Gas Stoichiometry Gas Stoichiometry In this particular section




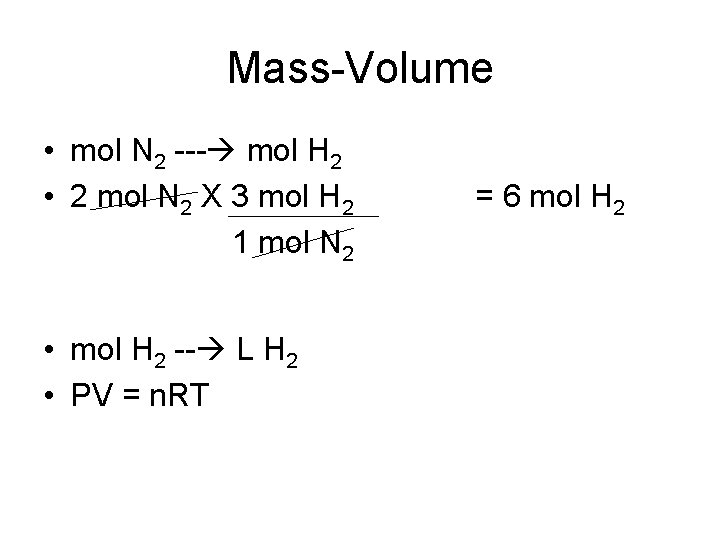
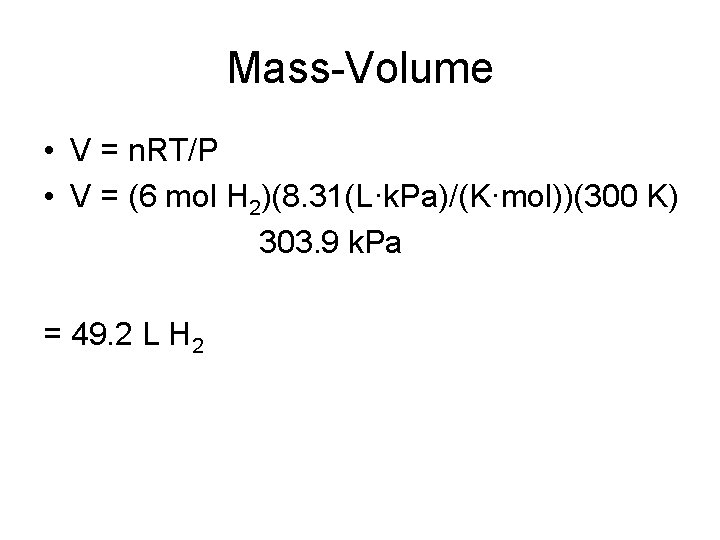

- Slides: 28

Gas Stoichiometry

Gas Stoichiometry • In this particular section of chemistry, you will be using the Ideal gas law to help you with your calculations. - In order to use this law properly, you will need to have enough information to solve for the variable that is needed. • You will also need to utilize what you learned in both the regular stoichiometry section and the gas law section. - In order to do so, you will need to use the Periodic table, the balanced chemical equation/reaction, and be able to manipulate equations.

Gas Stoichiometry • Most of all… • You will need to use your brain!!!!!!!!! • Think!

Gas Stoichiometry • • Remember: PV = n. RT P = pressure (k. Pa) V = volume (L) n = number of moles T = temperature (K) R = constant (8. 31 (L· k. Pa)/(K · mol) = 0. 0821 (L – atm)/(K-mol) = when using atm as pressure measurement

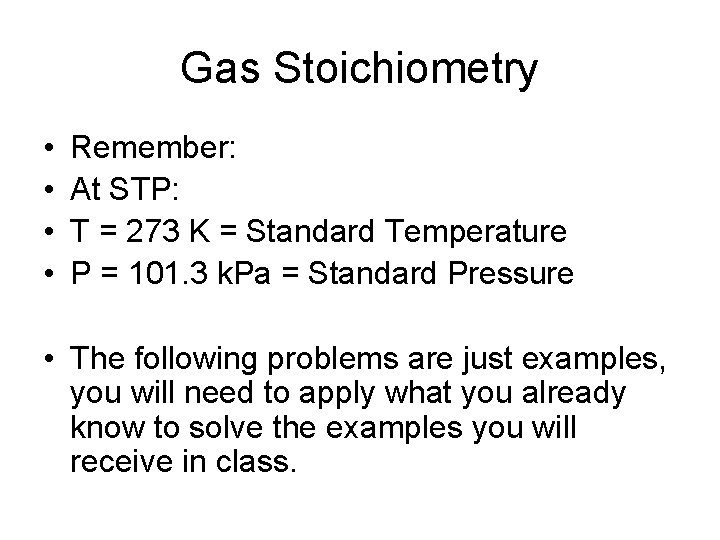
Gas Stoichiometry • • Remember: At STP: T = 273 K = Standard Temperature P = 101. 3 k. Pa = Standard Pressure • The following problems are just examples, you will need to apply what you already know to solve the examples you will receive in class.

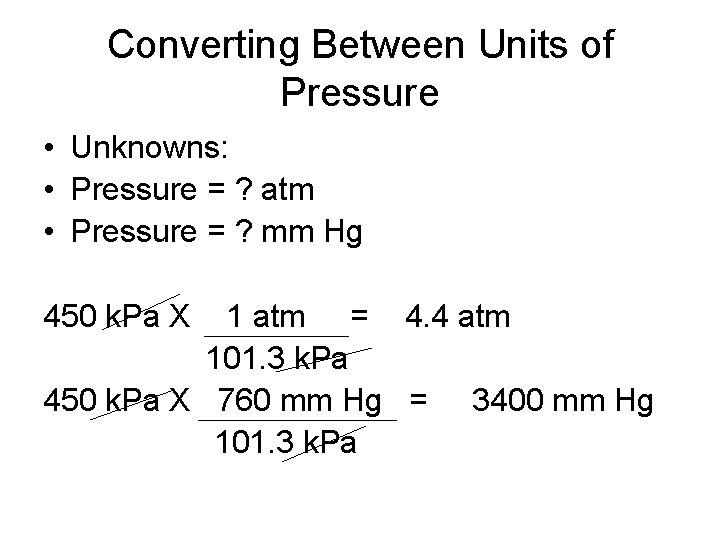
Converting Between Units of Pressure • A pressure gauge records a pressure of 450 k. Pa. What is this measurement expressed in atmospheres and millimeters of mercury? • Known: • Pressure = 450 k. Pa • 1 atm = 101. 3 k. Pa • 1 atm = 760 mm Hg

Converting Between Units of Pressure • Unknowns: • Pressure = ? atm • Pressure = ? mm Hg 450 k. Pa X 1 atm = 4. 4 atm 101. 3 k. Pa 450 k. Pa X 760 mm Hg = 3400 mm Hg 101. 3 k. Pa

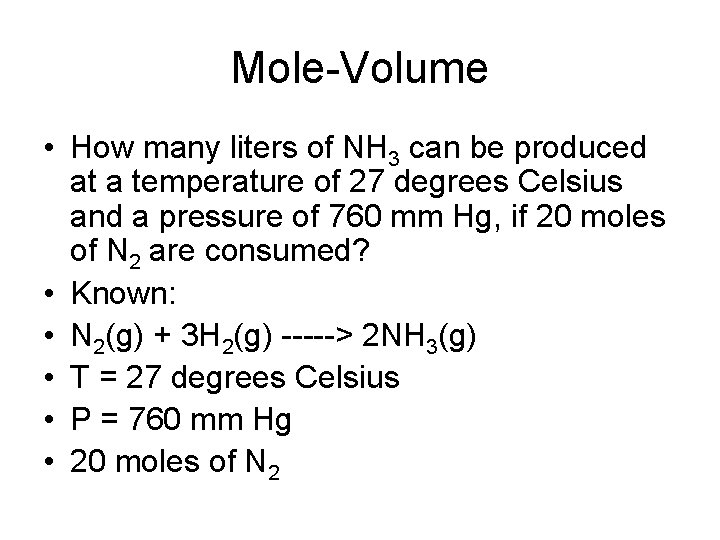
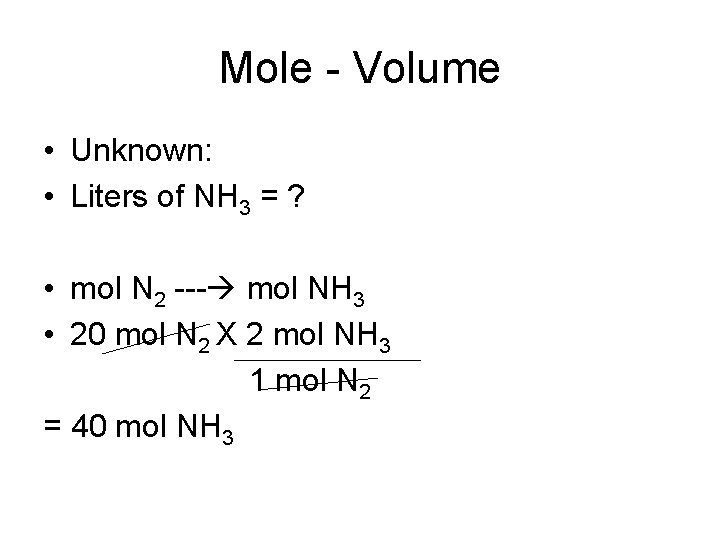
Mole-Volume • How many liters of NH 3 can be produced at a temperature of 27 degrees Celsius and a pressure of 760 mm Hg, if 20 moles of N 2 are consumed? • Known: • N 2(g) + 3 H 2(g) -----> 2 NH 3(g) • T = 27 degrees Celsius • P = 760 mm Hg • 20 moles of N 2

Mole - Volume • Unknown: • Liters of NH 3 = ? • mol N 2 --- mol NH 3 • 20 mol N 2 X 2 mol NH 3 1 mol N 2 = 40 mol NH 3

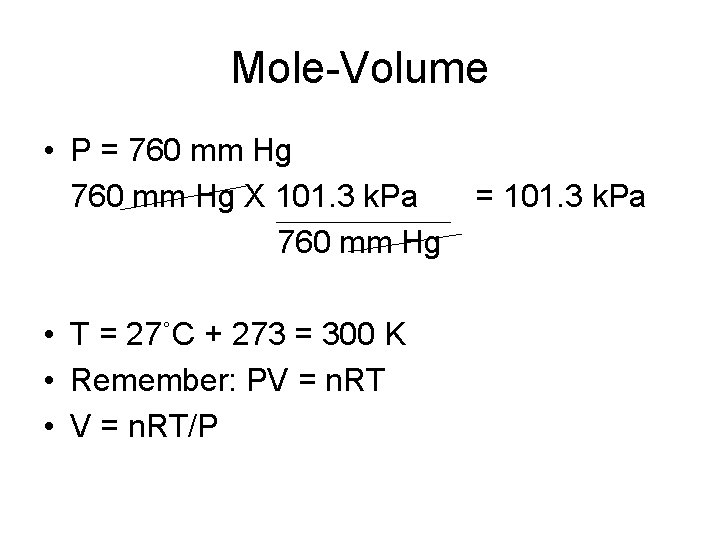
Mole-Volume • P = 760 mm Hg X 101. 3 k. Pa 760 mm Hg • T = 27˚C + 273 = 300 K • Remember: PV = n. RT • V = n. RT/P = 101. 3 k. Pa

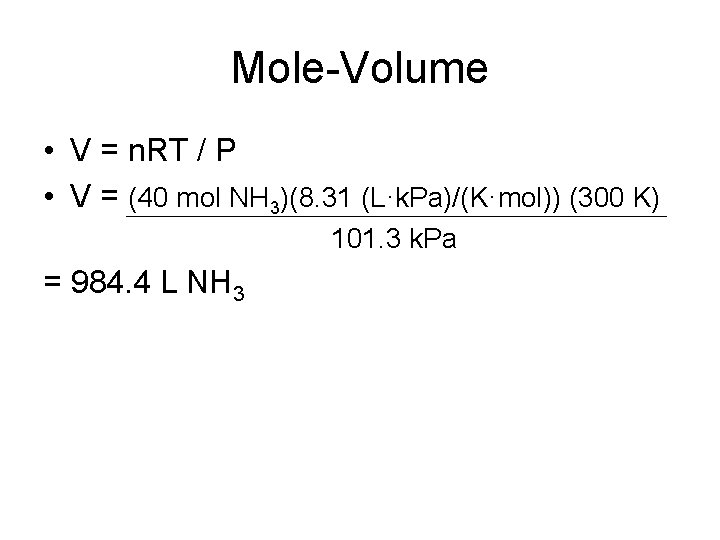
Mole-Volume • V = n. RT / P • V = (40 mol NH 3)(8. 31 (L·k. Pa)/(K·mol)) (300 K) 101. 3 k. Pa = 984. 4 L NH 3

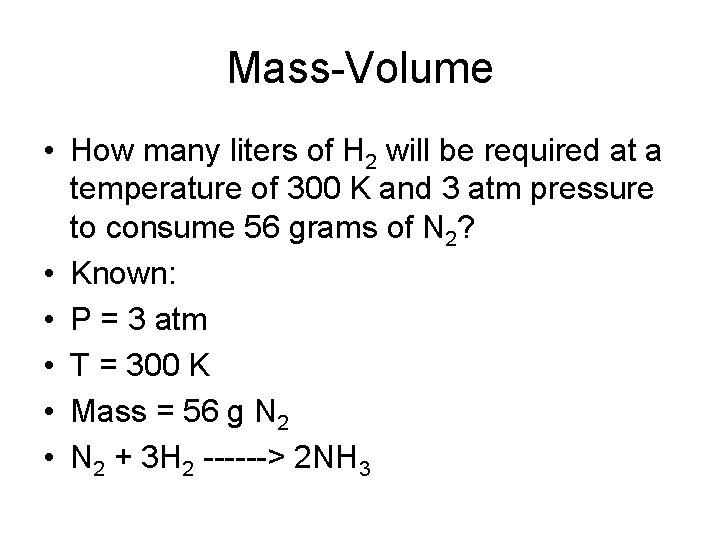
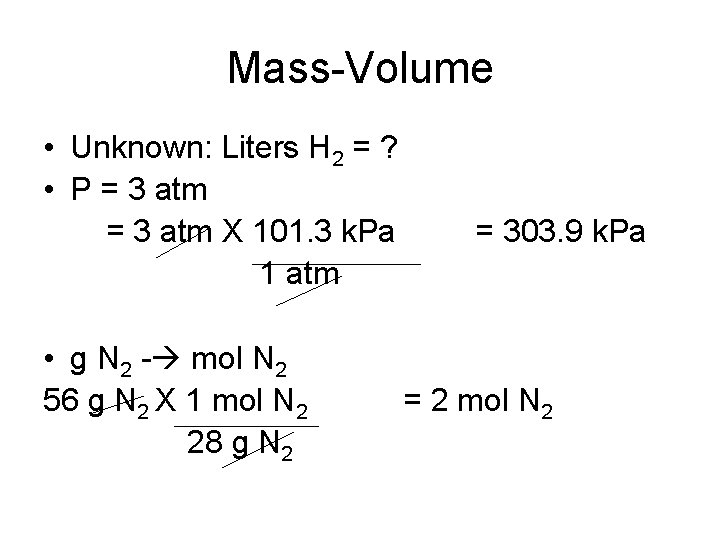
Mass-Volume • How many liters of H 2 will be required at a temperature of 300 K and 3 atm pressure to consume 56 grams of N 2? • Known: • P = 3 atm • T = 300 K • Mass = 56 g N 2 • N 2 + 3 H 2 ------> 2 NH 3

Mass-Volume • Unknown: Liters H 2 = ? • P = 3 atm X 101. 3 k. Pa 1 atm • g N 2 - mol N 2 56 g N 2 X 1 mol N 2 28 g N 2 = 303. 9 k. Pa = 2 mol N 2
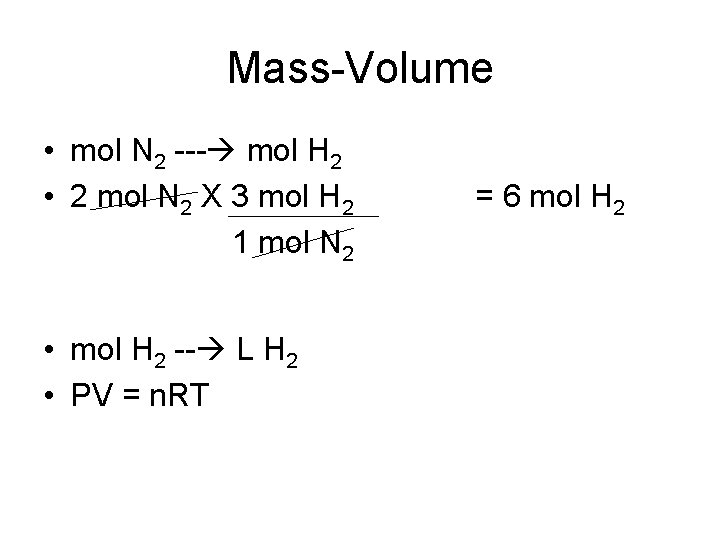
Mass-Volume • mol N 2 --- mol H 2 • 2 mol N 2 X 3 mol H 2 1 mol N 2 • mol H 2 -- L H 2 • PV = n. RT = 6 mol H 2
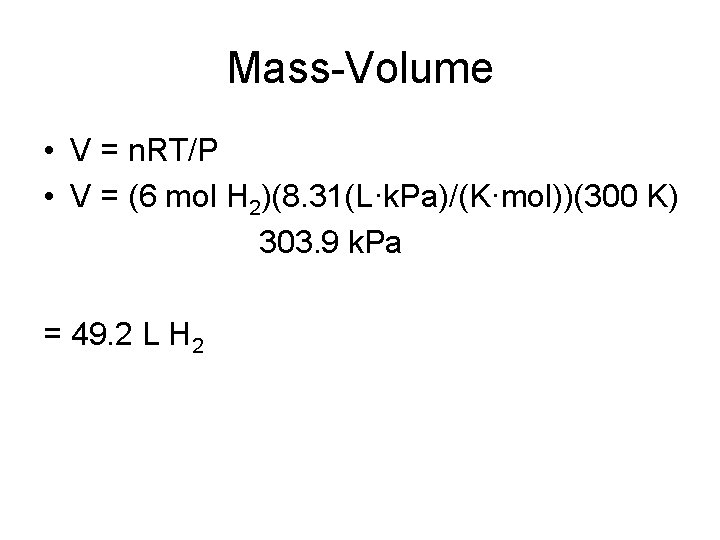
Mass-Volume • V = n. RT/P • V = (6 mol H 2)(8. 31(L·k. Pa)/(K·mol))(300 K) 303. 9 k. Pa = 49. 2 L H 2

Volume-Volume • How many liters of water vapor can be produced if 40 liters of H 2 is consumed in the below chemical reaction when all gases are at the same temperature and pressure? • 2 H 2(g) + O 2 (g) ----> 2 H 2 O (g) • Known: 40 L H 2 • 40 L H 2 X 2 L H 2 O = 40 L H 2 O 2 L H 2

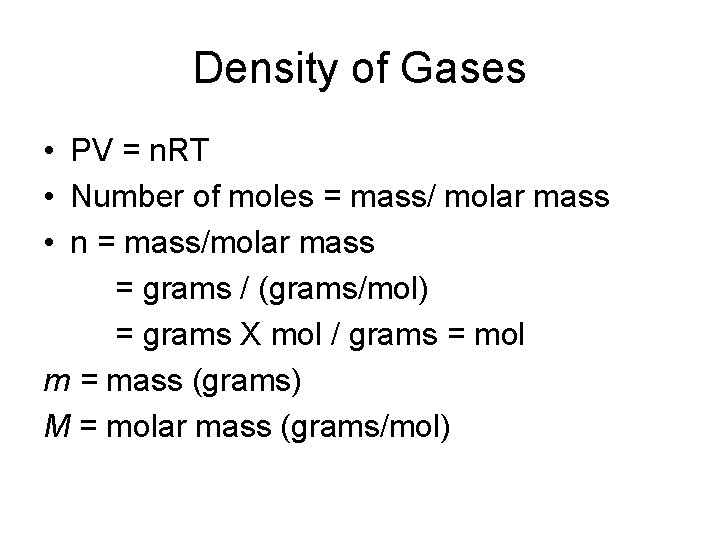
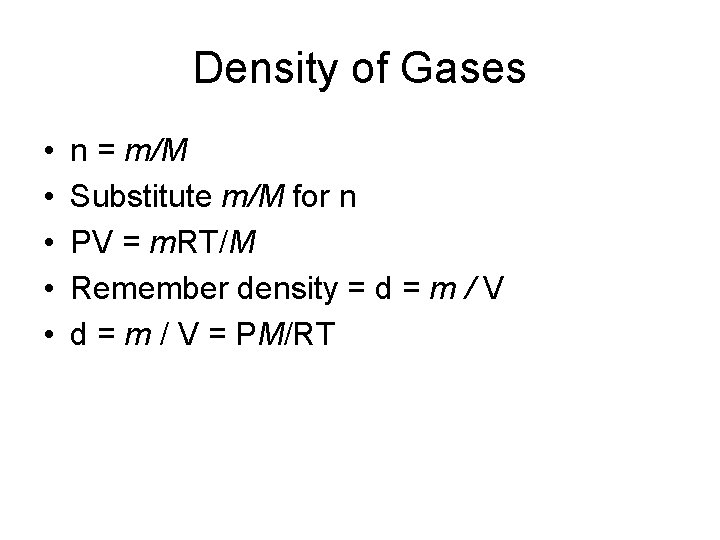
Density of Gases • PV = n. RT • Number of moles = mass/ molar mass • n = mass/molar mass = grams / (grams/mol) = grams X mol / grams = mol m = mass (grams) M = molar mass (grams/mol)

Density of Gases • • • n = m/M Substitute m/M for n PV = m. RT/M Remember density = d = m / V = PM/RT

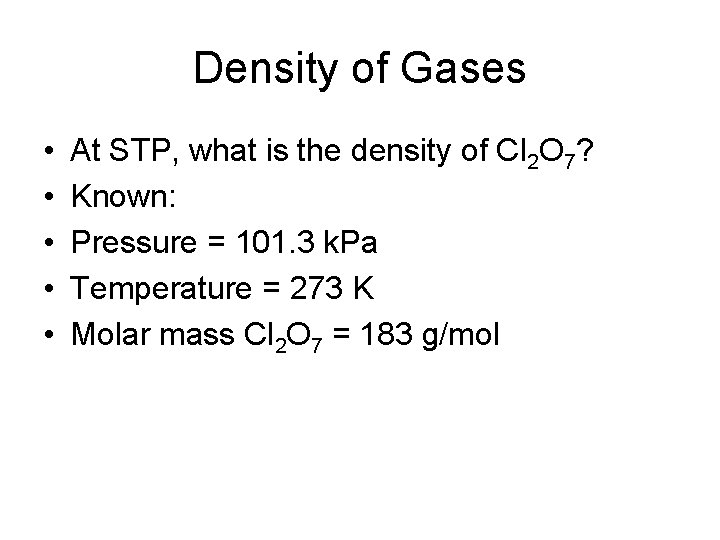
Density of Gases • • • At STP, what is the density of Cl 2 O 7? Known: Pressure = 101. 3 k. Pa Temperature = 273 K Molar mass Cl 2 O 7 = 183 g/mol

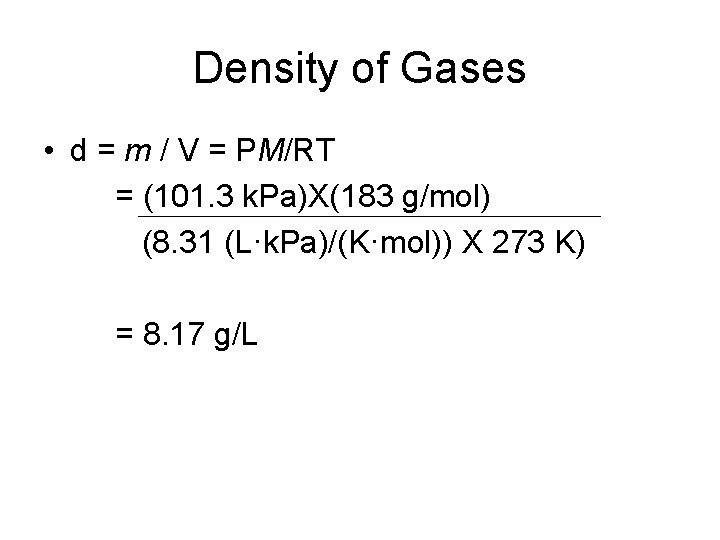
Density of Gases • d = m / V = PM/RT = (101. 3 k. Pa)X(183 g/mol) (8. 31 (L·k. Pa)/(K·mol)) X 273 K) = 8. 17 g/L

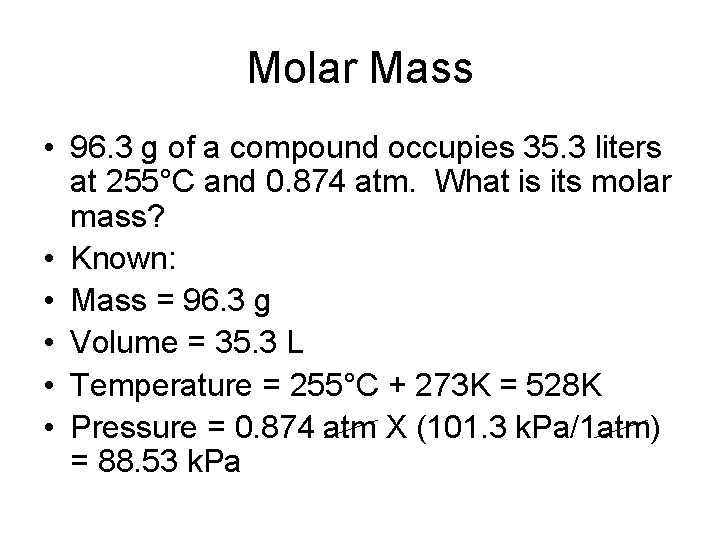
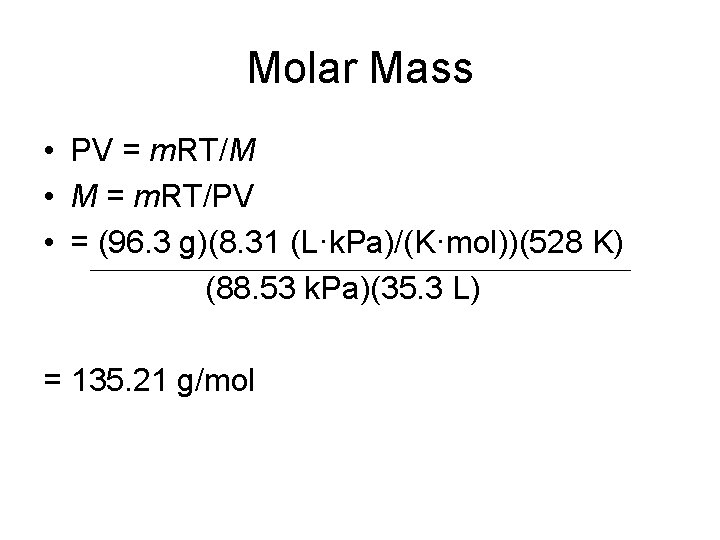
Molar Mass • 96. 3 g of a compound occupies 35. 3 liters at 255°C and 0. 874 atm. What is its molar mass? • Known: • Mass = 96. 3 g • Volume = 35. 3 L • Temperature = 255°C + 273 K = 528 K • Pressure = 0. 874 atm X (101. 3 k. Pa/1 atm) = 88. 53 k. Pa

Molar Mass • PV = m. RT/M • M = m. RT/PV • = (96. 3 g)(8. 31 (L·k. Pa)/(K·mol))(528 K) (88. 53 k. Pa)(35. 3 L) = 135. 21 g/mol

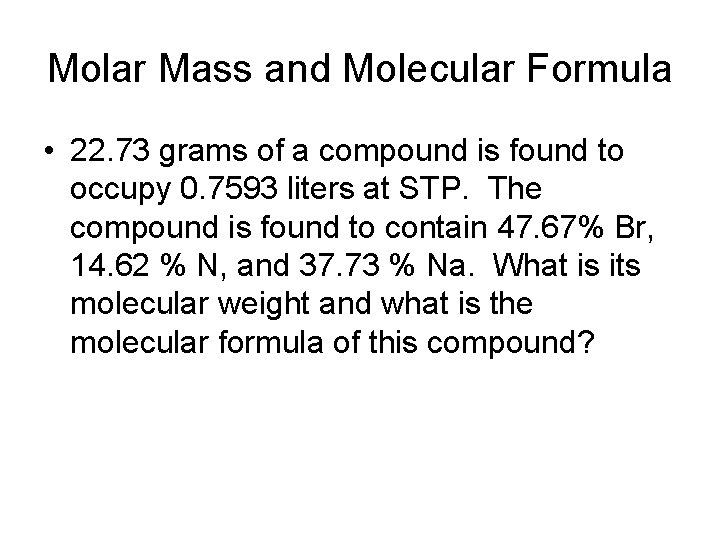
Molar Mass and Molecular Formula • 22. 73 grams of a compound is found to occupy 0. 7593 liters at STP. The compound is found to contain 47. 67% Br, 14. 62 % N, and 37. 73 % Na. What is its molecular weight and what is the molecular formula of this compound?

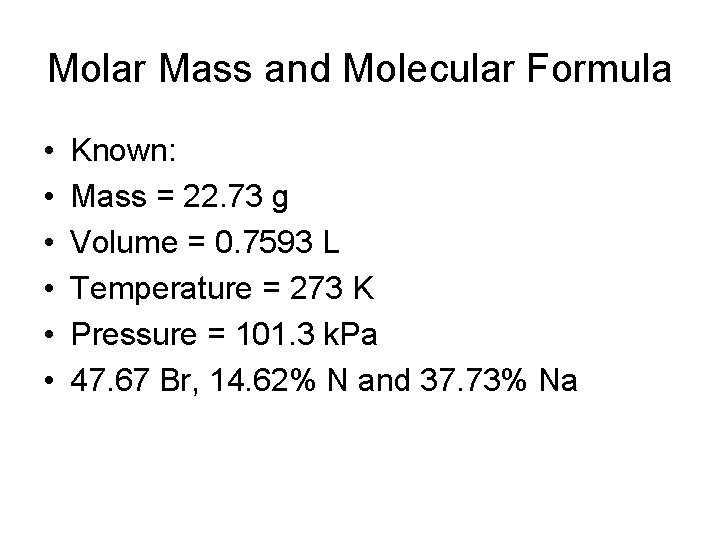
Molar Mass and Molecular Formula • • • Known: Mass = 22. 73 g Volume = 0. 7593 L Temperature = 273 K Pressure = 101. 3 k. Pa 47. 67 Br, 14. 62% N and 37. 73% Na

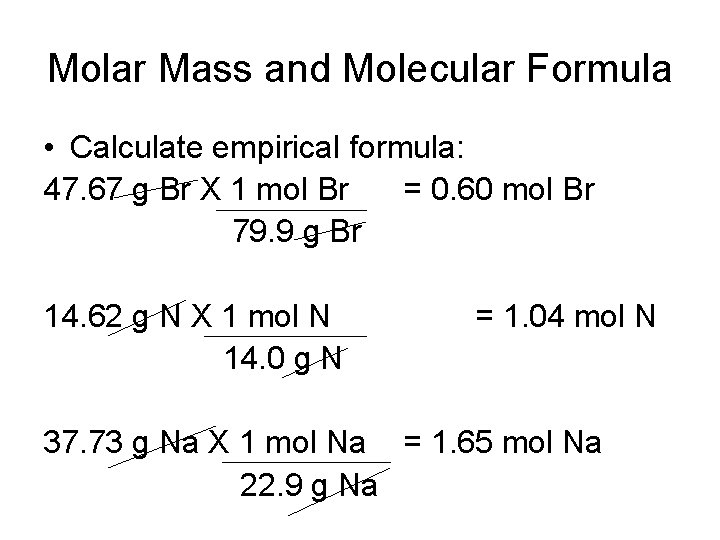
Molar Mass and Molecular Formula • Calculate empirical formula: 47. 67 g Br X 1 mol Br = 0. 60 mol Br 79. 9 g Br 14. 62 g N X 1 mol N 14. 0 g N = 1. 04 mol N 37. 73 g Na X 1 mol Na = 1. 65 mol Na 22. 9 g Na

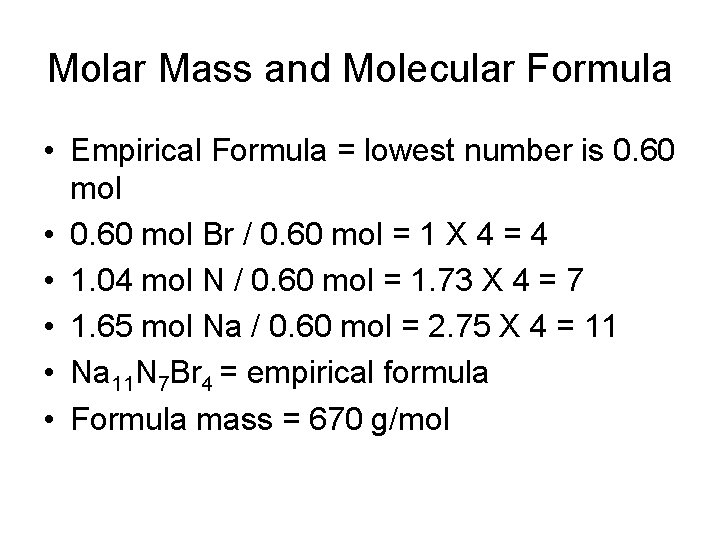
Molar Mass and Molecular Formula • Empirical Formula = lowest number is 0. 60 mol • 0. 60 mol Br / 0. 60 mol = 1 X 4 = 4 • 1. 04 mol N / 0. 60 mol = 1. 73 X 4 = 7 • 1. 65 mol Na / 0. 60 mol = 2. 75 X 4 = 11 • Na 11 N 7 Br 4 = empirical formula • Formula mass = 670 g/mol

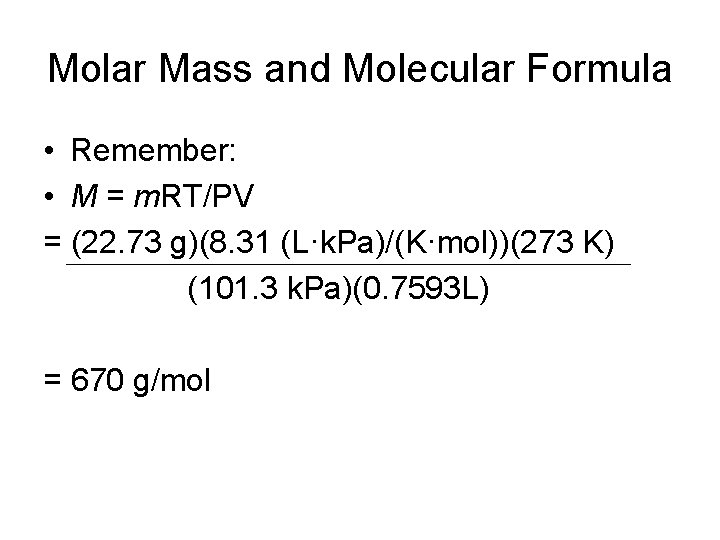
Molar Mass and Molecular Formula • Remember: • M = m. RT/PV = (22. 73 g)(8. 31 (L·k. Pa)/(K·mol))(273 K) (101. 3 k. Pa)(0. 7593 L) = 670 g/mol

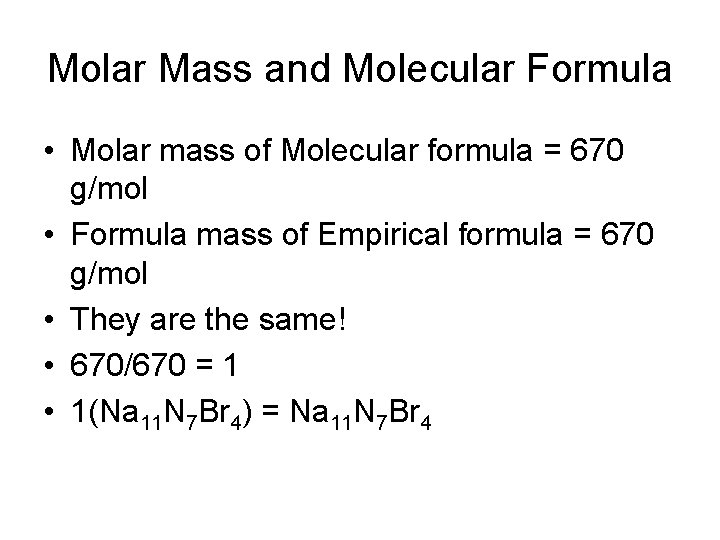
Molar Mass and Molecular Formula • Molar mass of Molecular formula = 670 g/mol • Formula mass of Empirical formula = 670 g/mol • They are the same! • 670/670 = 1 • 1(Na 11 N 7 Br 4) = Na 11 N 7 Br 4