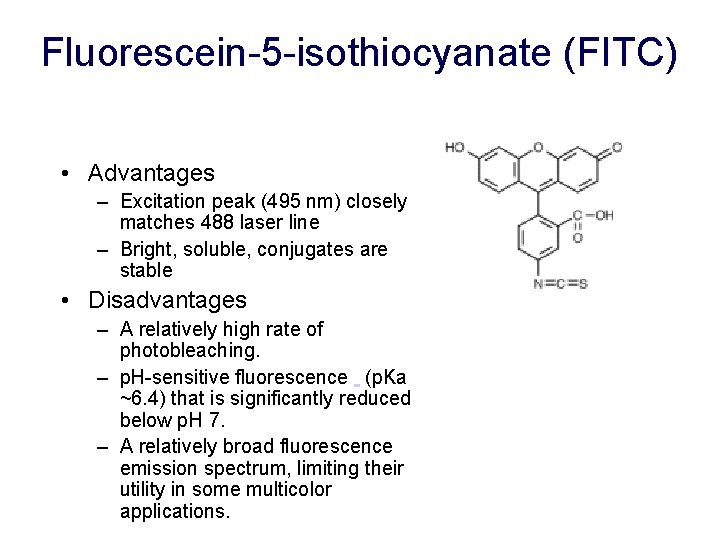
Fluorescein-5 -isothiocyanate (FITC) • Advantages – Excitation peak (495 nm) closely matches 488 laser line – Bright, soluble, conjugates are stable • Disadvantages – A relatively high rate of photobleaching. – p. H-sensitive fluorescence (p. Ka ~6. 4) that is significantly reduced below p. H 7. – A relatively broad fluorescence emission spectrum, limiting their utility in some multicolor applications.

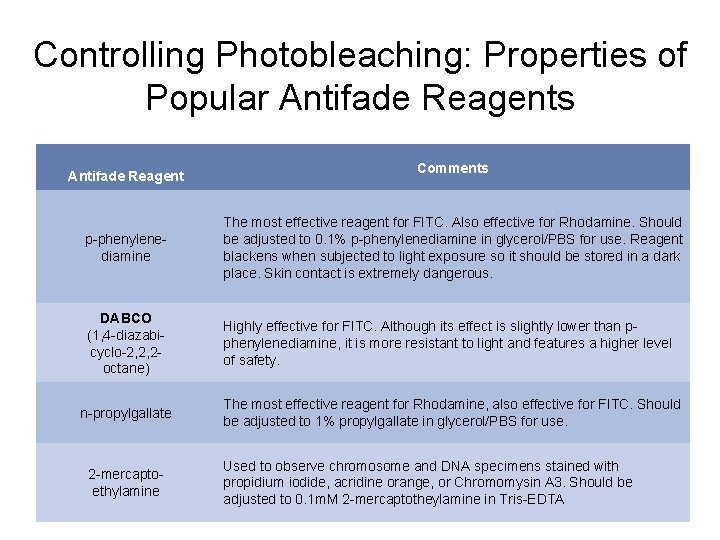
Controlling Photobleaching: Properties of Popular Antifade Reagents Antifade Reagent Comments p-phenylenediamine The most effective reagent for FITC. Also effective for Rhodamine. Should be adjusted to 0. 1% p-phenylenediamine in glycerol/PBS for use. Reagent blackens when subjected to light exposure so it should be stored in a dark place. Skin contact is extremely dangerous. DABCO (1, 4 -diazabicyclo-2, 2, 2 octane) Highly effective for FITC. Although its effect is slightly lower than pphenylenediamine, it is more resistant to light and features a higher level of safety. n-propylgallate 2 -mercaptoethylamine The most effective reagent for Rhodamine, also effective for FITC. Should be adjusted to 1% propylgallate in glycerol/PBS for use. Used to observe chromosome and DNA specimens stained with propidium iodide, acridine orange, or Chromomysin A 3. Should be adjusted to 0. 1 m. M 2 -mercaptotheylamine in Tris-EDTA

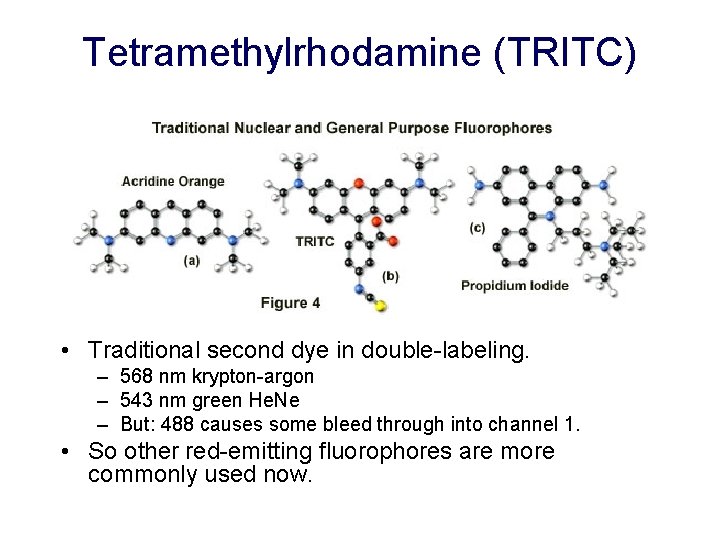
Tetramethylrhodamine (TRITC) • Traditional second dye in double-labeling. – 568 nm krypton-argon – 543 nm green He. Ne – But: 488 causes some bleed through into channel 1. • So other red-emitting fluorophores are more commonly used now.

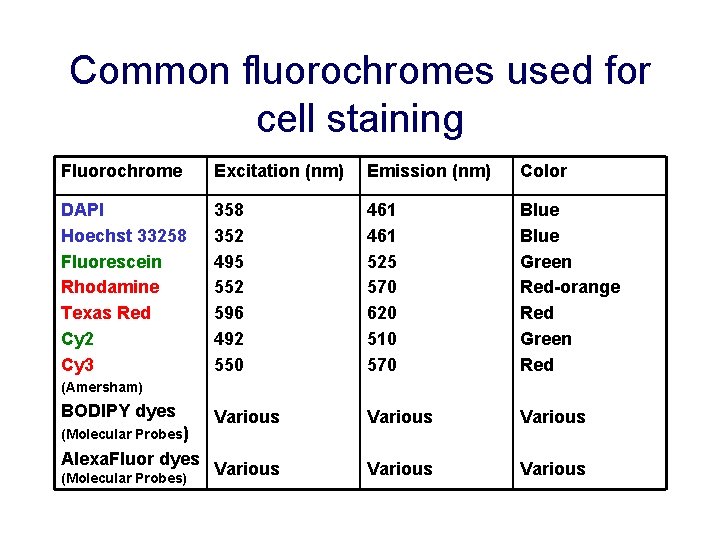
Common fluorochromes used for cell staining Fluorochrome Excitation (nm) Emission (nm) Color DAPI Hoechst 33258 Fluorescein Rhodamine Texas Red Cy 2 Cy 3 358 352 495 552 596 492 550 461 525 570 620 510 570 Blue Green Red-orange Red Green Red Various Various (Amersham) BODIPY dyes (Molecular Probes) Alexa. Fluor dyes (Molecular Probes)

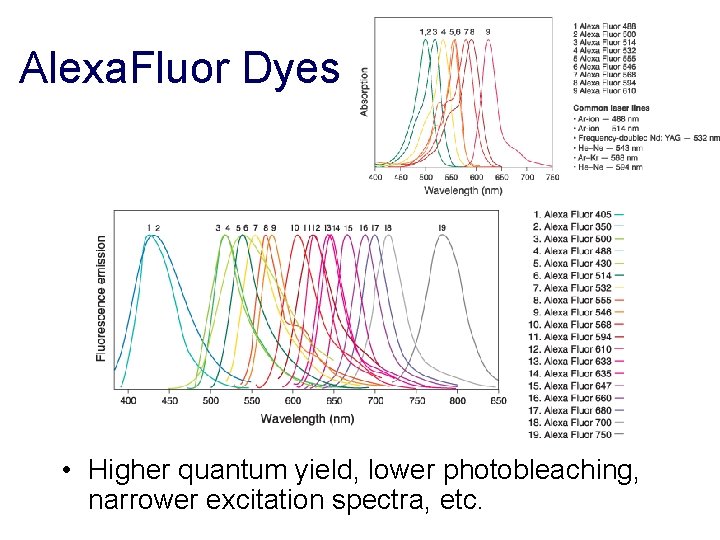
Alexa. Fluor Dyes • Higher quantum yield, lower photobleaching, narrower excitation spectra, etc.

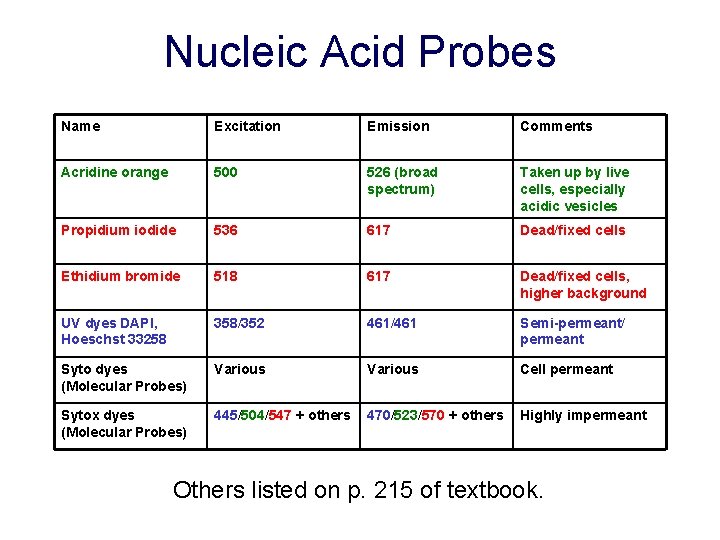
Nucleic Acid Probes Name Excitation Emission Comments Acridine orange 500 526 (broad spectrum) Taken up by live cells, especially acidic vesicles Propidium iodide 536 617 Dead/fixed cells Ethidium bromide 518 617 Dead/fixed cells, higher background UV dyes DAPI, Hoeschst 33258 358/352 461/461 Semi-permeant/ permeant Syto dyes (Molecular Probes) Various Cell permeant Sytox dyes (Molecular Probes) 445/504/547 + others 470/523/570 + others Highly impermeant Others listed on p. 215 of textbook.

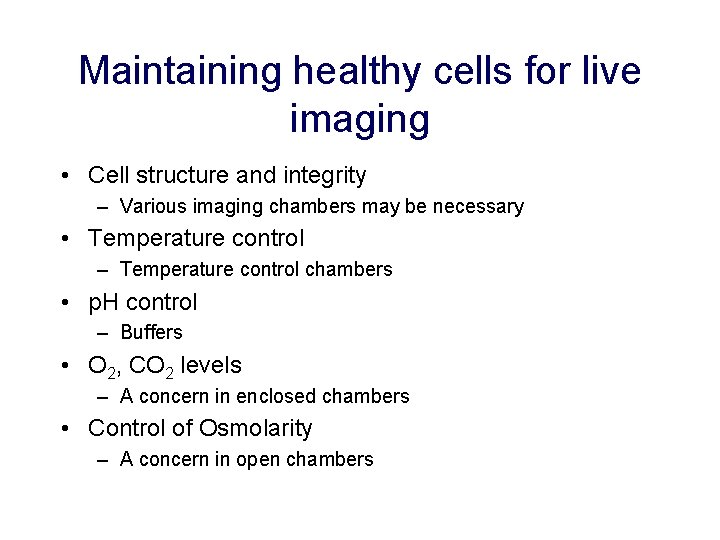
Maintaining healthy cells for live imaging • Cell structure and integrity – Various imaging chambers may be necessary • Temperature control – Temperature control chambers • p. H control – Buffers • O 2, CO 2 levels – A concern in enclosed chambers • Control of Osmolarity – A concern in open chambers

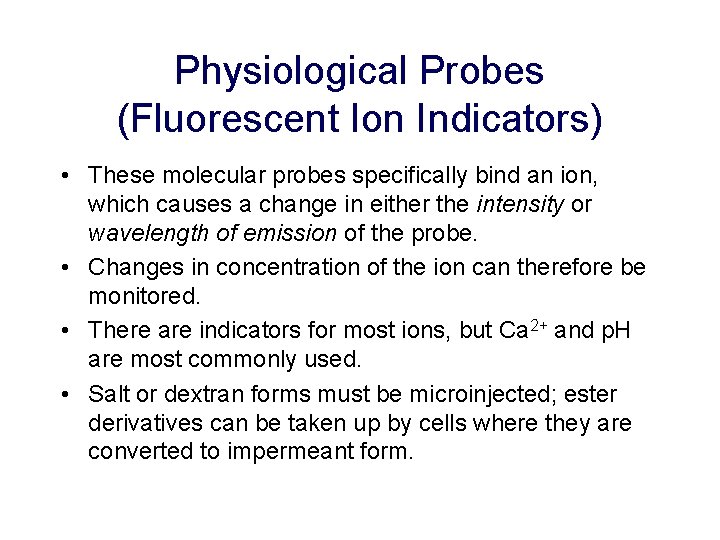
Physiological Probes (Fluorescent Ion Indicators) • These molecular probes specifically bind an ion, which causes a change in either the intensity or wavelength of emission of the probe. • Changes in concentration of the ion can therefore be monitored. • There are indicators for most ions, but Ca 2+ and p. H are most commonly used. • Salt or dextran forms must be microinjected; ester derivatives can be taken up by cells where they are converted to impermeant form.

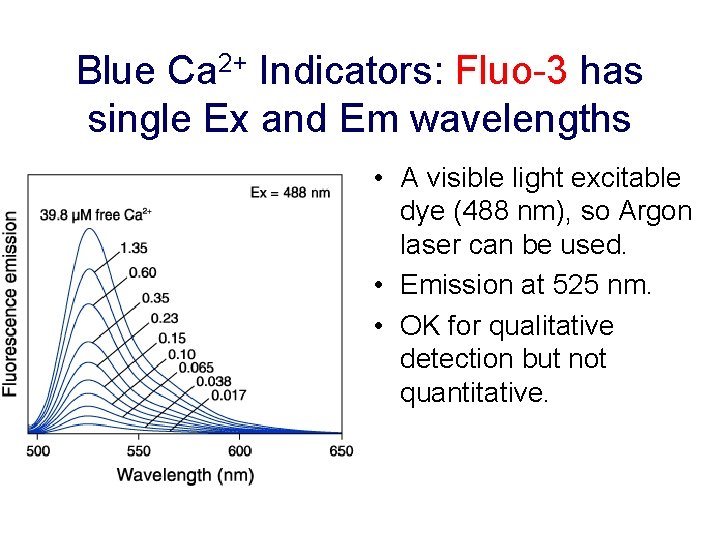
Blue Ca 2+ Indicators: Fluo-3 has single Ex and Em wavelengths • A visible light excitable dye (488 nm), so Argon laser can be used. • Emission at 525 nm. • OK for qualitative detection but not quantitative.

Ca 2+ Release During Shrimp Egg Activation • From Lindsay et al. (1992). Extracellular Mg 2+ Induces an Intracellular Ca 2+ Wave During Oocyte Activation in the Marine Shrimp Sicyonia ingentis. Dev. Biol. 152: 94 -102.

Problems with Quantitative Single Wavelength Fluorescence Imaging • Compartmentalization of probe in intracellular organelles. • Uneven loading of dye in the cell. • Instrumentation noise • Sample geometry (thickness) • Solution: ratio imaging of the dye

UV Ca 2+ Indicators: Indo-1 is a fixed excitation ratiometric dye Em at 400 Em at 475 • UV-excitable at fixed excitation wavelength (338 nm). • Collect at two emission wavelengths (400 and 475 nm). • [Ca 2+ ] ~ ratio of Em 400 / Em 475, independent of dye concentration, etc. – Big num/small dem > [hi] – Small num/big dem > [lo]

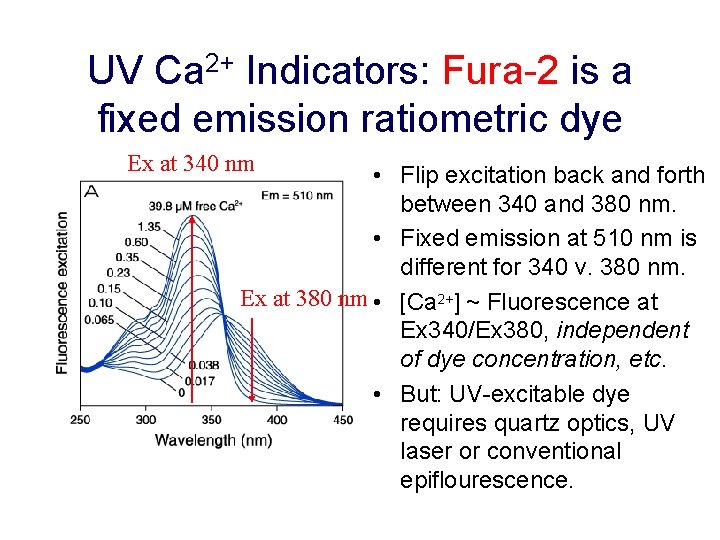
UV Ca 2+ Indicators: Fura-2 is a fixed emission ratiometric dye Ex at 340 nm • Flip excitation back and forth between 340 and 380 nm. • Fixed emission at 510 nm is different for 340 v. 380 nm. Ex at 380 nm • [Ca 2+] ~ Fluorescence at Ex 340/Ex 380, independent of dye concentration, etc. • But: UV-excitable dye requires quartz optics, UV laser or conventional epiflourescence.

Free Ca 2+ Concentration in a Purkinje Neuron from Embryonic Mouse Cerebellum • Neurons were loaded with fura-2. • Neurons were stimulated with glutamate receptor agonist. • The composite image represents the ratio of images obtained with excitation at 340 nm and 380 nm.

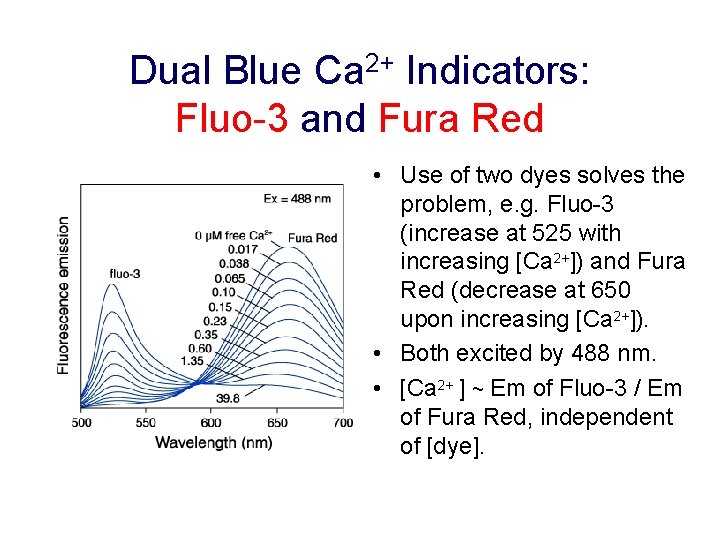
Dual Blue Ca 2+ Indicators: Fluo-3 and Fura Red • Use of two dyes solves the problem, e. g. Fluo-3 (increase at 525 with increasing [Ca 2+]) and Fura Red (decrease at 650 upon increasing [Ca 2+]). • Both excited by 488 nm. • [Ca 2+ ] ~ Em of Fluo-3 / Em of Fura Red, independent of [dye].

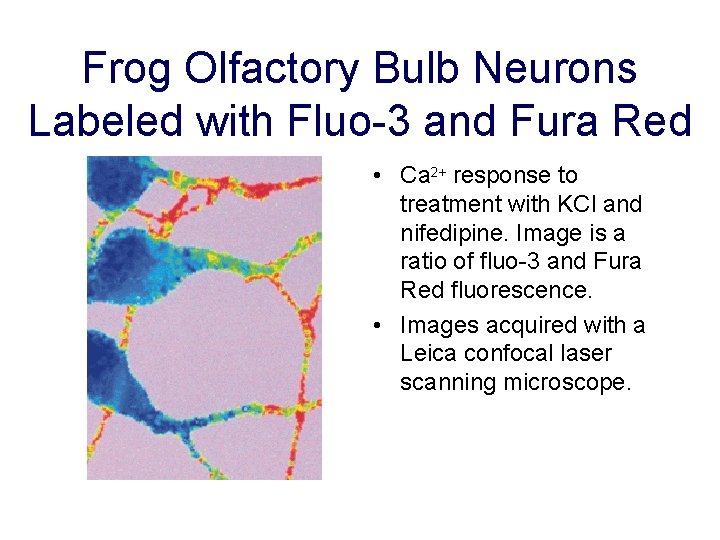
Frog Olfactory Bulb Neurons Labeled with Fluo-3 and Fura Red • Ca 2+ response to treatment with KCl and nifedipine. Image is a ratio of fluo-3 and Fura Red fluorescence. • Images acquired with a Leica confocal laser scanning microscope.

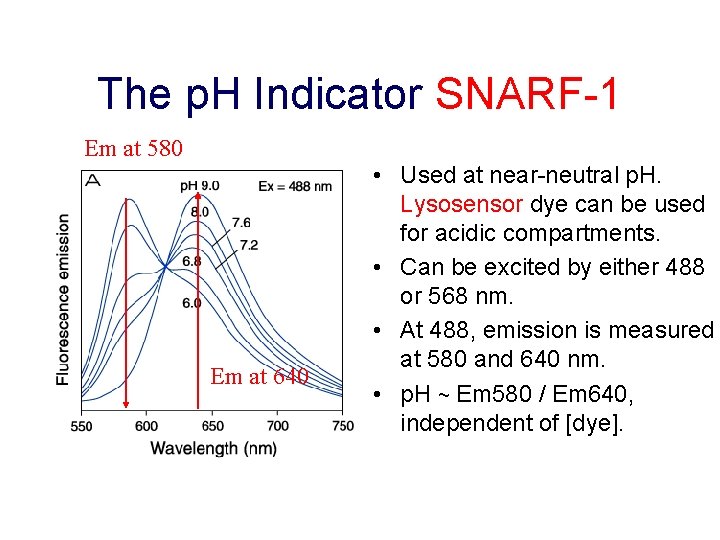
The p. H Indicator SNARF-1 Em at 580 Em at 640 • Used at near-neutral p. H. Lysosensor dye can be used for acidic compartments. • Can be excited by either 488 or 568 nm. • At 488, emission is measured at 580 and 640 nm. • p. H ~ Em 580 / Em 640, independent of [dye].

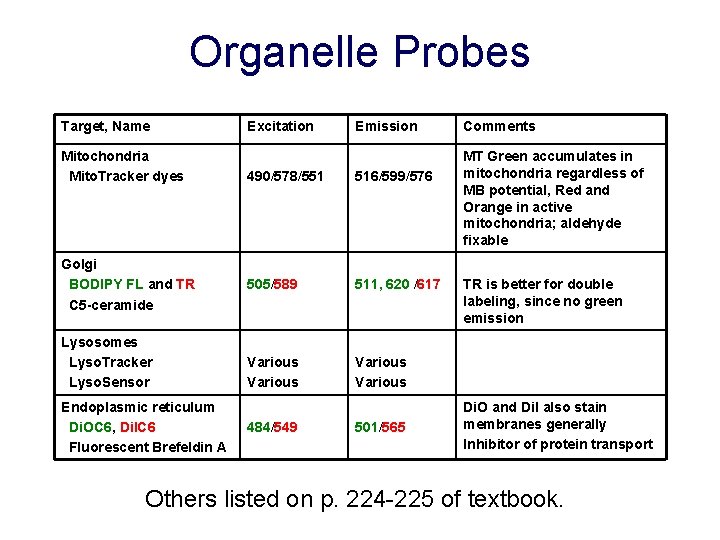
Organelle Probes Target, Name Mitochondria Mito. Tracker dyes Golgi BODIPY FL and TR C 5 -ceramide Lysosomes Lyso. Tracker Lyso. Sensor Endoplasmic reticulum Di. OC 6, Di. IC 6 Fluorescent Brefeldin A Excitation Emission 490/578/551 516/599/576 505/589 511, 620 /617 Various 484/549 501/565 Comments MT Green accumulates in mitochondria regardless of MB potential, Red and Orange in active mitochondria; aldehyde fixable TR is better for double labeling, since no green emission Di. O and Di. I also stain membranes generally Inhibitor of protein transport Others listed on p. 224 -225 of textbook.

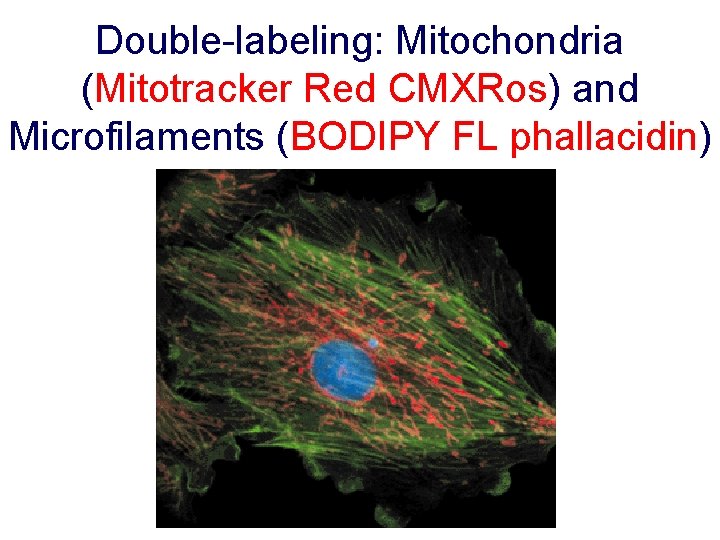
Double-labeling: Mitochondria (Mitotracker Red CMXRos) and Microfilaments (BODIPY FL phallacidin)

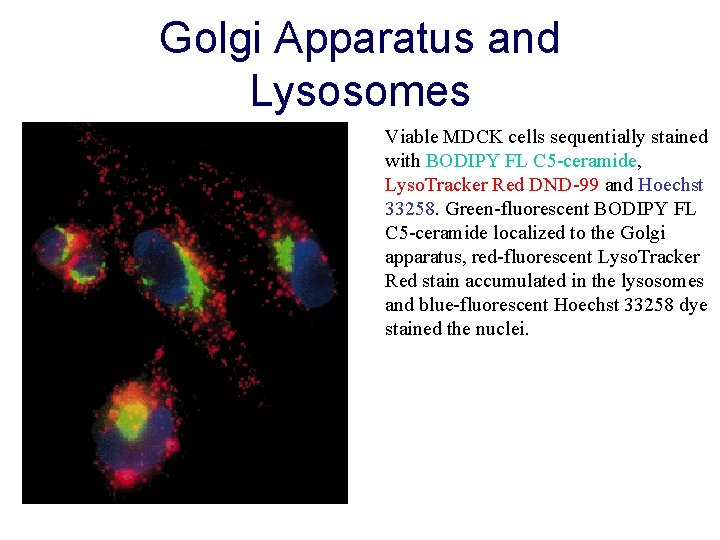
Golgi Apparatus and Lysosomes Viable MDCK cells sequentially stained with BODIPY FL C 5 -ceramide, Lyso. Tracker Red DND-99 and Hoechst 33258. Green-fluorescent BODIPY FL C 5 -ceramide localized to the Golgi apparatus, red-fluorescent Lyso. Tracker Red stain accumulated in the lysosomes and blue-fluorescent Hoechst 33258 dye stained the nuclei.

Membrane Probes • Useful for tracing cell fates without microinjecting • Di. I (red, D on right) • Di. O (green) • Many others available from Molecular Probes

Cell Tracers Name Ex Em Comments Cell. Tracker Various Permeant, then attaches to cellular proteins, aldehyde fixable Lucifer yellow 488 500 -600 Yellow Common gap junction & neuronal tracer, aldehyde fixable Membrane probes Various Can label by touching dye crystal against cell. Fluorescent dextrans Various Require microinjection Various

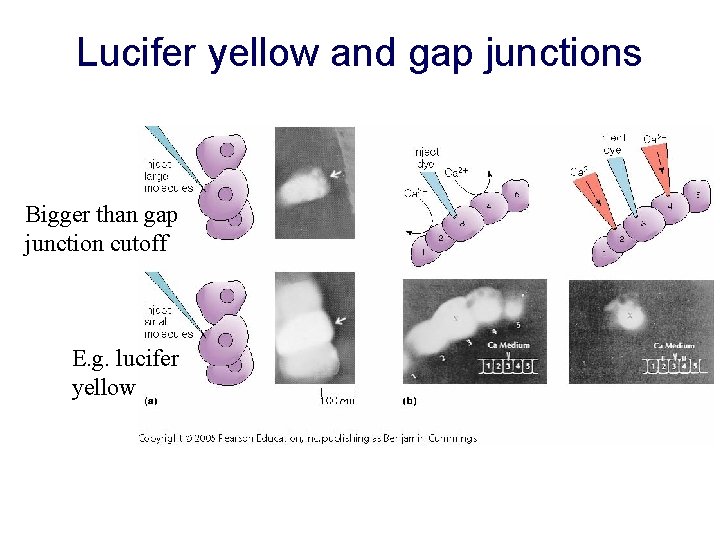
Lucifer yellow and gap junctions Bigger than gap junction cutoff E. g. lucifer yellow

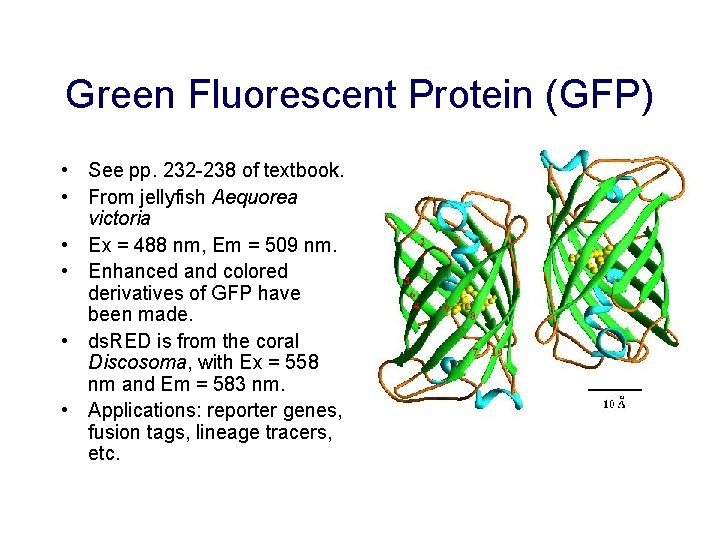
Green Fluorescent Protein (GFP) • See pp. 232 -238 of textbook. • From jellyfish Aequorea victoria • Ex = 488 nm, Em = 509 nm. • Enhanced and colored derivatives of GFP have been made. • ds. RED is from the coral Discosoma, with Ex = 558 nm and Em = 583 nm. • Applications: reporter genes, fusion tags, lineage tracers, etc.

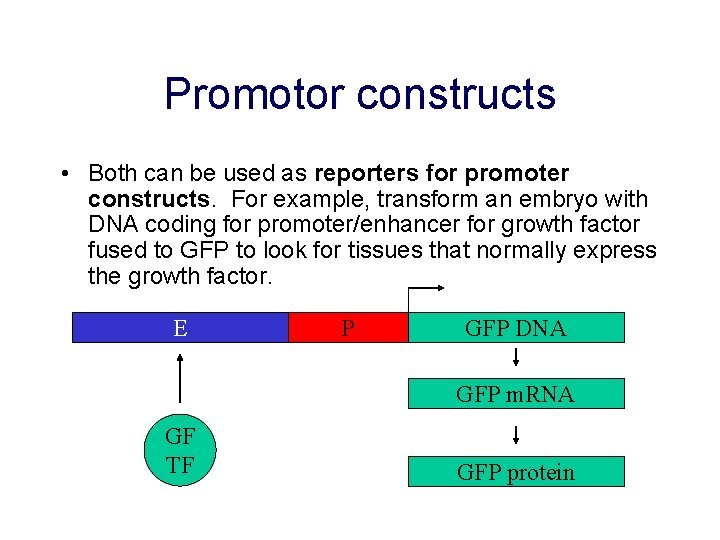
Promotor constructs • Both can be used as reporters for promoter constructs. For example, transform an embryo with DNA coding for promoter/enhancer for growth factor fused to GFP to look for tissues that normally express the growth factor. E P GFP DNA GFP m. RNA GF TF GFP protein

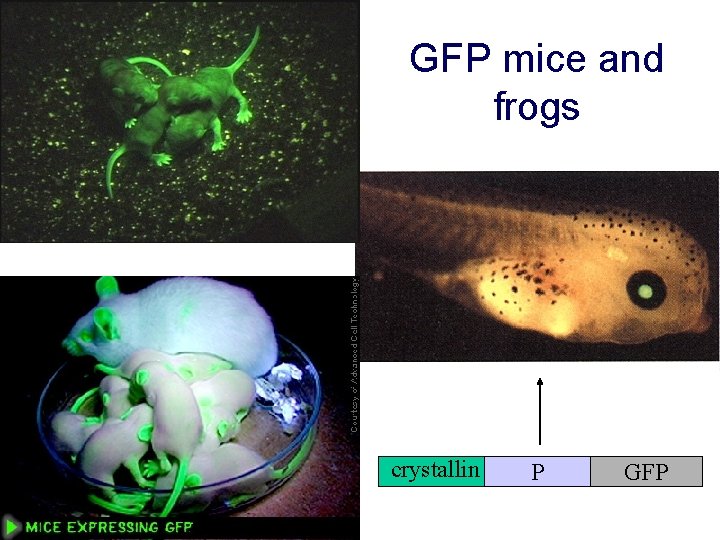
GFP mice and frogs crystallin P GFP

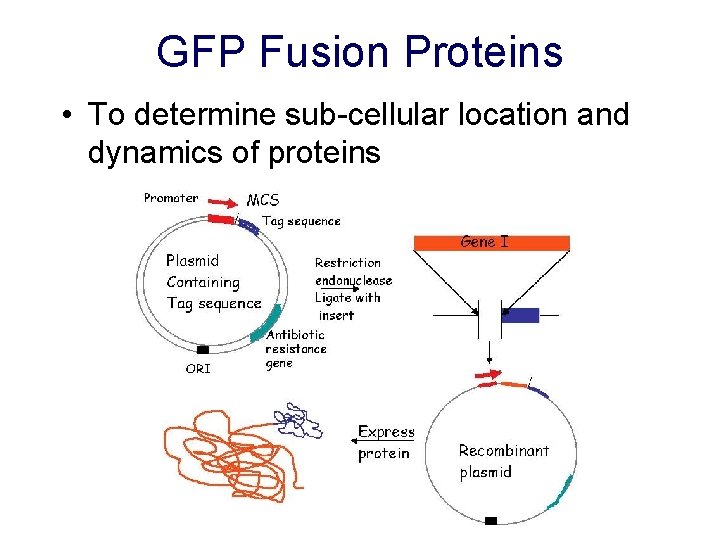
GFP Fusion Proteins • To determine sub-cellular location and dynamics of proteins

C. Lab: Onion epithelium prep (Dailey et al. 2007; Murphy, 2001) • • • Cut a small square of a layer from the onion using a razor blade. Use a forceps to peel off the thin epithelium on the inner surface of the onion layer. Put the epithelium on a microscope slide, cover with 1 -2 drops of staining solution. 1. Di. OC 6: mitochondria and endoplasmic reticulum • 0. 5 mg/ml stock in Et. OH, dilute 1: 1000 in water on day of expt. , stain 5 min, remove dye, rinse with water 2. Mitotracker Red for mitochondria 3. Lysotracker Red for lysosomes • Dilute both of these to 250 n. M in water, stain 5 -10 min, remove, rinse 4. BODIPY-ceramide for Golgi apparatus – Add 5 u. M BODIPY-ceramide, stain 15 min, replace with water; should be in endosomes after 30 min and Golgi after 60 min; can also fix and stain as below 5. (All) DAPI for nuclei • • • Fix w/ 0. 5% gluteraldehyde 5 min; remove fixative, rinse, 0. 5 mg/ml DAPI, stain Cover with a coverslip. Live motion (groups 1, 2, 3) can be collected in consecutive 1 s scans.

References • Murphy, D. B. 2001. Fundamentals of light microscopy and electronic imaging. New York: Wiley-Liss. • Dailey, M. E. et al. 2007. Confocal microscopy of living cells. In: Pawley, J. B. , (ed. ). Handbook of biological confocal microscopy, 3 rd ed. New York: Springer.