Nuclear Chemistry Notes Nuclear Chemistry 2 T A





























































- Slides: 121

Nuclear Chemistry Notes

Nuclear Chemistry 2

T A little history… • One of the pieces of evidence for the fact that atoms are made of smaller particles came from the work of Madame Curie (1876 -1934). • She discovered radioactivity, the spontaneous disintegration of some elements into smaller pieces. 3

4 Isotopes – Remember? • Not all atoms of the same element have the same mass due to different numbers of neutrons in those atoms. • There are three naturally occurring isotopes of uranium: – Uranium-234 – Uranium-235 – Uranium-238

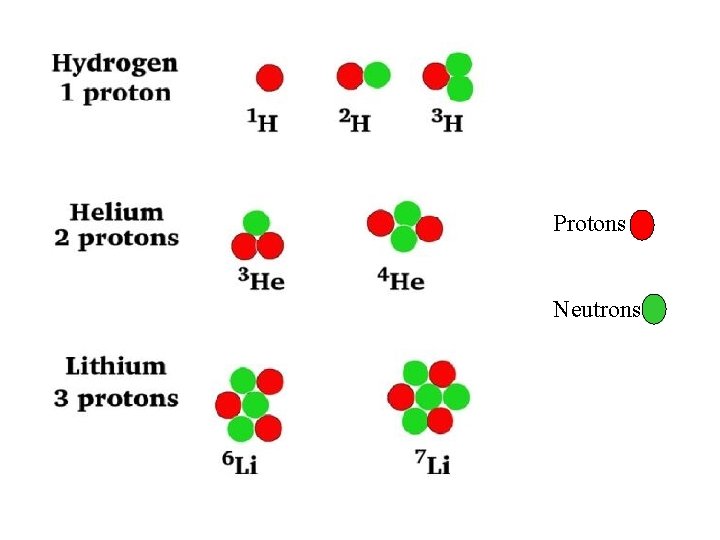
Protons Neutrons

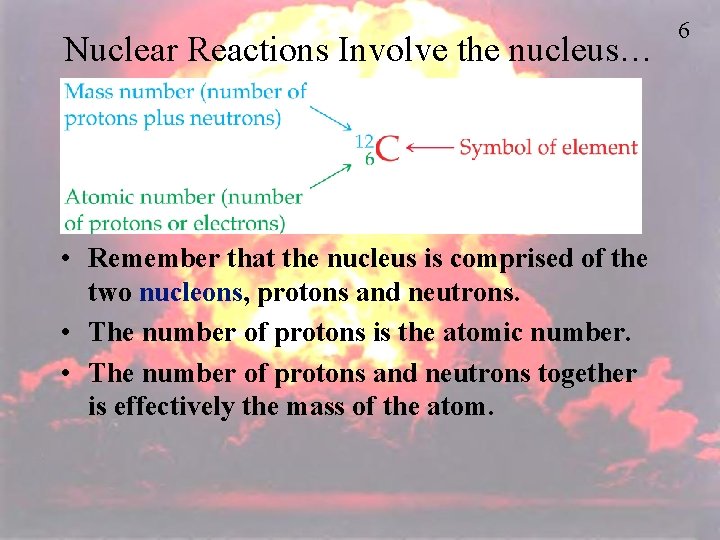
Nuclear Reactions Involve the nucleus… • Remember that the nucleus is comprised of the two nucleons, protons and neutrons. • The number of protons is the atomic number. • The number of protons and neutrons together is effectively the mass of the atom. 6

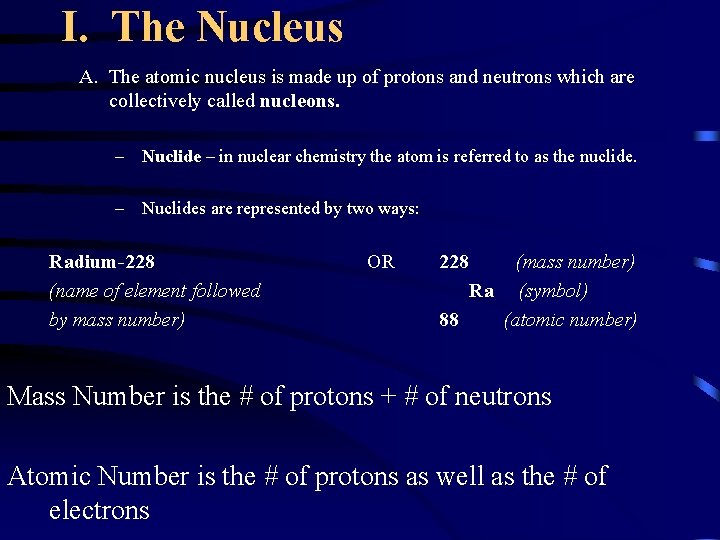
I. The Nucleus A. The atomic nucleus is made up of protons and neutrons which are collectively called nucleons. – Nuclide – in nuclear chemistry the atom is referred to as the nuclide. – Nuclides are represented by two ways: Radium-228 (name of element followed by mass number) OR 228 (mass number) Ra (symbol) 88 (atomic number) Mass Number is the # of protons + # of neutrons Atomic Number is the # of protons as well as the # of electrons

8 So… • Do nuclear reactions produce physical or chemical changes? – Is a NEW SUBSTANCE produced during a nuclear reaction? – Is the reactant the same substance as the product? 238 92 U 234 90 4 + 2 Th He

9 Yes, but… • You were taught that chemical changes result in a “new” substance, so it would seem these changes are chemical. – Classically, “chemical changes” involve changes in the electron cloud. – That is not what is happening here, this change involves the NUCLEUS. – These are neither chemical nor physical reactions – they are NUCLEAR reactions.

10 Furthermore… • Nuclear reactions don’t strictly obey the Law of Conservation of Mass the way “normal” chemical reactions do. – To be clear though, the mass is not necessarily conserved in these reactions – BUT THE PARTICLES ARE. • Some of the mass gets converted into energy, as described in a very famous equation…

You may have seen this equation… it describes Mass Defect • What this means is that mass is just a form of energy. • The difference between mass as energy and other forms of energy is that mass has gravity and inertia. E=mc 2 Energy Mass Speed of light constant 11

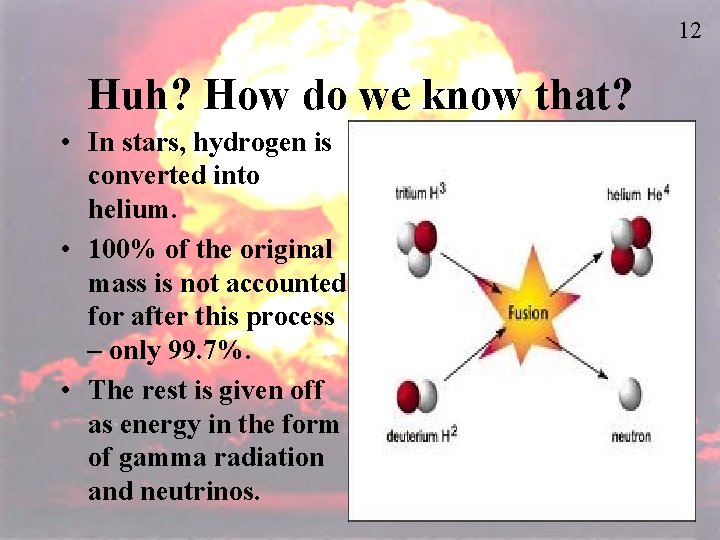
12 Huh? How do we know that? • In stars, hydrogen is converted into helium. • 100% of the original mass is not accounted for after this process – only 99. 7%. • The rest is given off as energy in the form of gamma radiation and neutrinos.

13 Wait, you said NEUTRINO? • A particle with very low mass, around that of an electron, and no electrical charge, the neutrino is an elusive subatomic particle. • Neutrinos travel at almost the speed of light, and many quadrillions of them penetrate your body every second. • But because neutrinos have such low mass, no charge, and interact only slightly with atoms, they can penetrate several light years of densely packed matter before interacting with an atom. For this reason they are very difficult to detect.

14

II. Mass Defect and Nuclear Stability A. Let’s look at helium-4: 2 protons (2 X 1. 007276 amu) = 2. 014552 amu 2 neutrons (2 X 1. 008665 amu) = 2. 017330 amu 2 electrons (2 X 0. 0005486 amu) =0. 001097 amu total combined mass: 4. 032979 amu The actual mass of the atom is measured to be 4. 00260 amu! That is 0. 03038 amu less then the sum of all of its particles, it is also called the mass defect.

B. The mass defect is caused by the conversion of mass (m) to energy (E) when the nucleus was originally formed. C. Using Einstein’s equation E=mc 2, we can actually calculate the energy that was formed when the nucleus was formed! This is called the nuclear binding energy. (It can also be thought of as the amount of energy required to break apart the nucleus; therefore, the nuclear binding energy is also a measure of the stability of a nucleus. )

How much energy was released when a single helium-4 atom was formed? (In other words, calculate the nuclear binding energy of one helium-4 atom. The measured atomic mass of helium-4 is 4. 00260 amu. ) Step 1: Find the mass defect. 4. 032979 amu – 4. 00260 amu = 0. 03038 amu Step 2: Using the conversion 1 amu =1. 6605 X 10 -27 kg convert the mass from amu’s to kilograms. ? kg 0. 03038 amu X 1. 6605 X 10 -27 kg 1 amu = 5. 0446 X 10 -29 kg Step 3: Calculate the energy using E = mc 2. c is a constant and it equals 3. 00 X 108 m/sec E = (5. 0446 X 10 -29 kg) (3. 00 X 108 m/sec)2 E = 4. 54 X 10 -12 kg m 2 or J (J = kg m 2 ) sec 2

Practice: Calculate the nuclear binding energy of a sulfur-32 atom. The measured atomic mass of sulfur-32 is 32. 080 amu.

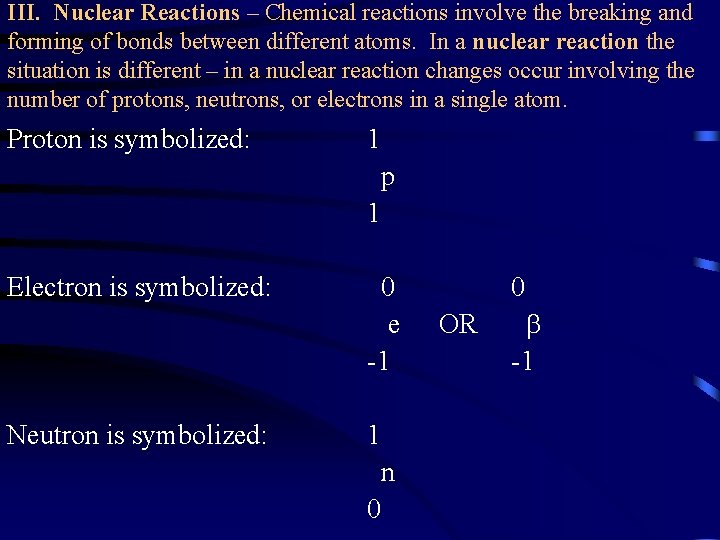
III. Nuclear Reactions – Chemical reactions involve the breaking and forming of bonds between different atoms. In a nuclear reaction the situation is different – in a nuclear reaction changes occur involving the number of protons, neutrons, or electrons in a single atom. Proton is symbolized: 1 p 1 Electron is symbolized: Neutron is symbolized: 0 e -1 1 n 0 OR 0 -1

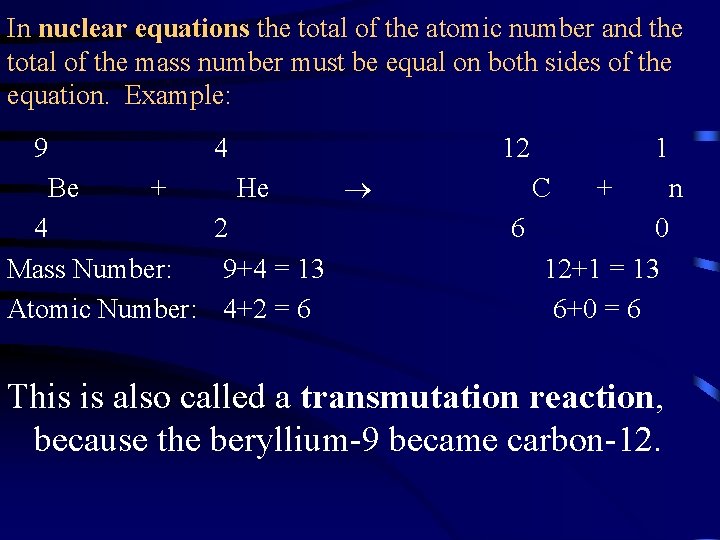
In nuclear equations the total of the atomic number and the total of the mass number must be equal on both sides of the equation. Example: 9 4 Be + 12 He 4 2 Mass Number: 9+4 = 13 Atomic Number: 4+2 = 6 1 C 6 + 0 12+1 = 13 6+0 = 6 n This is also called a transmutation reaction, because the beryllium-9 became carbon-12.

Example: 212 Po 84 4 208 He + 2 Pb 82 Mass Number: 212 4+208 = 212 Atomic Number: 84 2+82 = 84

Practice: Complete the following nuclear equations: 1. 218 Po 84 4 He 2 +

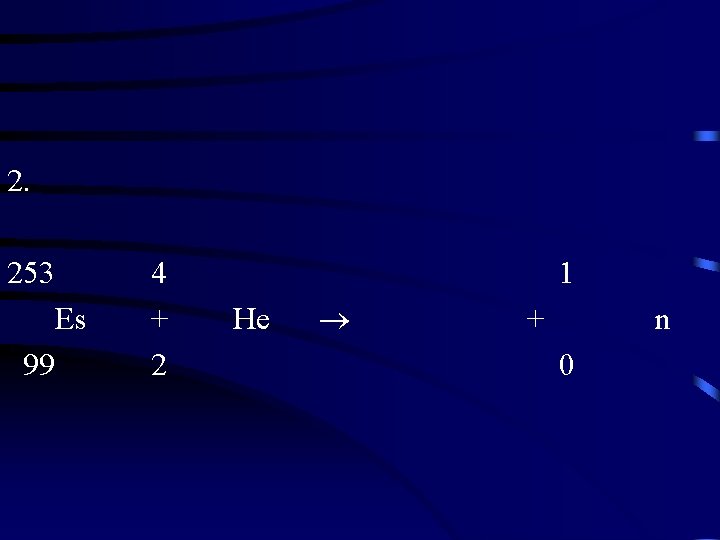
2. 253 Es 99 4 + 2 1 He + n 0

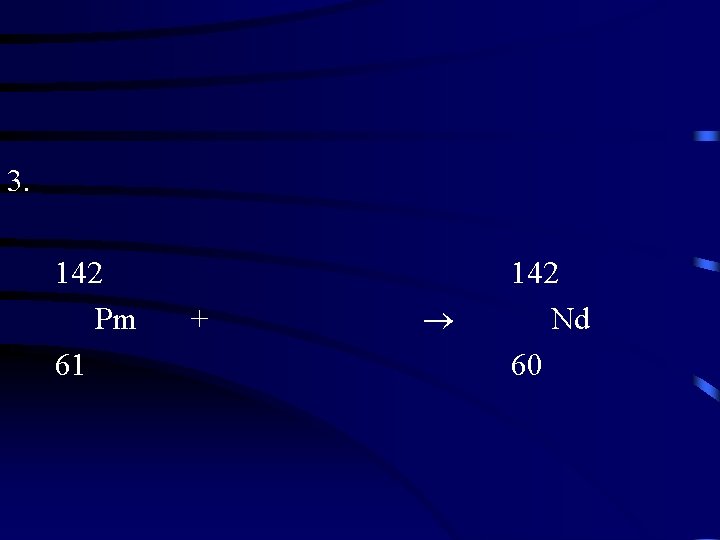
3. 142 Pm 61 + 142 Nd 60

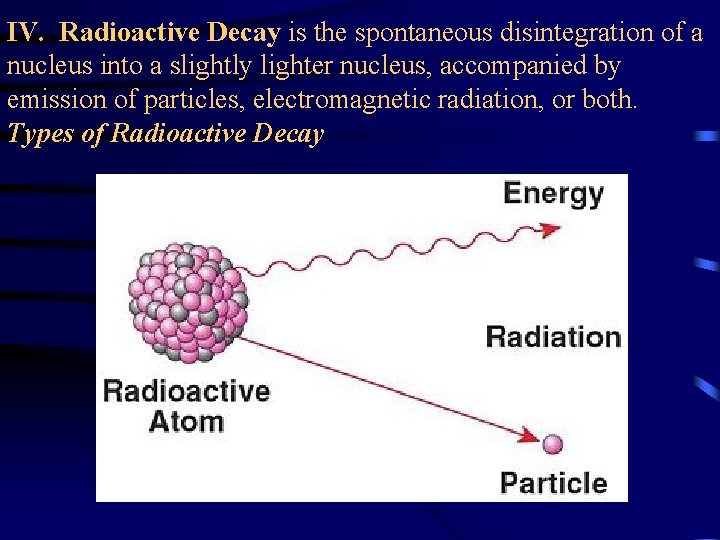
IV. Radioactive Decay is the spontaneous disintegration of a nucleus into a slightly lighter nucleus, accompanied by emission of particles, electromagnetic radiation, or both. Types of Radioactive Decay

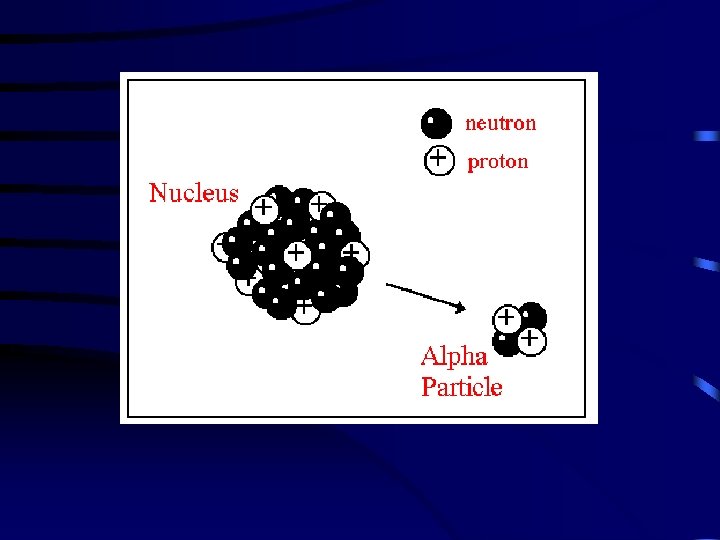
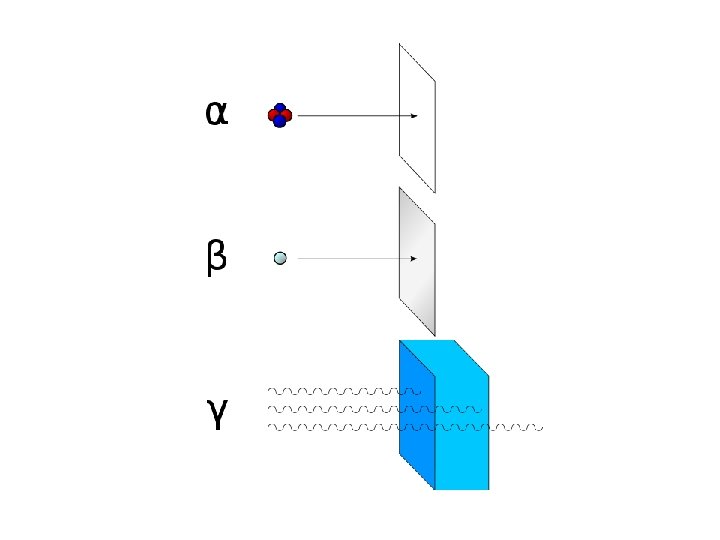
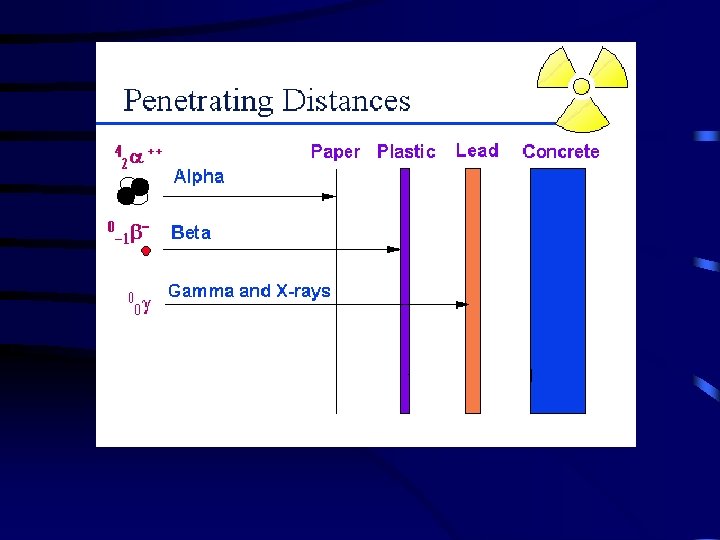
IV. Radioactive Decay is the spontaneous disintegration of a nucleus into a slightly lighter nucleus, accompanied by emission of particles, electromagnetic radiation, or both. Types of Radioactive Decay A. Alpha Emission – an alpha particle ( ) is 2 protons and 2 neutrons (or a helium atom) bound together and is emitted from the nucleus during some kinds of radioactive decay. 210 206 4 Po Pb + He 84 82 2 Clothes will shield you form alpha particles.


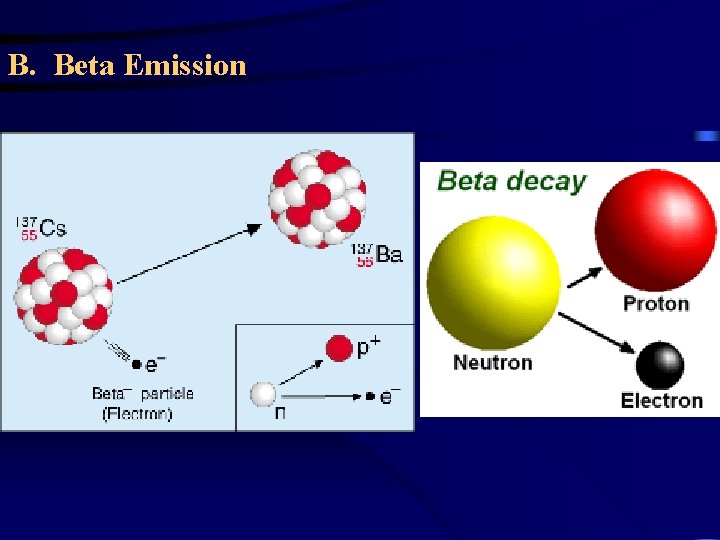
B. Beta Emission – a beta particle ( ) is an electron emitted from the nucleus when a neutron is converted to a proton. 1 1 0 n p + 0 1 -1 14 14 C 6 0 N 7 + -1 Metal foil will shield you from beta particles.

B. Beta Emission

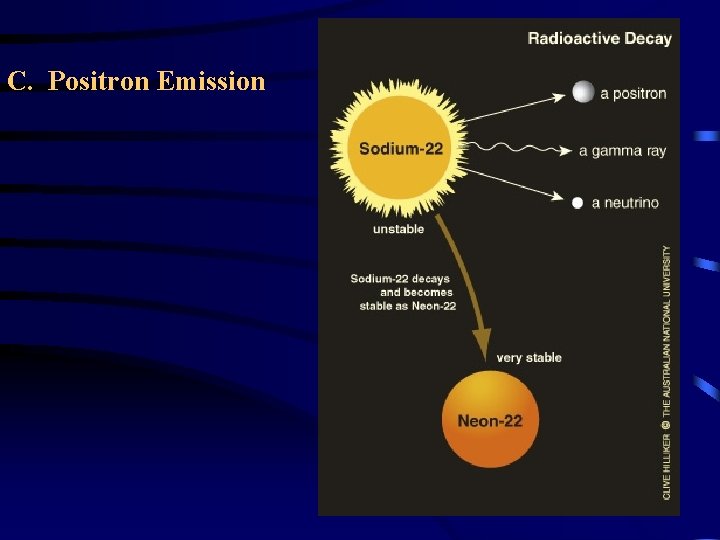
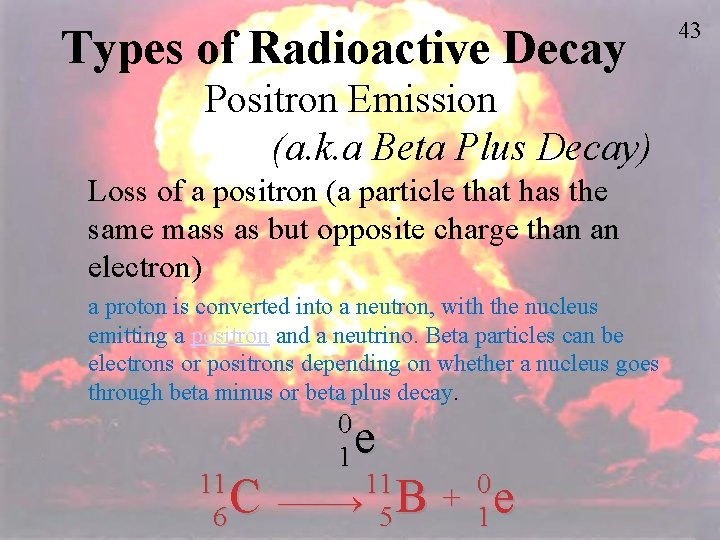
C. Positron Emission – a positron is a particle that is emitted from the nucleus when a proton is converted into a neutron. 1 1 0 p n + 1 0 1 38 38 Ar + 18 0 K 19 1

C. Positron Emission

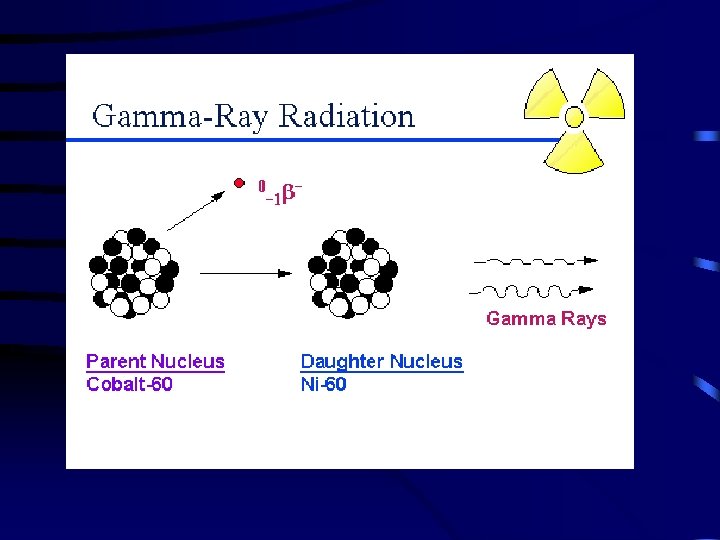
D. Gamma Emission – gamma rays ( ) are high-energy electromagnetic waves emitted from a nucleus as it changes from an excited state to a ground energy state. Very similar to light, but is much more dangerous. Gamma emission usually occurs immediately following other types of decay. (Page 713 ) Lead or concrete will protect you from gamma rays.




Practice: Complete the following nuclear reactions: 1. 239 Pu 94 242 + 1 Cm 96 + n 0

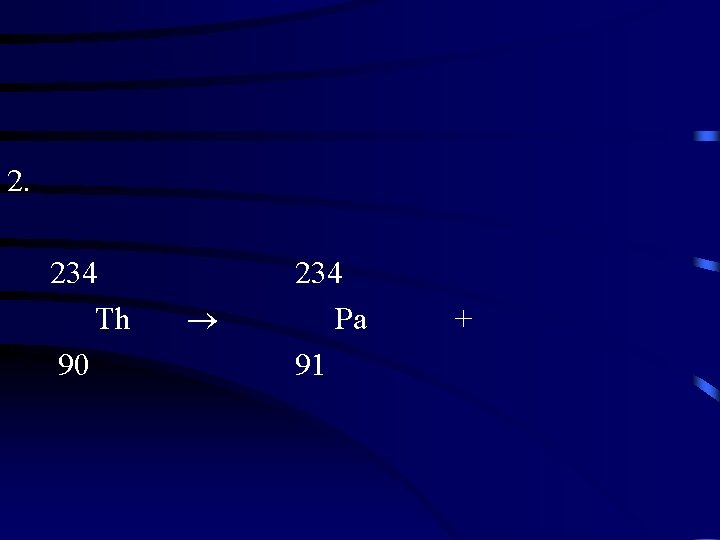
2. 234 Th 90 234 Pa 91 +

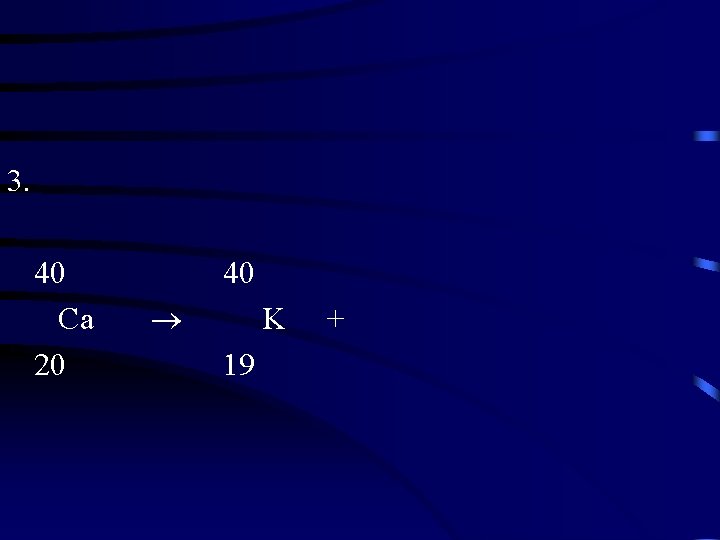
3. 40 Ca 20 40 K 19 +

4. Write an equation to represent the decay of thorium-230 by alpha emission.

40 Radioactivity • It is not uncommon for some nuclides of an element to be unstable, or radioactive. • We refer to these as radionuclides. • There are several ways radionuclides can decay into a different nuclide.

T 41 Types of Radioactive Decay Alpha Decay Loss of an -particle (a helium nucleus) 4 2 238 92 U He 234 90 4 + Th He 2

T Types of Radioactive Decay Beta Decay (a. k. a Beta Minus Decay) Loss of a -particle (a high energy electron) an excess neutron becomes a proton, and the nucleus emits an electron and an antineutrino. The electron is the beta particle, while the antineutrino is a particle with some unusual properties. 0 or 0 − 1 131 53 I 131 54 e Xe + 0 e − 1 42

Types of Radioactive Decay Positron Emission (a. k. a Beta Plus Decay) Loss of a positron (a particle that has the same mass as but opposite charge than an electron) a proton is converted into a neutron, with the nucleus emitting a positron and a neutrino. Beta particles can be electrons or positrons depending on whether a nucleus goes through beta minus or beta plus decay. 11 6 C 0 1 e 11 5 B 0 + 1 e 43

About this Beta particle… • The cause of beta emission is an excess of neutrons in the nucleus. • When there are way more neutrons than protons in a nucleus, a neutrons can split – or degenerate - into a proton and an electron This electron is then ejected from the nucleus at high speed. – …beta particles are not themselves radioactive, they cause damage ballistically, breaking chemical bonds and creating ions which do damage to tissue. • This increases the atomic number of the atom by 1 and also increases the atom’s stability. 44

T 45 Types of Radioactive Decay Gamma Emission Loss of a -ray (high-energy radiation that almost always accompanies the loss of a nuclear particle) 0 0

46 Types of Radioactive Decay Electron Capture (K-Capture) • During this process, one of the protons in the atom's nucleus pulls in an orbiting electron and neutralizes both the electron and itself. • This causes the atom to decay and become a different element with the same atomic mass. 1 0 1 + 0 1 − 1 This causes the atom to decay and become a different element (one less proton) with the same atomic mass. p e n

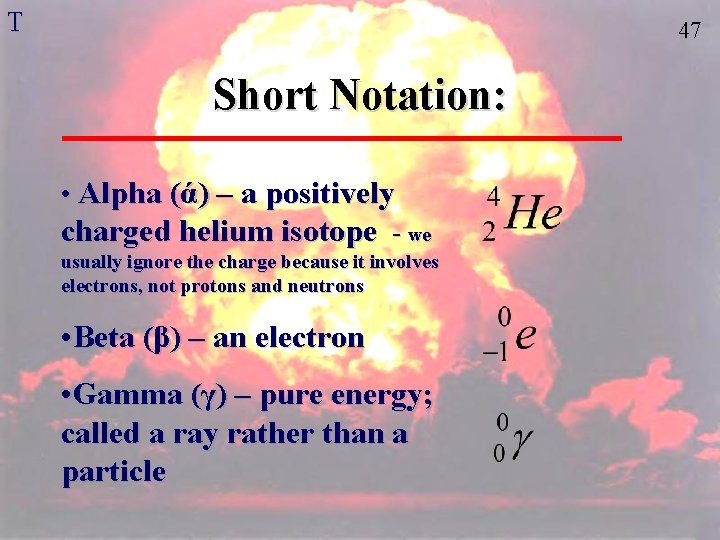
T 47 Short Notation: • Alpha (ά) – a positively charged helium isotope - we usually ignore the charge because it involves electrons, not protons and neutrons • Beta (β) – an electron • Gamma (γ) – pure energy; called a ray rather than a particle

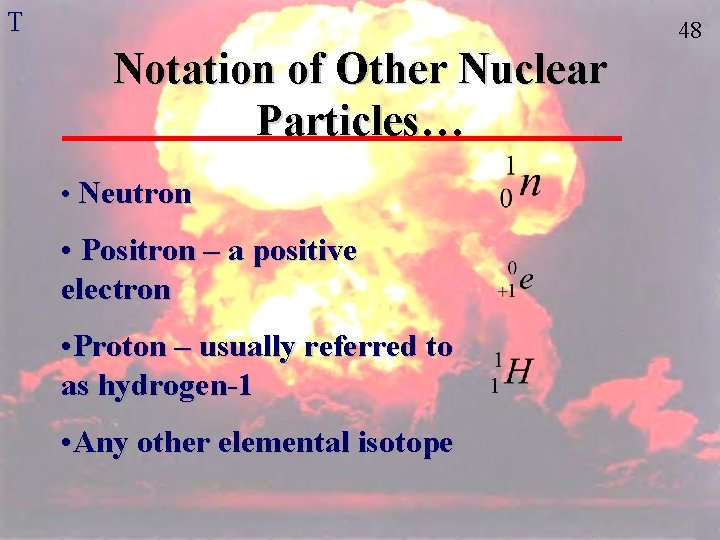
T 48 Notation of Other Nuclear Particles… • Neutron • Positron – a positive electron • Proton – usually referred to as hydrogen-1 • Any other elemental isotope

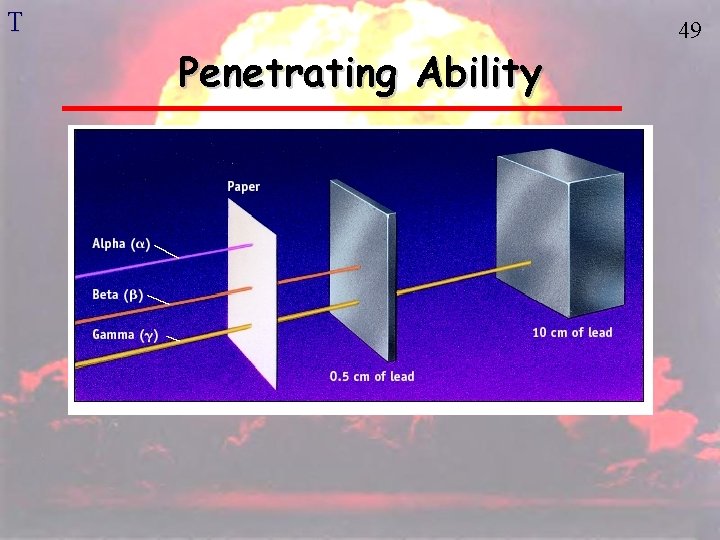
T Penetrating Ability 49

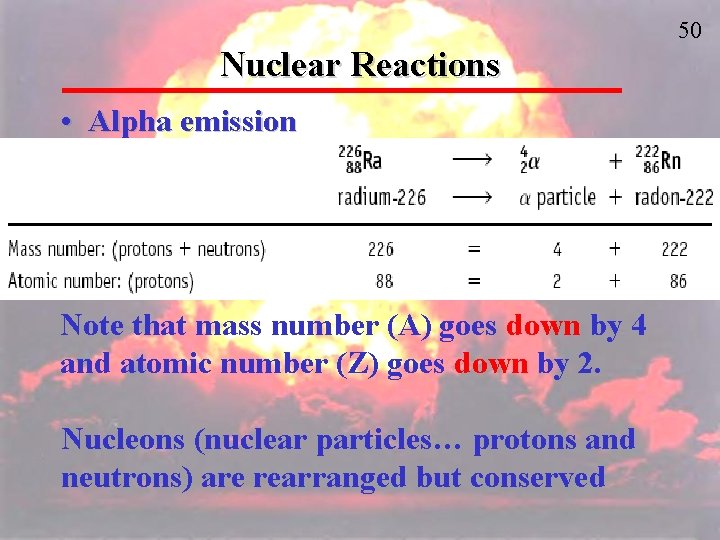
50 Nuclear Reactions • Alpha emission Note that mass number (A) goes down by 4 and atomic number (Z) goes down by 2. Nucleons (nuclear particles… protons and neutrons) are rearranged but conserved

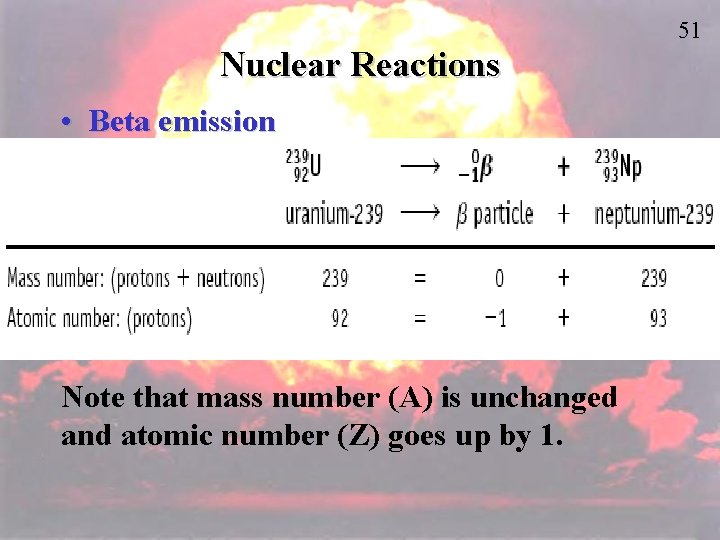
51 Nuclear Reactions • Beta emission Note that mass number (A) is unchanged and atomic number (Z) goes up by 1.

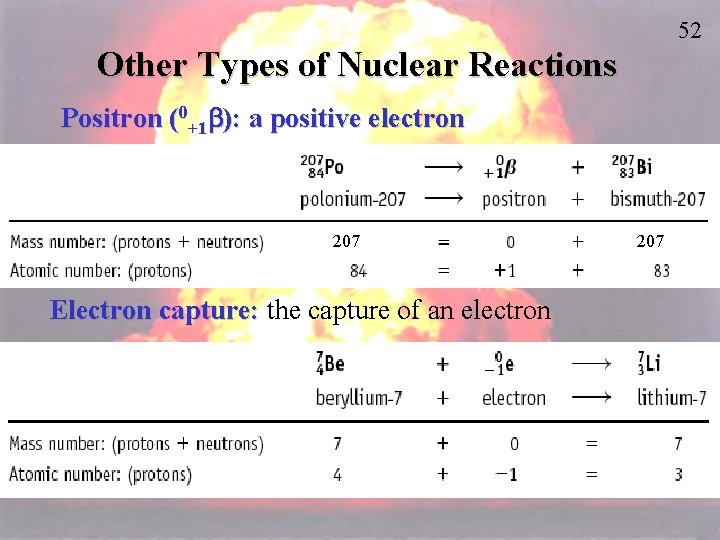
52 Other Types of Nuclear Reactions Positron (0+1 b): a positive electron 207 Electron capture: the capture of an electron 207

E. Effects of Radiation: The effects of radiation depends on the amount and exposure. Massive doses can be deadly. DNA molecules are sensitive to alpha, beta, positron, gamma, and x-rays.

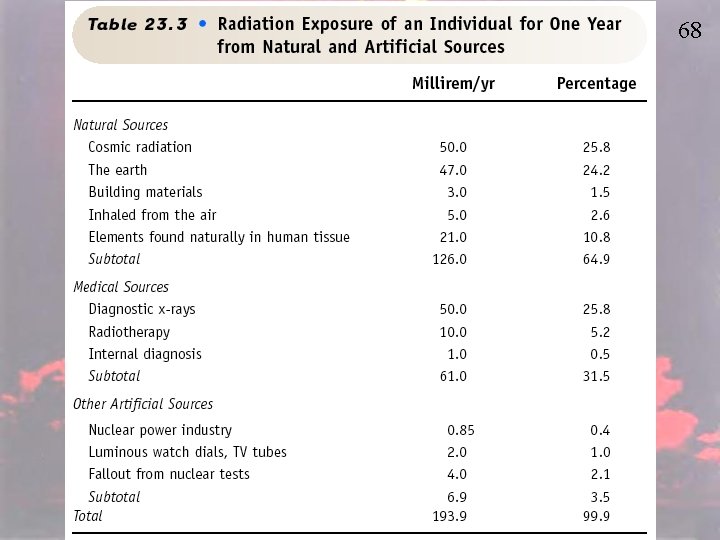
Estimate Your Personal Annual Radiation Dose http: //www. ans. org/pi/resources/dosechart/ We live in a radioactive world – humans always have. Radiation is part of our natural environment. We are exposed to radiation from materials in the earth itself, from naturally occurring radon in the air, from outer space, and from inside our own bodies (as a result of the food and water we consume). This radiation is measured in units called millirems (mrems). The average dose person from all sources is about 360 mrems in a given year (largely due to medical procedures we may undergo). International Standards allow exposure to as much as 5, 000 mrems a year for those who work with and around radioactive material.

Estimate Your Personal Annual Radiation Dose American Nuclear Society Factors Common Sources of Radiation Where Cosmic radiation (from outer space) You Exposure depends on your elevation (how much air Live is above you to block radiation). Amounts are listed in mrem (per year). At sea level 26 mrem 2 – 3000 ft 35 mrem 6 – 7000 ft 66 mrem 0 – 1000 ft 28 3 – 4000 ft 41 7 – 8000 ft 79 1 – 2000 ft 31 4 – 5000 ft 47 8 – 9000 ft 96 5 – 6000 ft 52 [Elevation of cities (in feet): Atlanta 1050; Chicago 595; Dallas 435; Denver 5280; Las Vegas 2000; Minneapolis 815; Pittsburgh 1200; St. Louis 455; Salt Lake City 4400; Spokane 1890. ] Your Annual Dose Total: 26 mrems

Estimate Your Personal Annual Radiation Dose American Nuclear Society Factors Common Sources of Radiation Where Terrestrial (from the ground) You • If you live in a state that borders the Live Gulf or Atlantic Coasts, add 16 mrem. • If you live in the Colorado Plateau area (around Denver), add 63 mrem. • If you live anywhere else in the continental U. S. , add 30 mrem. Your Annual Dose Total: 26 + 16 mrems

Estimate Your Personal Annual Radiation Dose American Nuclear Society Factors Common Sources of Radiation Where House Construction You If you live in a stone, adobe, brick, or concrete Live building, add 7 mrem. Power Plants • If you live within 50 miles of a nuclear power plant, add 0. 01 mrem. • If you live within 50 miles of a coal-fired power plant, add 0. 03 mrem. Your Annual Dose Total: _____mrems

Estimate Your Personal Annual Radiation Dose American Nuclear Society Factors Common Sources of Radiation Food Internal Radiation*** Water From food (Carbon-14 and Potassium-40) Air & from water (radon dissolved in water), add 40 mrem. From air (radon), add 200 mrem. Your Annual Dose Total: _____mrems

Estimate Your Personal Annual Radiation Dose American Nuclear Society Factors Common Sources of Radiation How You Live Weapons test fallout (less than 1*), add 1 mrem Jet Plane Travel, add 0. 5 mrem/hour in air If you have porcelain crowns or false teeth**, add 0. 07 mrem If you wear a luminous wristwatch, add 0. 06 mrem If you go through luggage inspection at airport, add 0. 02 mrem/pass Your Annual Dose Total: _____mrems

Estimate Your Personal Annual Radiation Dose American Nuclear Society Factors Common Sources of Radiation How You Live If you watch TV*, add 1 mrem If you use/view a computer screen*, add 1 mrem If you have a smoke detector, add 0. 08 mrem If you use a gas camping lantern, add 0. 2 mrem If you wear a plutonium-powered pacemaker, add 100 mrem Your Annual Dose Total: _____mrems

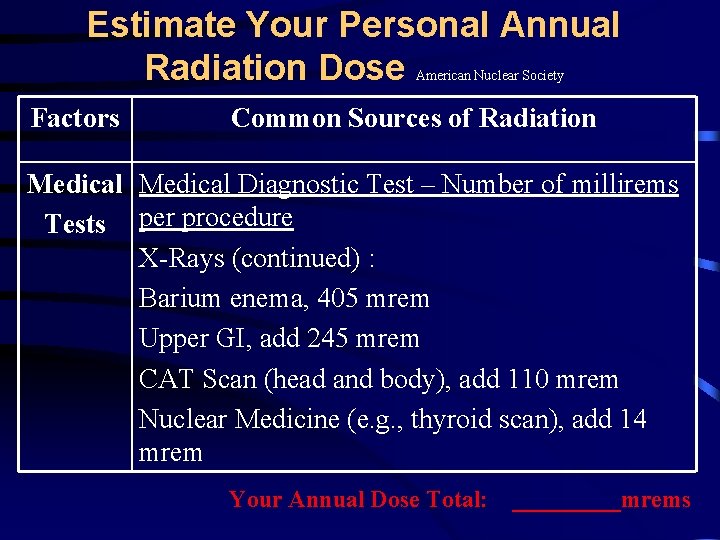
Estimate Your Personal Annual Radiation Dose American Nuclear Society Factors Common Sources of Radiation Medical Diagnostic Test – Number of millirems Tests per procedure X-Rays: Extremity (arm, hand, foot, or leg), add 1 mrem Dental, add 1 mrem Chest, add 6 mrem Pelvis/hip, add 65 mrem Skull/neck, add 20 mrem Your Annual Dose Total: _____mrems

Estimate Your Personal Annual Radiation Dose American Nuclear Society Factors Common Sources of Radiation Medical Diagnostic Test – Number of millirems Tests per procedure X-Rays (continued) : Barium enema, 405 mrem Upper GI, add 245 mrem CAT Scan (head and body), add 110 mrem Nuclear Medicine (e. g. , thyroid scan), add 14 mrem Your Annual Dose Total: _____mrems

Estimate Your Personal Annual Radiation Dose Test Info * The value is less than 1, but adding a value of 1 would be reasonable. **Some of the radiation sources listed in this chart result in an exposure to only part of the body. For example, false teeth or crowns result in a radiation dose to the mouth. The annual dose numbers given here represent the "effective dose" to the whole body. ***Average values. Primary sources for this information are National Council on Radiation and Measurements Reports: #92 Public Radiation Exposure from Nuclear Power Generation in the United States (1987); #93 Ionizing Radiation Exposure of the Population of the United States (1987); #94 Exposure of the Population in the United States and Canada from Natural Background Radiation (1987); #95 Radiation Exposure of the U. S. population from Consumer Products and Miscellaneous Sources, (1987); and #100 Exposure of the U. S. Population from Diagnostic Medical Radiation (1989). Copyright 2000 American Nuclear Society http: //www. ohio. edu/ehs/docs/10_persnl_rad_dose. pdf

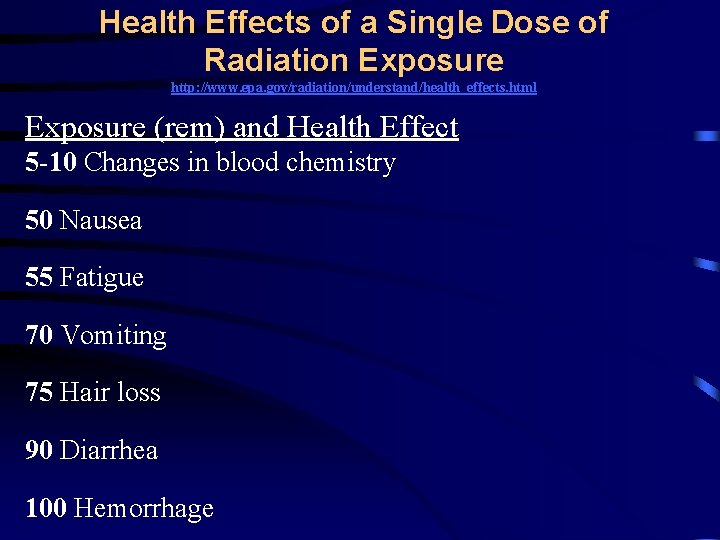
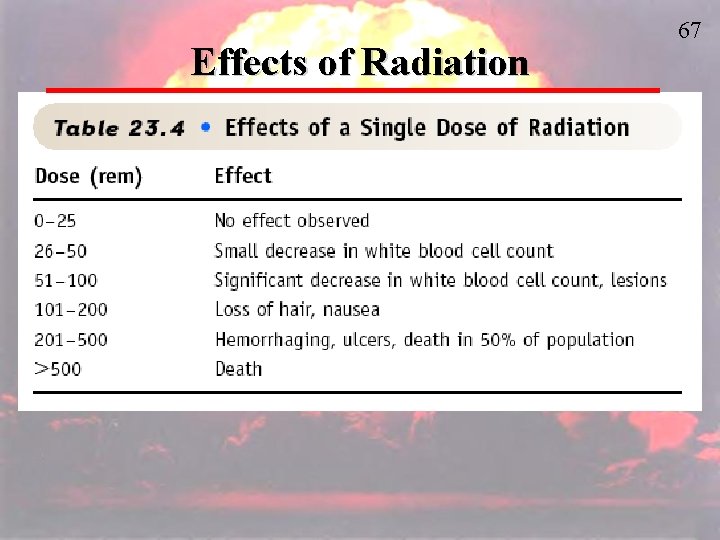
Health Effects of a Single Dose of Radiation Exposure http: //www. epa. gov/radiation/understand/health_effects. html Exposure (rem) and Health Effect 5 -10 Changes in blood chemistry 50 Nausea 55 Fatigue 70 Vomiting 75 Hair loss 90 Diarrhea 100 Hemorrhage

Health Effects of a Single Dose of Radiation Exposure http: //www. epa. gov/radiation/understand/health_effects. html Exposure (rem) and Health Effect 400 Possible death (within 2 months) 1, 000 Destruction of intestinal lining, internal bleeding, and death (within 1 -2 weeks) 2, 000 Damage to central nervous system, loss of consciousness; (within minutes) Death (hours to days)

66

Effects of Radiation 67

68

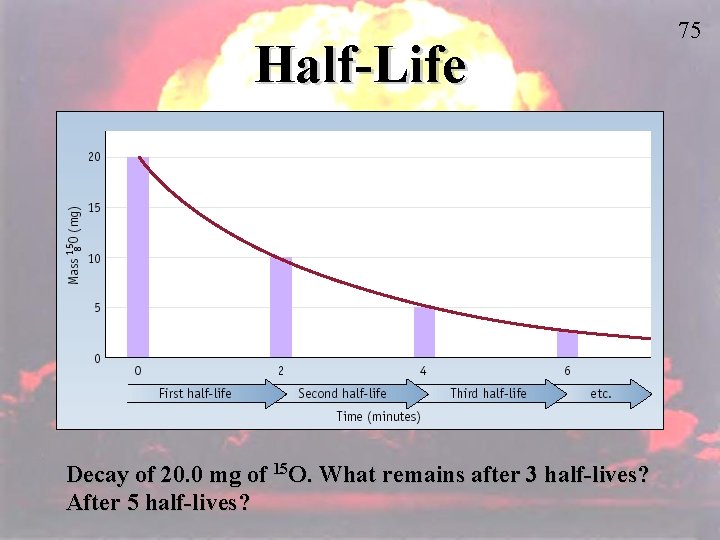
V. Half-life – is the time required for half the atoms of a radioactive nuclide to decay. Each radioactive nuclide has its own half-life. Example: Carbon-14 has a half-life of 57155730 years. Page 708 Example Problem: Phosphorus-32 has a half-life of 14. 3 days. How many mg of phosphorus-32 remain after 57. 2 days if you start with 4 mg of the isotope? Amount of Phosphorus-32 4 mg 2 mg 1 mg 0. 5 mg 0. 25 mg Time Elapsed 0 days have past 14. 3 days have passed 28. 6 days have passed 42. 9 days have passed 57. 2 days have passed

Another way to work half-life problems: # of half-lives = time elapsed X ratio for half-life # of half-lives = 57. 2 days X 1 half-life = 4 half-lives 14. 3 days So now we know the answer will be: 4 mg X ½ X ½ = 0. 25 mg

Write this down: Original Mass(½)x = Final Mass x = Number of Half Lives x= Time Elapsed One Half Life

Practice: 1. The half-life of polonium-210 is 138. 4 days. How many mg of polonium-210 remain after 415. 2 days if you start with 2 mg of the isotope? # of half-lives = 415. 2 days X 1 half-life = 3 half-lives 138. 4 days 2 mg X ½ X ½ = 0. 25 mg

2. The half-life of radon-222 is 3. 824 days. After what time will ¼ of a given amount of radon-222 remain? To obtain ¼ of any amount, it will have to go through 2 half-lives: ½ X ½ = ¼ Therefore to get ¼ of any amount of radon-222 it will take 7. 648 days (enough time for 2 half-lives).

74 Half-Life • HALF-LIFE is the time that it takes for 1/2 a sample to decompose. • The rate of a nuclear transformation depends only on the “reactant” concentration.

Half-Life Decay of 20. 0 mg of 15 O. What remains after 3 half-lives? After 5 half-lives? 75

Kinetics of Radioactive Decay For each duration (half-life), one half of the substance decomposes. For example: Ra-234 has a half-life of 3. 6 days If you start with 50 grams of Ra-234 After 3. 6 days > 25 grams After 7. 2 days > 12. 5 grams After 10. 8 days > 6. 25 grams 76

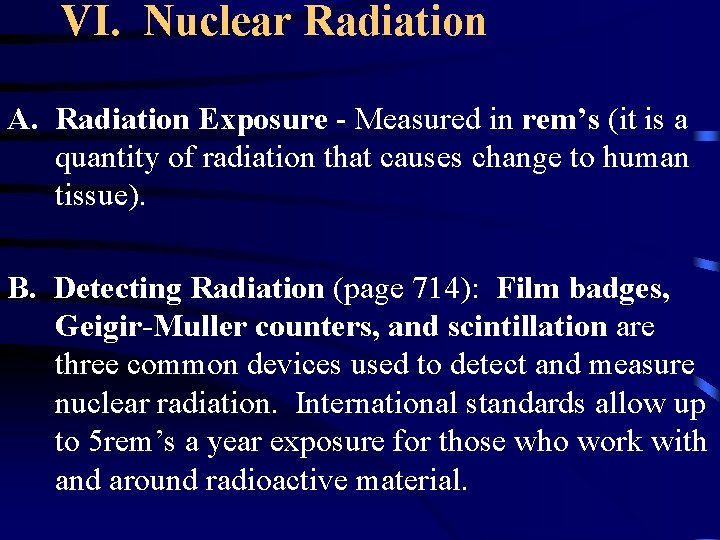
VI. Nuclear Radiation A. Radiation Exposure - Measured in rem’s (it is a quantity of radiation that causes change to human tissue). B. Detecting Radiation (page 714): Film badges, Geigir-Muller counters, and scintillation are three common devices used to detect and measure nuclear radiation. International standards allow up to 5 rem’s a year exposure for those who work with and around radioactive material.

Film Badges: Film badges are worn the entire time a person is at work. At specific times the film is removed and developed. The strength and type or radiation exposure are determined from the darkening of the film.

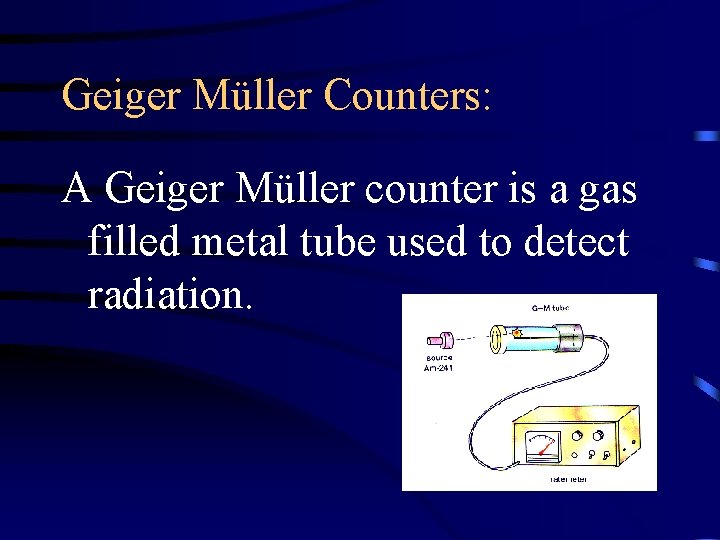
Geiger Müller Counters: A Geiger Müller counter is a gas filled metal tube used to detect radiation.

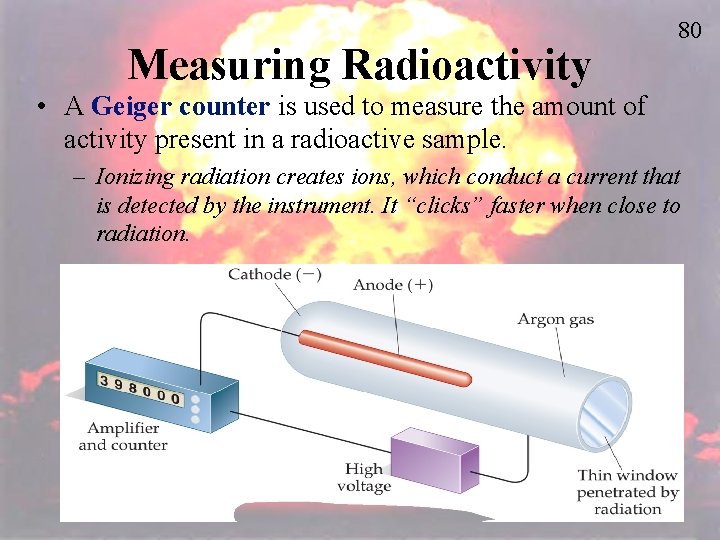
Measuring Radioactivity 80 • A Geiger counter is used to measure the amount of activity present in a radioactive sample. – Ionizing radiation creates ions, which conduct a current that is detected by the instrument. It “clicks” faster when close to radiation.

Scintillation Counters: This is a device that uses a phosphorus coated surface to detect radiation.

C. Applications of Nuclear Radiation 1. 2. 3. 4. Radioactive Dating Radioactive Tracers – Medical uses Radioactive Tracers – Agricultural uses Radiation Therapy

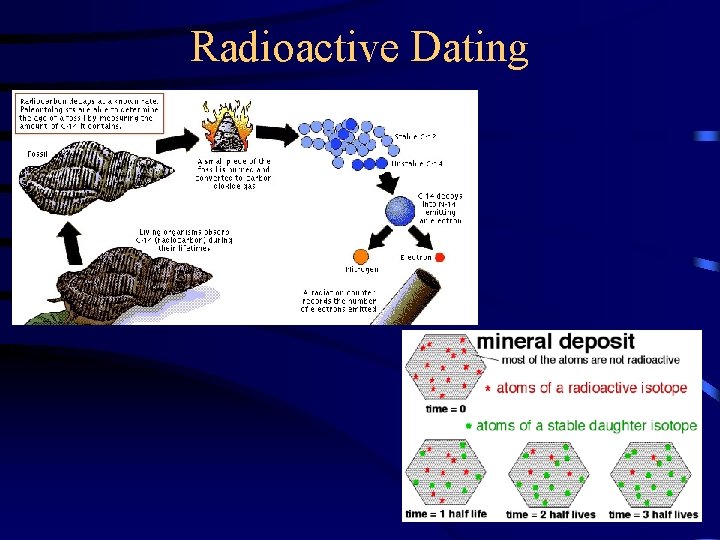
Radioactive Dating

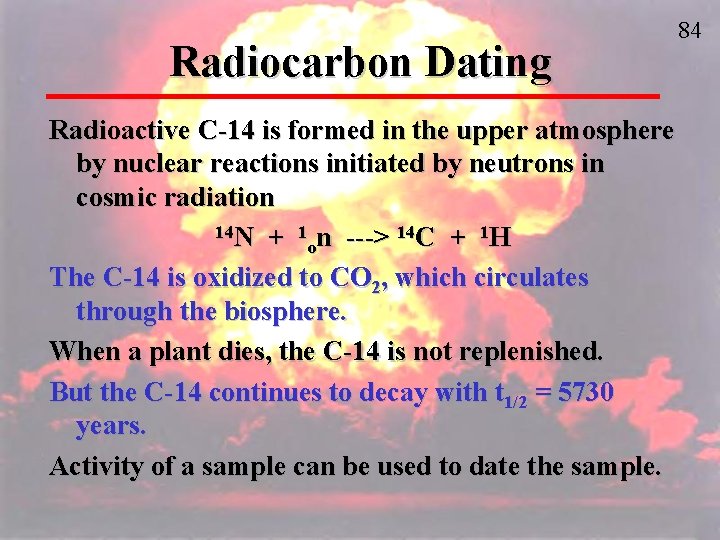
Radiocarbon Dating Radioactive C-14 is formed in the upper atmosphere by nuclear reactions initiated by neutrons in cosmic radiation 14 N + 1 n ---> 14 C + 1 H o The C-14 is oxidized to CO 2, which circulates through the biosphere. When a plant dies, the C-14 is not replenished. But the C-14 continues to decay with t 1/2 = 5730 years. Activity of a sample can be used to date the sample. 84

Radioactive Tracers: These are incorporated into substances so that movement of the substance can be followed by radiation detectors. (Page 715) Detection of radioactive tracers can be used to diagnose cancer and other diseases.

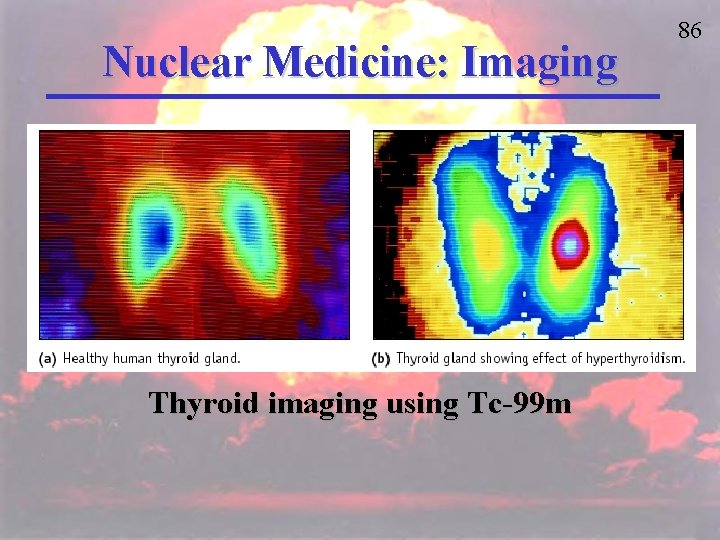
Nuclear Medicine: Imaging Thyroid imaging using Tc-99 m 86

Radioactive Tracers – Agricultural Uses: In agriculture they are used to test the effect of fertilizers, pesticides, and herbicides. During the procedure the tracer is first introduced into a substance being tested to make the substance radioactive. Next, plants are treated with the radioactive substance. The radioactivity of the plant is measured to determine location of the substance.

88 Food Irradiation • Food can be irradiated with g rays from 60 Co or 137 Cs. • Irradiated milk has a shelf life of 3 mo. without refrigeration. • USDA has approved irradiation of meats and eggs.

Radiation Therapy: This is commonly used to treat cancer. The cancerous area can be treated with radiation to kill the caner cells. (Some healthy cells are killed as well, so the benefit and risks of the treatment must be carefully considered prior to treatment. ) Gamma and beta particles are most commonly used.

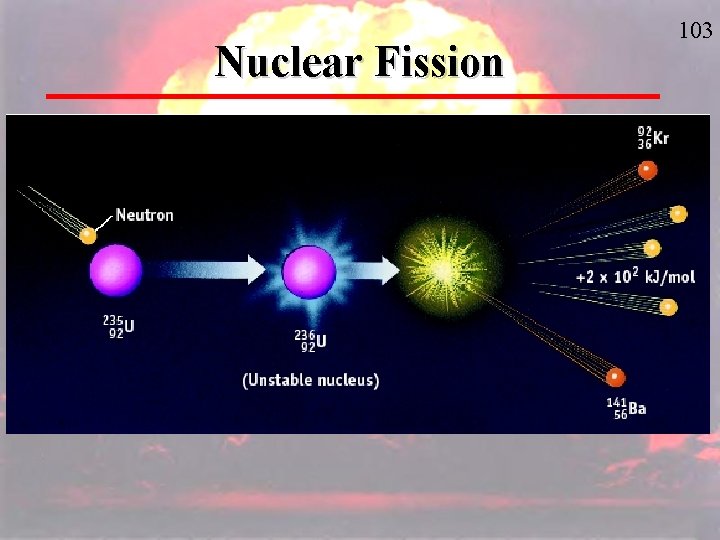
VII. Nuclear Fission and Fusion A. Nuclear Fission – the splitting of a nucleus into smaller fragments (the splitting is caused by bombarding the nucleus with neutrons). This process releases enormous amounts of energy. (page 717) A nuclear chain reaction is a reaction in which the material that starts the reaction (neutron) is also one of the products and can be used to start another reaction. 1. Nuclear Reactors use controlled – fission chain reactions to produce energy or radioactive nuclides.

Fission Chain Reaction

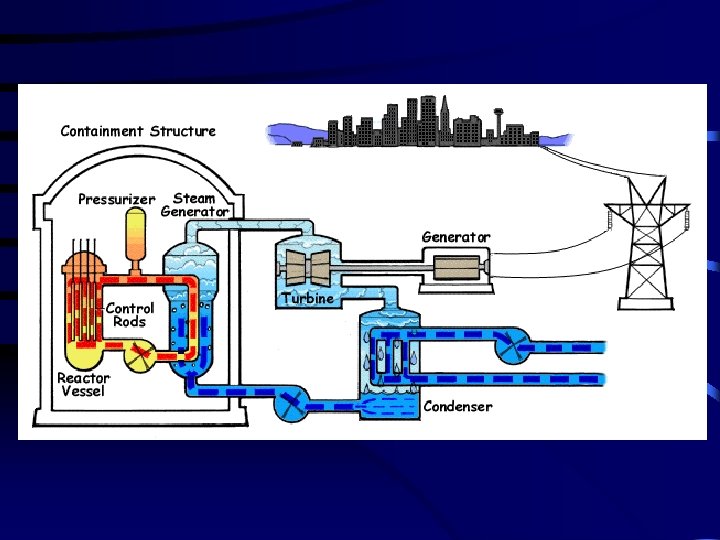
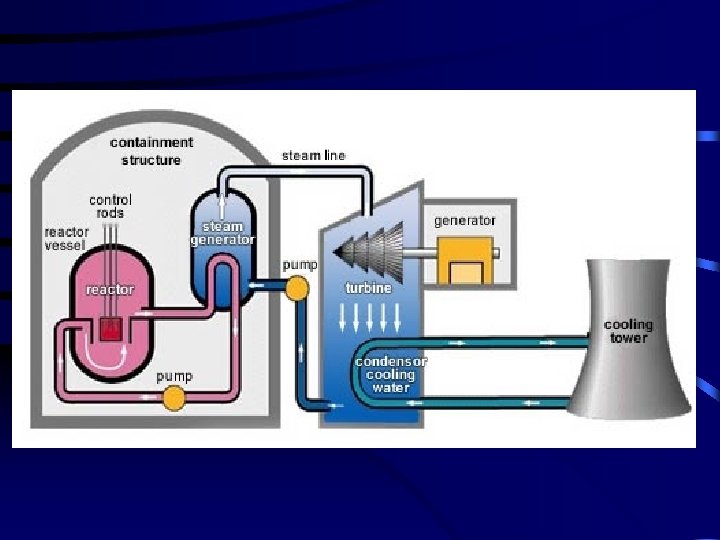
2. Nuclear Power Plants use heat from nuclear reactors to produce electrical energy. They have 5 main components: page 718 a. shielding – radiation absorbing material that is used to decrease exposure to radiation. b. fuel – uranium is most often used c. control rods – neutron absorbing rods that help control the reaction by limiting the number of free neutrons d. moderator – water (sometimes carbon) is used to slow down the fast neutrons produced by fission e. coolant – water acts as a coolant and transports heat between the reaction and the steam turbines to produce electric current Nuclear Power Plants produce a great deal of energy, the current problems with nuclear power plants include environmental requirements, safety of operation, plant construction costs, and storage and disposal of spent fuel and radioactive waste.




3. Atomic Bomb – fission reaction

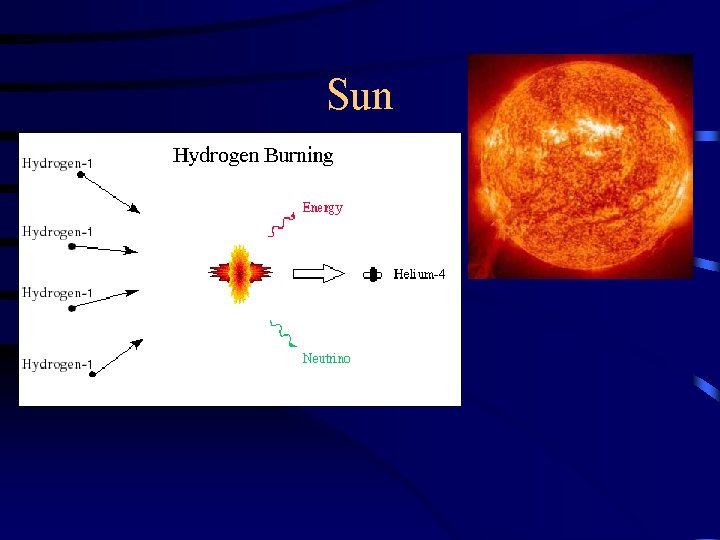
B. Nuclear Fusion – light mass nuclei combine to form a heavier, more stable nucleus. Nuclear fusion releases even more energy per gram of fuel then nuclear fission!! 1. Sun/Stars – four hydrogen nuclei combine at extremely high temperatures and pressures to form a helium nucleus – this is a fusion reaction. 2. Hydrogen Bomb (page 719)- uncontrolled fusion reactions of hydrogen are the source of energy for the hydrogen bomb. Hydrogen bombs generate a great deal more of energy then an atomic bomb. 3. Fusion as a Source of Energy: because fusion reactions generate a great deal more energy and their products are less harmful then fission reactions. Research is being done to try to use fusion instead of fission, there a few problems: temperature of 108 Kelvin is required and no known material can withstand the temperature.


Sun

C. Nuclear Waste (produced from fission and fusion reactions) 1. Types of Nuclear Waste -spent fuel rods -dismanteled nuclear power plants -military -radioisotopes used in many hospitals


2. Containment – on-site storage and off-site disposal (Remember that every radioactive substance has a half-life, some only a few months, others hundreds of thousands of years. ) a. On-Site Storage – most common nuclear waste is spent fuel rods from nuclear power plants -water pools -dry casks (concrete or steel) b. Off-Site Disposal – disposal of nuclear waste is done with the intention of never retrieving the materials. -77 disposal sites around the United States -new site near Las Vegas, Nevada Yucca Mountain

Nuclear Fission 103

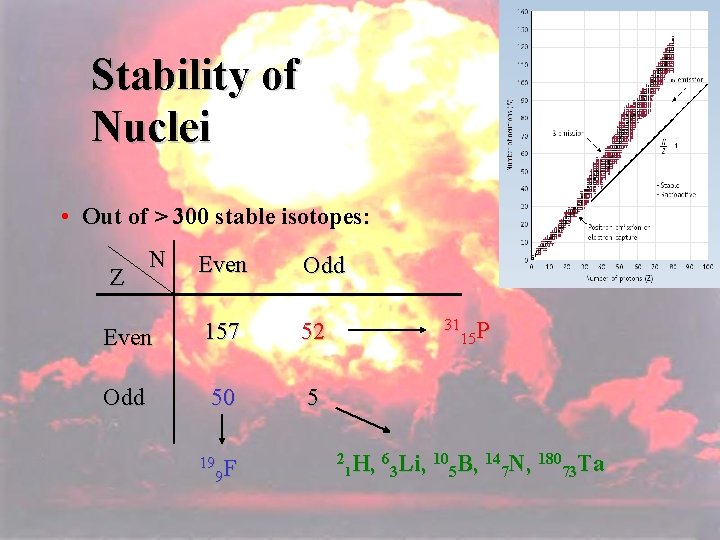
104 Stability of Nuclei • Out of > 300 stable isotopes: N Even Odd Even 157 52 Odd 50 5 Z 19 F 9 31 P 15 2 H, 63 Li, 105 B, 147 N, 18073 Ta 1

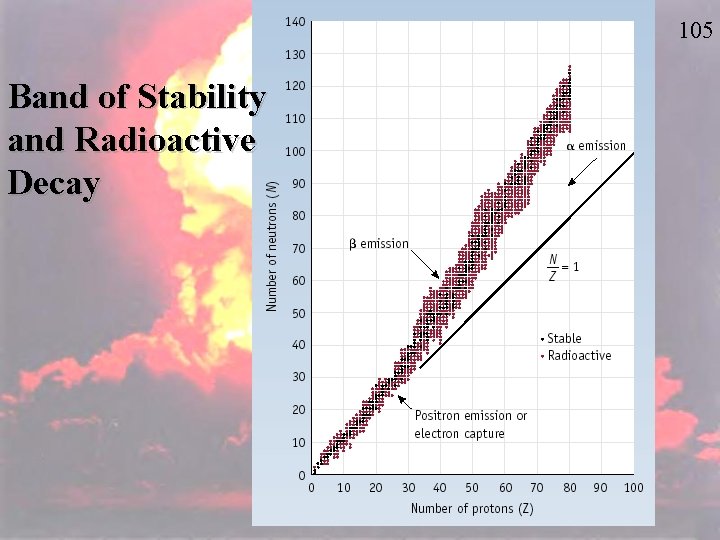
105 Band of Stability and Radioactive Decay

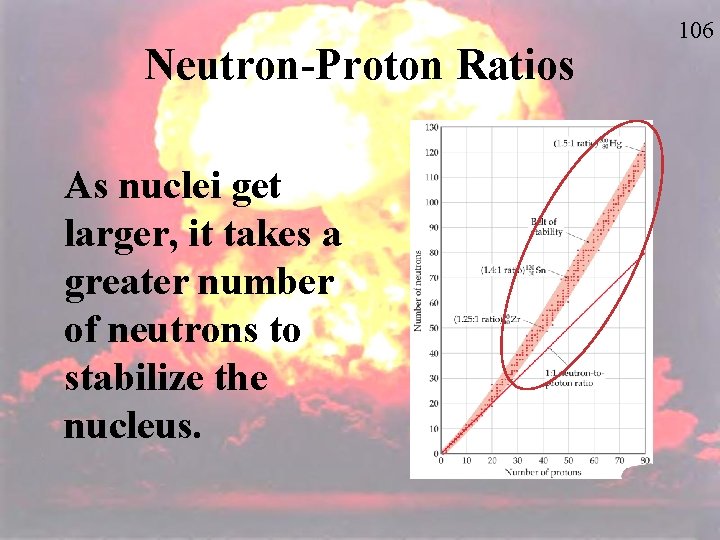
Neutron-Proton Ratios As nuclei get larger, it takes a greater number of neutrons to stabilize the nucleus. 106

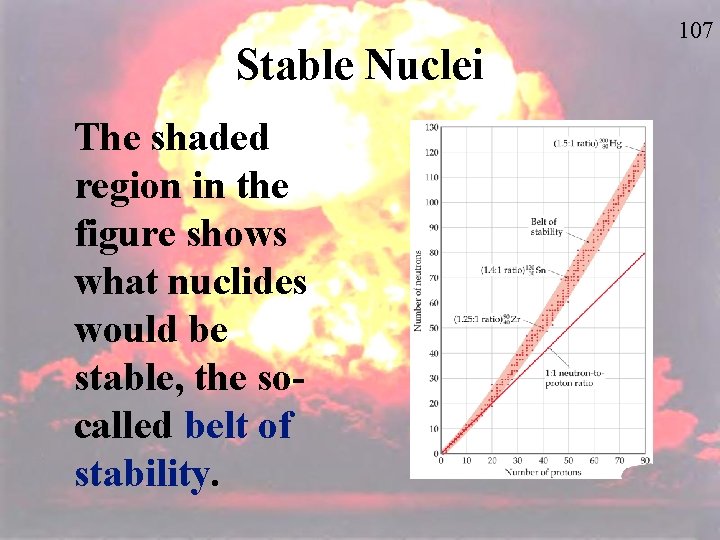
Stable Nuclei The shaded region in the figure shows what nuclides would be stable, the socalled belt of stability. 107

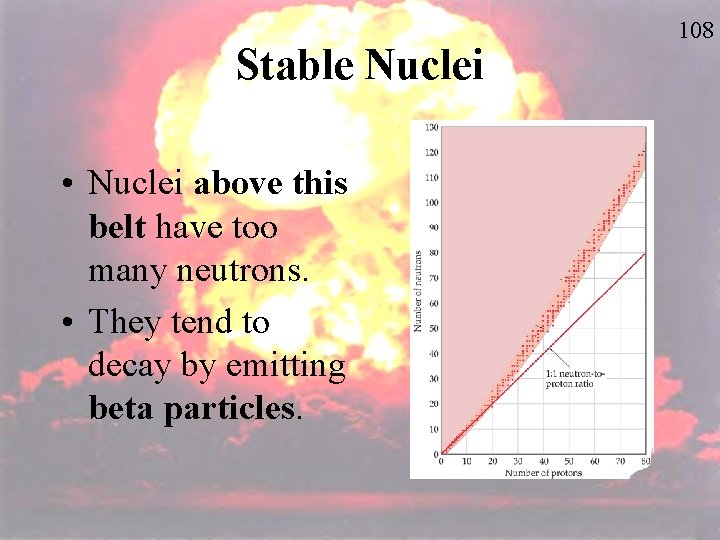
Stable Nuclei • Nuclei above this belt have too many neutrons. • They tend to decay by emitting beta particles. 108

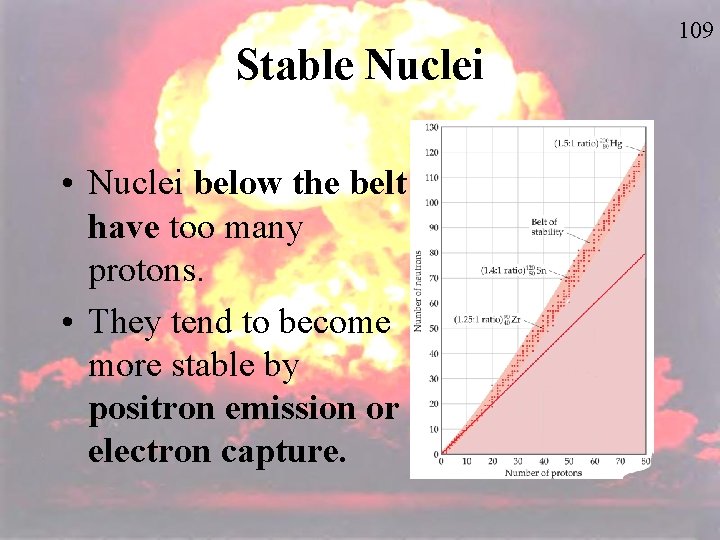
Stable Nuclei • Nuclei below the belt have too many protons. • They tend to become more stable by positron emission or electron capture. 109

110 Stable Nuclei • There are no stable nuclei with an atomic number greater than 83. – These nuclei tend to decay by alpha emission, emitting alpha particles.

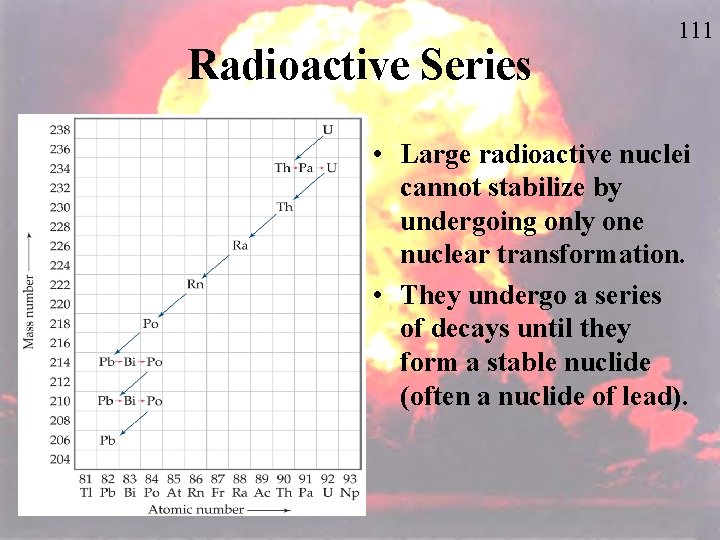
Radioactive Series 111 • Large radioactive nuclei cannot stabilize by undergoing only one nuclear transformation. • They undergo a series of decays until they form a stable nuclide (often a nuclide of lead).

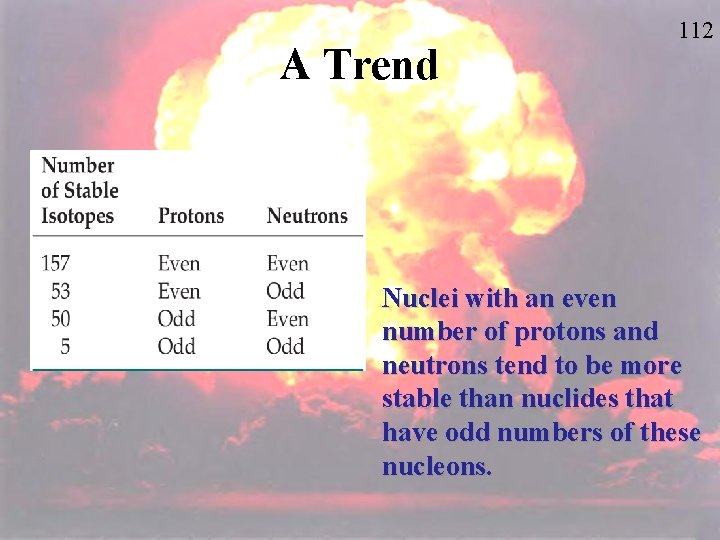
A Trend 112 Nuclei with an even number of protons and neutrons tend to be more stable than nuclides that have odd numbers of these nucleons.

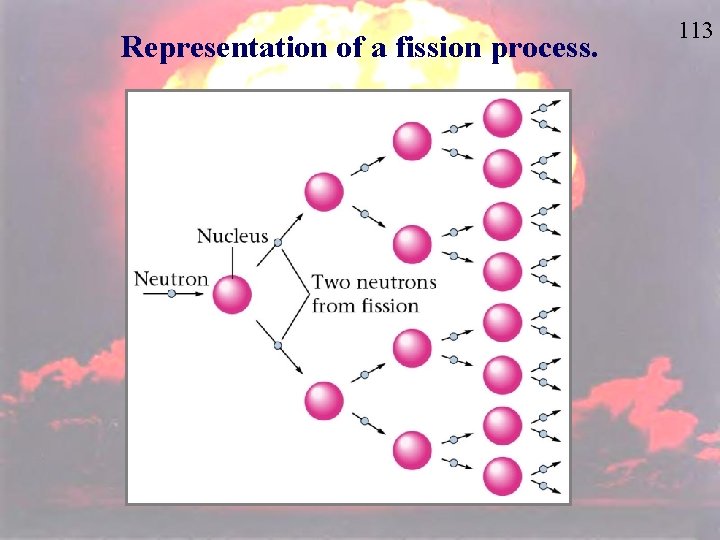
Representation of a fission process. 113

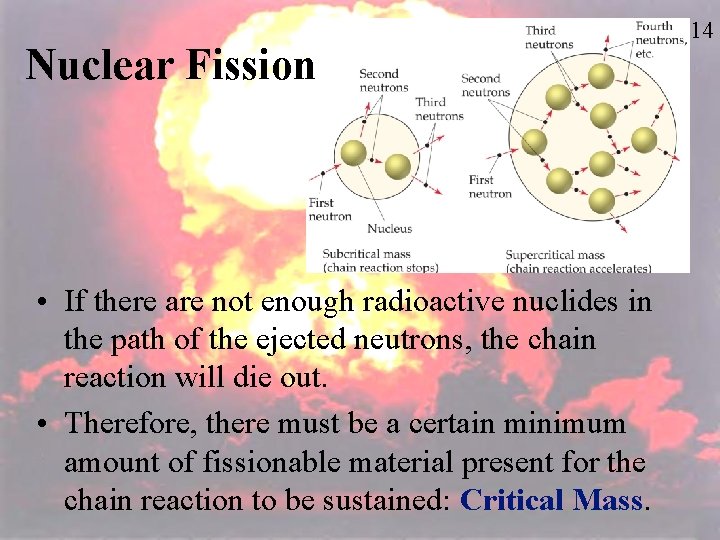
Nuclear Fission • If there are not enough radioactive nuclides in the path of the ejected neutrons, the chain reaction will die out. • Therefore, there must be a certain minimum amount of fissionable material present for the chain reaction to be sustained: Critical Mass. 114

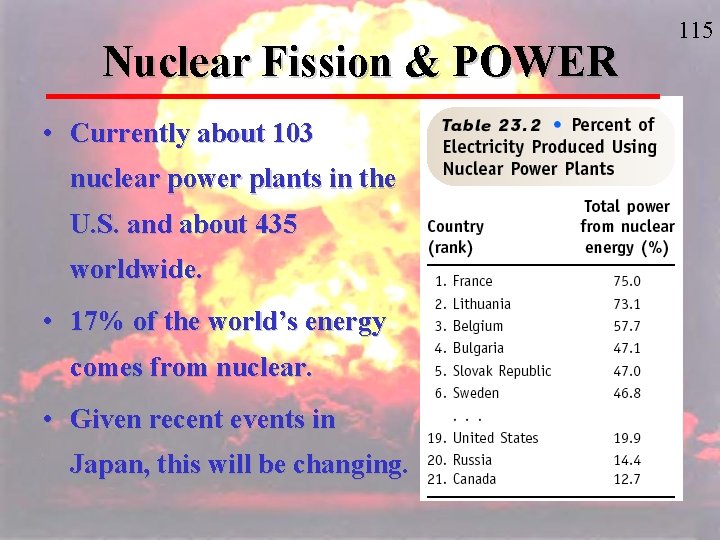
Nuclear Fission & POWER • Currently about 103 nuclear power plants in the U. S. and about 435 worldwide. • 17% of the world’s energy comes from nuclear. • Given recent events in Japan, this will be changing. 115

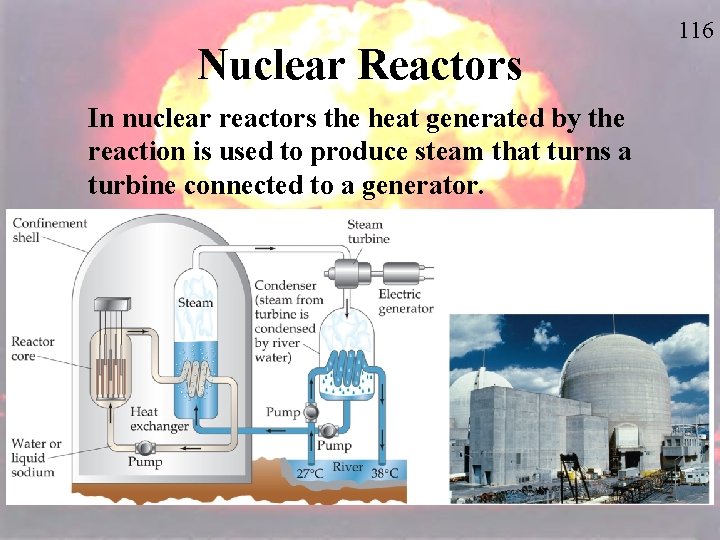
Nuclear Reactors In nuclear reactors the heat generated by the reaction is used to produce steam that turns a turbine connected to a generator. 116

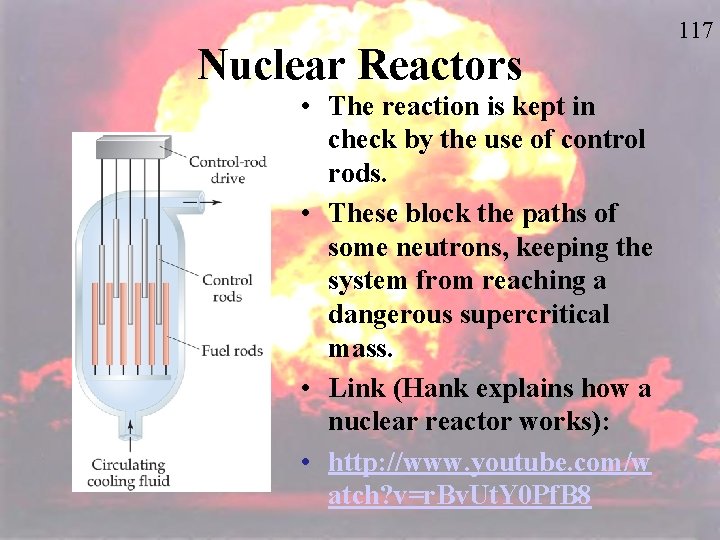
Nuclear Reactors • The reaction is kept in check by the use of control rods. • These block the paths of some neutrons, keeping the system from reaching a dangerous supercritical mass. • Link (Hank explains how a nuclear reactor works): • http: //www. youtube. com/w atch? v=r. Bv. Ut. Y 0 Pf. B 8 117

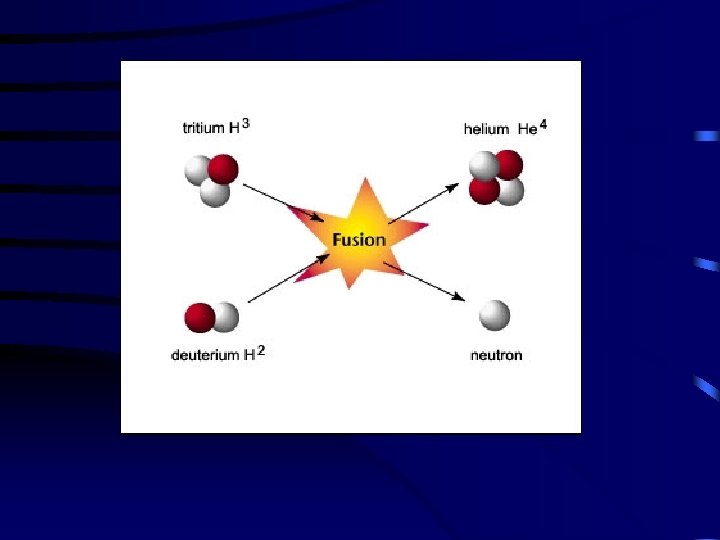
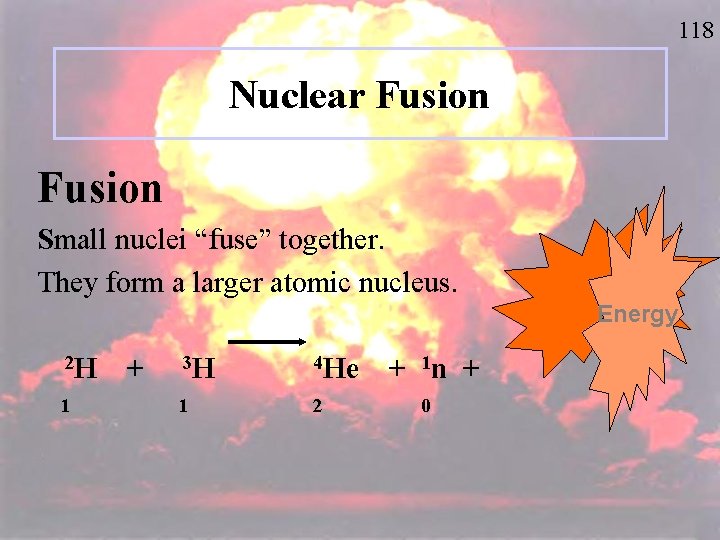
118 Nuclear Fusion Small nuclei “fuse” together. They form a larger atomic nucleus. Energy 2 H 1 + 3 H 4 He 1 2 + 1 n + 0

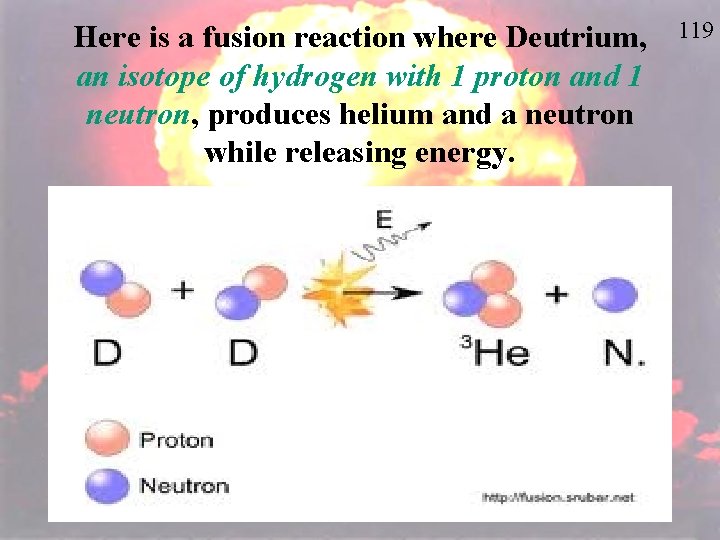
Here is a fusion reaction where Deutrium, an isotope of hydrogen with 1 proton and 1 neutron, produces helium and a neutron while releasing energy. 119

Where does fusion naturally occur? Fusion • Since excessive amounts of heat can not be contained, • To date, all attempts at “cold” fusion have FAILED. • “Hot” fusion is difficult to contain, but happens in the stars. 120

Nuclear Fusion 121 • Fusion would be a superior method of generating power, if we could make it happen, and if we could control it here on EARTH. – The products of the reaction are not radioactive, not dangerous! – However, in order to achieve fusion, the material must be in the plasma state at several million kelvins.