Homework 10 due today Homework 11 posted and





- Slides: 17

• Homework 10 due today • Homework 11 posted and due Friday Monday…Ick

Limiting yield and % yield calculations are the last stop on the mole bus trip

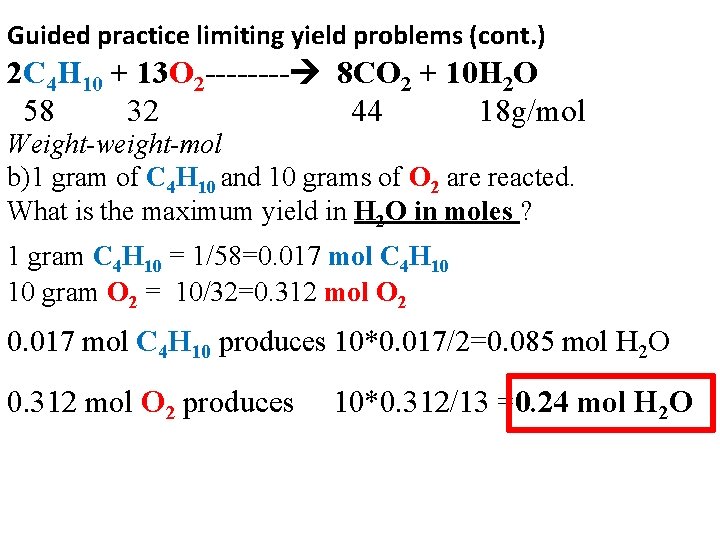
Guided practice limiting yield problems (cont. ) 2 C 4 H 10 + 13 O 2 ---- 8 CO 2 + 10 H 2 O 58 32 44 18 g/mol Weight-weight-mol b)1 gram of C 4 H 10 and 10 grams of O 2 are reacted. What is the maximum yield in H 2 O in moles ? 1 gram C 4 H 10 = 1/58=0. 017 mol C 4 H 10 10 gram O 2 = 10/32=0. 312 mol O 2 0. 017 mol C 4 H 10 produces 10*0. 017/2=0. 085 mol H 2 O 0. 312 mol O 2 produces 10*0. 312/13 =0. 24 mol H 2 O

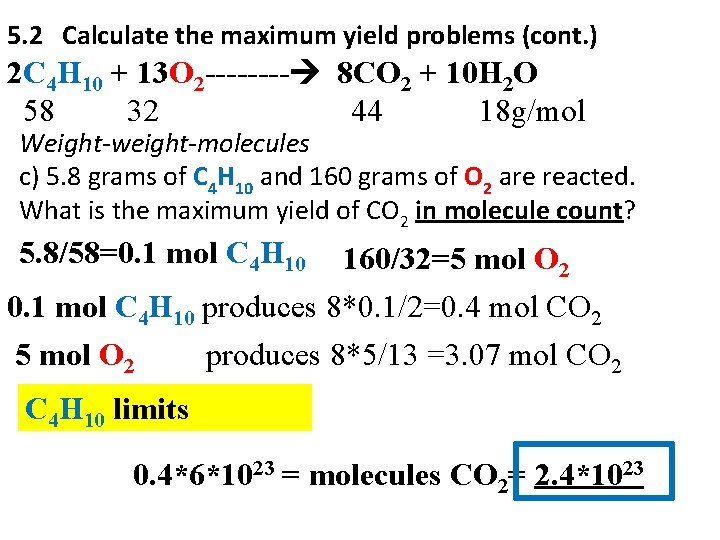
5. 2 Calculate the maximum yield problems (cont. ) 2 C 4 H 10 + 13 O 2 ---- 8 CO 2 + 10 H 2 O 58 32 44 18 g/mol Weight-weight-molecules c) 5. 8 grams of C 4 H 10 and 160 grams of O 2 are reacted. What is the maximum yield of CO 2 in molecule count? 5. 8/58=0. 1 mol C 4 H 10 160/32=5 mol O 2 0. 1 mol C 4 H 10 produces 8*0. 1/2=0. 4 mol CO 2 5 mol O 2 produces 8*5/13 =3. 07 mol CO 2 C 4 H 10 limits 0. 4*6*1023 = molecules CO 2= 2. 4*1023

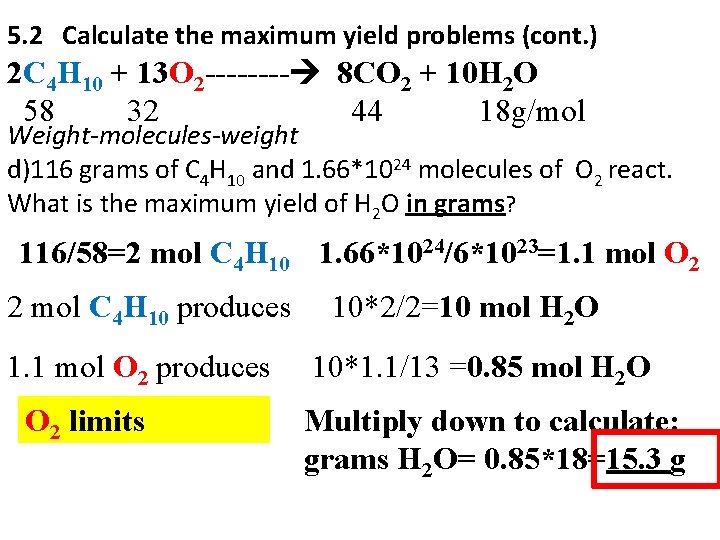
5. 2 Calculate the maximum yield problems (cont. ) 2 C 4 H 10 + 13 O 2 ---- 8 CO 2 + 10 H 2 O 58 32 44 18 g/mol Weight-molecules-weight d)116 grams of C 4 H 10 and 1. 66*1024 molecules of O 2 react. What is the maximum yield of H 2 O in grams? 116/58=2 mol C 4 H 10 1. 66*1024/6*1023=1. 1 mol O 2 2 mol C 4 H 10 produces 1. 1 mol O 2 produces O 2 limits 10*2/2=10 mol H 2 O 10*1. 1/13 =0. 85 mol H 2 O Multiply down to calculate: grams H 2 O= 0. 85*18=15. 3 g

U-Try-It on your own Clicker Examples

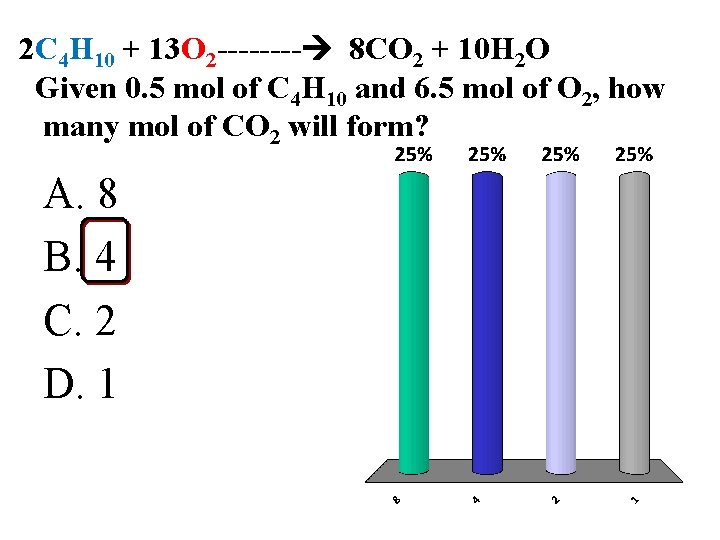
2 C 4 H 10 + 13 O 2 ---- 8 CO 2 + 10 H 2 O Given 0. 5 mol of C 4 H 10 and 6. 5 mol of O 2, how many mol of CO 2 will form? A. 8 B. 4 C. 2 D. 1

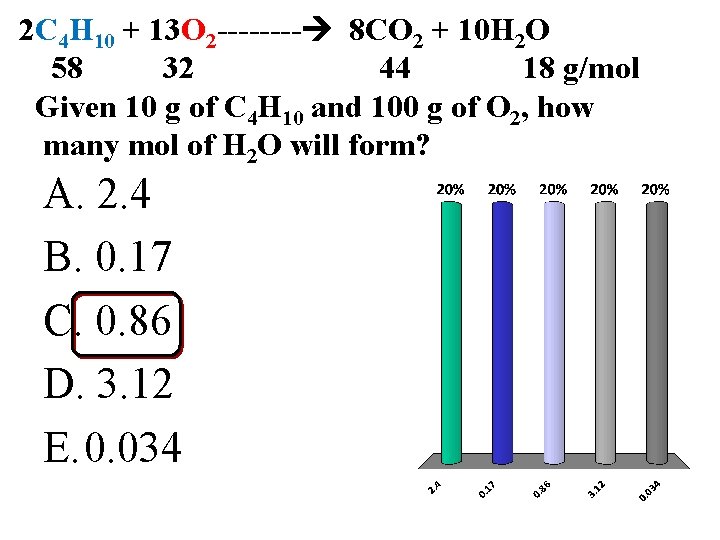
2 C 4 H 10 + 13 O 2 ---- 8 CO 2 + 10 H 2 O 58 32 44 18 g/mol Given 10 g of C 4 H 10 and 100 g of O 2, how many mol of H 2 O will form? A. 2. 4 B. 0. 17 C. 0. 86 D. 3. 12 E. 0. 034

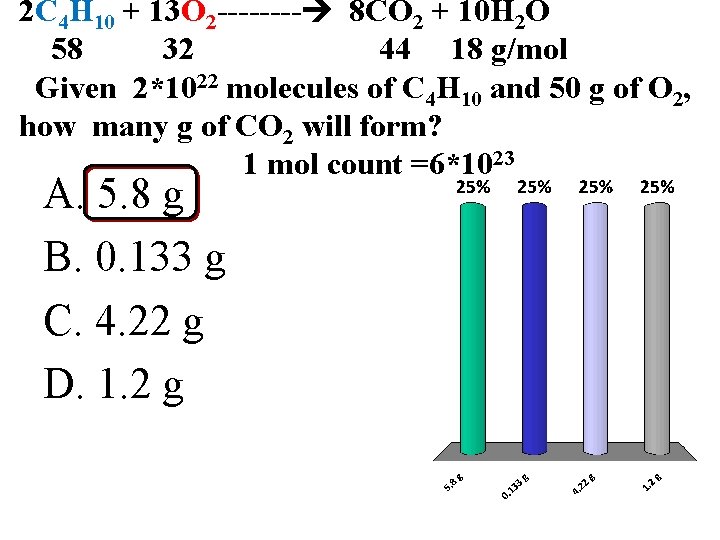
2 C 4 H 10 + 13 O 2 ---- 8 CO 2 + 10 H 2 O 58 32 44 18 g/mol Given 2*1022 molecules of C 4 H 10 and 50 g of O 2, how many g of CO 2 will form? 1 mol count =6*1023 A. 5. 8 g B. 0. 133 g C. 4. 22 g D. 1. 2 g

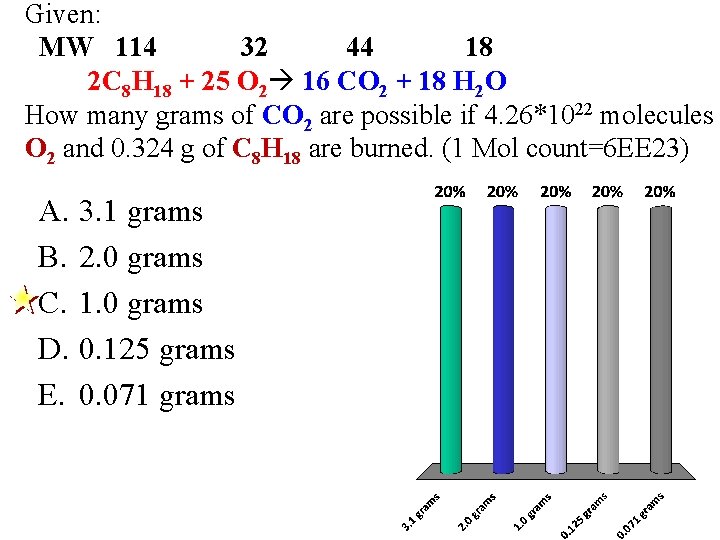
Given: MW 114 32 44 18 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O How many grams of CO 2 are possible if 4. 26*1022 molecules O 2 and 0. 324 g of C 8 H 18 are burned. (1 Mol count=6 EE 23) A. 3. 1 grams B. 2. 0 grams C. 1. 0 grams D. 0. 125 grams E. 0. 071 grams

Need more practice ? ? ?

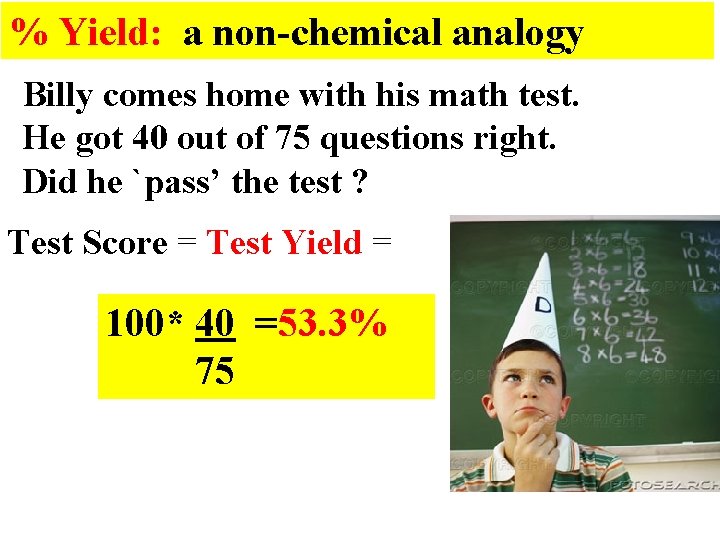
% Yield: a non-chemical analogy Billy comes home with his math test. He got 40 out of 75 questions right. Did he `pass’ the test ? Test Score = Test Yield = 100* 40 =53. 3% 75

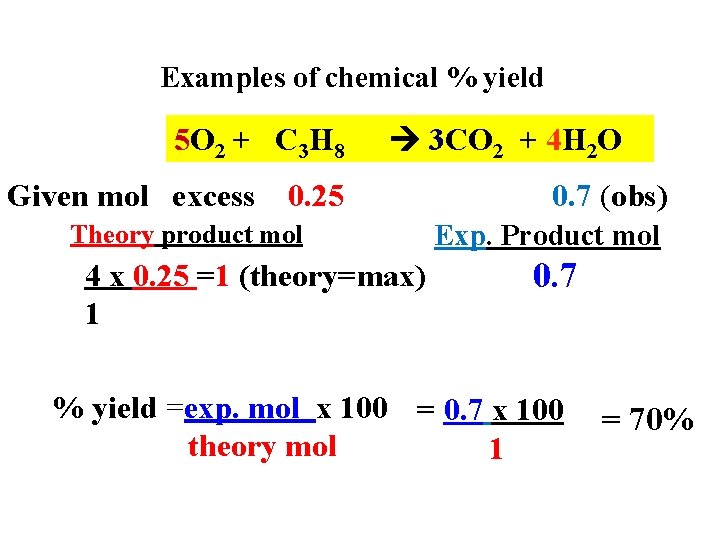
Examples of chemical % yield 5 O 2 + C 3 H 8 Given mol excess 3 CO 2 + 4 H 2 O 0. 25 Theory product mol 4 x 0. 25 =1 (theory=max) 1 0. 7 (obs) Exp. Product mol 0. 7 % yield =exp. mol x 100 = 0. 7 x 100 theory mol 1 = 70%

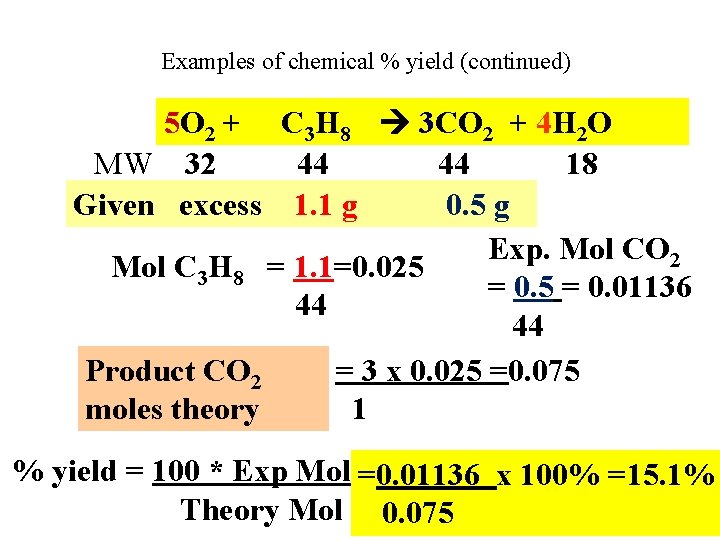
Examples of chemical % yield (continued) 5 O 2 + C 3 H 8 3 CO 2 + 4 H 2 O MW 32 44 44 18 Given excess 1. 1 g 0. 5 g Exp. Mol CO 2 Mol C 3 H 8 = 1. 1=0. 025 = 0. 01136 44 44 Product CO 2 = 3 x 0. 025 =0. 075 moles theory 1 % yield = 100 * Exp Mol =0. 01136 x 100% =15. 1% Theory Mol 0. 075

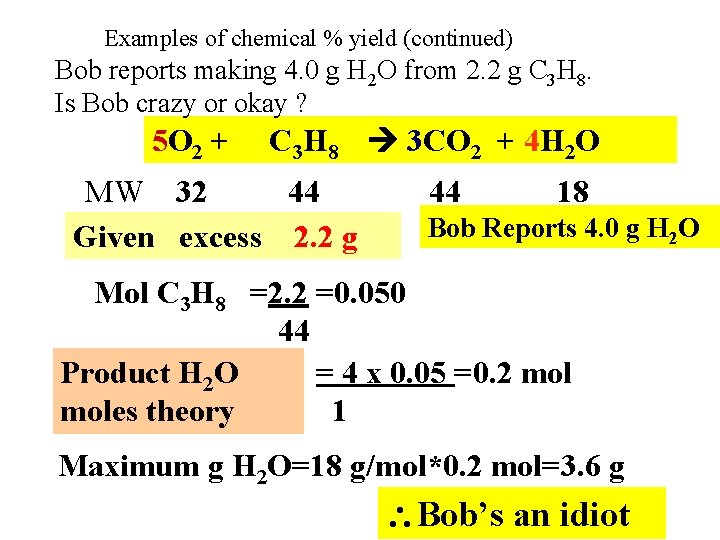
Examples of chemical % yield (continued) Bob reports making 4. 0 g H 2 O from 2. 2 g C 3 H 8. Is Bob crazy or okay ? 5 O 2 + C 3 H 8 3 CO 2 + 4 H 2 O MW 32 44 Given excess 2. 2 g 44 18 Bob Reports 4. 0 g H 2 O Mol C 3 H 8 =2. 2 =0. 050 44 Product H 2 O = 4 x 0. 05 =0. 2 moles theory 1 Maximum g H 2 O=18 g/mol*0. 2 mol=3. 6 g Bob’s an idiot

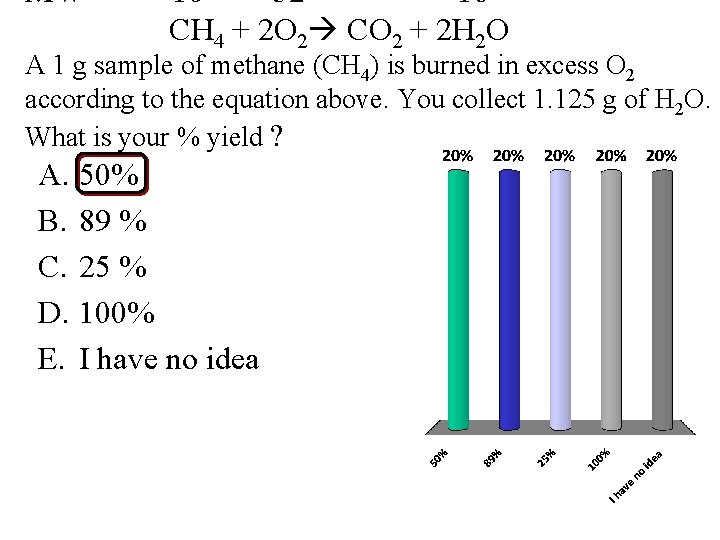
MW 16 32 18 CH 4 + 2 O 2 CO 2 + 2 H 2 O A 1 g sample of methane (CH 4) is burned in excess O 2 according to the equation above. You collect 1. 125 g of H 2 O. What is your % yield ? A. 50% B. 89 % C. 25 % D. 100% E. I have no idea

Need more practice ? ? ?
 Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram Homework due today
Homework due today Homework due today
Homework due today Homework
Homework 3 weeks from today
3 weeks from today Astr
Astr Homework due today
Homework due today Today meeting or today's meeting
Today meeting or today's meeting Today's class was amazing
Today's class was amazing Proposal kickoff meeting agenda
Proposal kickoff meeting agenda Fingerprint galton details
Fingerprint galton details Today's lesson or today lesson
Today's lesson or today lesson Example of repitition
Example of repitition Assignment due today
Assignment due today Assignment due today
Assignment due today Assignment due today
Assignment due today Reports due today
Reports due today Blood supply of liver
Blood supply of liver