Periodic Relationships Among the Elements Copyright The Mc

![Electron Configurations of Cations and Anions Representative Elements Na [Ne]3 s 1 Na+ [Ne] Electron Configurations of Cations and Anions Representative Elements Na [Ne]3 s 1 Na+ [Ne]](https://slidetodoc.com/presentation_image/f2c655eaaa1f5494e687a01db3f481df/image-11.jpg)
![Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or](https://slidetodoc.com/presentation_image/f2c655eaaa1f5494e687a01db3f481df/image-13.jpg)
![Write the electronic configuration for Ni 2+ ? (a) [Ar] 4 s 2 3 Write the electronic configuration for Ni 2+ ? (a) [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image/f2c655eaaa1f5494e687a01db3f481df/image-16.jpg)





- Slides: 55

Periodic Relationships Among the Elements Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Chapter 8 • 8. 2 -Periodic classification of the elements (326 -330). • 8. 3 -Periodic variation in physical properties (330 -335). • 8. 4 -Ionization energy- Electron affinity. (337 -343). • 9. 5 -Electronegativity (377 -378).

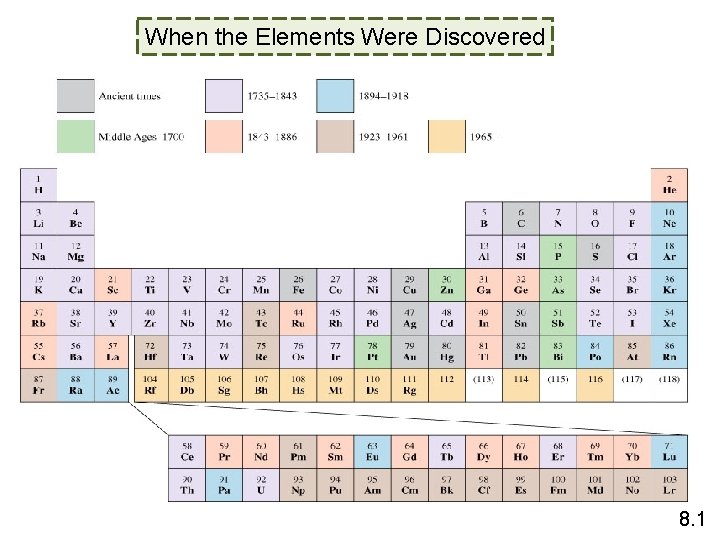
When the Elements Were Discovered 8. 1

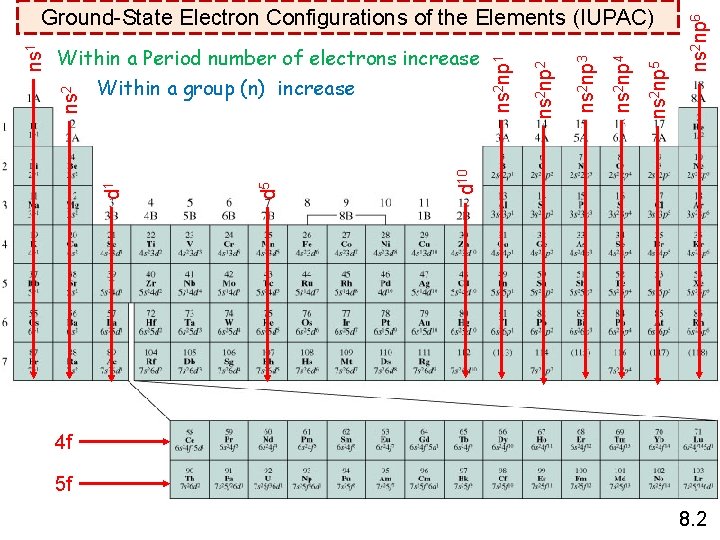
ns 2 np 6 ns 2 np 5 ns 2 np 4 ns 2 np 3 ns 2 np 2 d 10 d 5 d 1 ns 2 np 1 Within a Period number of electrons increase Within a group (n) increase ns 2 ns 1 Ground-State Electron Configurations of the Elements (IUPAC) 4 f 5 f 8. 2

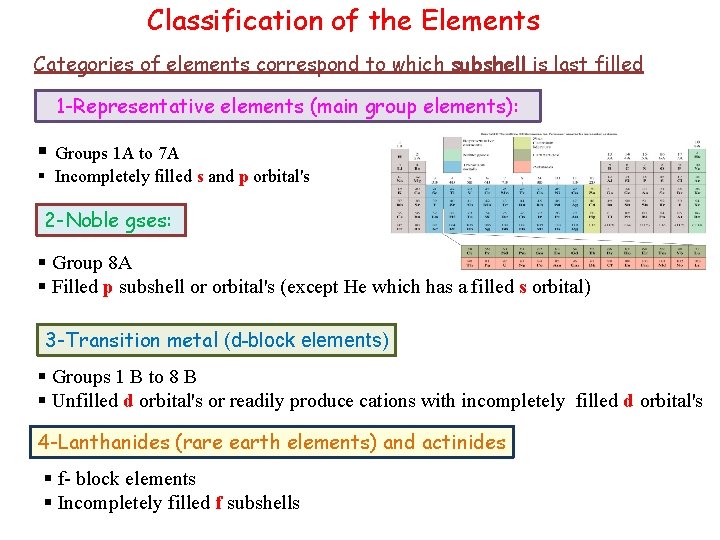
Classification of the Elements Categories of elements correspond to which subshell is last filled 1 -Representative elements (main group elements): § Groups 1 A to 7 A § Incompletely filled s and p orbital's 2 -Noble gses: § Group 8 A § Filled p subshell or orbital's (except He which has a filled s orbital) 3 -Transition metal (d-block elements) § Groups 1 B to 8 B § Unfilled d orbital's or readily produce cations with incompletely filled d orbital's 4 -Lanthanides (rare earth elements) and actinides § f- block elements § Incompletely filled f subshells

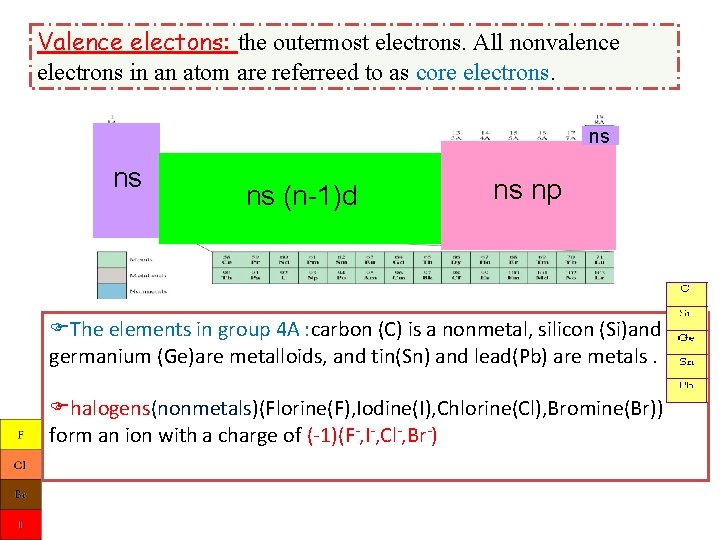
Valence electons: the outermost electrons. All nonvalence electrons in an atom are referreed to as core electrons. ns ns ns (n-1)d ns np The elements in group 4 A : carbon (C) is a nonmetal, silicon (Si)and germanium (Ge)are metalloids, and tin(Sn) and lead(Pb) are metals. halogens(nonmetals)(Florine(F), Iodine(I), Chlorine(Cl), Bromine(Br)) form an ion with a charge of (-1)(F-, I-, Cl-, Br-)

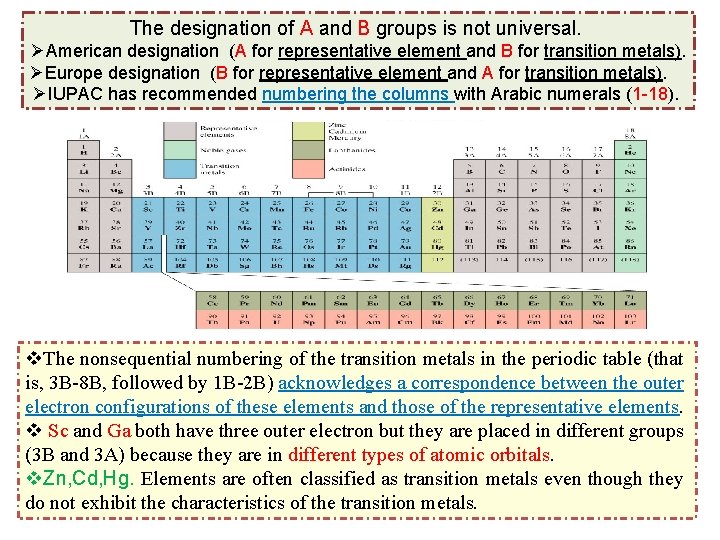
The designation of A and B groups is not universal. ØAmerican designation (A for representative element and B for transition metals). ØEurope designation (B for representative element and A for transition metals). ØIUPAC has recommended numbering the columns with Arabic numerals (1 -18). v. The nonsequential numbering of the transition metals in the periodic table (that is, 3 B-8 B, followed by 1 B-2 B) acknowledges a correspondence between the outer electron configurations of these elements and those of the representative elements. v Sc and Ga both have three outer electron but they are placed in different groups (3 B and 3 A) because they are in different types of atomic orbitals. v. Zn, Cd, Hg. Elements are often classified as transition metals even though they do not exhibit the characteristics of the transition metals.

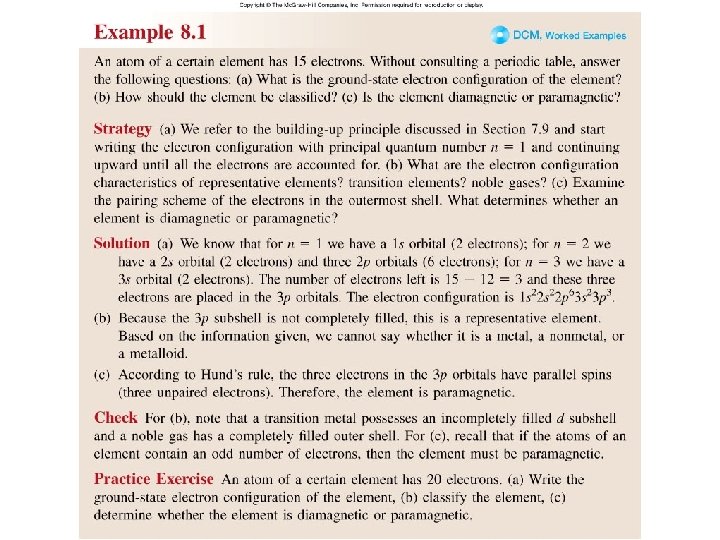
Worked Example 8. 1

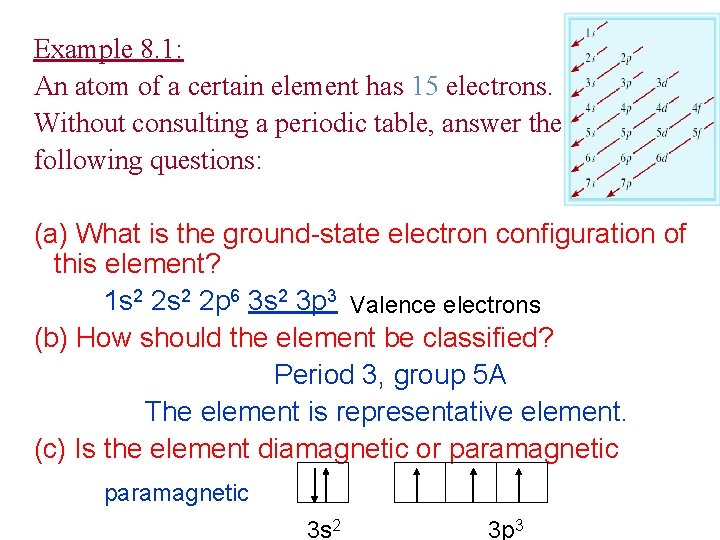
Example 8. 1: An atom of a certain element has 15 electrons. Without consulting a periodic table, answer the following questions: (a) What is the ground-state electron configuration of this element? 1 s 2 2 p 6 3 s 2 3 p 3 Valence electrons (b) How should the element be classified? Period 3, group 5 A The element is representative element. (c) Is the element diamagnetic or paramagnetic 3 s 2 3 p 3

Representing Free Elements in Chemical Equations 1. Chemists always use the empirical formulas to represent metals and metalloids in chemical equations, such as Fe, Na, and B, Si, Ge. (the empirical formulas are the same as the symbols that represent the element). 2. For nonmetals there is no single rule. - Carbon, for example, exists in its empirical formula (C) but hydrogen, nitrogen, oxygen, and halogens exist as diatomic molecules, so we use their molecular formulas [H 2, N 2, O 2, and X 2 (F 2, Cl 2, Br 2…. ] in equations. - The stable form of phosphorus is (P 4 )and so we use P 4. for sulfur the stable from is S 8, but chemists often use the empirical formula (S) in chemical equations, rather than S 8 S + O 2 SO 2 3. All the noble gases are monoatomic species.
![Electron Configurations of Cations and Anions Representative Elements Na Ne3 s 1 Na Ne Electron Configurations of Cations and Anions Representative Elements Na [Ne]3 s 1 Na+ [Ne]](https://slidetodoc.com/presentation_image/f2c655eaaa1f5494e687a01db3f481df/image-11.jpg)
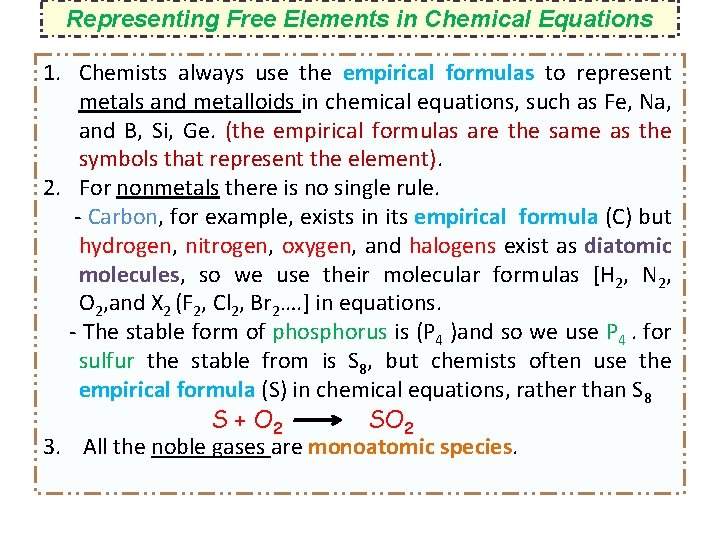
Electron Configurations of Cations and Anions Representative Elements Na [Ne]3 s 1 Na+ [Ne] Ca [Ar]4 s 2 Ca 2+ [Ar] Al [Ne]3 s 23 p 1 Al 3+ [Ne] H 1 s 1 Atoms lose electrons so that cation has a noble-gas outer electron configuration. Atoms gain electrons so 2 2 5 that anion has a noble-gas F 1 s 2 s 2 p outer electron O 1 s 22 p 4 configuration. N 1 s 22 p 3 H- 1 s 2 or [He] F- 1 s 22 p 6 or [Ne] O 2 - 1 s 22 p 6 or [Ne] N 3 - 1 s 22 p 6 or [Ne]

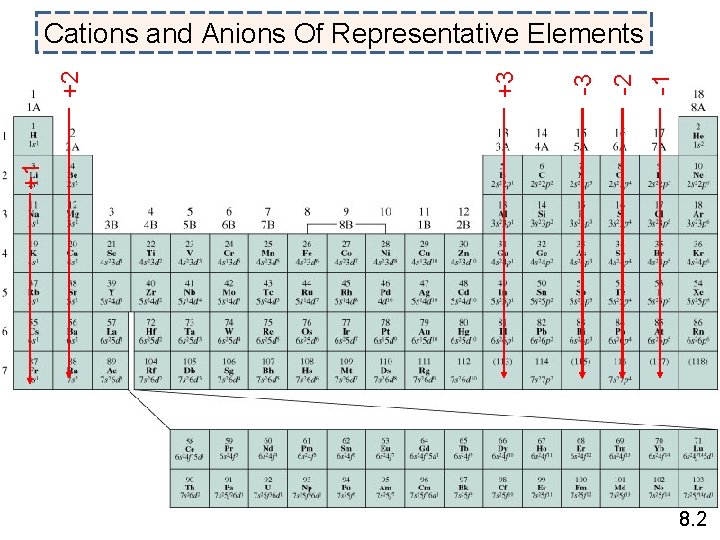
-1 -2 -3 +3 +1 +2 Cations and Anions Of Representative Elements 8. 2
![Na Ne Al 3 Ne O 2 1 s 22 p 6 or Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or](https://slidetodoc.com/presentation_image/f2c655eaaa1f5494e687a01db3f481df/image-13.jpg)
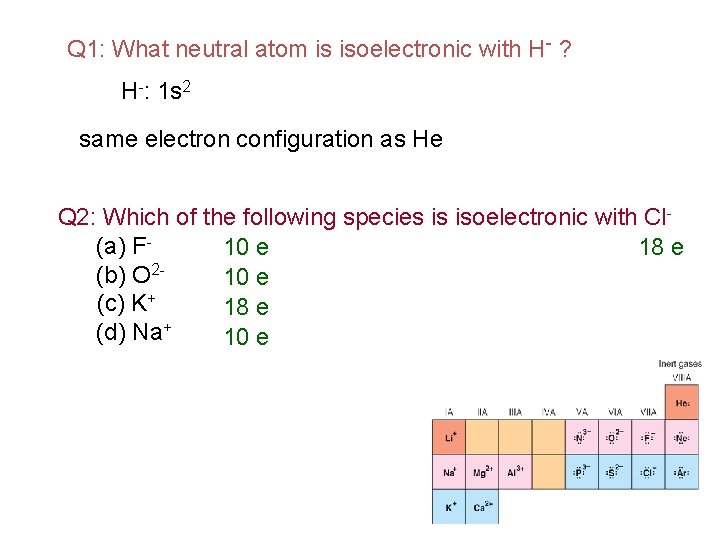
Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or [Ne] F-: 1 s 22 p 6 or [Ne] N 3 -: 1 s 22 p 6 or [Ne] ﺑﺪﻭﻥ ﺍﻟﻨﻈﺮ ﺍﻟﻰ ﺍﻟﺘﻮﺯﻳﻊ ﺍﻻﻟﻜﺘﺮﻭﻧﻲ Na+, Al 3+, F-, O 2 -, and N 3 - are all isoelectronic !! ﻓﻘﻂ ﺑﺤﺴﺎﺏ ﻋﺪﺩ ﺍﻻﻟﻜﺘﺮﻭﻧﺎﺕ with Ne. ﺍﻻﻟﻜﺘﺮﻭﻧﺎﺕ ﻣﺘﺴﺎﻭﻳﺔ ﺑﻤﻌﺮﻓﺔ ﺍﻟﻌﺪﺩ ﺍﻟﺬﺭﻱ ﻟﻠﻌﻨﺼﺮ isoelectronic is the species (atoms and Na (11 e) → Na+ (10 e) ions) have the same number of electrons, and hence the same ground- state electron Al (13 e) →Al 3+ (10 e) configuration. N (7 e) → N 3 - (10 e) Different isotopes of a particular element contain the same number of electrons. F (9 e) → F- (10 e) O (8 e) → O 2 - (10 e) Ne (10 e) Thus: All are isolelctronic to Ne

Q 1: What neutral atom is isoelectronic with H- ? H-: 1 s 2 same electron configuration as He Q 2: Which of the following species is isoelectronic with Cl (a) F- 10 e 18 e (b) O 2 - 10 e (c) K+ 18 e (d) Na+ 10 e

Electron Configurations of Cations of Transition Metals -When a cation is formed from an atom of a transition metal, electrons are always removed: Ø First from the ns orbital and Ø Then from the (n – 1)d orbital's. Why? Because the 3 d orbital's is more stable than the 4 s orbital in transition metal ions -The cations for transition metal are not isoelectronic with the preceding noble gases. Why? Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]4 s 03 d 6 or [Ar]3 d 6 Mn: [Ar]4 s 23 d 5 Fe 3+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Mn 2+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Because the transition metals can form more than one cation.
![Write the electronic configuration for Ni 2 a Ar 4 s 2 3 Write the electronic configuration for Ni 2+ ? (a) [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image/f2c655eaaa1f5494e687a01db3f481df/image-16.jpg)
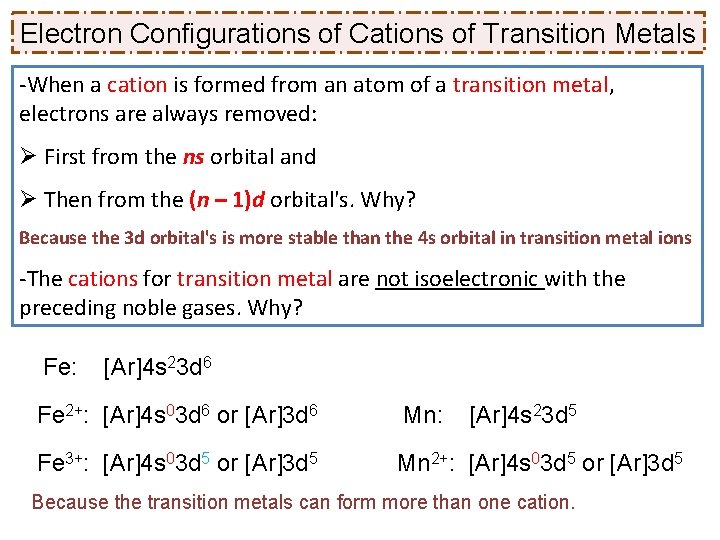
Write the electronic configuration for Ni 2+ ? (a) [Ar] 4 s 2 3 d 5 (b) [Ar] 3 d 8 (c) [Ar] 4 s 1 3 d 6 Ni: [Ar] 4 s 2 3 d 8 (transition metal) → Ni 2+: [Ar] 3 d 8 Transition metals cations: First: electrons lost from s orbital Then: from the (n-1)d orbital

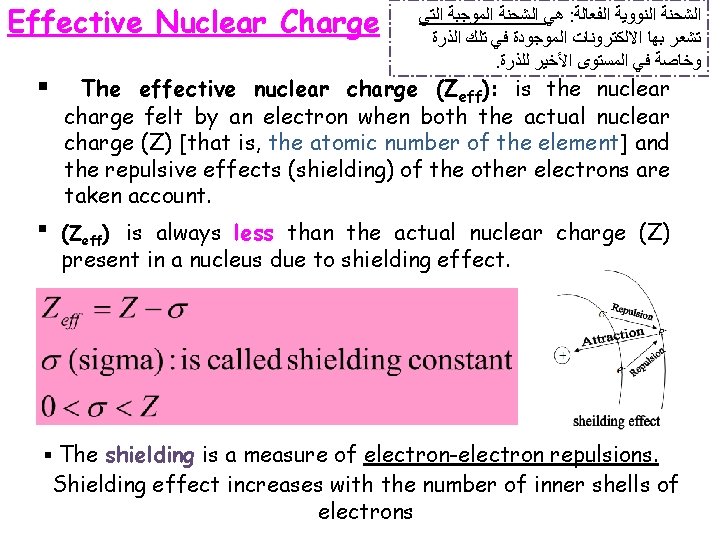
Periodic Variation in Physical Properties q Effective nuclear charge ﺷﺤﻨﻪ ﺍﻟﻨﻮﺍﻩ ﺍﻟﻔﻌﺎﻟﺔ q Atomic Radius ﻧﺼﻒ ﺍﻟﻘﻄﺮ ﺍﻟﺬﺭﻱ q Ionic Radius ﺍﻻﻳﻮﻧﻲ ﻧﺼﻒ ﺍﻟﻘﻄﺮ q Ionization Energy ﻃﺎﻗﺔ ﺍﻟﺘﺄﻴﻦ q Electron Affinity ﺍﻻﻟﻜﺘﺮﻭﻧﻴﺔ ﺍﻷﻠﻔﺔ q Electronegativity ﺍﻟﺴﺎﻟﺒﻴﺔ ﺍﻟﻜﻬﺮﺑﻴﺔ

Effective Nuclear Charge § ﺍﻟﺘﻲ ﺍﻟﻤﻮﺟﺒﺔ ﺍﻟﺸﺤﻨﺔ ﻫﻲ : ﺍﻟﻔﻌﺎﻟﺔ ﺍﻟﻨﻮﻭﻳﺔ ﺍﻟﺸﺤﻨﺔ ﺍﻟﺬﺭﺓ ﺗﻠﻚ ﻓﻲ ﺍﻟﻤﻮﺟﻮﺩﺓ ﺍﻻﻟﻜﺘﺮﻭﻧﺎﺕ ﺑﻬﺎ ﺗﺸﻌﺮ . ﻟﻠﺬﺭﺓ ﺍﻷﺨﻴﺮ ﺍﻟﻤﺴﺘﻮﻯ ﻓﻲ ﻭﺧﺎﺻﺔ The effective nuclear charge (Zeff): is the nuclear charge felt by an electron when both the actual nuclear charge (Z) [that is, the atomic number of the element] and the repulsive effects (shielding) of the other electrons are taken account. § (Zeff) is always less than the actual nuclear charge (Z) present in a nucleus due to shielding effect. § The shielding is a measure of electron-electron repulsions. Shielding effect increases with the number of inner shells of electrons

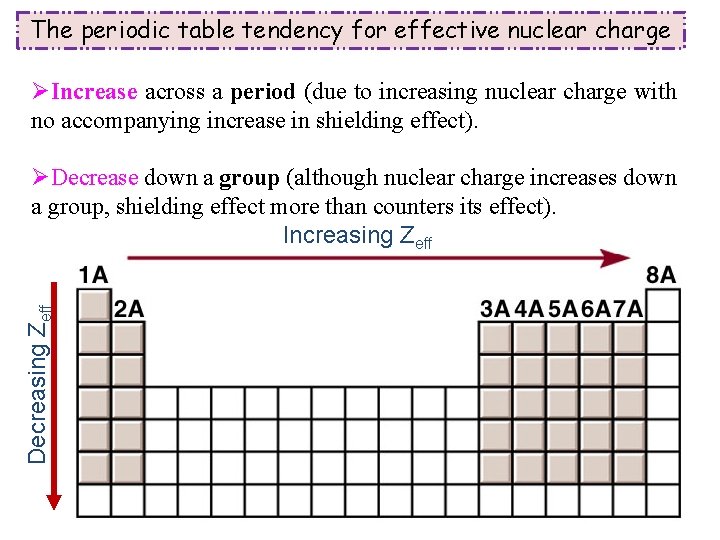
The periodic table tendency for effective nuclear charge ØIncrease across a period (due to increasing nuclear charge with no accompanying increase in shielding effect). Decreasing Zeff ØDecrease down a group (although nuclear charge increases down a group, shielding effect more than counters its effect). Increasing Zeff

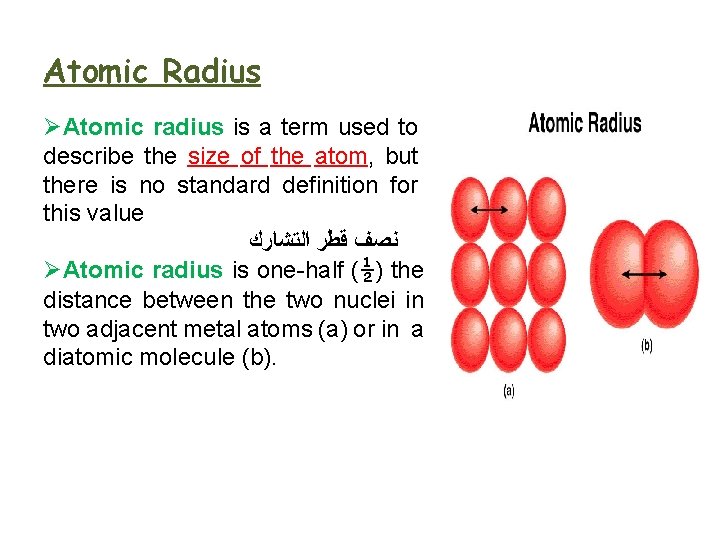
Atomic Radius ØAtomic radius is a term used to describe the size of the atom, but there is no standard definition for this value ﻧﺼﻒ ﻗﻄﺮ ﺍﻟﺘﺸﺎﺭﻙ ØAtomic radius is one-half (½) the distance between the two nuclei in two adjacent metal atoms (a) or in a diatomic molecule (b).

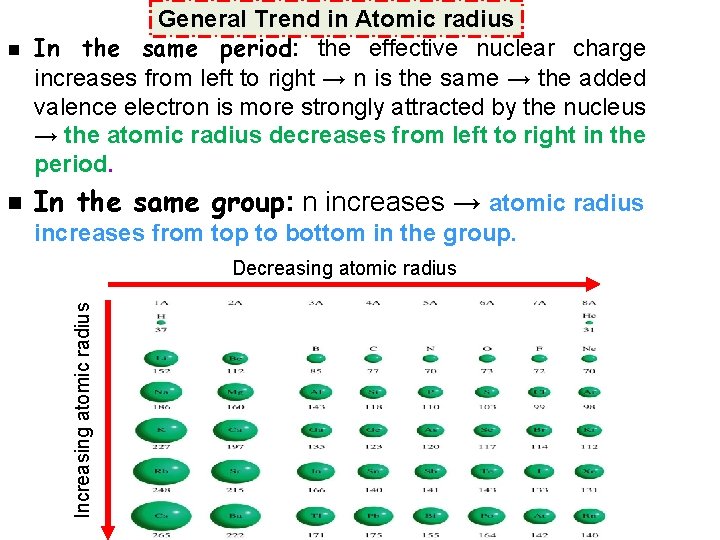
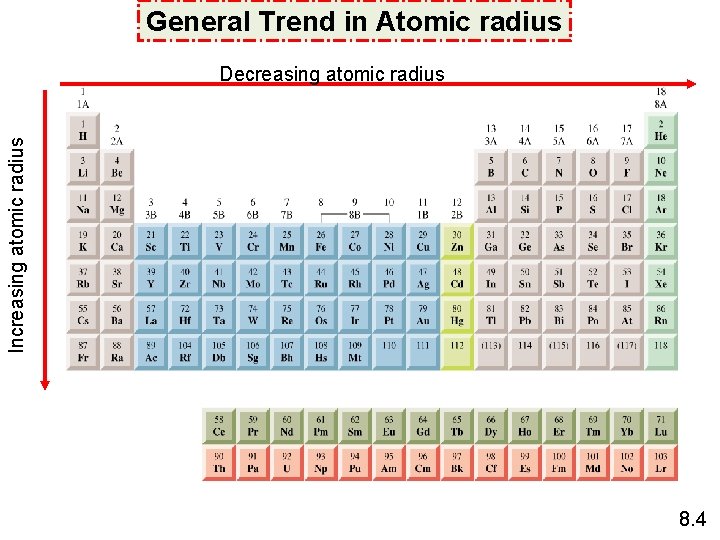
n In the same group: n increases → atomic radius increases from top to bottom in the group. Decreasing atomic radius Increasing atomic radius n General Trend in Atomic radius In the same period: the effective nuclear charge increases from left to right → n is the same → the added valence electron is more strongly attracted by the nucleus → the atomic radius decreases from left to right in the period.

General Trend in Atomic radius Increasing atomic radius Decreasing atomic radius 8. 4

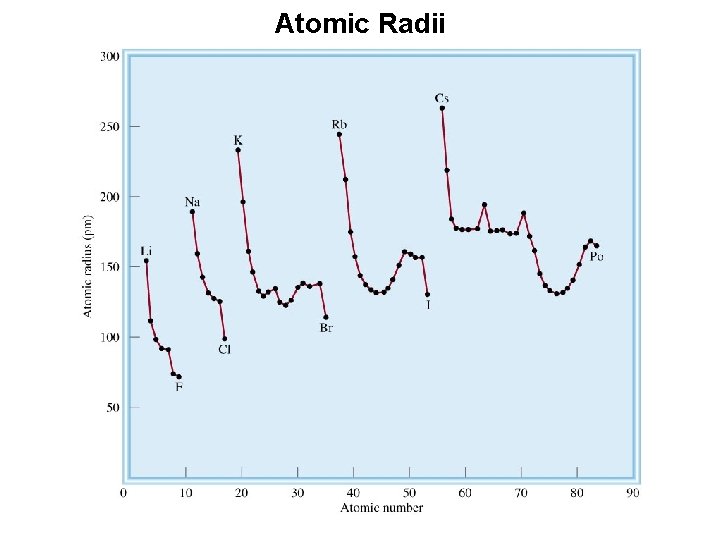
Atomic Radii

Worked Example 8. 2

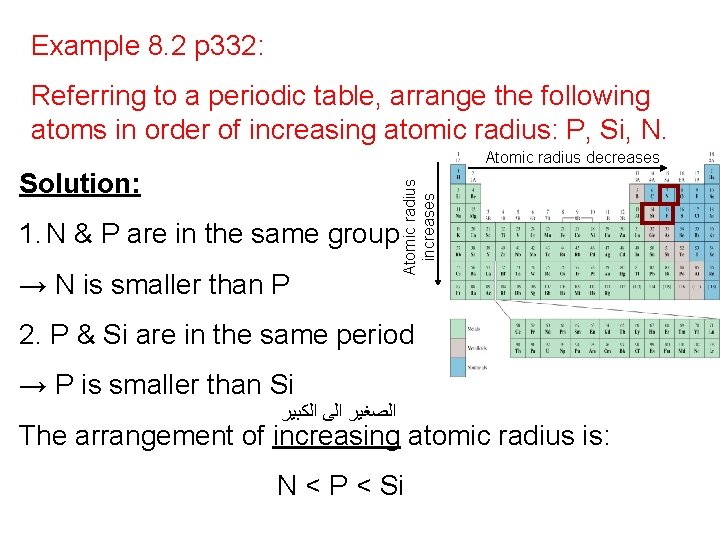
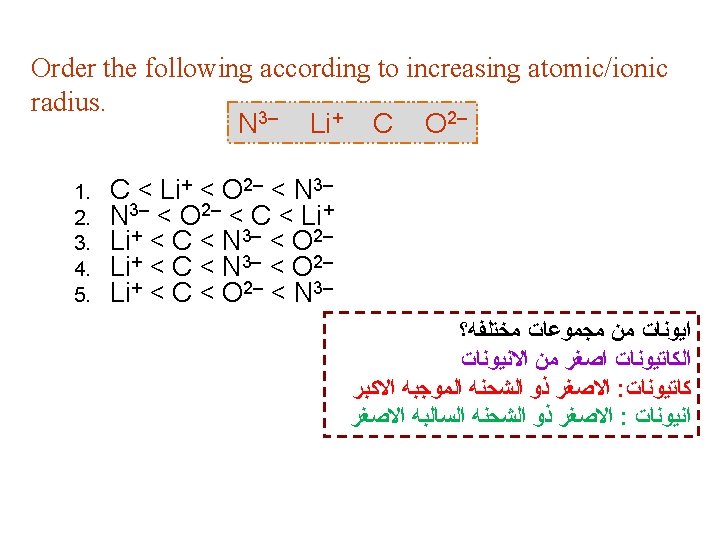
Example 8. 2 p 332: Referring to a periodic table, arrange the following atoms in order of increasing atomic radius: P, Si, N. Solution: 1. N & P are in the same group → N is smaller than P Atomic radius increases Atomic radius decreases 2. P & Si are in the same period → P is smaller than Si ﺍﻟﻜﺒﻴﺮ ﺍﻟﻰ ﺍﻟﺼﻐﻴﺮ The arrangement of increasing atomic radius is: N < P < Si

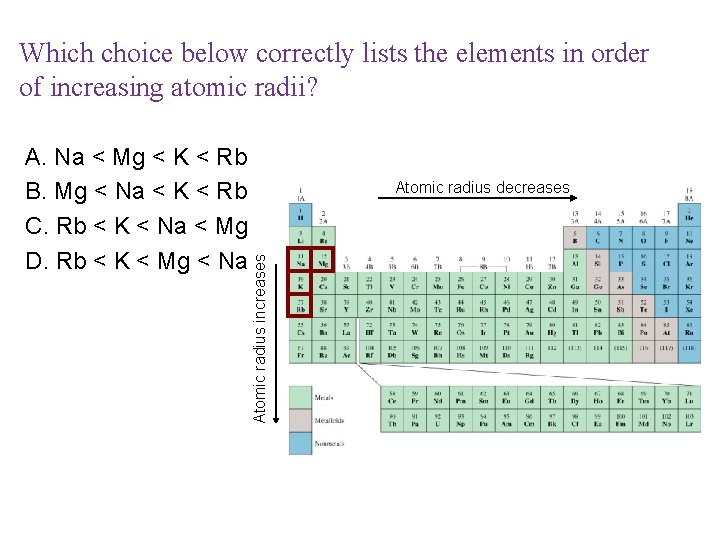
Which choice below correctly lists the elements in order of increasing atomic radii? Atomic radius decreases Atomic radius increases A. Na < Mg < K < Rb B. Mg < Na < K < Rb C. Rb < K < Na < Mg D. Rb < K < Mg < Na

Ionic Radius Ionic radius: is the radius of a cation or an anion. Anions: gain electrons → ionic radius increase because the nuclear charge remain the same but the repulsion resulting from the additional electrons increases the size (Atom <Anions) n Cations: lose electron → ionic radius decrease because removing one or more electron from an atom reduces electron-electron repulsion but the nuclear charge remains the same. (Cations <Atom) n Cation is always smaller than atom from which it is formed. Anion is always larger than atom from which it is formed. Cations <Anions

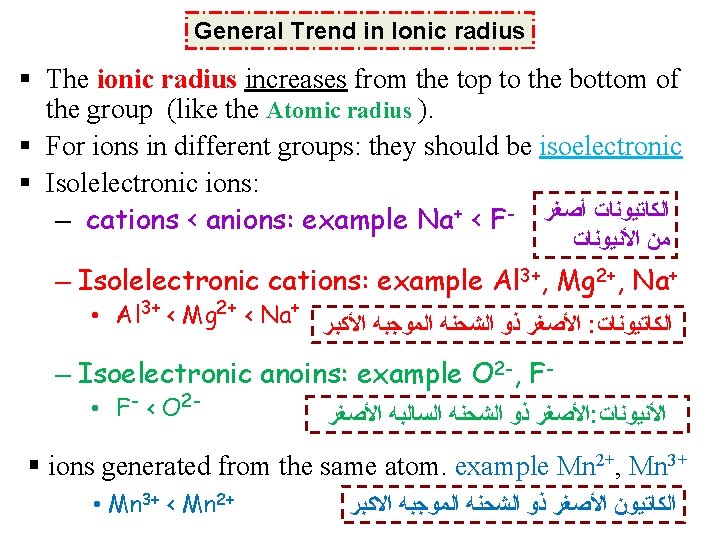
General Trend in Ionic radius § The ionic radius increases from the top to the bottom of the group (like the Atomic radius ). § For ions in different groups: they should be isoelectronic § Isolelectronic ions: – cations < anions: example Na+ < F- ﺍﻟﻜﺎﺗﻴﻮﻧﺎﺕ ﺃﺼﻐﺮ ﻣﻦ ﺍﻷﻨﻴﻮﻧﺎﺕ – Isolelectronic cations: example Al 3+, Mg 2+, Na+ • Al 3+ < Mg 2+ < Na+ ﺍﻷﺼﻐﺮ ﺫﻭ ﺍﻟﺸﺤﻨﻪ ﺍﻟﻤﻮﺟﺒﻪ ﺍﻷﻜﺒﺮ : ﺍﻟﻜﺎﺗﻴﻮﻧﺎﺕ – Isoelectronic anoins: example O 2 -, F • F- < O 2 - ﺍﻷﺼﻐﺮ ﺫﻭ ﺍﻟﺸﺤﻨﻪ ﺍﻟﺴﺎﻟﺒﻪ ﺍﻷﺼﻐﺮ : ﺍﻷﻨﻴﻮﻧﺎﺕ § ions generated from the same atom. example Mn 2+, Mn 3+ • Mn 3+ < Mn 2+ ﺍﻟﻜﺎﺗﻴﻮﻥ ﺍﻷﺼﻐﺮ ﺫﻭ ﺍﻟﺸﺤﻨﻪ ﺍﻟﻤﻮﺟﺒﻪ ﺍﻻﻛﺒﺮ

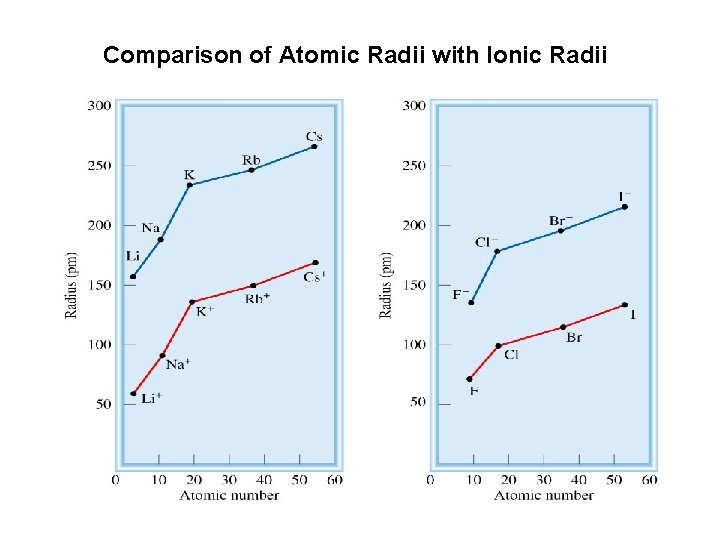
Comparison of Atomic Radii with Ionic Radii

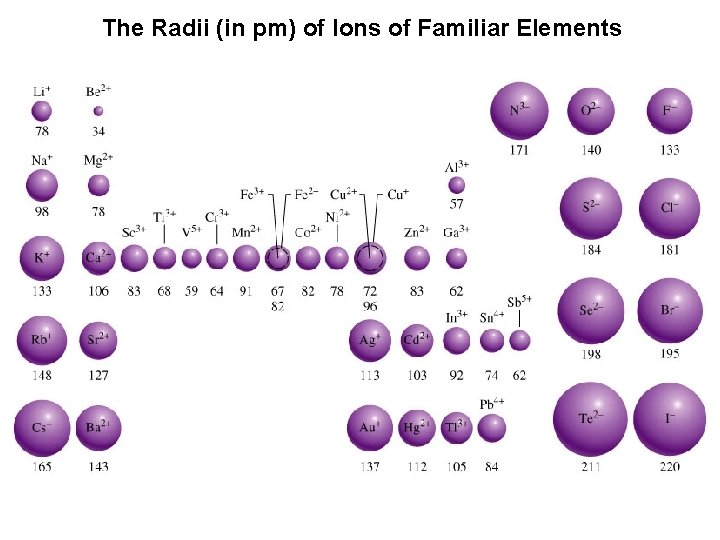
The Radii (in pm) of Ions of Familiar Elements


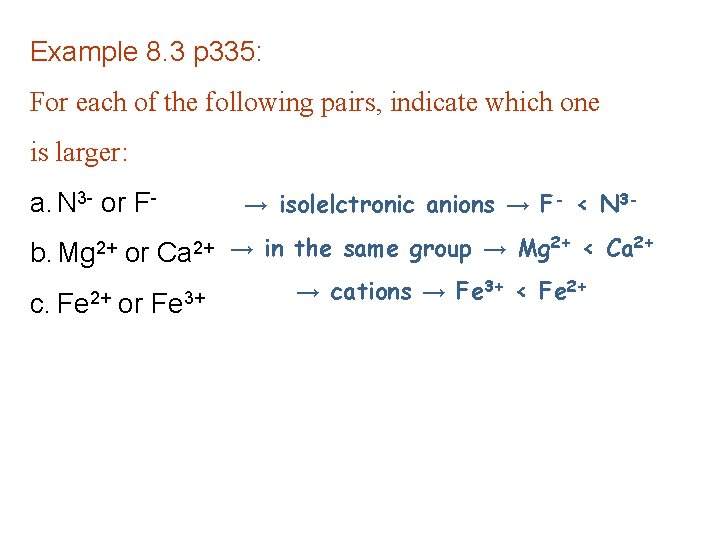
Example 8. 3 p 335: For each of the following pairs, indicate which one is larger: a. N 3 - or F- → isolelctronic anions → F- < N 3 - b. Mg 2+ or Ca 2+ → in the same group → Mg 2+ < Ca 2+ c. Fe 2+ or Fe 3+ → cations → Fe 3+ < Fe 2+


Variation of physical properties across a period and within a group. From left to right across a period there is a transition from metals to metalloids to nonmetals and within a periodic group the physical properties vary more predictably. Chemistry in Action: The 3 rd Liquid Element? 113 elements, 2 are liquids at 250 C – Br 2 and Hg 223 Fr, t = 21 minutes 1/2 Liquid?

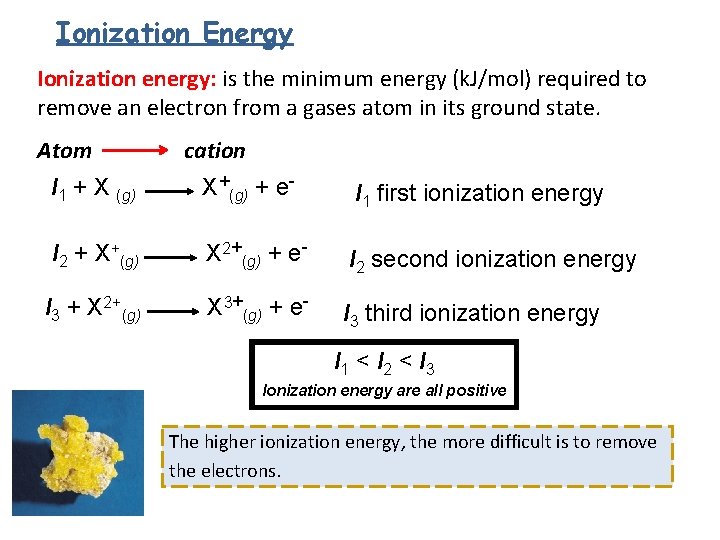
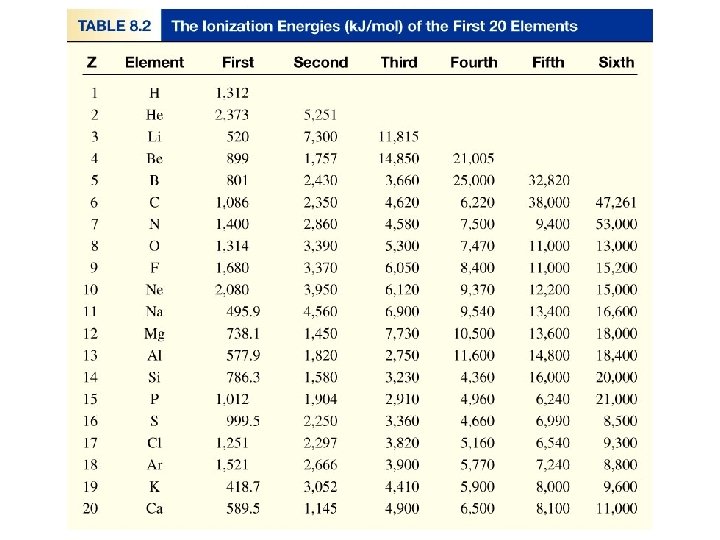
Ionization Energy Ionization energy: is the minimum energy (k. J/mol) required to remove an electron from a gases atom in its ground state. Atom cation I 1 + X (g) X+(g) + e- I 1 first ionization energy I 2 + X+(g) X 2+(g) + e- I 2 second ionization energy I 3 + X 2+(g) X 3+(g) + e- I 3 third ionization energy I 1 < I 2 < I 3 Ionization energy are all positive The higher ionization energy, the more difficult is to remove the electrons.


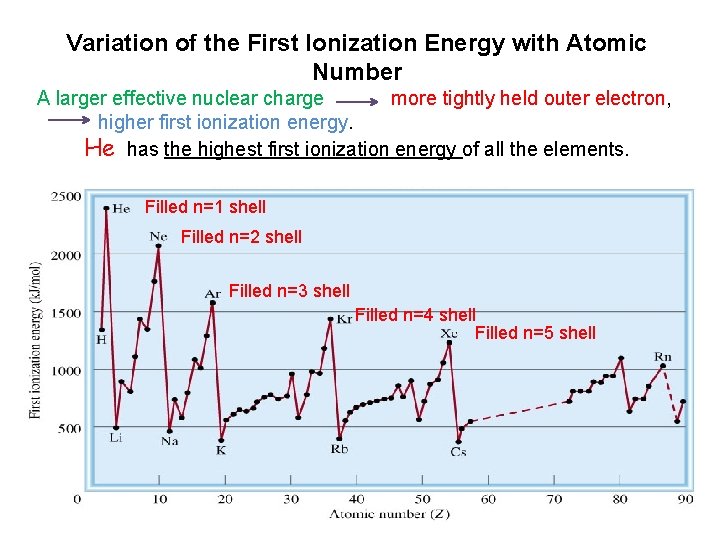
Variation of the First Ionization Energy with Atomic Number A larger effective nuclear charge more tightly held outer electron, higher first ionization energy. He has the highest first ionization energy of all the elements. Filled n=1 shell Filled n=2 shell Filled n=3 shell Filled n=4 shell Filled n=5 shell

General Trend in First Ionization Energies Decreasing First Ionization Energy Increasing First Ionization Energy 8. 4

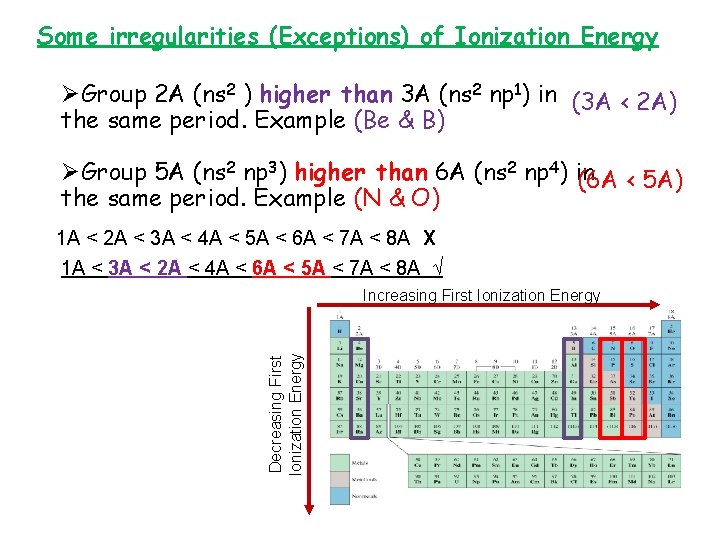
Some irregularities (Exceptions) of Ionization Energy ØGroup 2 A (ns 2 ) higher than 3 A (ns 2 np 1) in (3 A < 2 A) the same period. Example (Be & B) ØGroup 5 A (ns 2 np 3) higher than 6 A (ns 2 np 4) in (6 A < 5 A) the same period. Example (N & O) 1 A < 2 A < 3 A < 4 A < 5 A < 6 A < 7 A < 8 A X 1 A < 3 A < 2 A < 4 A < 6 A < 5 A < 7 A < 8 A √ Decreasing First Ionization Energy Increasing First Ionization Energy

Worked Example 8. 4

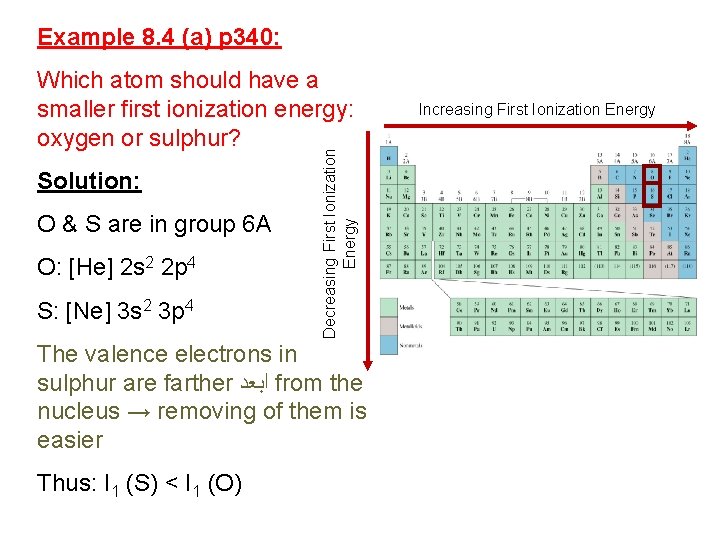
Example 8. 4 (a) p 340: Solution: O & S are in group 6 A O: [He] 2 s 2 2 p 4 S: [Ne] 3 s 2 3 p 4 Decreasing First Ionization Energy Which atom should have a smaller first ionization energy: oxygen or sulphur? The valence electrons in sulphur are farther ﺍﺑﻌﺪ from the nucleus → removing of them is easier Thus: I 1 (S) < I 1 (O) Increasing First Ionization Energy

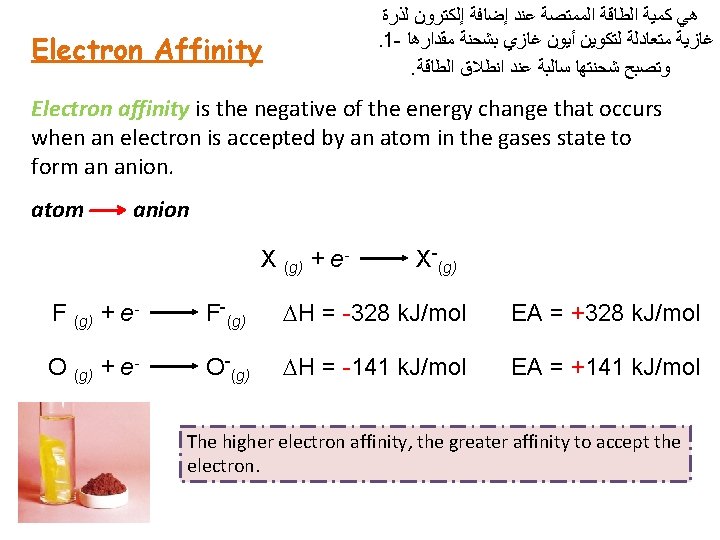
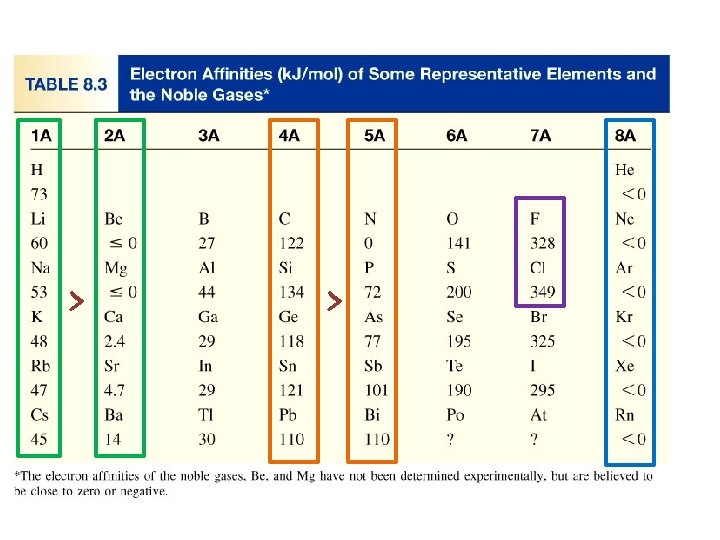
Electron Affinity ﻟﺬﺭﺓ ﺇﻟﻜﺘﺮﻭﻥ ﺇﺿﺎﻓﺔ ﻋﻨﺪ ﺍﻟﻤﻤﺘﺼﺔ ﺍﻟﻄﺎﻗﺔ ﻛﻤﻴﺔ ﻫﻲ . 1 - ﻣﻘﺪﺍﺭﻫﺎ ﺑﺸﺤﻨﺔ ﻏﺎﺯﻱ ﺃﻴﻮﻥ ﻟﺘﻜﻮﻳﻦ ﻣﺘﻌﺎﺩﻟﺔ ﻏﺎﺯﻳﺔ . ﺍﻟﻄﺎﻗﺔ ﺍﻧﻄﻼﻕ ﻋﻨﺪ ﺳﺎﻟﺒﺔ ﺷﺤﻨﺘﻬﺎ ﻭﺗﺼﺒﺢ Electron affinity is the negative of the energy change that occurs when an electron is accepted by an atom in the gases state to form an anion. atom anion X (g) + e- X-(g) F (g) + e- F-(g) DH = -328 k. J/mol EA = +328 k. J/mol O (g) + e- O-(g) DH = -141 k. J/mol EA = +141 k. J/mol The higher electron affinity, the greater affinity to accept the electron.

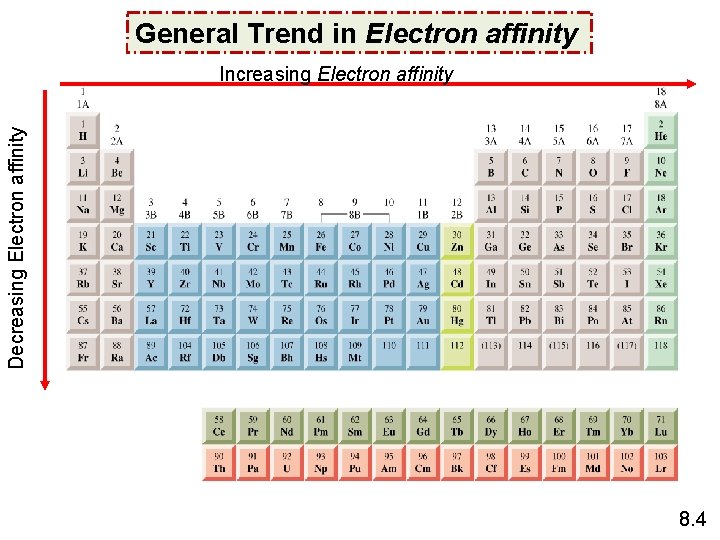
General Trend in Electron affinity Decreasing Electron affinity Increasing Electron affinity 8. 4

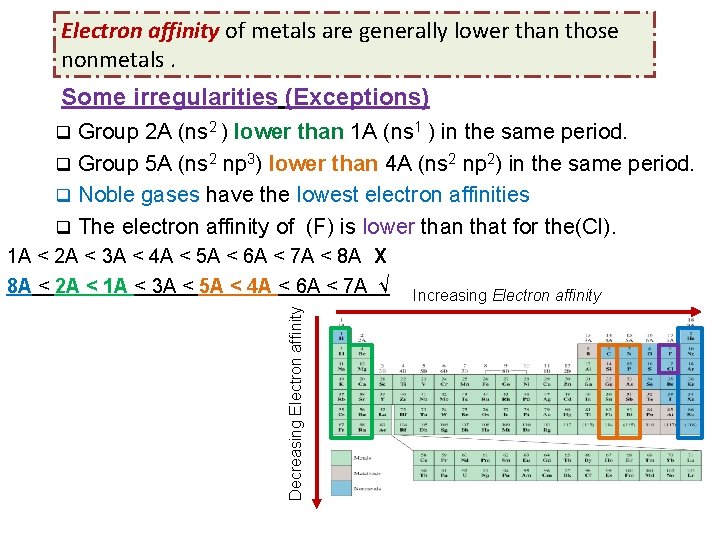
Electron affinity of metals are generally lower than those nonmetals. Some irregularities (Exceptions) Group 2 A (ns 2 ) lower than 1 A (ns 1 ) in the same period. q Group 5 A (ns 2 np 3) lower than 4 A (ns 2 np 2) in the same period. q Noble gases have the lowest electron affinities q The electron affinity of (F) is lower than that for the(Cl). q Decreasing Electron affinity 1 A < 2 A < 3 A < 4 A < 5 A < 6 A < 7 A < 8 A X 8 A < 2 A < 1 A < 3 A < 5 A < 4 A < 6 A < 7 A √ Increasing Electron affinity


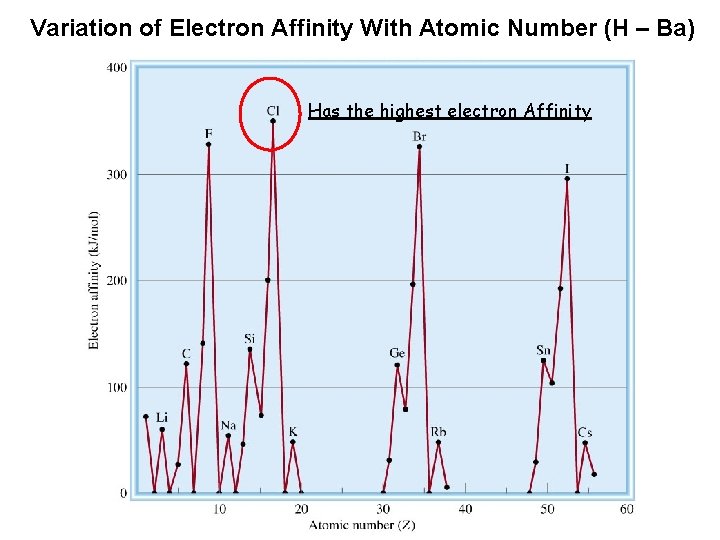
Variation of Electron Affinity With Atomic Number (H – Ba) Has the highest electron Affinity

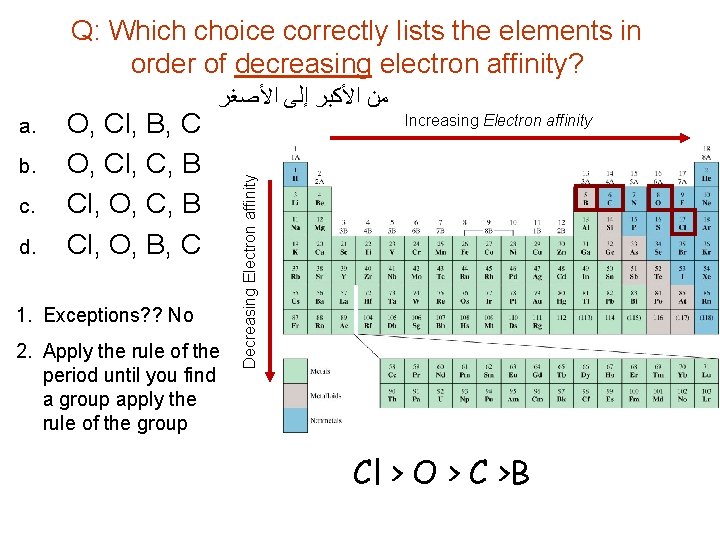
Q: Which choice correctly lists the elements in order of decreasing electron affinity? b. c. d. O, Cl, B, C O, Cl, C, B Cl, O, B, C 1. Exceptions? ? No 2. Apply the rule of the period until you find a group apply the rule of the group Increasing Electron affinity Decreasing Electron affinity a. ﺍﻷﺼﻐﺮ ﺇﻟﻰ ﺍﻷﻜﺒﺮ ﻣﻦ Cl > O > C >B

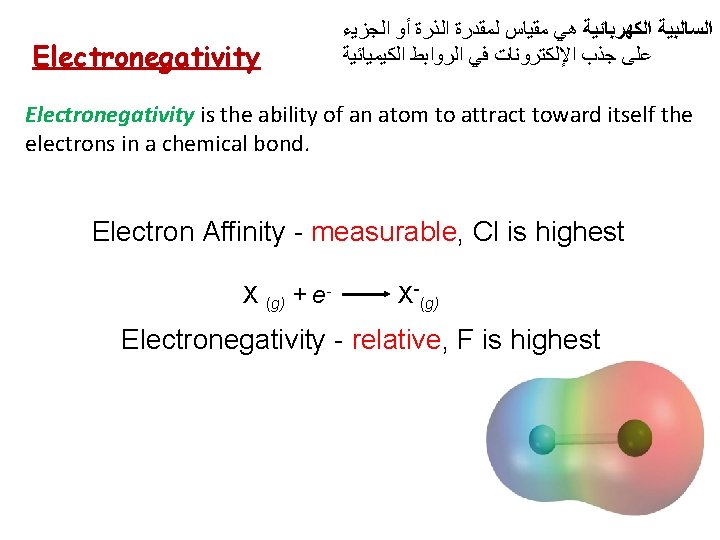
Electronegativity ﺍﻟﺠﺰﻳﺀ ﺃﻮ ﺍﻟﺬﺭﺓ ﻟﻤﻘﺪﺭﺓ ﻣﻘﻴﺎﺱ ﻫﻲ ﺍﻟﺴﺎﻟﺒﻴﺔ ﺍﻟﻜﻬﺮﺑﺎﺋﻴﺔ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﺍﻟﺮﻭﺍﺑﻂ ﻓﻲ ﺍﻹﻟﻜﺘﺮﻭﻧﺎﺕ ﺟﺬﺏ ﻋﻠﻰ Electronegativity is the ability of an atom to attract toward itself the electrons in a chemical bond. Electron Affinity - measurable, Cl is highest X (g) + e- X-(g) Electronegativity - relative, F is highest

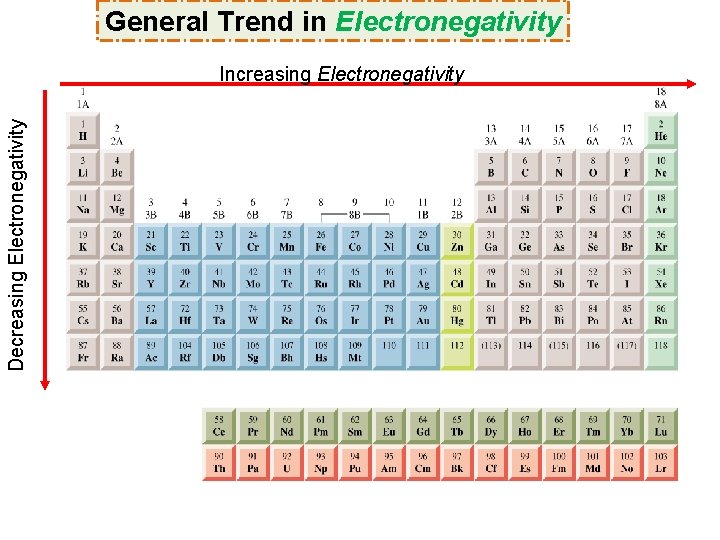
General Trend in Electronegativity Decreasing Electronegativity Increasing Electronegativity

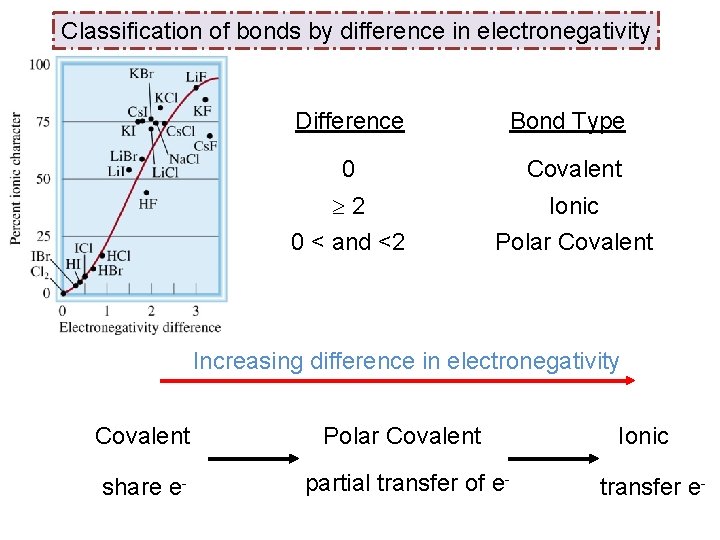
Classification of bonds by difference in electronegativity Difference Bond Type 0 Covalent 2 0 < and <2 Ionic Polar Covalent Increasing difference in electronegativity Covalent Polar Covalent share e- partial transfer of e- Ionic transfer e-

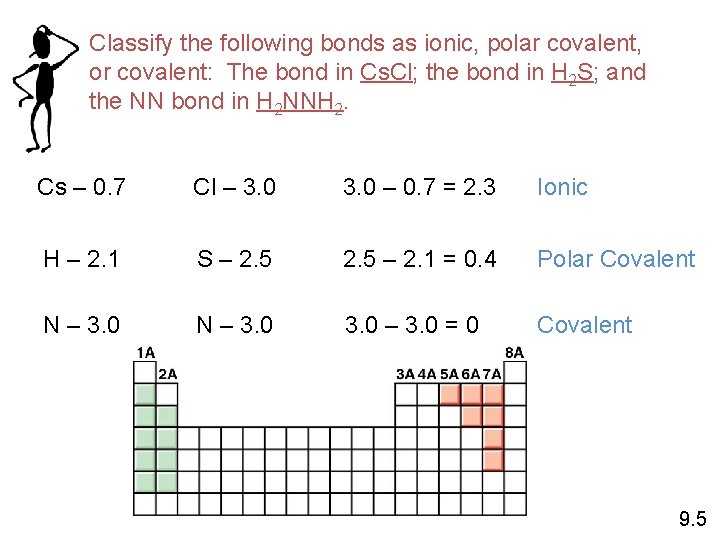
Classify the following bonds as ionic, polar covalent, or covalent: The bond in Cs. Cl; the bond in H 2 S; and the NN bond in H 2 NNH 2. Cs – 0. 7 Cl – 3. 0 – 0. 7 = 2. 3 Ionic H – 2. 1 S – 2. 5 – 2. 1 = 0. 4 Polar Covalent N – 3. 0 – 3. 0 = 0 Covalent 9. 5

Worked Example 9. 2

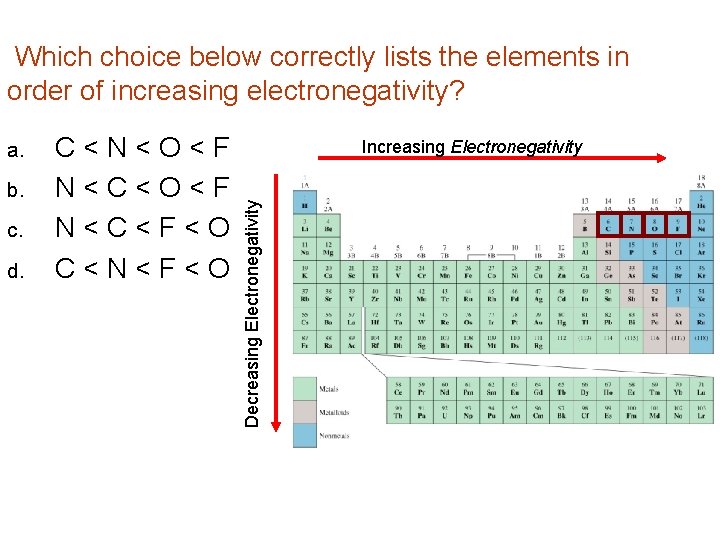
Which choice below correctly lists the elements in order of increasing electronegativity? b. c. d. C < N < O < F N < C < F < O C < N < F < O Increasing Electronegativity Decreasing Electronegativity a.

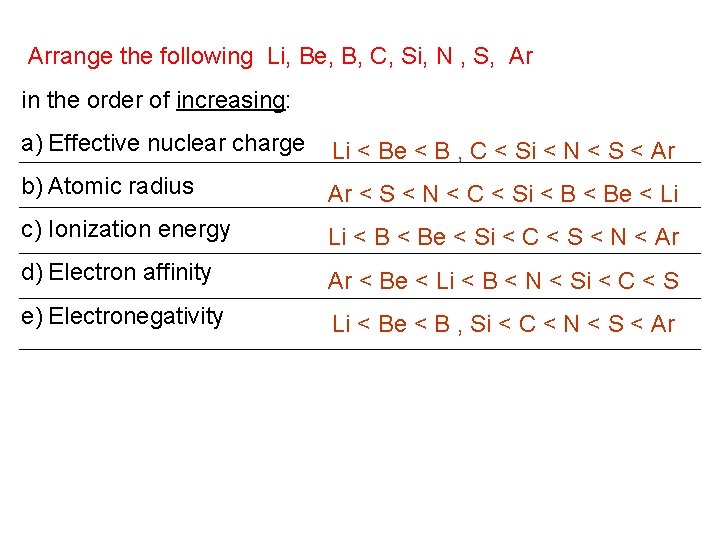
Arrange the following Li, Be, B, C, Si, N , S, Ar in the order of increasing: a) Effective nuclear charge Li < Be < B , C < Si < N < S < Ar b) Atomic radius Ar < S < N < C < Si < Be < Li c) Ionization energy Li < Be < Si < C < S < N < Ar d) Electron affinity Ar < Be < Li < B < N < Si < C < S e) Electronegativity Li < Be < B , Si < C < N < S < Ar

• Problems • 8. 5 – 8. 8 – 8. 12 – 8. 20 – 8. 24 - 8. 26 – 8. 28 – 8. 30 – 8. 32 • 8. 36 – 8. 38 – 8. 40 – 8. 44 – 8. 46 • 8. 52 – 8. 54 – 8. 62 – 8. 64