Homework 4 due today Homework 5 posted and





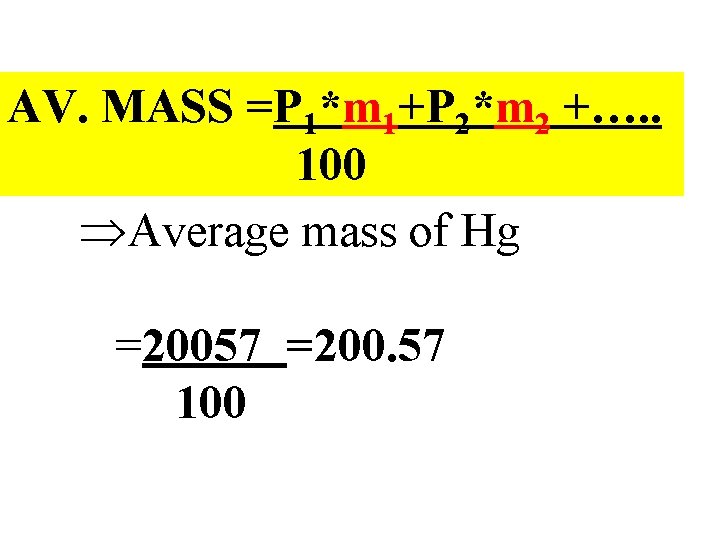







- Slides: 45

Homework 4 due today Homework 5 posted and due this Friday It’s hump day !!! Two days till the 3 rd week is over !!

Homework 4… • another look at prefix assignment • Boo boos in naming

metals Naming Inorganic compounds: in-class practicing Non-metals Transition metals Lanthanides Actinides

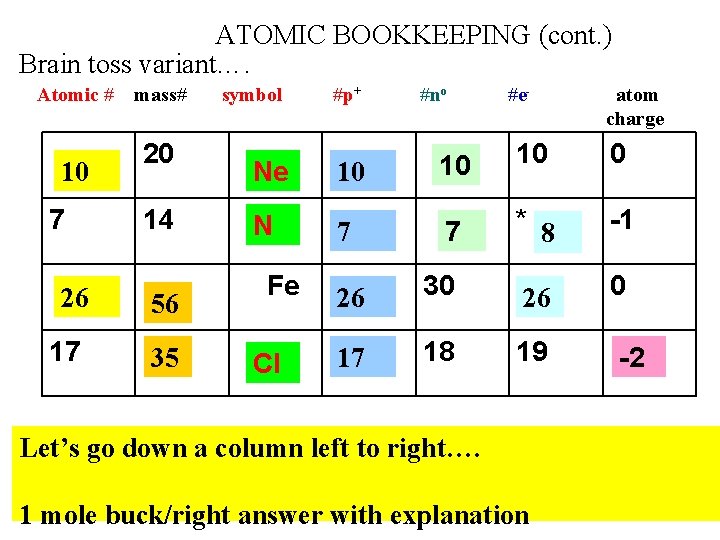
ATOMIC BOOKKEEPING (cont. ) Brain toss variant…. Atomic # 10 7 26 17 mass# 20 14 56 35 symbol #p+ #no #e- atom charge 10 0 Ne 10 10 N 7 7 * 8 -1 26 30 26 0 17 18 19 Fe Cl Let’s go down a column left to right…. 1 mole buck/right answer with explanation -2

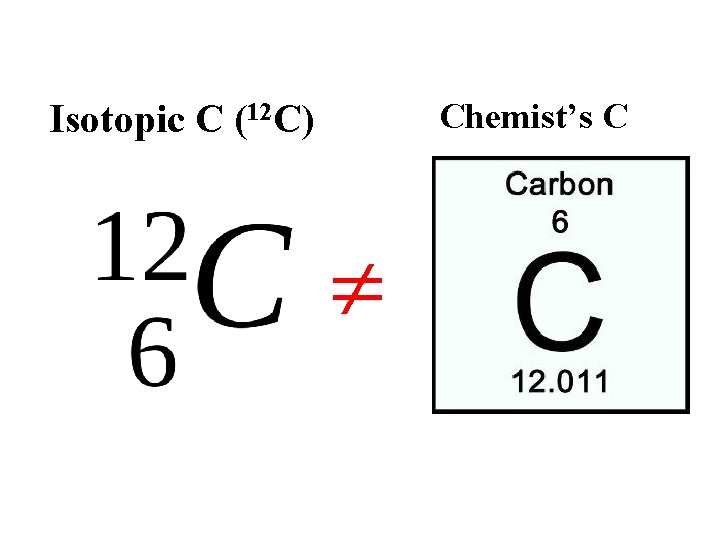
Chemist’s C Isotopic C (12 C)

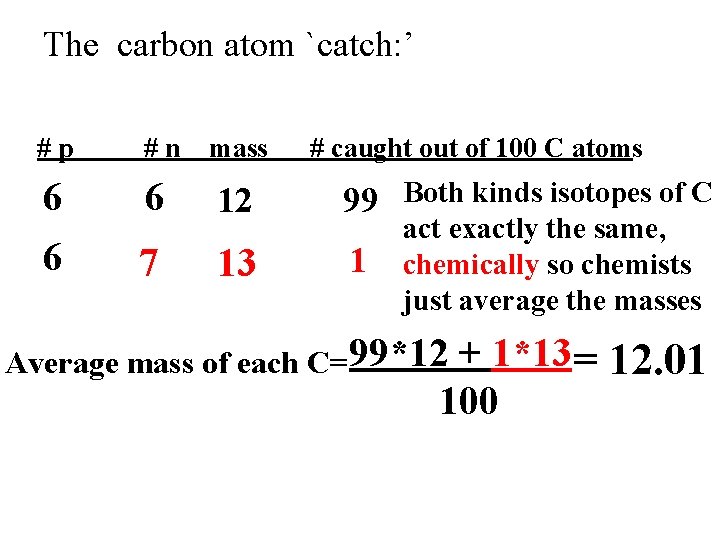
Why the Chemist’s C lists 12. 01 and not 12 Imagine `fishing’ out 100 atoms of Carbon from a sample of graphite (pure carbon). What would you catch ?

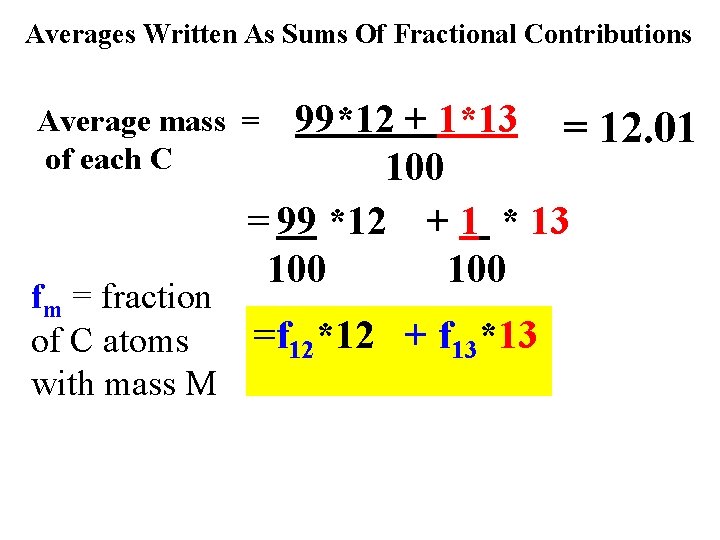
The carbon atom `catch: ’ #p #n mass # caught out of 100 C atoms 6 6 6 12 99 Both kinds isotopes of C 7 13 1 act exactly the same, chemically so chemists just average the masses Average mass of each C=99*12 + 1*13 = 12. 01 100

Averages Written As Sums Of Fractional Contributions 99*12 + 1*13 = 12. 01 100 = 99 *12 + 1 * 13 100 Average mass = of each C fm = fraction of C atoms with mass M =f 12*12 + f 13*13

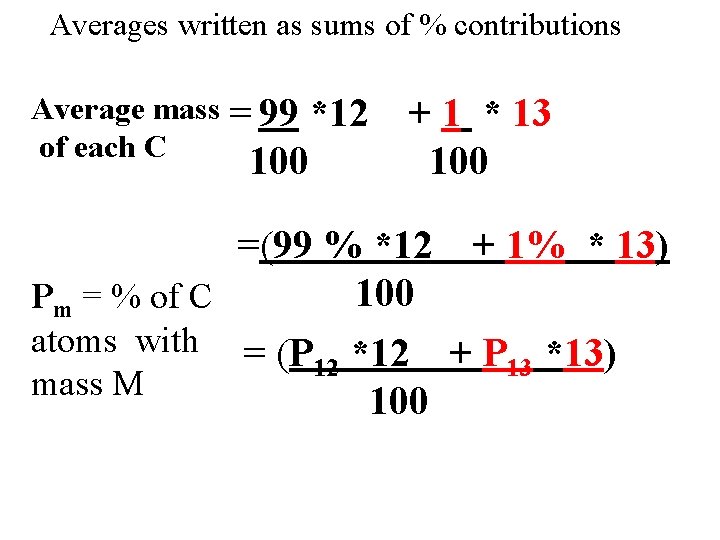
Averages written as sums of % contributions Average mass = 99 of each C *12 100 + 1 * 13 100 =(99 % *12 + 1% * 13) 100 Pm = % of C atoms with = (P *12 + P *13) 12 13 mass M 100

Take home lesson : Average mass is computable from fractional abundances fk and mk so: AV. MASS =f 1*m 1+f 2*m 2 +…. . Or, from % abundances Pk and mk so: AV. MASS =P 1*m 1+P 2*m 2 +…. . 100

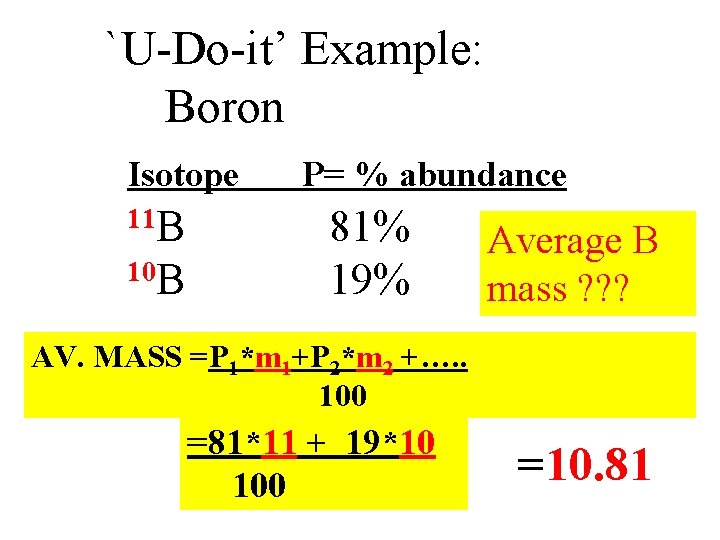
`U-Do-it’ Example: Boron Isotope 11 B 10 B P= % abundance 81% 19% Average B mass ? ? ? AV. MASS =P 1*m 1+P 2*m 2 +…. . 100 =81*11 + 19*10 100 =10. 81

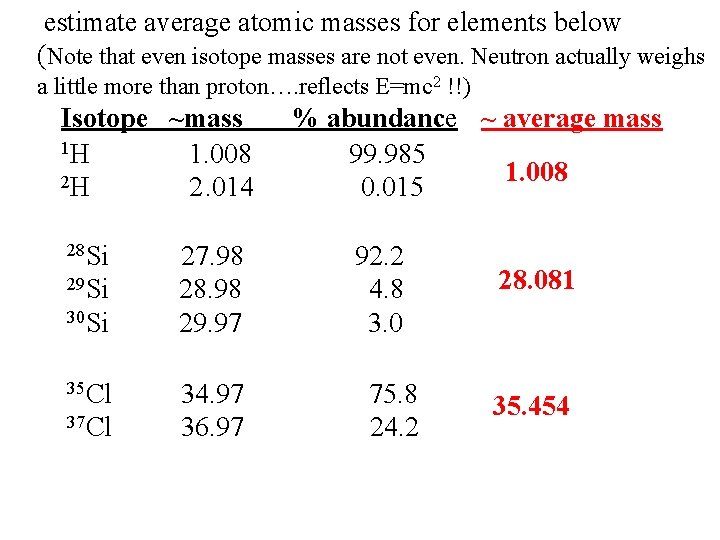
estimate average atomic masses for elements below (Note that even isotope masses are not even. Neutron actually weighs a little more than proton…. reflects E=mc 2 !!) Isotope ~mass 1 H 1. 008 2 H 2. 014 28 Si 29 Si 30 Si 35 Cl 37 Cl 27. 98 28. 98 29. 97 34. 97 36. 97 % abundance ~ average mass 99. 985 1. 008 0. 015 92. 2 4. 8 3. 0 75. 8 24. 2 28. 081 35. 454

Need more, or are you exhausted ? ? ?

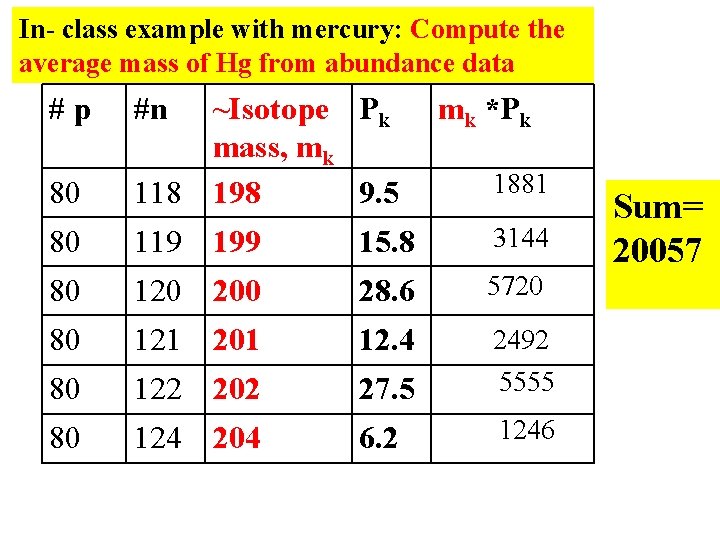
In- class example with mercury: Compute the average mass of Hg from abundance data #p #n 80 ~Isotope Pk mass, mk 118 198 9. 5 mk *Pk 80 119 199 15. 8 3144 80 120 200 28. 6 5720 80 121 201 12. 4 80 122 202 27. 5 2492 5555 80 124 204 6. 2 1246 1881 Sum= 20057
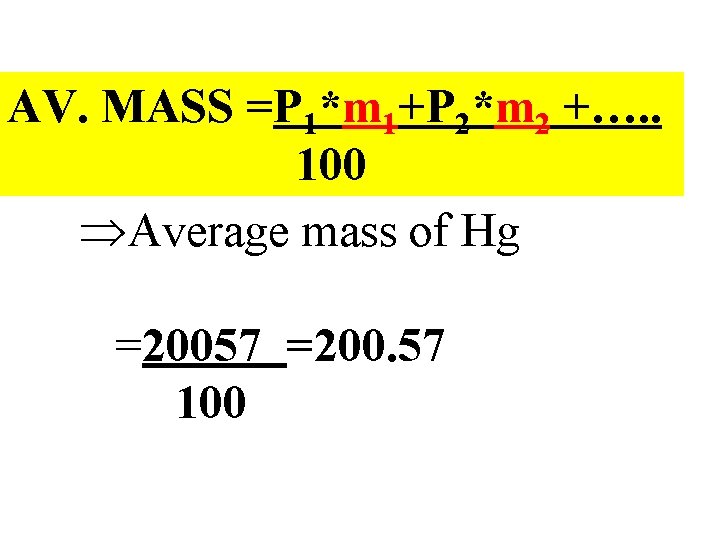
AV. MASS =P 1*m 1+P 2*m 2 +…. . 100 ÞAverage mass of Hg =20057 =200. 57 100

Chapter 3: MOLES- Every science involves counting Astronomers count …… Animal biologists count … Bacteriologists count. . . Chemists count…….

Astronomers can see…. Animal biologists can see … Bacteriologists can see… Chemists can’t see…. molecules

Even worse news…even if we could see molecules…guess long would it take to count the number of water molecules in a tea cup ? ?

Let’s use the Chinese Tihane-2 computer (world’s current fastest computer) to count all the molecules of water in a teacup assuming it counted at its maximum processor rate = 34, 000, 000 molecules/second (=3400 THz=3. 4*106 GHz 1 ) ? How long would it take to count them all ? Answer: ~ 3. 2 years 1 ~ 1 million times faster than a typical laptop (It houses 3 million processors in parallel)

From your lecture syllabus…. Student Learning Outcome 5 Students should be able to perform basic chemical calculations connected to: • • • mole-weight-count conversions reaction stoichiometry limiting yields. Translation: How do chemists count atoms and molecules without actually having to count them ? ? ?

Chemists count atoms and molecules using… Moles

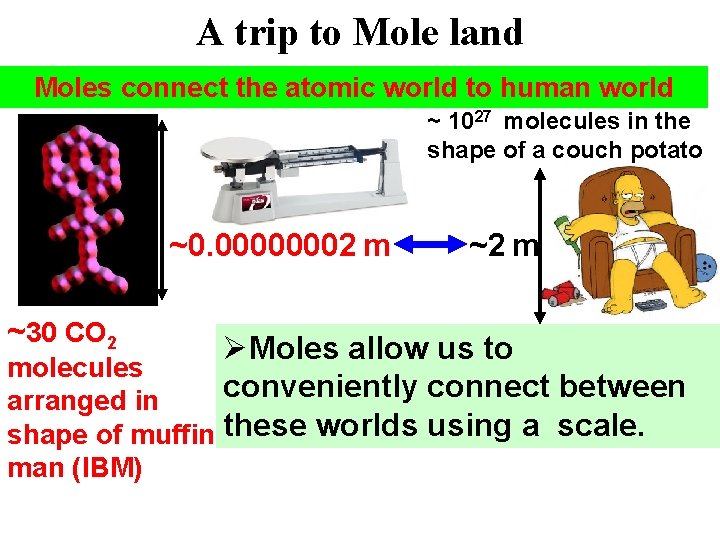
A trip to Mole land Moles connect the atomic world to human world ~ 1027 molecules in the shape of a couch potato ~0. 00000002 m ~30 CO 2 ~2 m ØMoles allow us to molecules conveniently connect between arranged in shape of muffin these worlds using a scale. man (IBM)

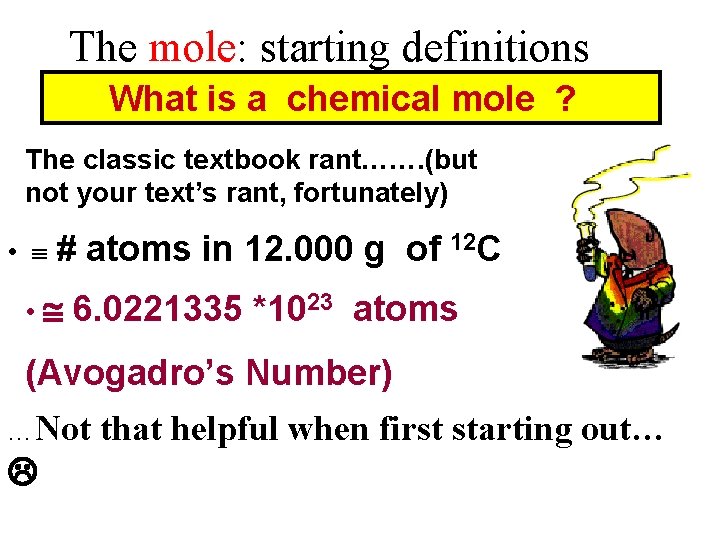
The mole: starting definitions What is a chemical mole ? The classic textbook rant……. (but not your text’s rant, fortunately) • # • atoms in 12. 000 g of 12 C 6. 0221335 *1023 atoms (Avogadro’s Number) …Not that helpful when first starting out…

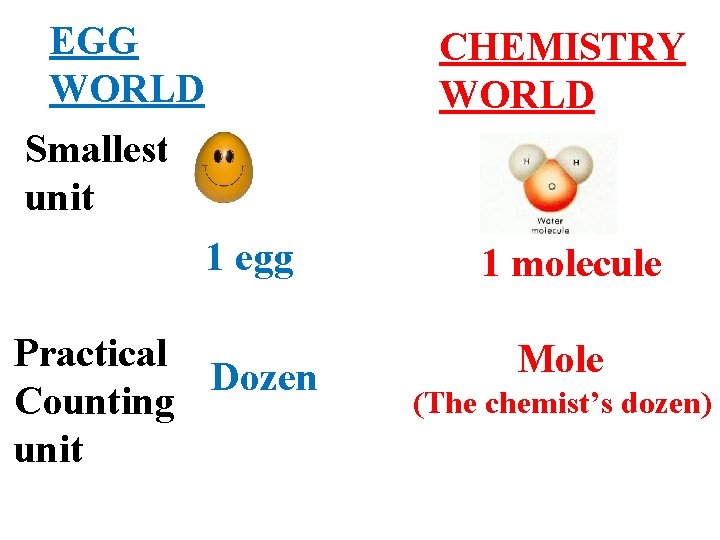
A better beginning: the mole concept is really … the same idea as a `dozen. ’

IN-CLASS EGG CALCULATIONS

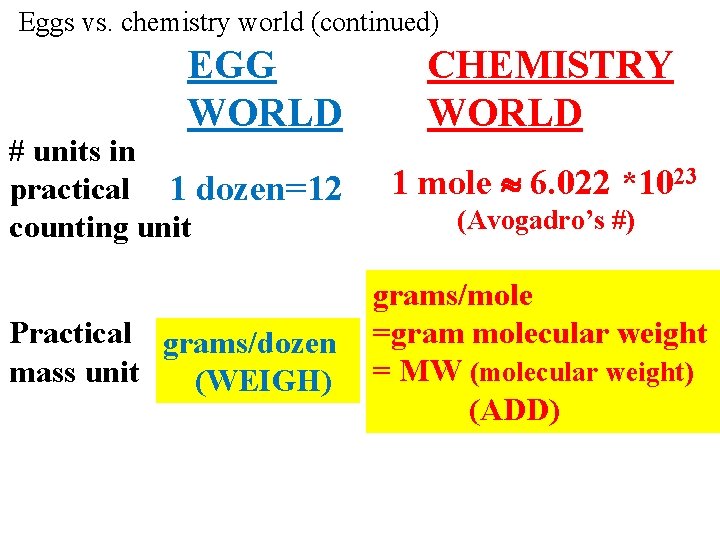
EGG WORLD CHEMISTRY WORLD Smallest unit 1 egg Practical Dozen Counting unit 1 molecule Mole (The chemist’s dozen)

Eggs vs. chemistry world (continued) EGG WORLD CHEMISTRY WORLD # units in practical 1 dozen=12 counting unit 1 mole 6. 022 *1023 Practical grams/dozen mass unit (WEIGH) grams/mole =gram molecular weight = MW (molecular weight) (ADD) (Avogadro’s #)

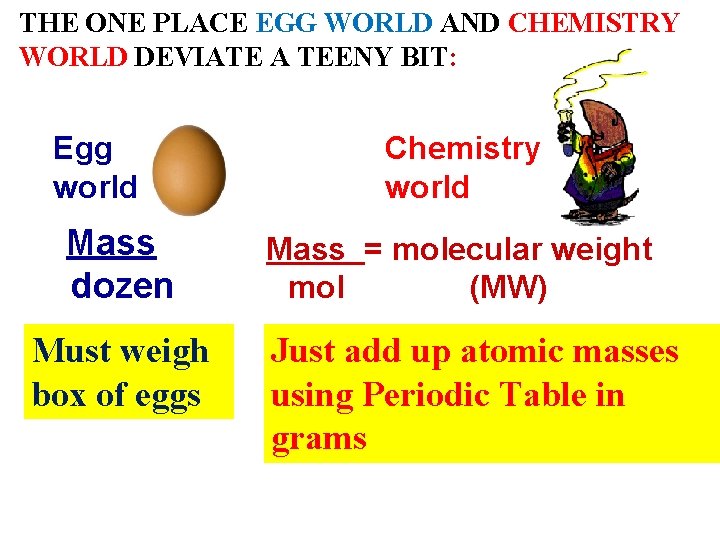
THE ONE PLACE EGG WORLD AND CHEMISTRY WORLD DEVIATE A TEENY BIT: Egg world Mass dozen Must weigh box of eggs Chemistry world Mass = molecular weight mol (MW) Just add up atomic masses using Periodic Table in grams

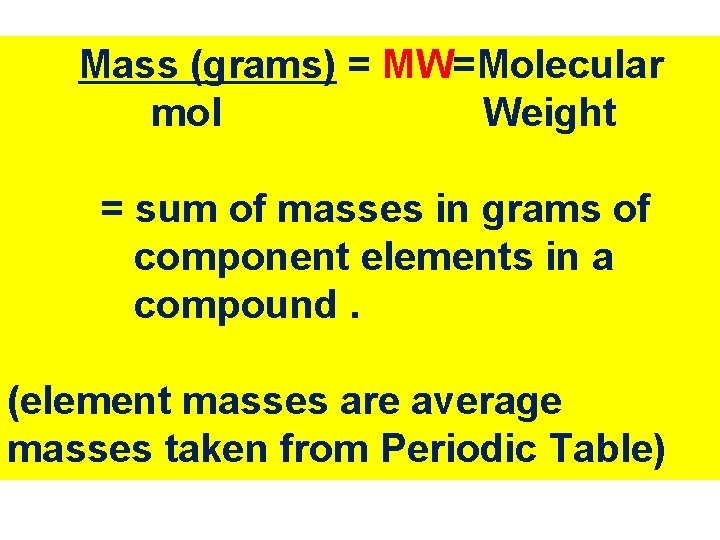
Mass (grams) = MW=Molecular mol Weight = sum of masses in grams of component elements in a compound. (element masses are average masses taken from Periodic Table)

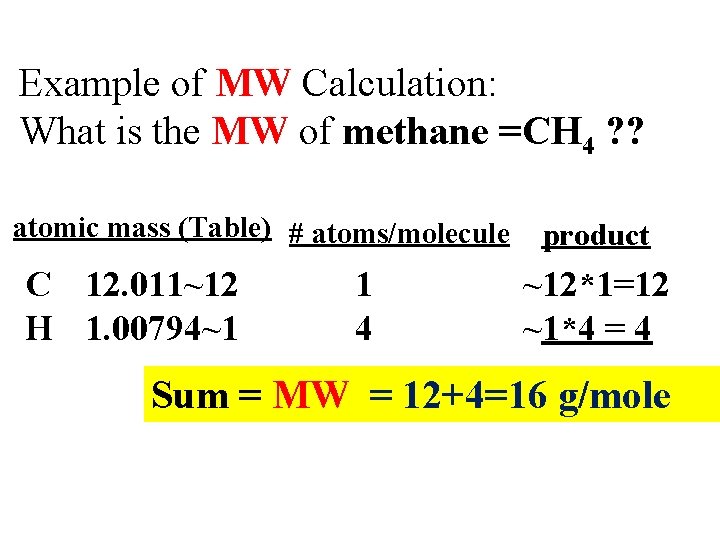
Example of MW Calculation: What is the MW of methane =CH 4 ? ? atomic mass (Table) # atoms/molecule C 12. 011~12 H 1. 00794~1 1 4 product ~12*1=12 ~1*4 = 4 Sum = MW = 12+4=16 g/mole

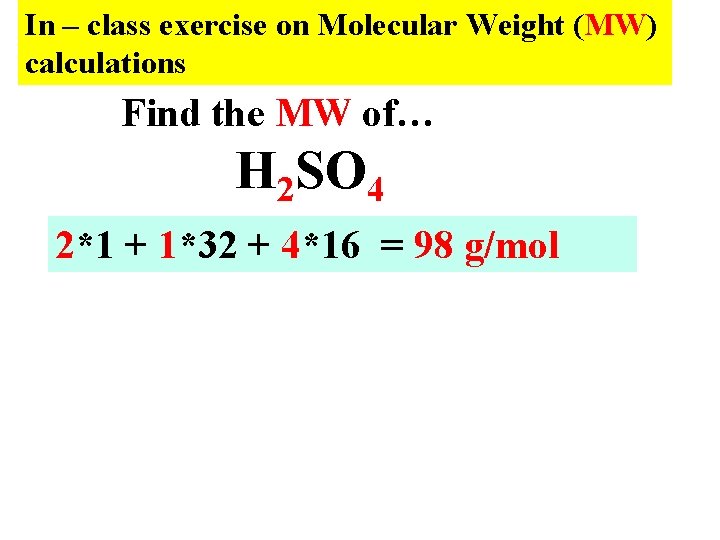
In – class exercise on Molecular Weight (MW) calculations Find the MW of… H 2 SO 4 2*1 + 1*32 + 4*16 = 98 g/mol

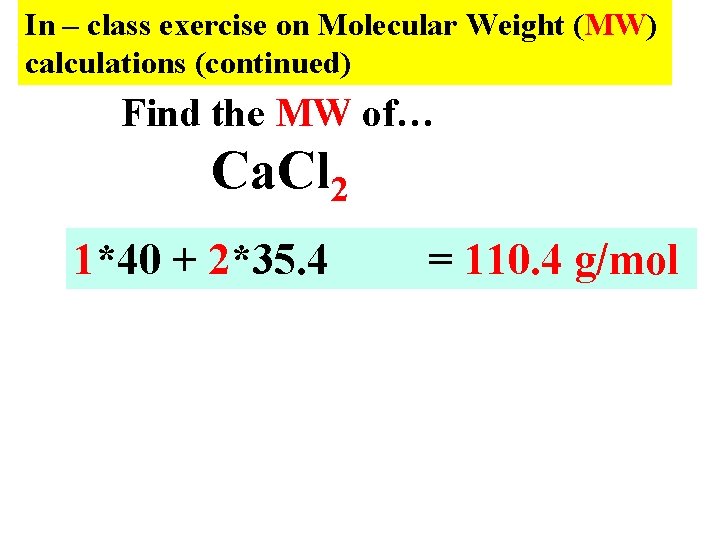
In – class exercise on Molecular Weight (MW) calculations (continued) Find the MW of… Ca. Cl 2 1*40 + 2*35. 4 = 110. 4 g/mol

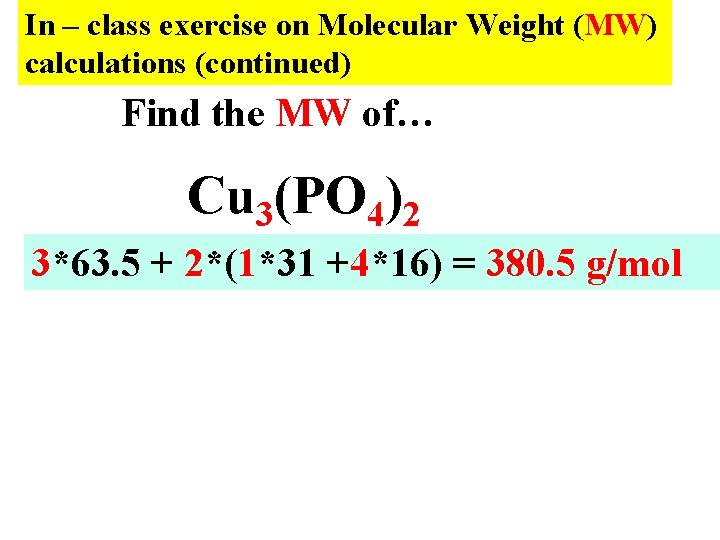
In – class exercise on Molecular Weight (MW) calculations (continued) Find the MW of… Cu 3(PO 4)2 3*63. 5 + 2*(1*31 +4*16) = 380. 5 g/mol

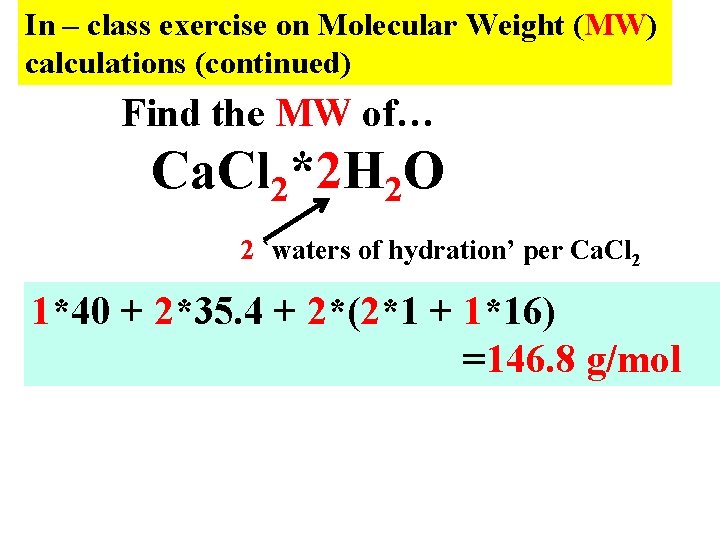
In – class exercise on Molecular Weight (MW) calculations (continued) Find the MW of… Ca. Cl 2*2 H 2 O 2 `waters of hydration’ per Ca. Cl 2 1*40 + 2*35. 4 + 2*(2*1 + 1*16) =146. 8 g/mol

IN-CLASS simple mole calculations (on board) the dozen method way

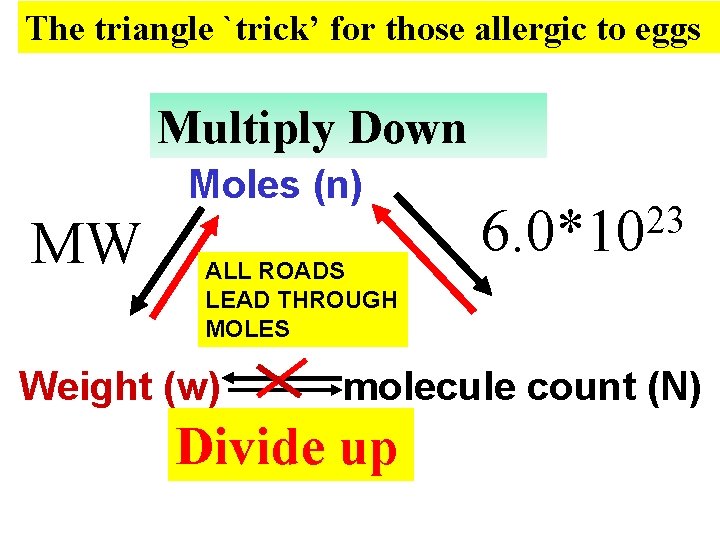
The triangle `trick’ for those allergic to eggs Multiply Down Moles (n) MW ALL ROADS LEAD THROUGH MOLES Weight (w) 23 6. 0*10 molecule count (N) Divide up

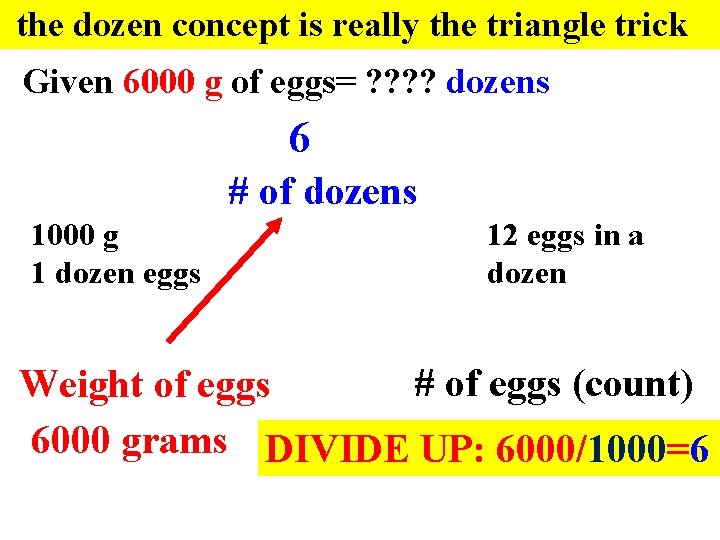
the dozen concept is really the triangle trick Given 6000 g of eggs= ? ? dozens 6 # of dozens 1000 g 1 dozen eggs 12 eggs in a dozen # of eggs (count) Weight of eggs 6000 grams DIVIDE UP: 6000/1000=6

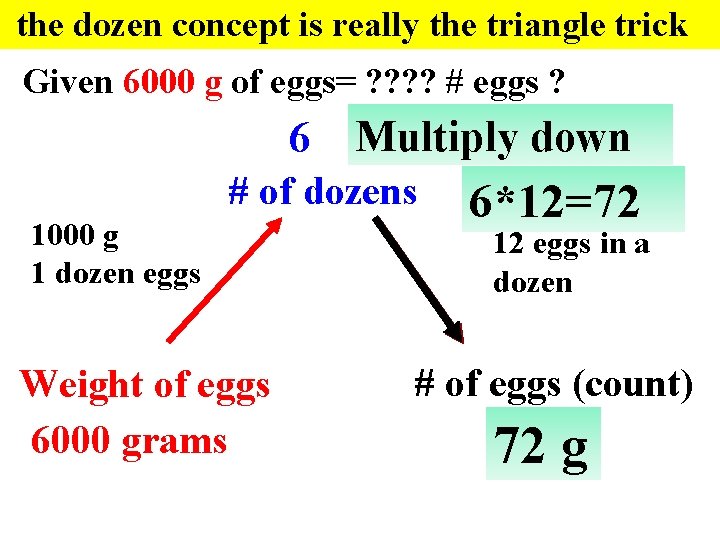
the dozen concept is really the triangle trick Given 6000 g of eggs= ? ? # eggs ? 6 Multiply down # of dozens 1000 g 1 dozen eggs Weight of eggs 6000 grams 6*12=72 12 eggs in a dozen # of eggs (count) 72 g

On the triangle…, . DIVIDE UP MULTIPLY DOWN

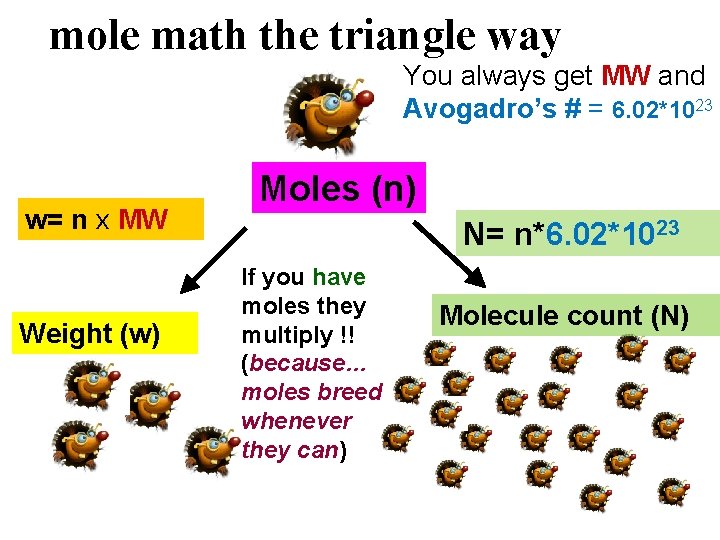
mole math the triangle way You always get MW and Avogadro’s # = 6. 02*1023 w= n x MW Weight (w) Moles (n) N= n*6. 02*1023 If you have moles they multiply !! (because… moles breed whenever they can) Molecule count (N)

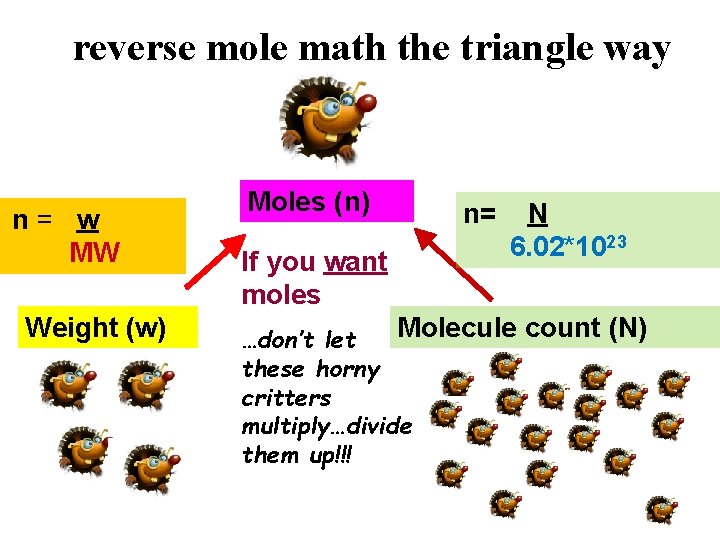
reverse mole math the triangle way n= w MW Weight (w) Moles (n) If you want moles n= N 6. 02*1023 Molecule count (N) …don’t let these horny critters multiply…divide them up!!!

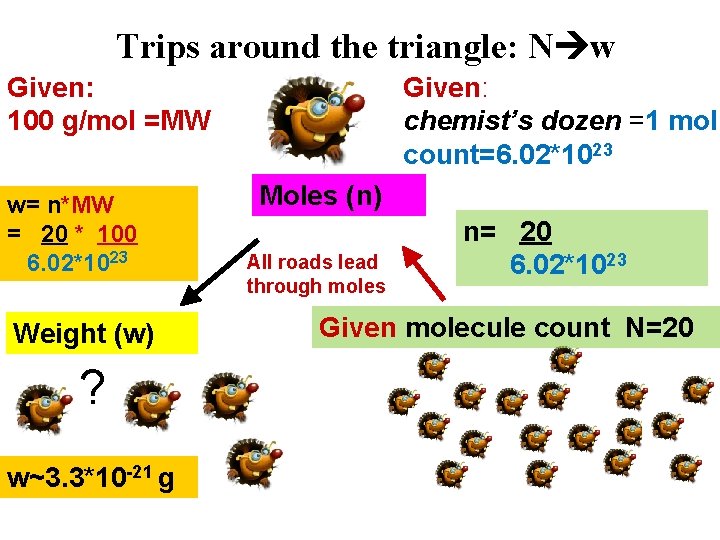
Trips around the triangle: N w Given: 100 g/mol =MW w= n*MW = 20 * 100 6. 02*1023 Weight (w) ? w~3. 3*10 -21 g Given: chemist’s dozen =1 mol count=6. 02*1023 Moles (n) All roads lead through moles n= 20 6. 02*1023 Given molecule count N=20

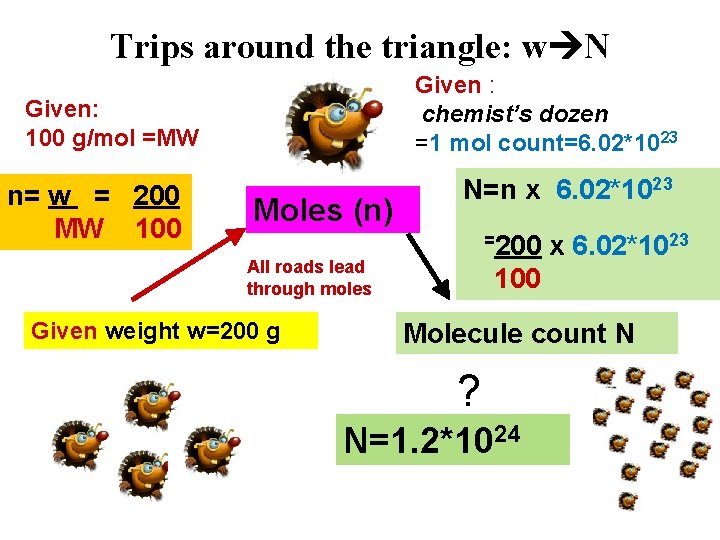
Trips around the triangle: w N Given : chemist’s dozen =1 mol count=6. 02*1023 Given: 100 g/mol =MW n= w = 200 MW 100 Moles (n) N=n x 6. 02*1023 =200 All roads lead through moles Given weight w=200 g x 6. 02*1023 100 Molecule count N ? N=1. 2*1024

IN-CLASS simple mole calculations (on board) the triangle trick way

AN ANECDOTE An `out-of-towner’ visiting NYC for the first time asks Joshua Bell (world’s greatest violinist): “How do you get to the Lincoln Center for the Performing Arts ? ” Joshua’s answer (supposedly): “Practice, practice, practice…. ”