Homework 10 due today Homework 11 posted and













- Slides: 39

• Homework 10 due today • Homework 11 posted and due Friday Monday…Ick

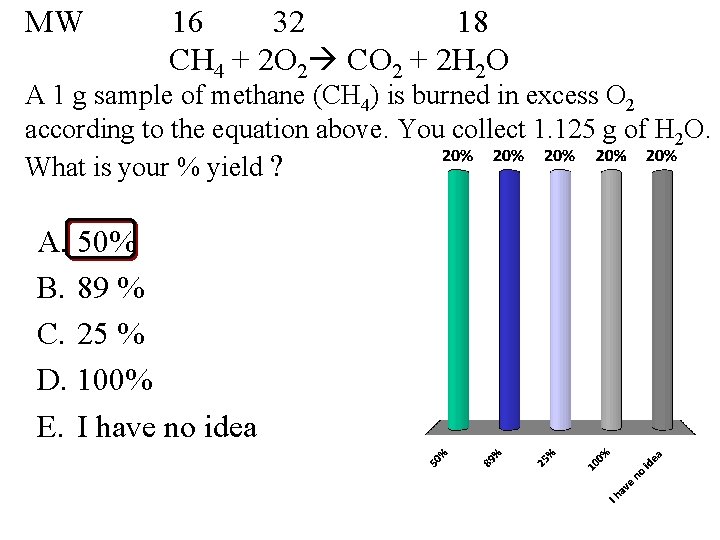
MW 16 32 18 CH 4 + 2 O 2 CO 2 + 2 H 2 O A 1 g sample of methane (CH 4) is burned in excess O 2 according to the equation above. You collect 1. 125 g of H 2 O. What is your % yield ? A. 50% B. 89 % C. 25 % D. 100% E. I have no idea

Where we are on the mole road trip…. We is done with moles !!!

And now, after many weeks of &^%!! Moles …. for something completely different…. CLASSICAL REACTIONs

Today’s Student Learning Objective (SLO): SLO #6 Students will be able to: write, balance, identify and predict common reaction classes (metatheses, acidbase, redox). Translation: What happens when I add this to that ?


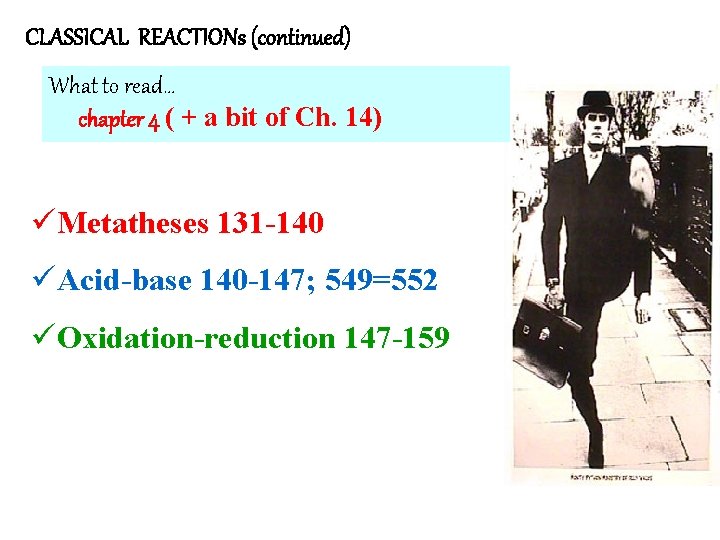
CLASSICAL REACTIONs (continued) What to read… chapter 4 ( + a bit of Ch. 14) üMetatheses 131 -140 üAcid-base 140 -147; 549=552 üOxidation-reduction 147 -159

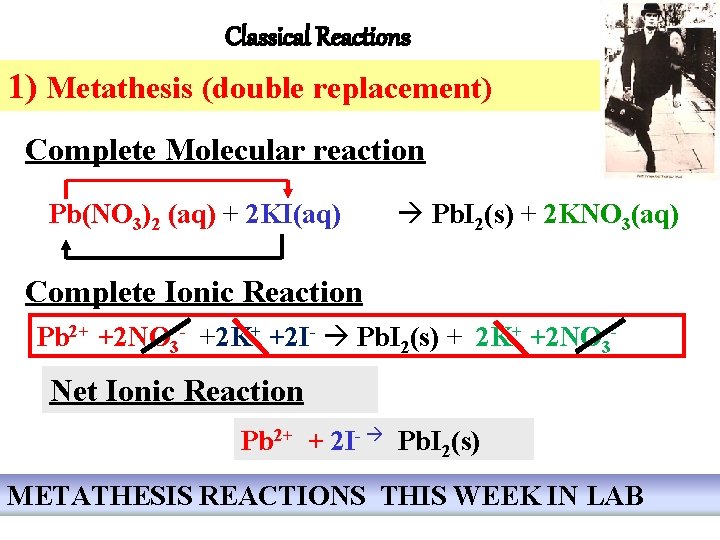
Classical Reactions 1) Metathesis (double replacement) Complete Molecular reaction Pb(NO 3)2 (aq) + 2 KI(aq) Pb. I 2(s) + 2 KNO 3(aq) Complete Ionic Reaction Pb 2+ +2 NO 3 - +2 K+ +2 I- Pb. I 2(s) + 2 K+ +2 NO 3 - Net Ionic Reaction Pb 2+ + 2 I- Pb. I 2(s) METATHESIS REACTIONS THIS WEEK IN LAB

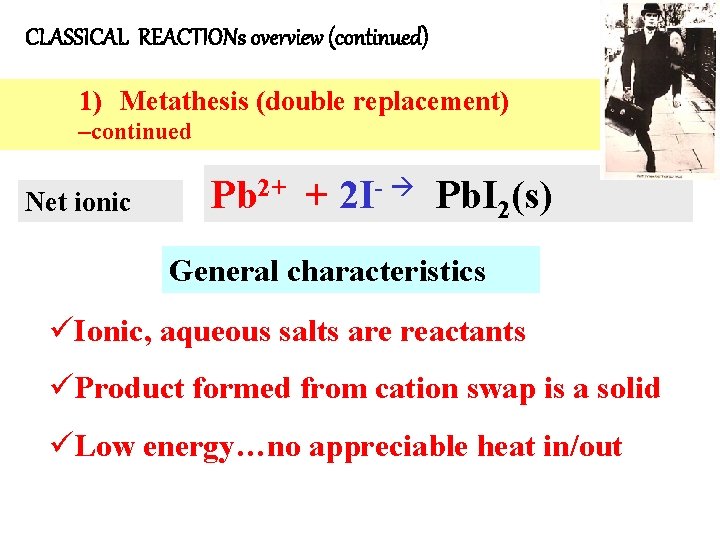
CLASSICAL REACTIONs overview (continued) 1) Metathesis (double replacement) –continued Net ionic Pb 2+ + 2 I- Pb. I 2(s) General characteristics üIonic, aqueous salts are reactants üProduct formed from cation swap is a solid üLow energy…no appreciable heat in/out

Let’s practice a few on the board, kids !

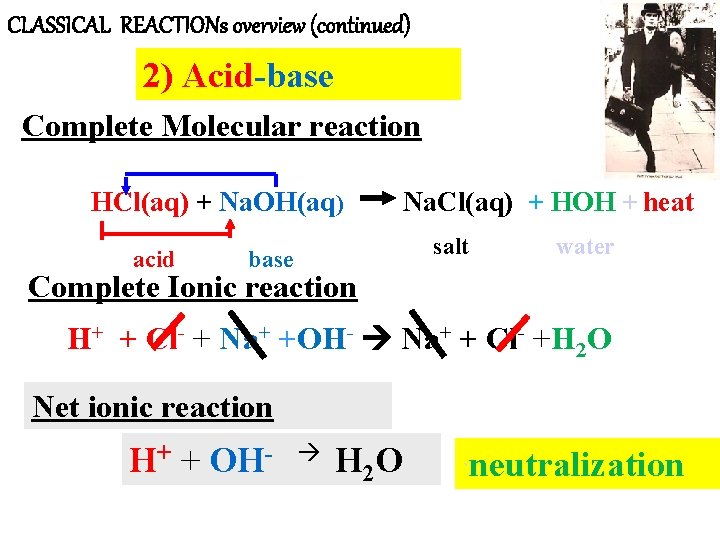
CLASSICAL REACTIONs overview (continued) 2) Acid-base Complete Molecular reaction HCl(aq) + Na. OH(aq) acid Na. Cl(aq) + HOH + heat salt base water Complete Ionic reaction H+ + Cl- + Na+ +OH- Na+ + Cl- +H 2 O Net ionic reaction H+ + OH- H 2 O ` neutralization

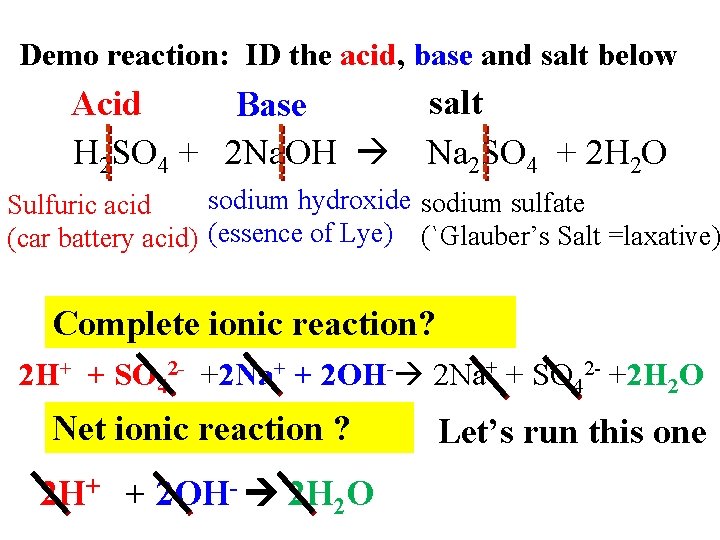
Demo reaction: ID the acid, base and salt below Acid Base H 2 SO 4 + 2 Na. OH salt Na 2 SO 4 + 2 H 2 O sodium hydroxide sodium sulfate Sulfuric acid (car battery acid) (essence of Lye) (`Glauber’s Salt =laxative) Complete ionic reaction? 2 H+ + SO 42 - +2 Na+ + 2 OH- 2 Na+ + SO 42 - +2 H 2 O Net ionic reaction ? 2 H+ + 2 OH- 2 H 2 O Let’s run this one

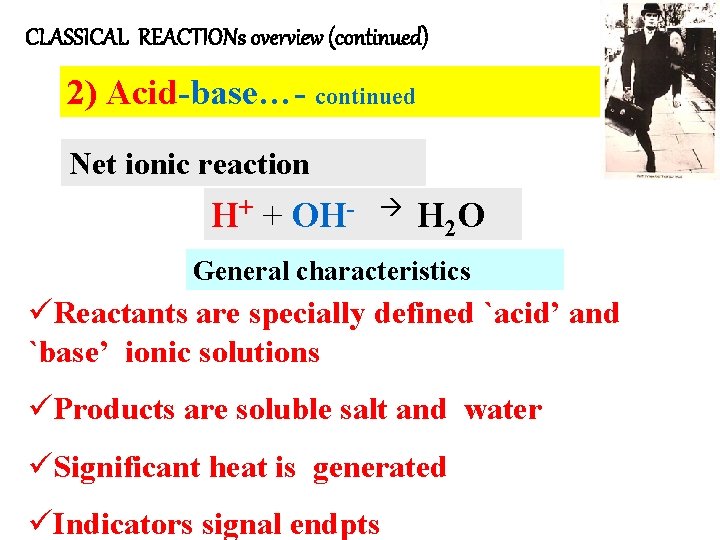
CLASSICAL REACTIONs overview (continued) 2) Acid-base…- continued Net ionic reaction H+ + OH- H 2 O General characteristics üReactants are specially defined `acid’ and `base’ ionic solutions üProducts are soluble salt and water üSignificant heat is generated üIndicators signal endpts

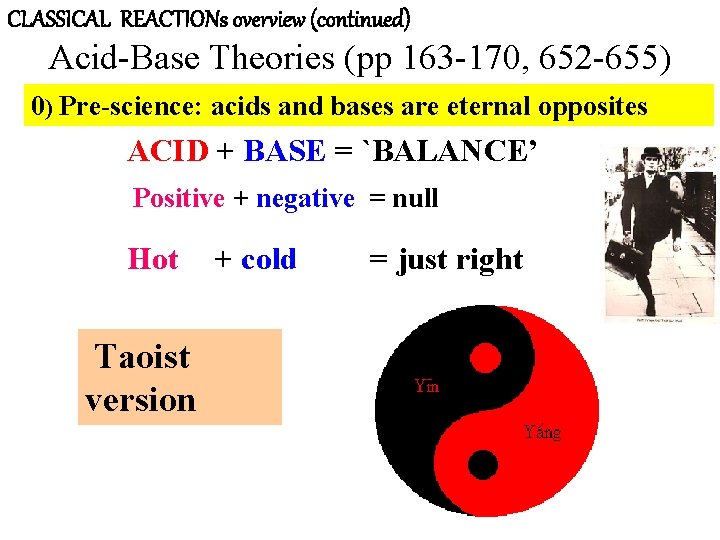
CLASSICAL REACTIONs overview (continued) Acid-Base Theories (pp 163 -170, 652 -655) 0) Pre-science: acids and bases are eternal opposites ACID + BASE = `BALANCE’ Positive + negative = null Hot Taoist version + cold = just right

CLASSICAL REACTIONs overview (continued) Acid-Base Theories (pp 653 -656) Modern Theory: Try #1 Svante Arrhenius: Father of the first modern acid/base theory Thesis on Acids & Bases derided by his research committee… Graduates with Ph. D ordinare (no distinction) see p. 144 Young Arrhenius (not considered promising) Old Arrhenius (wins Nobel prize in 1903 for same acid base theory)

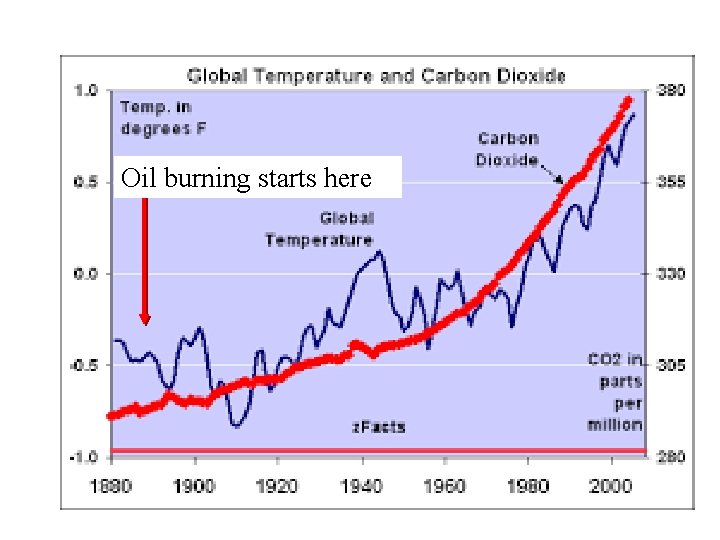
CLASSICAL REACTIONs OVERVIEW(continued) A measure of this Ph. D `ordinaire’’s brilliance… In 1896 Arrhenius predicted green house gas (CO 2) from profligate burning of the newly popular fuel source …petroleum oil… would cause (gasp !) measurable and catastrophic global warming… …starting in 1990 -2000 AD …the entire scientific establishment (and Standard Oil) laughed at him (again)

FYI…. Until 2013 not a single current Republican member of the US Congress admitted that global warming is caused by burning of fossil fuels. The current President thinks it is a Chinese hoax perpetrated to make us less competitive


Oil burning starts here

There are currently 748 major fires burning in the US http: //activefiremaps. fed. us/

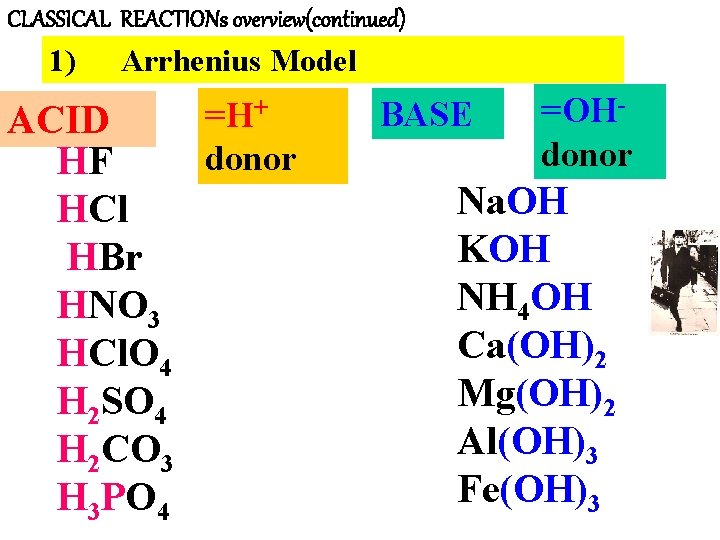
CLASSICAL REACTIONs overview(continued) 1) Arrhenius Model =H+ ACID donor HF HCl HBr HNO 3 HCl. O 4 H 2 SO 4 H 2 CO 3 H 3 PO 4 BASE =OHdonor Na. OH KOH NH 4 OH Ca(OH)2 Mg(OH)2 Al(OH)3 Fe(OH)3

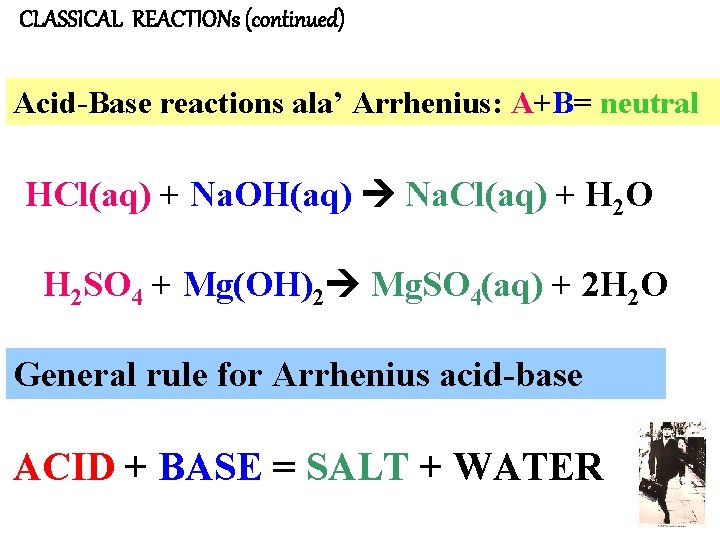
CLASSICAL REACTIONs (continued) Acid-Base reactions ala’ Arrhenius: A+B= neutral HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O H 2 SO 4 + Mg(OH)2 Mg. SO 4(aq) + 2 H 2 O General rule for Arrhenius acid-base ACID + BASE = SALT + WATER

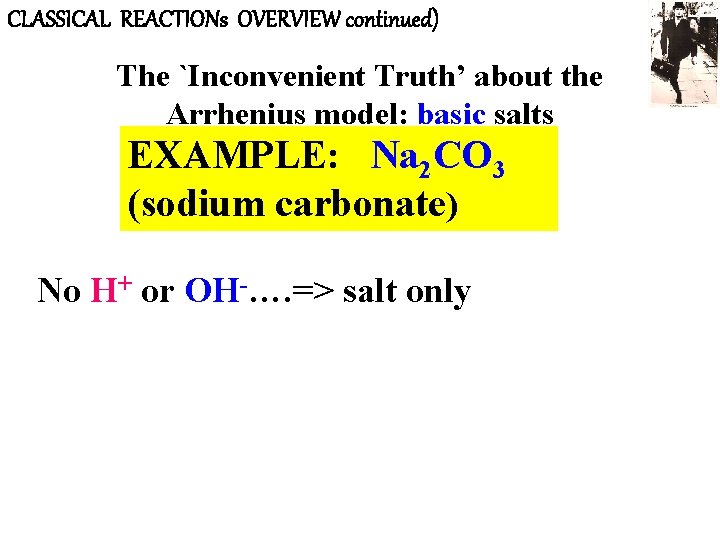
CLASSICAL REACTIONs OVERVIEW continued) The `Inconvenient Truth’ about the Arrhenius model: basic salts EXAMPLE: Na 2 CO 3 (sodium carbonate) No H+ or OH-…. => salt only

CLASSICAL REACTIONs OVERVIEW(continued) The `Inconvenient Truth’ about the Arrhenius model: basic salts (continued) EXAMPLE: Na 2 CO 3 (sodium carbonate) experimental results of adding to water: • Turns pink in presence of phenolphthalein • gas-forming reaction with HCl, pink disappears => A base !!!!? ? ? Where’s OH ? ? ?

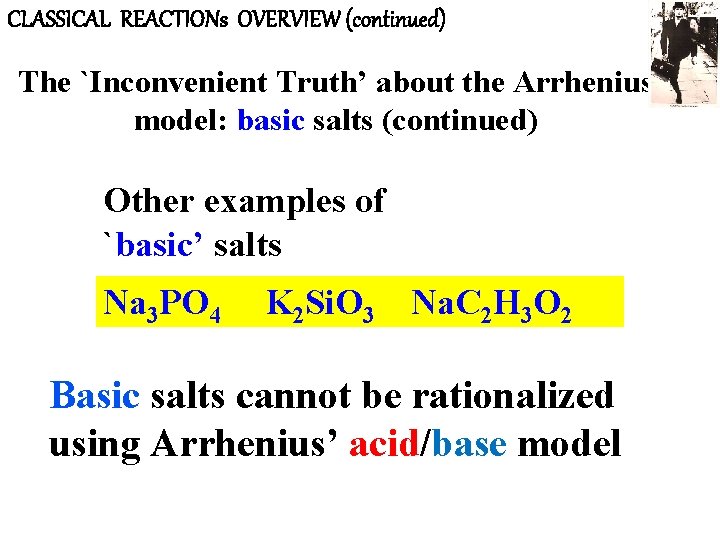
CLASSICAL REACTIONs OVERVIEW (continued) The `Inconvenient Truth’ about the Arrhenius model: basic salts (continued) Other examples of `basic’ salts Na 3 PO 4 K 2 Si. O 3 Na. C 2 H 3 O 2 Basic salts cannot be rationalized using Arrhenius’ acid/base model

CLASSICAL REACTIONs (continued) Bronsted to the rescue… Winner of the Bronsted look alike contest…. Young Bronsted…Swedis h chemist circa 1910… Young James Dean…American actor circa 1955…(“Rebel Without a Cause, ”“East of Eden”, “Giant” ) Bronsted a few years after marriage and kids

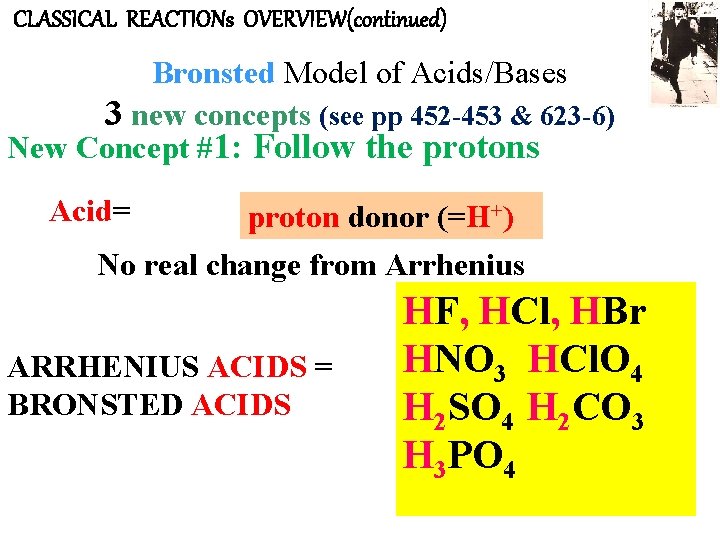
CLASSICAL REACTIONs OVERVIEW(continued) Bronsted Model of Acids/Bases 3 new concepts (see pp 452 -453 & 623 -6) New Concept #1: Follow the protons Acid= proton donor (=H+) No real change from Arrhenius ARRHENIUS ACIDS = BRONSTED ACIDS HF, HCl, HBr HNO 3 HCl. O 4 H 2 SO 4 H 2 CO 3 H 3 PO 4

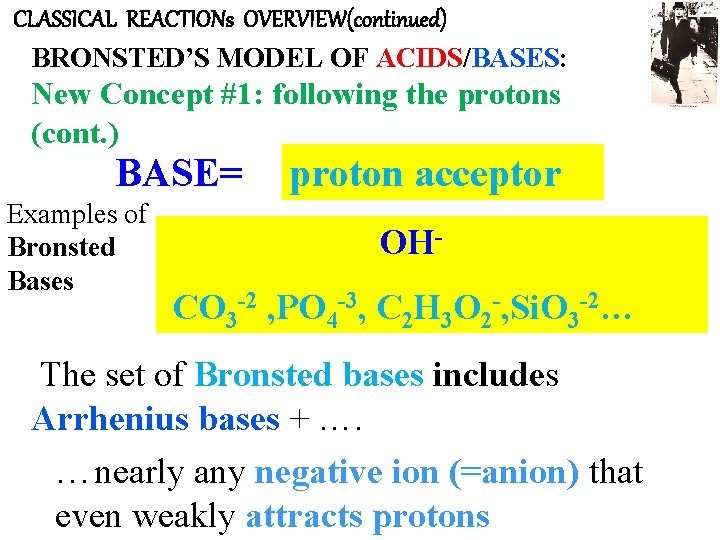
CLASSICAL REACTIONs OVERVIEW(continued) BRONSTED’S MODEL OF ACIDS/BASES: New Concept #1: following the protons (cont. ) BASE= Examples of Bronsted Bases proton acceptor OH- CO 3 -2 , PO 4 -3, C 2 H 3 O 2 -, Si. O 3 -2… The set of Bronsted bases includes Arrhenius bases + …. …nearly any negative ion (=anion) that even weakly attracts protons

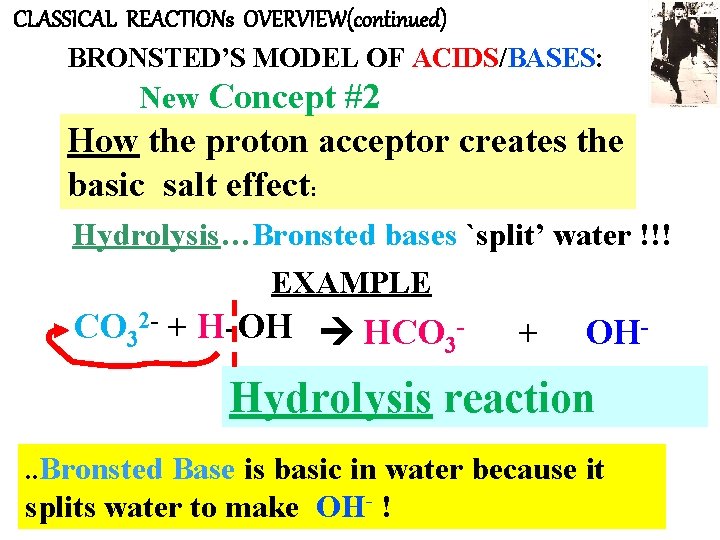
CLASSICAL REACTIONs OVERVIEW(continued) BRONSTED’S MODEL OF ACIDS/BASES: New Concept #2 How the proton acceptor creates the basic salt effect: Hydrolysis…Bronsted bases `split’ water !!! EXAMPLE CO 32 - + H-OH HCO 3 - + OH- Hydrolysis reaction. . Bronsted Base is basic in water because it splits water to make OH- !

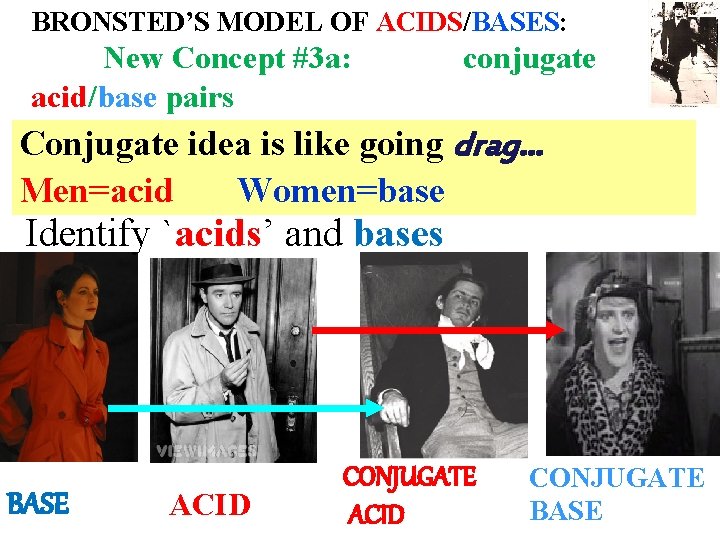
BRONSTED’S MODEL OF ACIDS/BASES: New Concept #3 a: acid/base pairs conjugate Conjugate idea is like going drag… Men=acid Women=base Identify `acids’ and bases BASE ACID CONJUGATE BASE

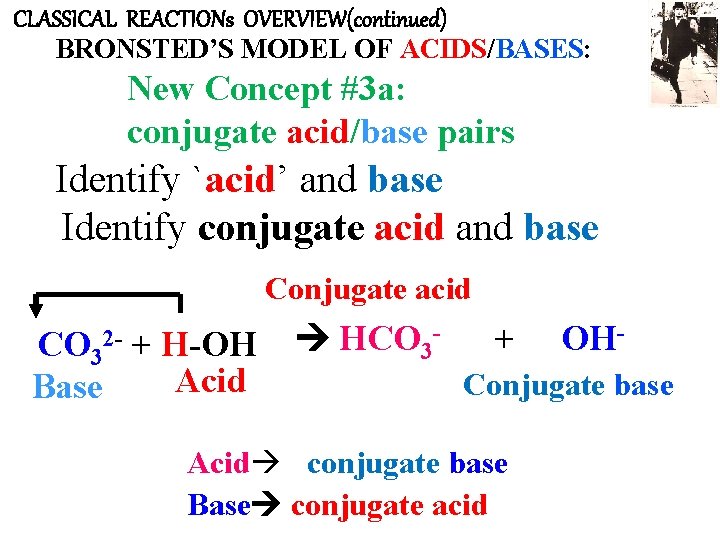
CLASSICAL REACTIONs OVERVIEW(continued) BRONSTED’S MODEL OF ACIDS/BASES: New Concept #3 a: conjugate acid/base pairs Identify `acid’ and base Identify conjugate acid and base Conjugate acid CO 32 - + H-OH Acid Base HCO 3 - + OH- Conjugate base Acid conjugate base Base conjugate acid

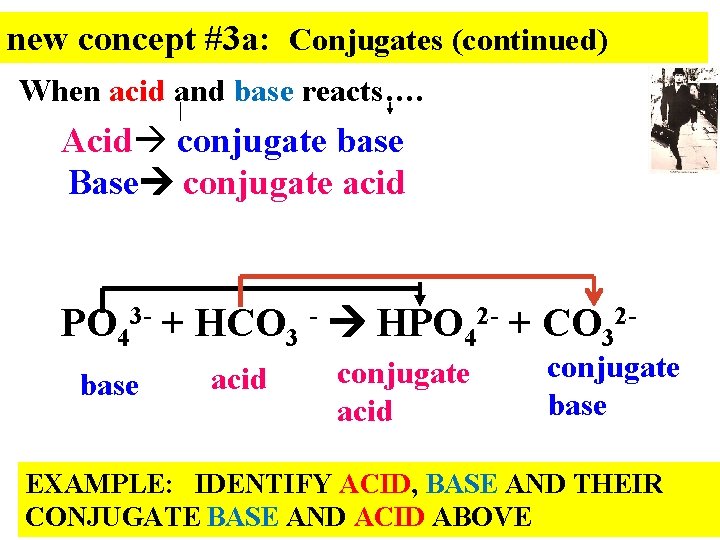
new concept #3 a: Conjugates (continued) When acid and base reacts…. Acid conjugate base Base conjugate acid PO 43 - + HCO 3 - HPO 42 - + CO 32 base acid conjugate base EXAMPLE: IDENTIFY ACID, BASE AND THEIR CONJUGATE BASE AND ACID ABOVE

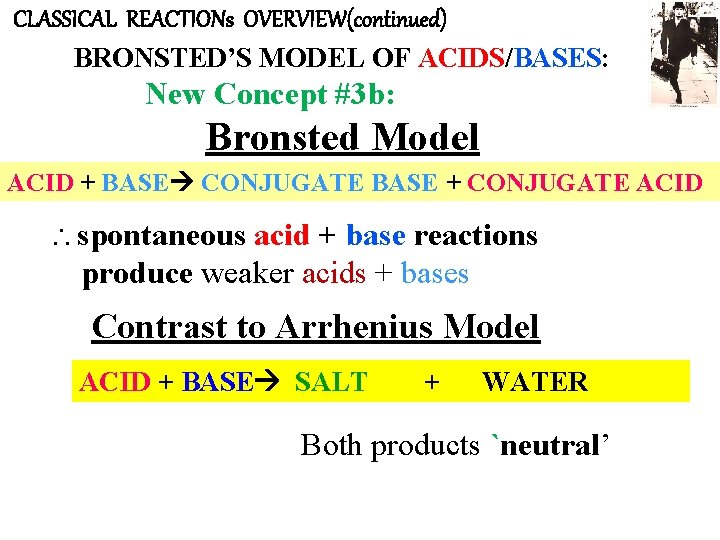
CLASSICAL REACTIONs OVERVIEW(continued) BRONSTED’S MODEL OF ACIDS/BASES: New Concept #3 b: Bronsted Model ACID + BASE CONJUGATE BASE + CONJUGATE ACID spontaneous acid + base reactions produce weaker acids + bases Contrast to Arrhenius Model ACID + BASE SALT + WATER Both products `neutral’

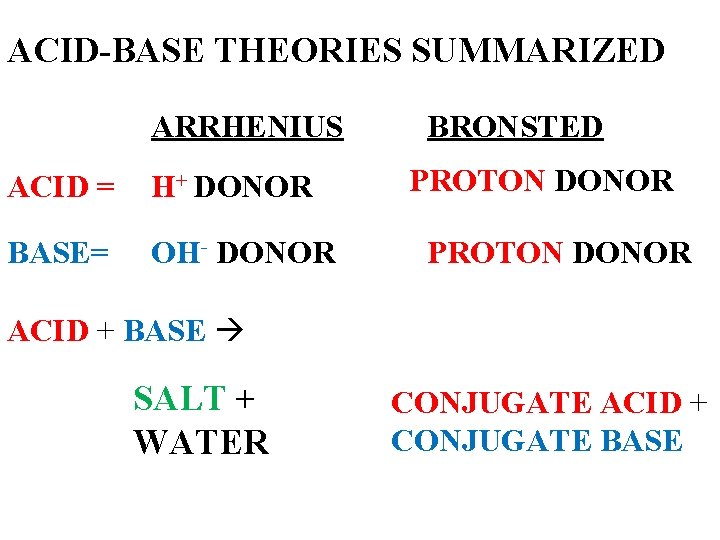
ACID-BASE THEORIES SUMMARIZED ARRHENIUS ACID = H+ DONOR BASE= OH- DONOR BRONSTED PROTON DONOR ACID + BASE SALT + WATER CONJUGATE ACID + CONJUGATE BASE

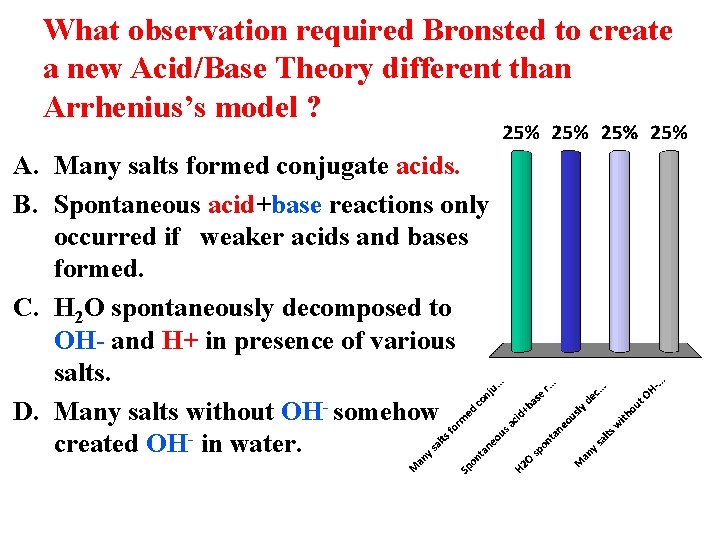
What observation required Bronsted to create a new Acid/Base Theory different than Arrhenius’s model ? A. Many salts formed conjugate acids. B. Spontaneous acid+base reactions only occurred if weaker acids and bases formed. C. H 2 O spontaneously decomposed to OH- and H+ in presence of various salts. D. Many salts without OH- somehow created OH- in water.

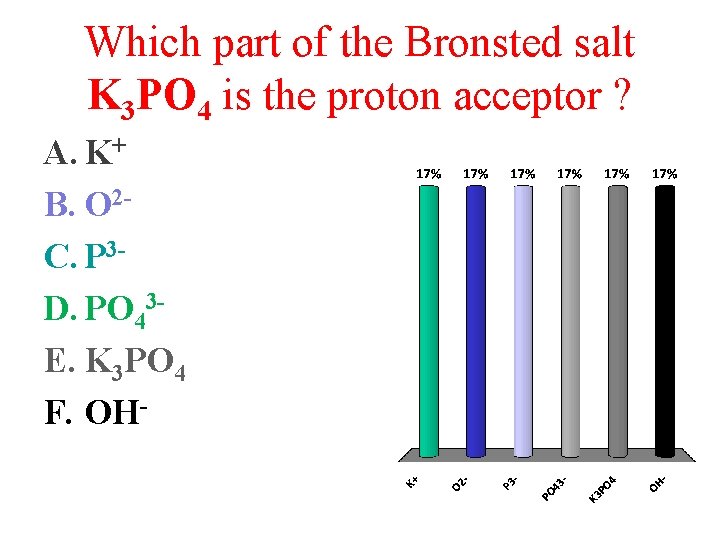
Which part of the Bronsted salt K 3 PO 4 is the proton acceptor ? A. K+ B. O 2 C. P 3 D. PO 43 E. K 3 PO 4 F. OH-

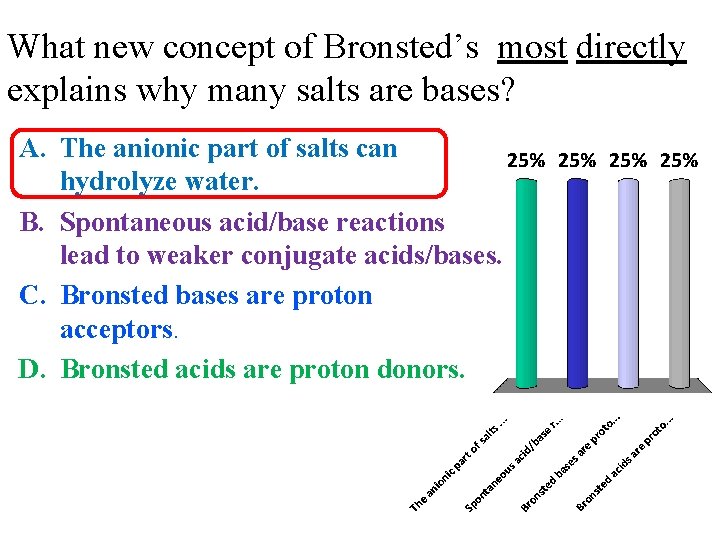
What new concept of Bronsted’s most directly explains why many salts are bases? A. The anionic part of salts can hydrolyze water. B. Spontaneous acid/base reactions lead to weaker conjugate acids/bases. C. Bronsted bases are proton acceptors. D. Bronsted acids are proton donors.

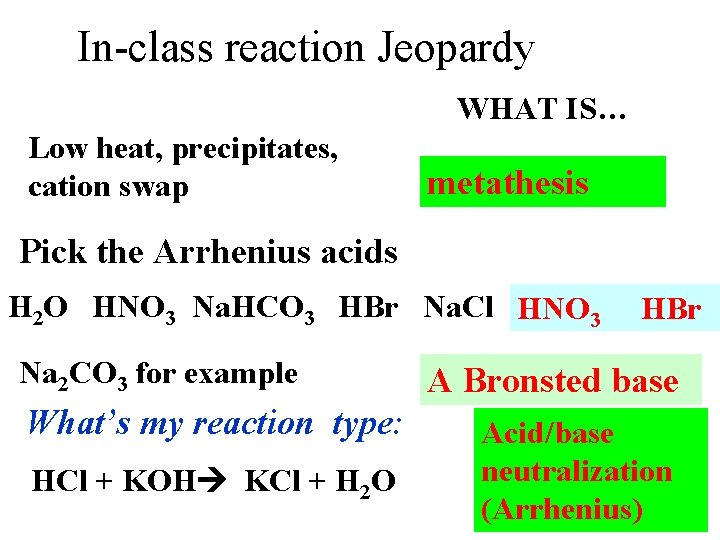
In-class reaction Jeopardy WHAT IS… Low heat, precipitates, cation swap metathesis Pick the Arrhenius acids H 2 O HNO 3 Na. HCO 3 HBr Na. Cl HNO 3 Na 2 CO 3 for example What’s my reaction type: HCl + KOH KCl + H 2 O HBr A Bronsted base Acid/base neutralization (Arrhenius)

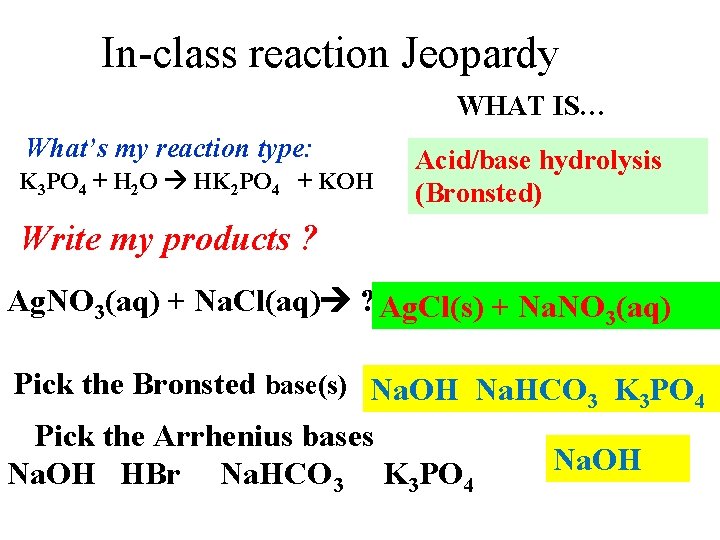
In-class reaction Jeopardy WHAT IS… What’s my reaction type: K 3 PO 4 + H 2 O HK 2 PO 4 + KOH Acid/base hydrolysis (Bronsted) Write my products ? Ag. NO 3(aq) + Na. Cl(aq) ? Ag. Cl(s) + Na. NO 3(aq) Pick the Bronsted base(s) Na. OH Na. HCO 3 K 3 PO 4 Pick the Arrhenius bases Na. OH HBr Na. HCO 3 K 3 PO 4
 Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram Homework due today
Homework due today Homework due today
Homework due today Homework due today
Homework due today Iron core
Iron core Homework due today
Homework due today Homework due today
Homework due today For today's meeting
For today's meeting Todaysclass
Todaysclass Today meeting or today's meeting
Today meeting or today's meeting Fingerprint galton details
Fingerprint galton details Today's lesson or today lesson
Today's lesson or today lesson Today's lesson or today lesson
Today's lesson or today lesson Assignment due today
Assignment due today Assignment due today
Assignment due today Assignment due today
Assignment due today Reports due today!
Reports due today! Liver blood supply diagram
Liver blood supply diagram That was due today
That was due today Figura geometrica con lati disuguali
Figura geometrica con lati disuguali Liberty chapter 20
Liberty chapter 20 Pentacosiomedimni cavalieri zeugiti teti
Pentacosiomedimni cavalieri zeugiti teti Due piccole sfere identiche sono sospese a due punti
Due piccole sfere identiche sono sospese a due punti What is an infrared thermometer best used for servsafe
What is an infrared thermometer best used for servsafe A food handler drops the end of a hose into a mop bucket
A food handler drops the end of a hose into a mop bucket Impulse examples
Impulse examples What do errors discovered after posting require?
What do errors discovered after posting require? Post the journal’s special amount column totals.
Post the journal’s special amount column totals. Room rack front office
Room rack front office Look mom i posted it again
Look mom i posted it again Q: when do you do your homework? a: …
Q: when do you do your homework? a: … Homework is due on friday
Homework is due on friday Homework is due on friday
Homework is due on friday Homework due tomorrow
Homework due tomorrow Homework is due
Homework is due Jack prelutsky homework oh homework
Jack prelutsky homework oh homework Homework oh homework i hate you you stink
Homework oh homework i hate you you stink Jack prelutsky homework oh homework
Jack prelutsky homework oh homework Homework oh homework jack prelutsky
Homework oh homework jack prelutsky Alitteration definition
Alitteration definition