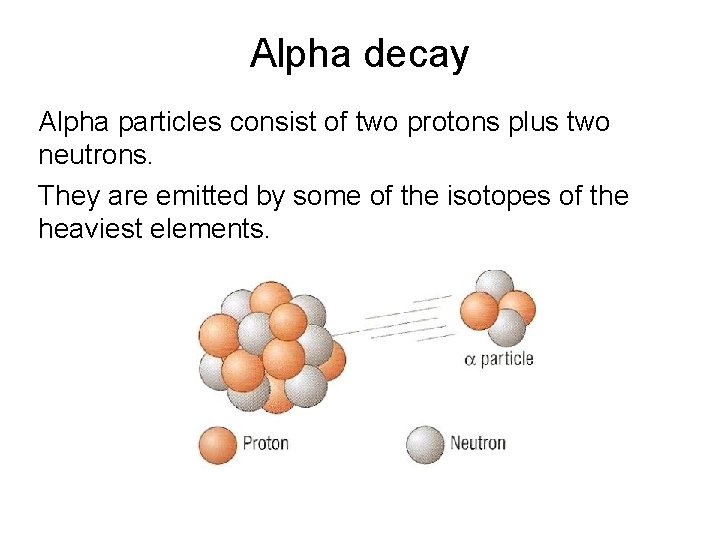
Alpha decay Alpha particles consist of two protons


















- Slides: 45

Alpha decay Alpha particles consist of two protons plus two neutrons. They are emitted by some of the isotopes of the heaviest elements.

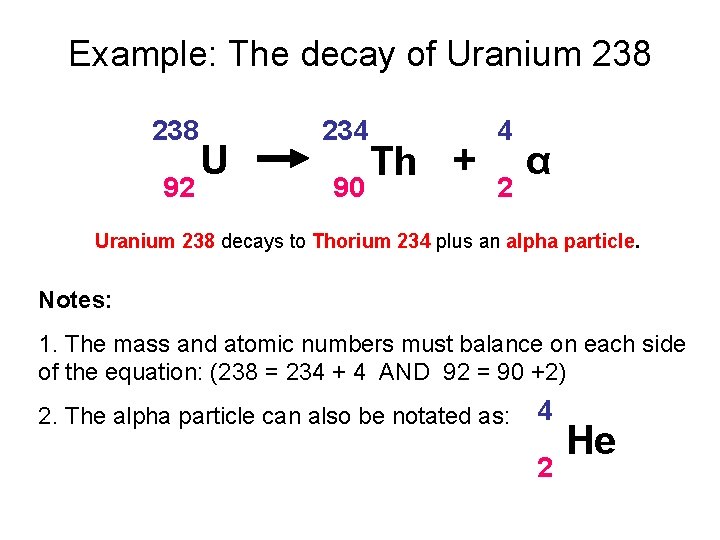
Example: The decay of Uranium 238 92 U 234 90 Th + 4 2 α Uranium 238 decays to Thorium 234 plus an alpha particle. Notes: 1. The mass and atomic numbers must balance on each side of the equation: (238 = 234 + 4 AND 92 = 90 +2) 2. The alpha particle can also be notated as: 4 2 He

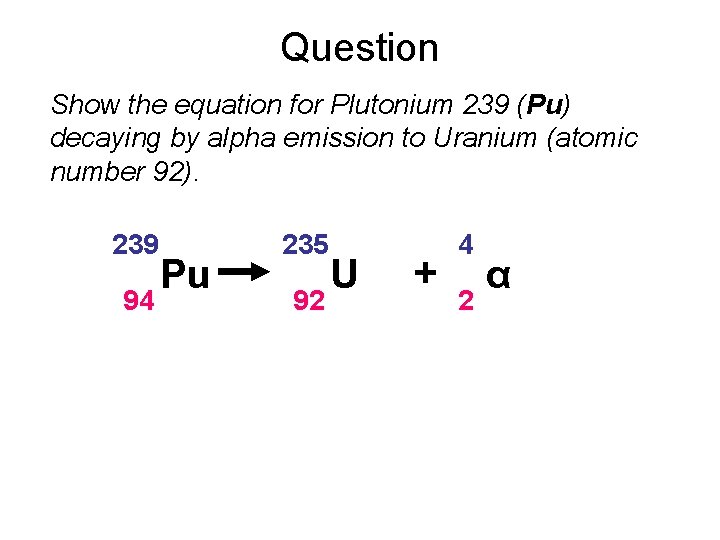
Question Show the equation for Plutonium 239 (Pu) decaying by alpha emission to Uranium (atomic number 92). 239 94 Pu 235 92 U + 4 2 α

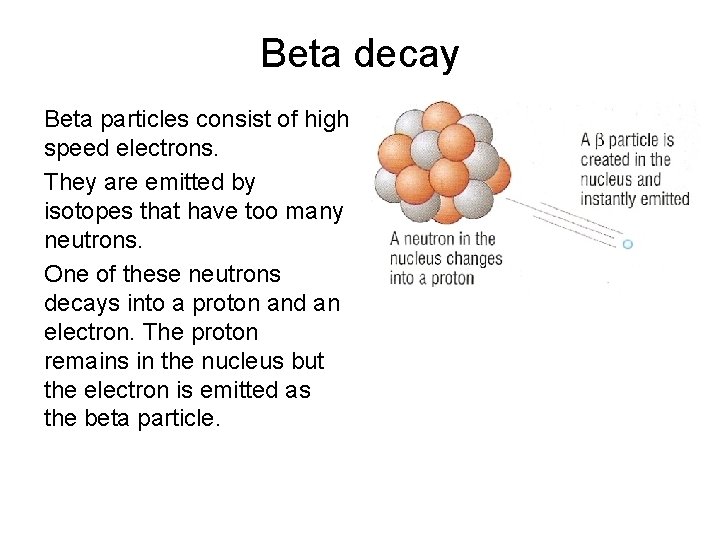
Beta decay Beta particles consist of high speed electrons. They are emitted by isotopes that have too many neutrons. One of these neutrons decays into a proton and an electron. The proton remains in the nucleus but the electron is emitted as the beta particle.

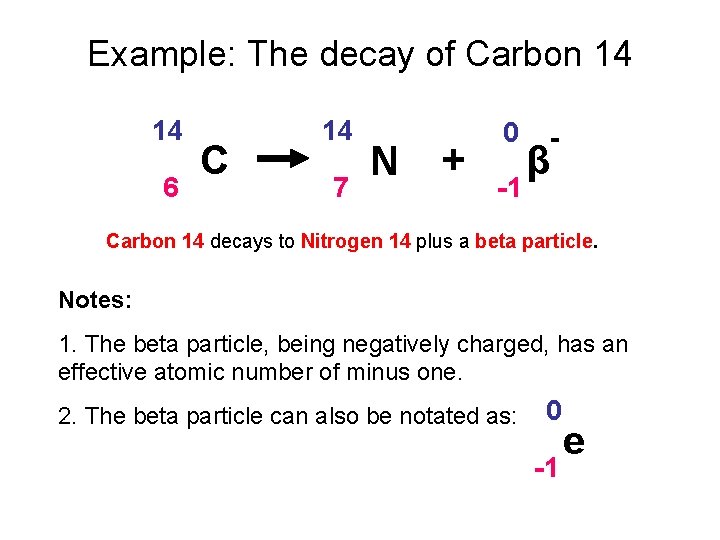
Example: The decay of Carbon 14 14 6 C 14 7 N + 0 -1 β - Carbon 14 decays to Nitrogen 14 plus a beta particle. Notes: 1. The beta particle, being negatively charged, has an effective atomic number of minus one. 2. The beta particle can also be notated as: 0 -1 e

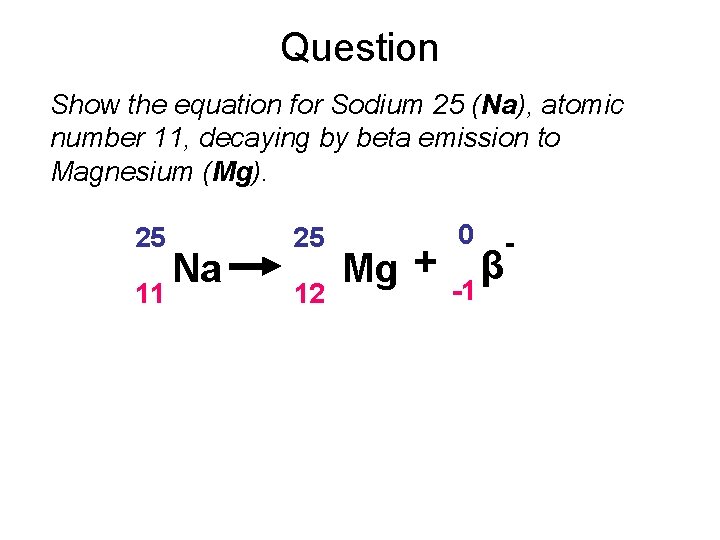
Question Show the equation for Sodium 25 (Na), atomic number 11, decaying by beta emission to Magnesium (Mg). 25 11 Na 25 12 Mg + 0 -1 β -

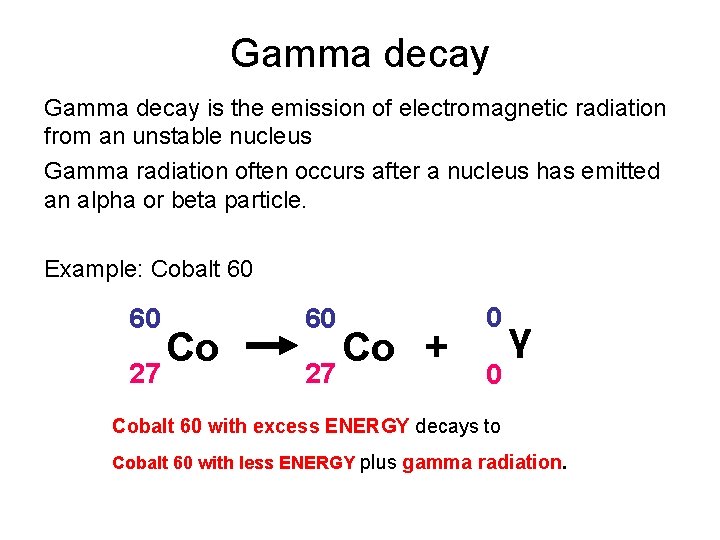
Gamma decay is the emission of electromagnetic radiation from an unstable nucleus Gamma radiation often occurs after a nucleus has emitted an alpha or beta particle. Example: Cobalt 60 60 27 Co + 0 0 γ Cobalt 60 with excess ENERGY decays to Cobalt 60 with less ENERGY plus gamma radiation.

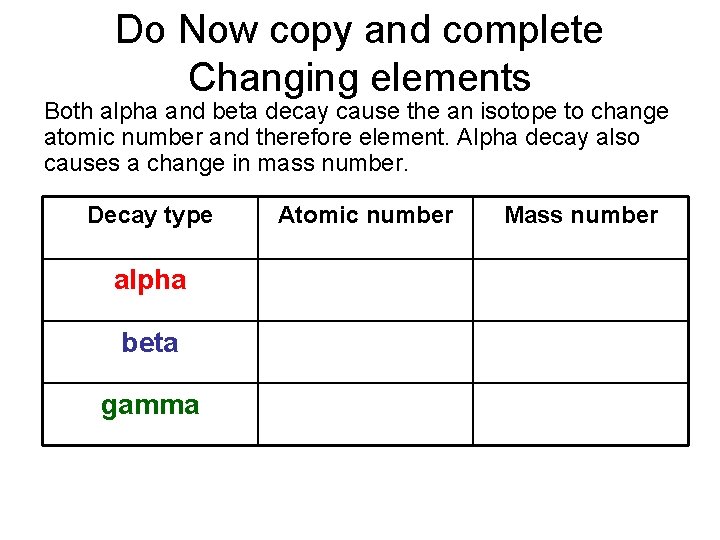
Do Now copy and complete Changing elements Both alpha and beta decay cause the an isotope to change atomic number and therefore element. Alpha decay also causes a change in mass number. Decay type Atomic number Mass number alpha DOWN by 2 DOWN by 4 beta UP by 1 NO CHANGE gamma NO CHANGE

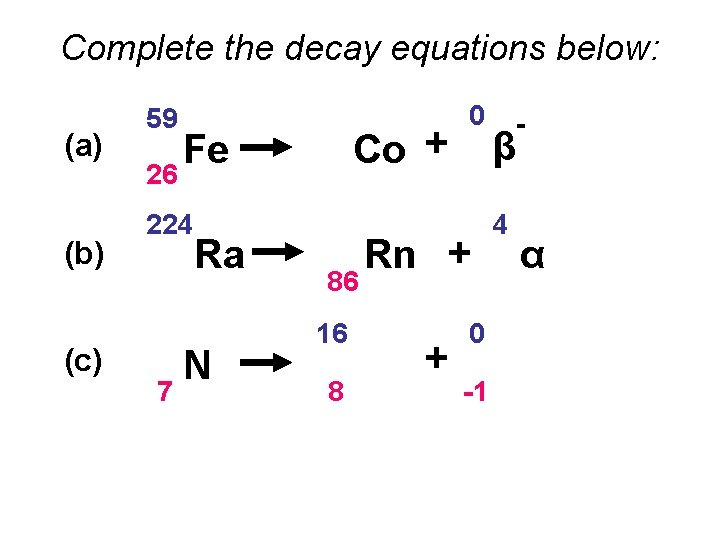
Complete the decay equations below: (a) (b) (c) 59 26 Fe 224 88 16 7 Ra N 59 27 Co + 220 86 16 8 0 -1 Rn + O + 0 -1 β 4 2 β - α -

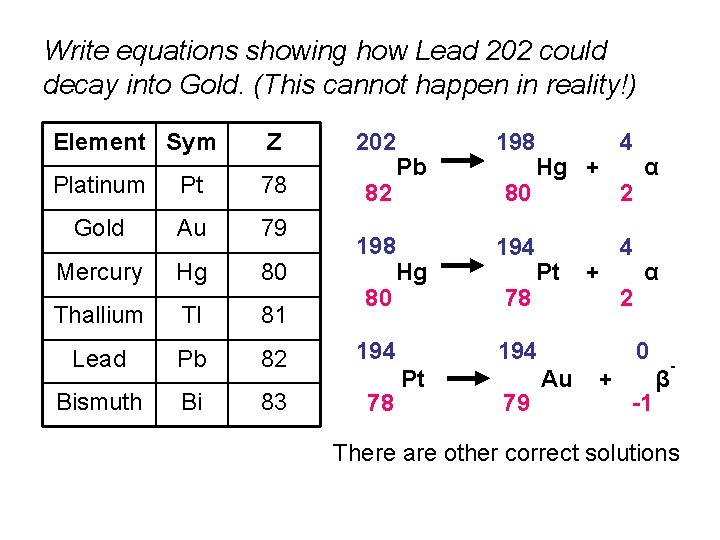
Write equations showing how Lead 202 could decay into Gold. (This cannot happen in reality!) Element Sym Z 202 Platinum Pt 78 82 Gold Au 79 Mercury Hg 80 Thallium Tl 81 Lead Pb 82 194 Bismuth Bi 83 78 198 80 Pb Hg 198 80 194 78 Hg + Pt + 194 Pt 79 4 2 α α 0 Au + -1 β - There are other correct solutions

Choose appropriate words to fill in the gaps below: When an unstable nucleus emits an alpha particle its atomic two four number falls by _______ and its mass number by ______. neutrons Beta particles are emitted by nuclei with too many ____. one In this case the atomic number increases by ______ while the mass ____ number remains unchanged. electromagnetic radiation that is Gamma rays consist of _______ energy emitted from a nucleus when it loses ____, often after undergoing alpha or beta decay. WORD SELECTION: four one energy two neutrons mass electromagnetic

Today’s lesson • Use the term half-life in simple calculations, including the use of information in tables or decay curves. • Give and explain examples of practical applications of isotopes. • Title Half-life

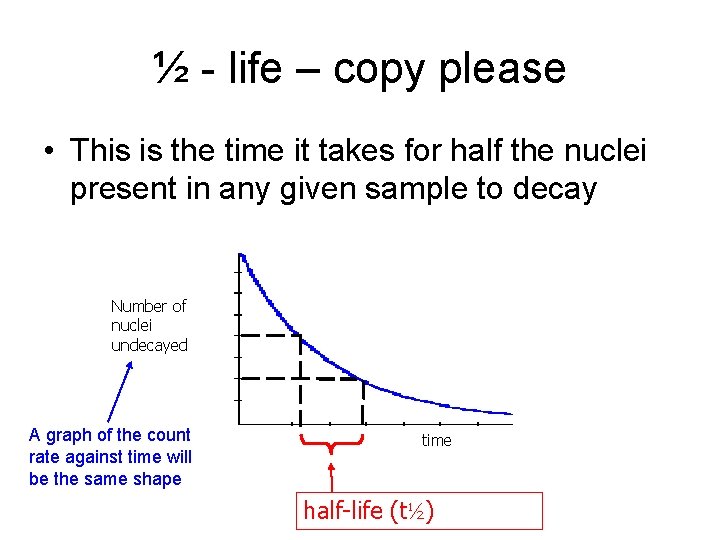
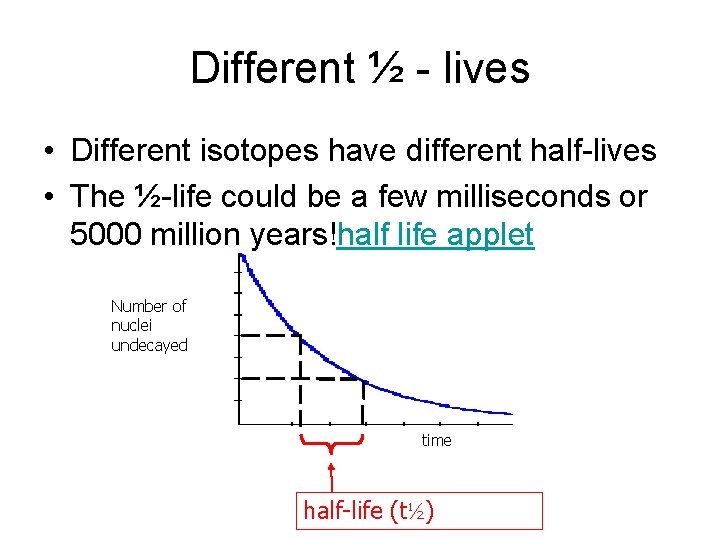
½ - life – copy please • This is the time it takes for half the nuclei present in any given sample to decay Number of nuclei undecayed A graph of the count rate against time will be the same shape time half-life (t½)

Different ½ - lives • Different isotopes have different half-lives • The ½-life could be a few milliseconds or 5000 million years!half life applet Number of nuclei undecayed time half-life (t½)

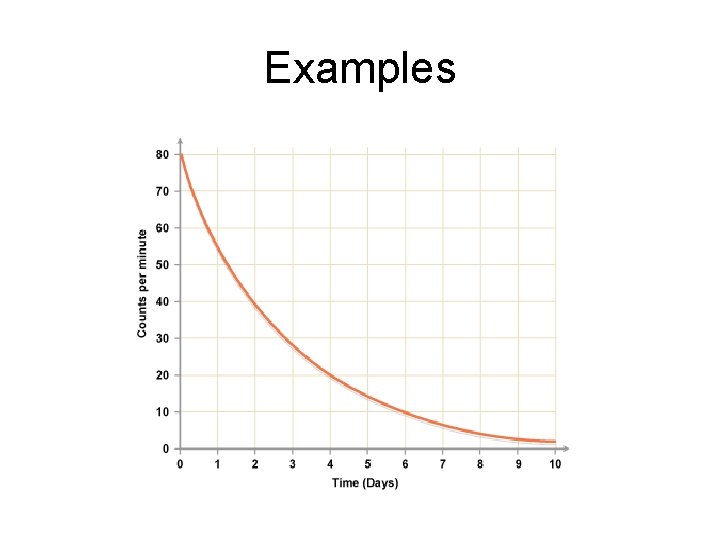
Examples • A sample of a radioactive isotope of half life 2 hours has a count rate of 30 000 counts per second. What will the count rate be after 8 hours?

Examples

Activity The activity of a radioactive source is equal to the number of decays per second. Activity is measured in bequerels (Bq) 1 becquerel = 1 decay per second Half life Henri Becquerel discovered radioactivity in 1896

Question 1 At 10 am in the morning a radioactive sample contains 80 g of a radioactive isotope. If the isotope has a halflife of 20 minutes calculate the mass of the isotope remaining at 11 am. 10 am to 11 am = 60 minutes = 3 x 20 minutes = 3 half-lives mass of isotope = ½ x ½ x 80 g mass at 11 am = 10 g

Question 2 Calculate the half-life of the radioactive isotope in a source if its mass decreases from 24 g to 6 g over a period of 60 days. 24 g x ½ = 12 g x ½ = 6 g therefore TWO half-lives occur in 60 days half-life = 30 days

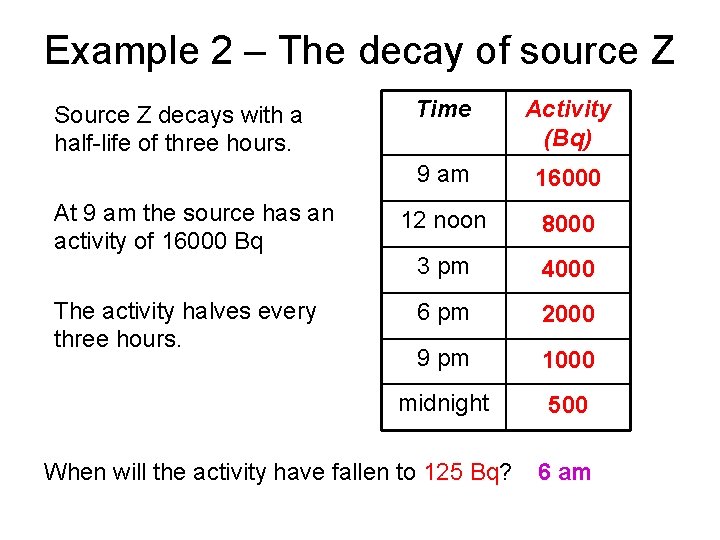
Example 2 – The decay of source Z Source Z decays with a half-life of three hours. At 9 am the source has an activity of 16000 Bq The activity halves every three hours. Time Activity (Bq) 9 am 16000 12 noon 8000 3 pm 4000 6 pm 2000 9 pm 1000 midnight 500 When will the activity have fallen to 125 Bq? 6 am

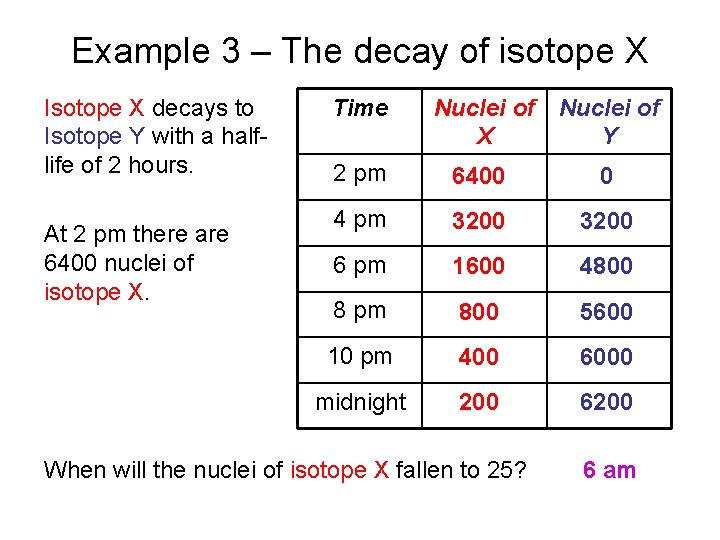
Example 3 – The decay of isotope X Isotope X decays to Isotope Y with a halflife of 2 hours. At 2 pm there are 6400 nuclei of isotope X. Time Nuclei of X Y 2 pm 6400 0 4 pm 3200 6 pm 1600 4800 8 pm 800 5600 10 pm 400 6000 midnight 200 6200 When will the nuclei of isotope X fallen to 25? 6 am

Question 3 A radioactive source has a half-life of 3 hours. At 8 am it has an activity of 600 Bq. What will be its activity at 2 pm? at 8 am activity = 600 Bq 2 pm is 6 hours later this is 2 half-lives later therefore the activity will halve twice that is: 600 300 150 activity at 2 pm = 150 Bq

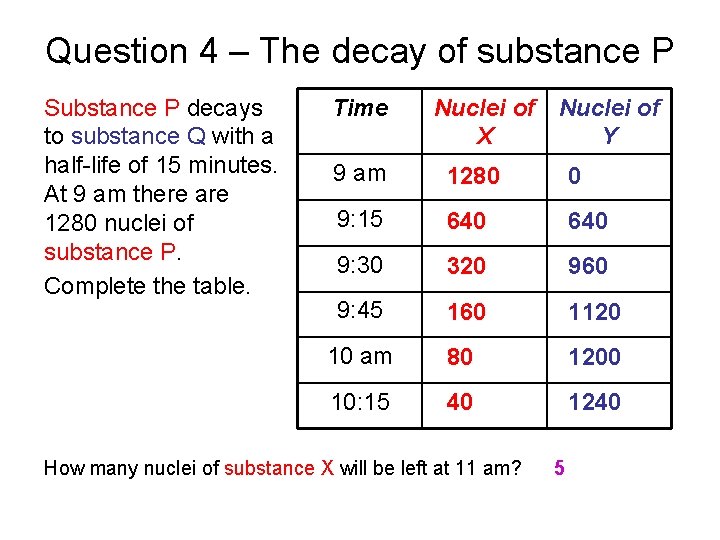
Question 4 – The decay of substance P Substance P decays to substance Q with a half-life of 15 minutes. At 9 am there are 1280 nuclei of substance P. Complete the table. Time Nuclei of X Y 9 am 1280 0 9: 15 640 9: 30 320 960 9: 45 160 1120 10 am 80 1200 10: 15 40 1240 How many nuclei of substance X will be left at 11 am? 5

Question 5 A sample contains 8 billion nuclei of hydrogen 3 atoms. Hydrogen 3 has a half-life of 12 years. How many nuclei should remain after a period 48 years? 48 years = 4 x 12 years = FOUR half-lives nuclei left = ½ x ½ x 8 billion nuclei left = 500 million

Experiment Dicium 25

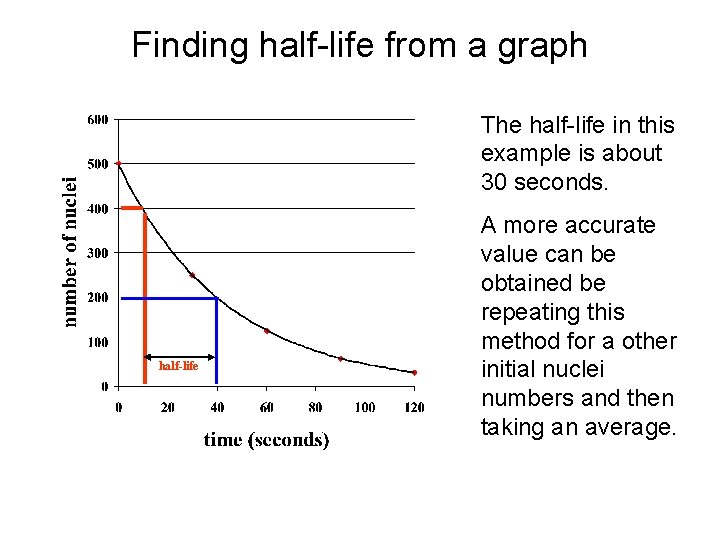
Finding half-life from a graph The half-life in this example is about 30 seconds. half-life A more accurate value can be obtained be repeating this method for a other initial nuclei numbers and then taking an average.

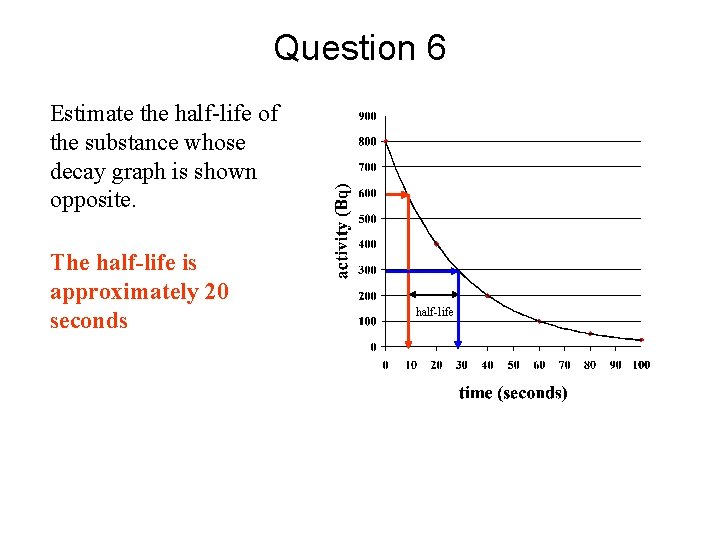
Question 6 Estimate the half-life of the substance whose decay graph is shown opposite. The half-life is approximately 20 seconds half-life

Question 7 The mass of a radioactive substance over a 8 hour period is shown in the table below. Draw a graph of mass against time and use it to determine the half-life of the substance. Time (hours) Mass (g) 0 1 2 3 4 5 6 7 8 650 493 373 283 214 163 123 93 71 The half-life should be about 2 hours:

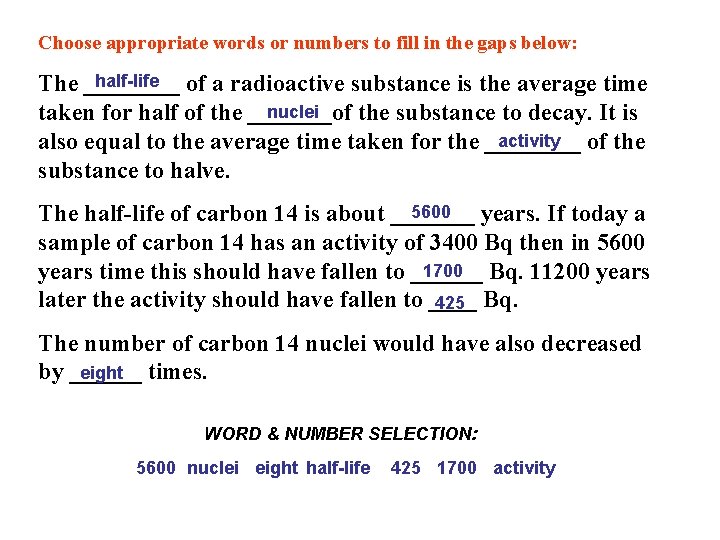
Choose appropriate words or numbers to fill in the gaps below: half-life The ____ of a radioactive substance is the average time nuclei taken for half of the _______of the substance to decay. It is activity also equal to the average time taken for the ____ of the substance to halve. 5600 The half-life of carbon 14 is about _______ years. If today a sample of carbon 14 has an activity of 3400 Bq then in 5600 1700 Bq. 11200 years time this should have fallen to ______ later the activity should have fallen to ____ 425 Bq. The number of carbon 14 nuclei would have also decreased eight times. by ______ WORD & NUMBER SELECTION: 5600 nuclei eight half-life 425 1700 activity

Revision Simulations Half-Life - S-Cool section on half-life and uses of radioactivity including an onscreen half-life calculation and an animation showing thickness control. BBC AQA GCSE Bitesize Revision: Detecting radiation Natural sources of background radiation Artificial radiation Half life Alpha Decay - Ph. ET - Watch alpha particles escape from a Polonium nucleus, causing radioactive alpha decay. See how random decay times relate to the half life.

Uses of radioactive isotopes

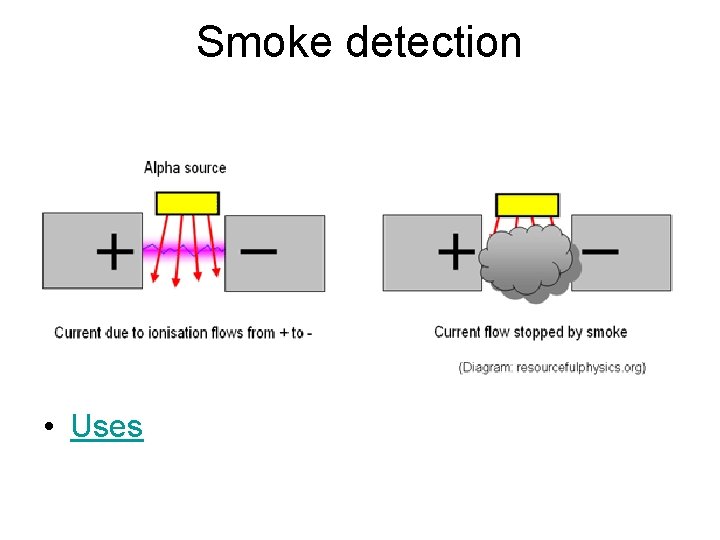
Smoke detection • Uses

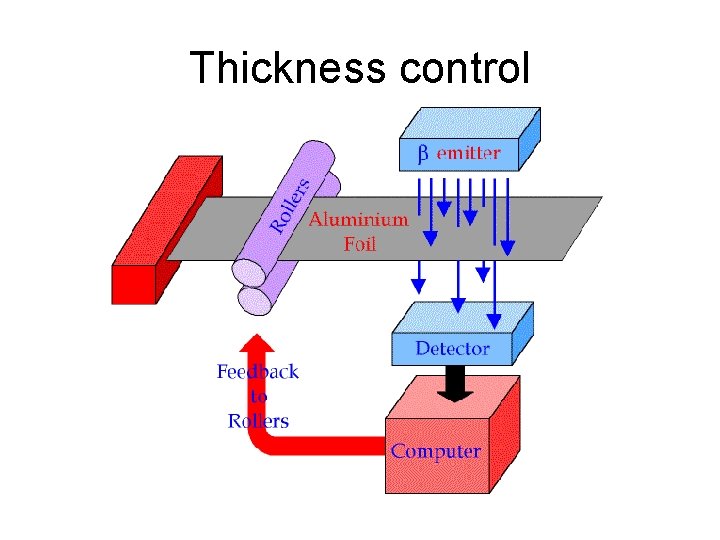
Thickness control

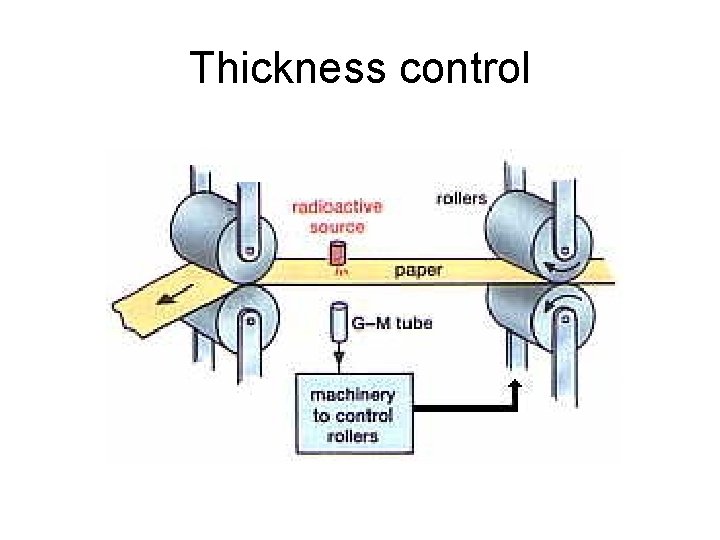
Thickness control

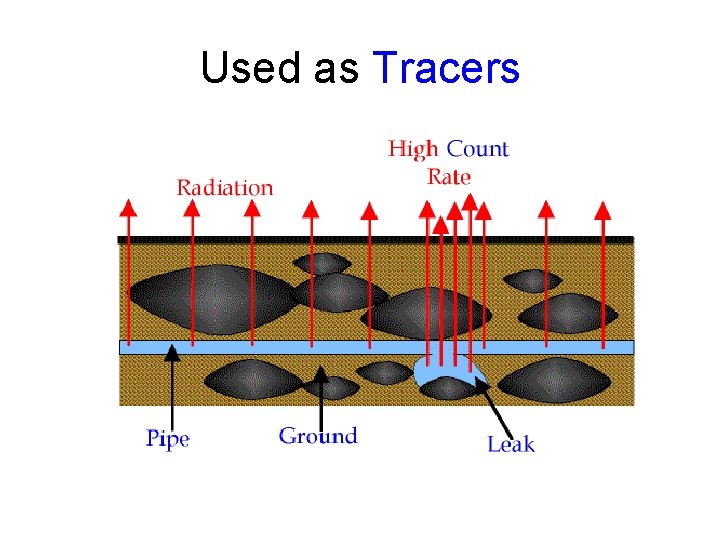
Used as Tracers

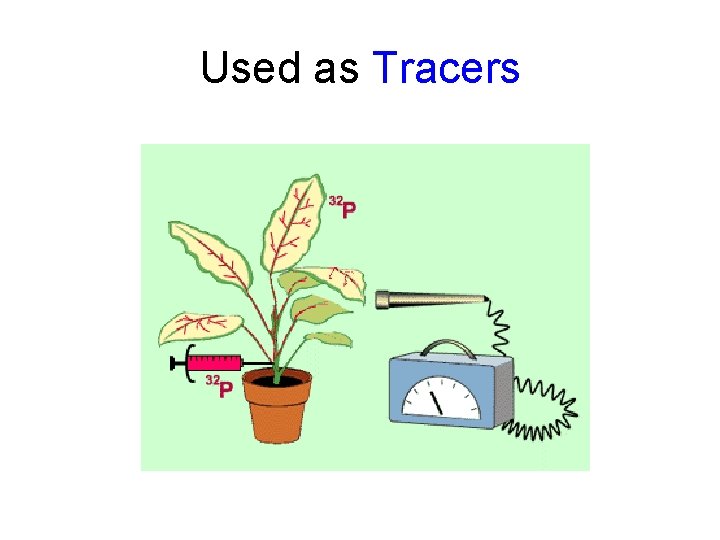
Used as Tracers

Killing microbes

Killing microbes

Checking welds

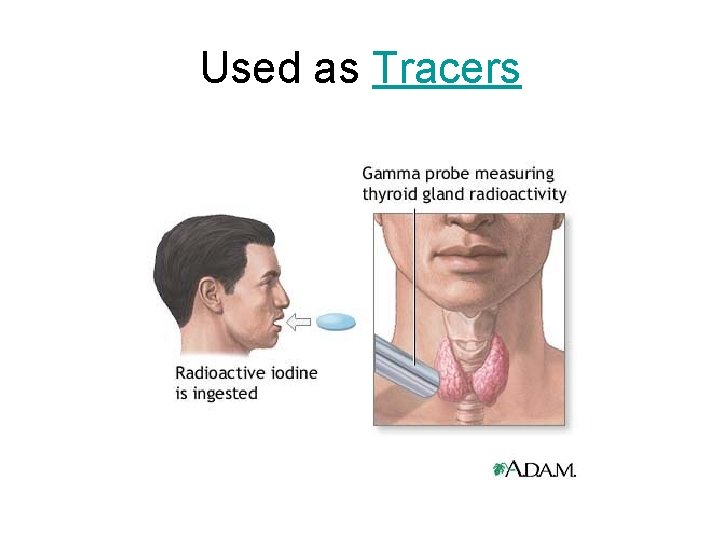
Used as Tracers

Carbon dating – write notes using the book page 265

Summary sheet


Test! Thursday 27 th September 2012

Can you answer the questions on pages 261 and 265?