Gas Stoichiometry Moles Liters of a Gas STP
- Slides: 10

Gas Stoichiometry Moles Liters of a Gas: – STP - use 22. 4 L/mol – Non-STP - use ideal gas law Non-STP – Given liters of gas? • start with ideal gas law – Looking for liters of gas? • start with stoichiometry conversion Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

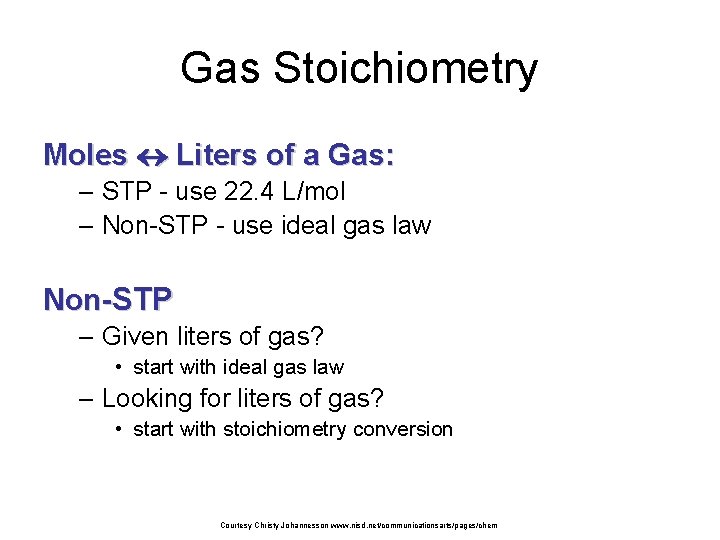
Gas Stoichiometry Problem What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? Ca. CO 3 5. 25 g Ca. O + Looking for liters: Start with stoich and calculate moles of CO 2. 5. 25 g 1 mol Ca. CO 3 1 mol CO 2 100. 09 g 1 mol Ca. CO 3 CO 2 ? L non-STP = 1. 26 mol CO 2 Plug this into the Ideal Gas Law to find liters. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

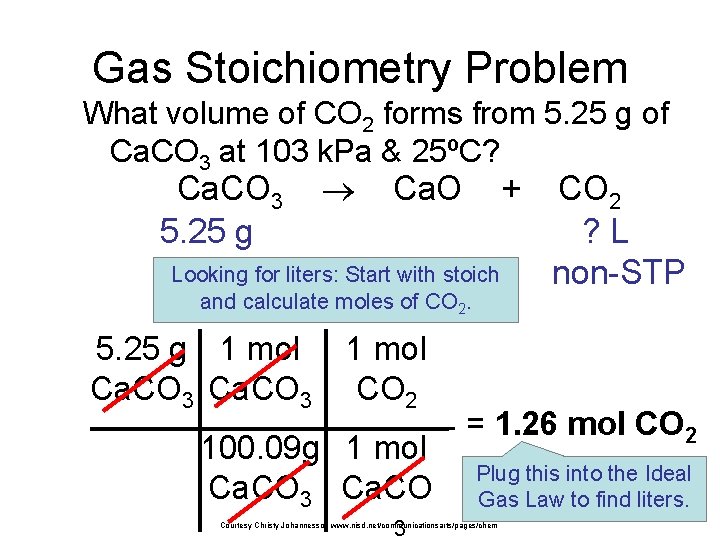
Gas Stoichiometry Problem • What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? GIVEN: P = 103 k. Pa V=? n = 1. 26 mol T = 25°C = 298 K R = 8. 315 dm 3 k. Pa/mol K WORK: PV = n. RT (103 k. Pa)V =(1 mol)(8. 315 dm 3 k. Pa/mol K)( 298 K) V = 1. 26 dm 3 CO 2 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

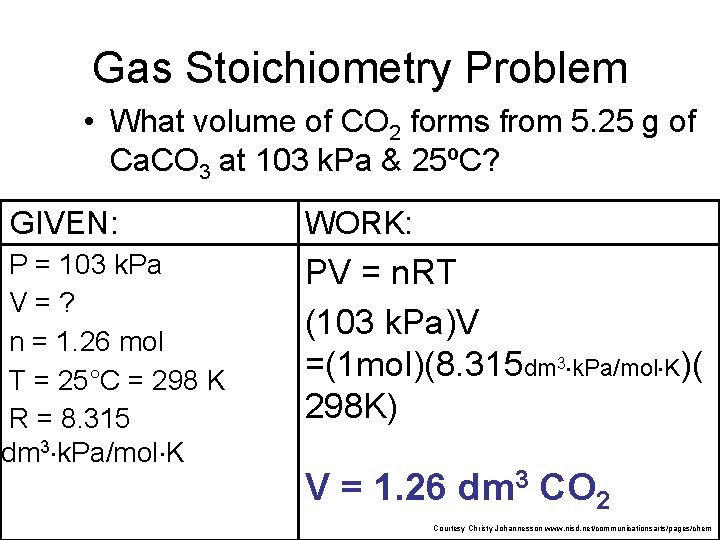
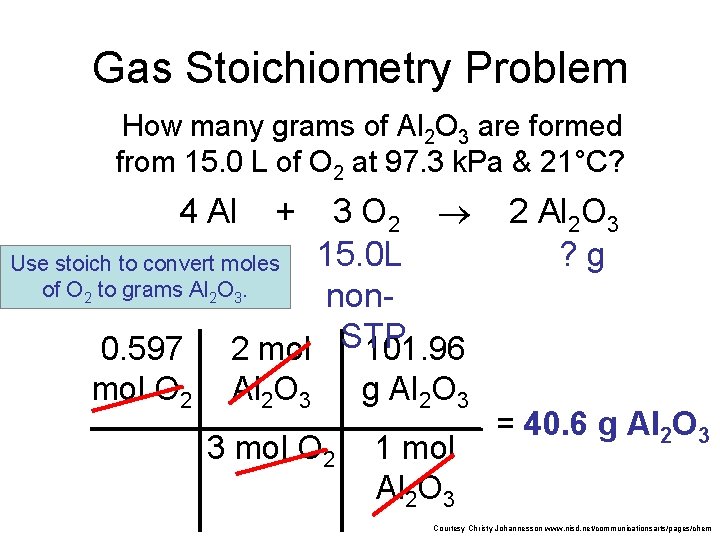
Gas Stoichiometry Problem How many grams of Al 2 O 3 are formed from 15. 0 L O 2 at 97. 3 k. Pa & 21°C? 4 Al + 3 O 2 15. 0 L non. STP 2 Al 2 O 3 ? g GIVEN: WORK: P = 97. 3 k. Pa V = 15. 0 L n=? T = 21°C = 294 K R = 8. 315 PV = n. RT (97. 3 k. Pa) (15. 0 L) = n (8. 315 dm 3 k. Pa/mol K) NEXT (294 K) dm 3 k. Pa/mol K of Given liters: Start with Ideal Gas Law and calculate moles of O 2. n = 0. 597 mol O Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Gas Stoichiometry Problem How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 97. 3 k. Pa & 21°C? 3 O 2 15. 0 L Use stoich to convert moles of O 2 to grams Al 2 O 3. non. STP 0. 597 2 mol 101. 96 4 Al mol O 2 + Al 2 O 3 g Al 2 O 3 3 mol O 2 1 mol Al 2 O 3 2 Al 2 O 3 ? g = 40. 6 g Al 2 O 3 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

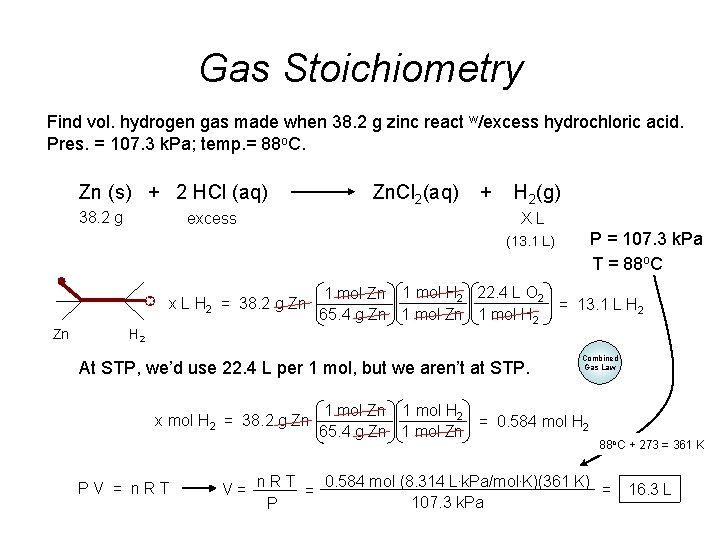
Gas Stoichiometry Find vol. hydrogen gas made when 38. 2 g zinc react w/excess hydrochloric acid. Pres. = 107. 3 k. Pa; temp. = 88 o. C. Zn (s) + 2 HCl (aq) 38. 2 g excess Zn. Cl 2(aq) + H 2(g) XL P = 107. 3 k. Pa T = 88 o. C (13. 1 L) x L H 2 = 38. 2 g Zn Zn 1 mol H 2 22. 4 L O 2 = 13. 1 L H 2 65. 4 g Zn 1 mol H 2 At STP, we’d use 22. 4 L per 1 mol, but we aren’t at STP. x mol H 2 = 38. 2 g Zn PV = n. RT Combined Gas Law 1 mol Zn 1 mol H 2 = 0. 584 mol H 2 65. 4 g Zn 1 mol Zn 88 o. C + 273 = 361 K 0. 584 mol (8. 314 L. k. Pa/mol. K)(361 K) V= n. RT = = 107. 3 k. Pa P 16. 3 L

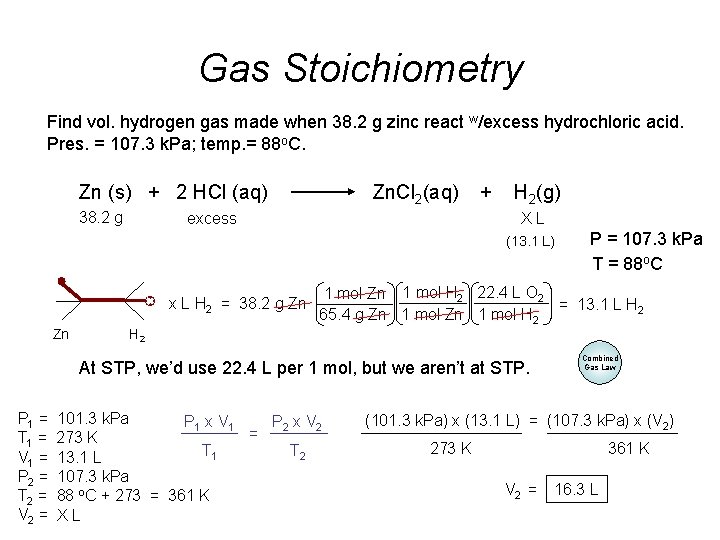
Gas Stoichiometry Find vol. hydrogen gas made when 38. 2 g zinc react w/excess hydrochloric acid. Pres. = 107. 3 k. Pa; temp. = 88 o. C. Zn (s) + 2 HCl (aq) 38. 2 g Zn. Cl 2(aq) excess + H 2(g) XL (13. 1 L) x L H 2 = 38. 2 g Zn Zn 1 mol H 2 22. 4 L O 2 = 13. 1 L H 2 65. 4 g Zn 1 mol H 2 At STP, we’d use 22. 4 L per 1 mol, but we aren’t at STP. P 1 = T 1 = V 1 = P 2 = T 2 = V 2 = P = 107. 3 k. Pa T = 88 o. C 101. 3 k. Pa P 1 x V 1 P 2 x V 2 = 273 K T 1 T 2 13. 1 L 107. 3 k. Pa 88 o. C + 273 = 361 K XL Combined Gas Law (101. 3 k. Pa) x (13. 1 L) = (107. 3 k. Pa) x (V 2) 273 K 361 K V 2 = 16. 3 L

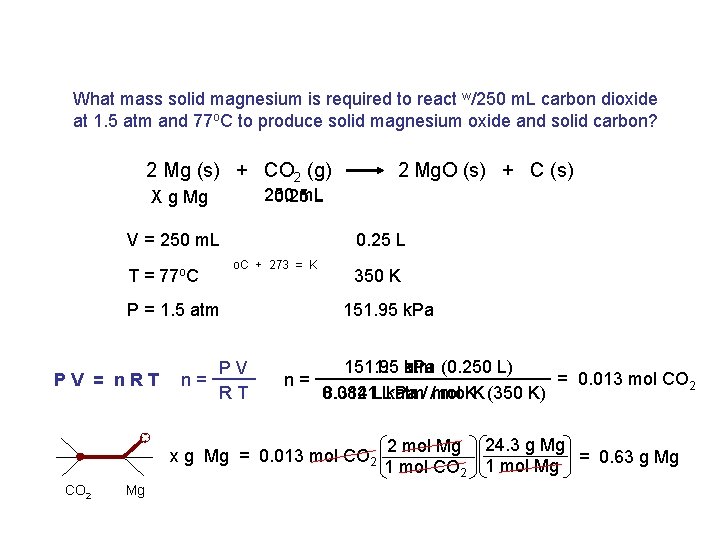
What mass solid magnesium is required to react w/250 m. L carbon dioxide at 1. 5 atm and 77 o. C to produce solid magnesium oxide and solid carbon? 2 Mg (s) + CO 2 (g) 250 m. L 0. 25 L X g Mg 0. 25 L V = 250 m. L o. C + 273 = K T = 77 o. C n= PV RT 350 K 151. 95 k. Pa P = 1. 5 atm PV = n. RT 2 Mg. O (s) + C (s) n= 151. 95 1. 5 k. Pa atm (0. 250 L) = 0. 013 mol CO 2. atm/ /mol 0. 0821 mol. K. K (350 K) 8. 314 LL. k. Pa x g Mg = 0. 013 mol CO 2 Mg 2 mol Mg 1 mol CO 2 24. 3 g Mg = 0. 63 g Mg 1 mol Mg

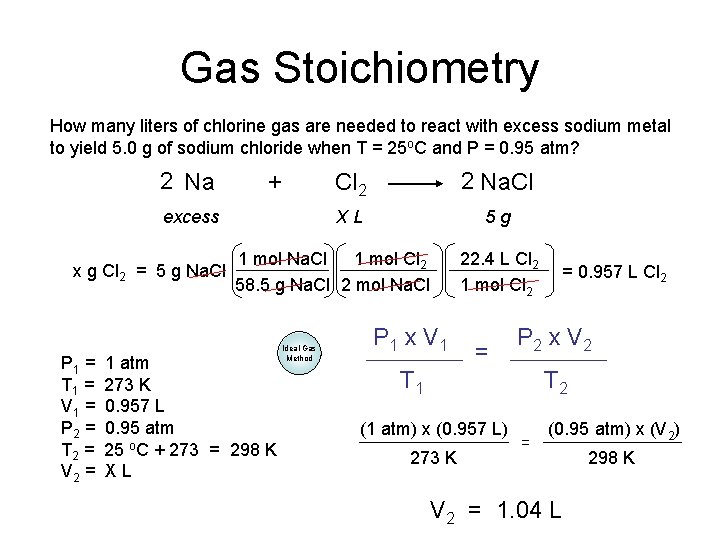
Gas Stoichiometry How many liters of chlorine gas are needed to react with excess sodium metal to yield 5. 0 g of sodium chloride when T = 25 o. C and P = 0. 95 atm? 2 Na + excess x g Cl 2 = 5 g Na. Cl P 1 = T 1 = V 1 = P 2 = T 2 = V 2 = Cl 2 2 Na. Cl XL 5 g 1 mol Na. Cl 1 mol Cl 2 58. 5 g Na. Cl 2 mol Na. Cl 1 atm 273 K 0. 957 L 0. 95 atm 25 o. C + 273 = 298 K XL Ideal Gas Method 22. 4 L Cl 2 1 mol Cl 2 P 1 x V 1 = = 0. 957 L Cl 2 P 2 x V 2 T 1 T 2 (1 atm) x (0. 957 L) 273 K = (0. 95 atm) x (V 2) V 2 = 1. 04 L 298 K

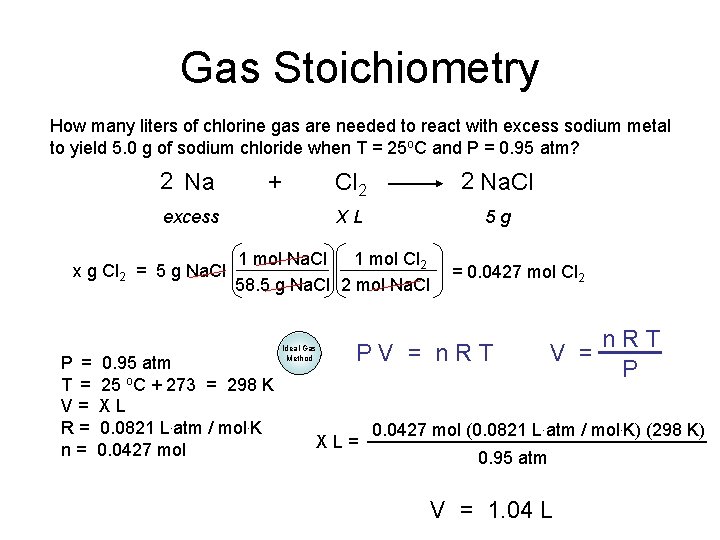
Gas Stoichiometry How many liters of chlorine gas are needed to react with excess sodium metal to yield 5. 0 g of sodium chloride when T = 25 o. C and P = 0. 95 atm? 2 Na + excess x g Cl 2 = 5 g Na. Cl Cl 2 2 Na. Cl XL 5 g 1 mol Na. Cl 1 mol Cl 2 58. 5 g Na. Cl 2 mol Na. Cl P = 0. 95 atm T = 25 o. C + 273 = 298 K V= XL R = 0. 0821 L. atm / mol. K n = 0. 0427 mol Ideal Gas Method = 0. 0427 mol Cl 2 PV = n. RT XL= n. RT V = P 0. 0427 mol (0. 0821 L. atm / mol. K) (298 K) 0. 95 atm V = 1. 04 L