Periodic Relationships Among the Elements Chapter 8 Copyright

![Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+ Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+](https://slidetodoc.com/presentation_image/2889827b4d5ef2f766179841987f5bfd/image-5.jpg)







- Slides: 64

Periodic Relationships Among the Elements Chapter 8 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

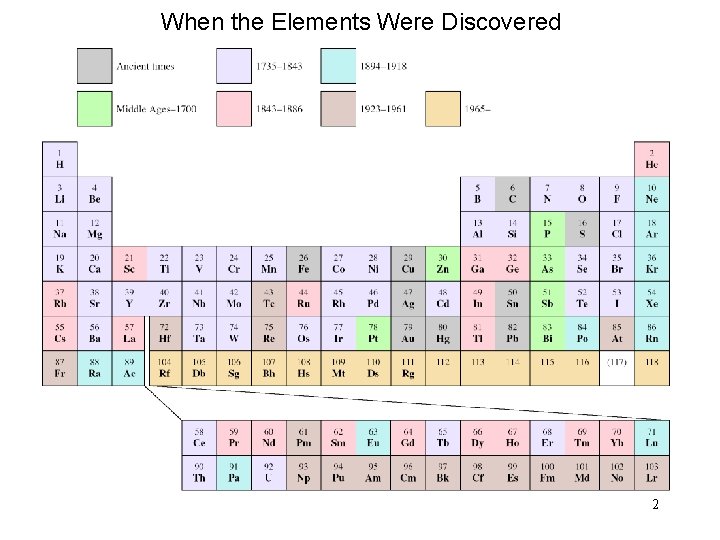
When the Elements Were Discovered 2

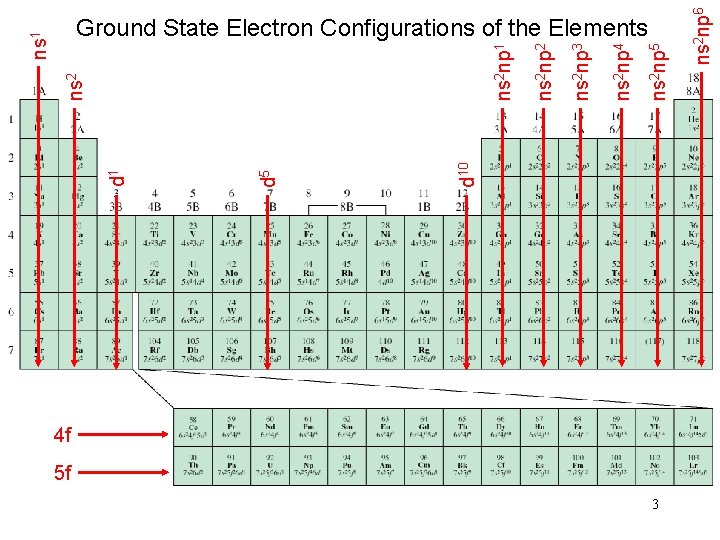
4 f 5 f 3 ns 2 np 6 ns 2 np 5 ns 2 np 4 ns 2 np 3 ns 2 np 2 ns 2 np 1 d 10 d 5 d 1 ns 2 ns 1 Ground State Electron Configurations of the Elements

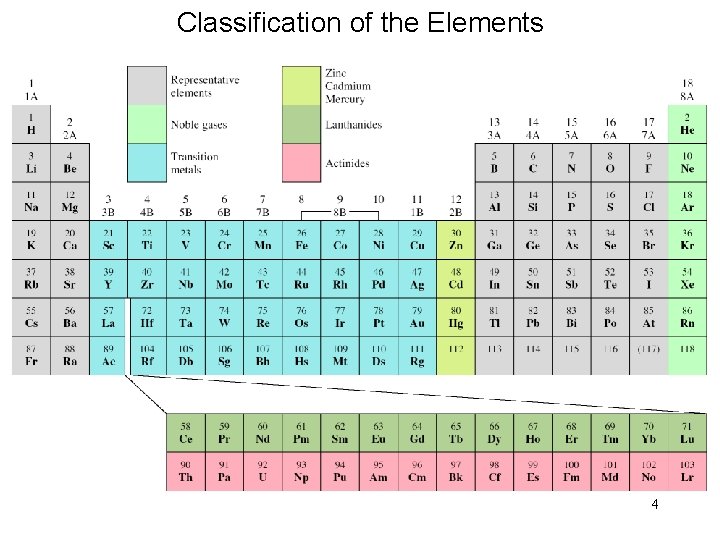
Classification of the Elements 4
![Electron Configurations of Cations and Anions Of Representative Elements Na Ne3 s 1 Na Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+](https://slidetodoc.com/presentation_image/2889827b4d5ef2f766179841987f5bfd/image-5.jpg)
Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+ [Ne] Ca [Ar]4 s 2 Ca 2+ [Ar] Al [Ne]3 s 23 p 1 Al 3+ [Ne] Atoms lose electrons so that cation has a noble-gas outer electron configuration. H 1 s 1 Atoms gain electrons so F 1 s 22 p 5 that anion has a noblegas outer electron O 1 s 22 p 4 configuration. N 1 s 22 p 3 H- 1 s 2 or [He] F- 1 s 22 p 6 or [Ne] O 2 - 1 s 22 p 6 or [Ne] N 3 - 1 s 22 p 6 or [Ne] 5

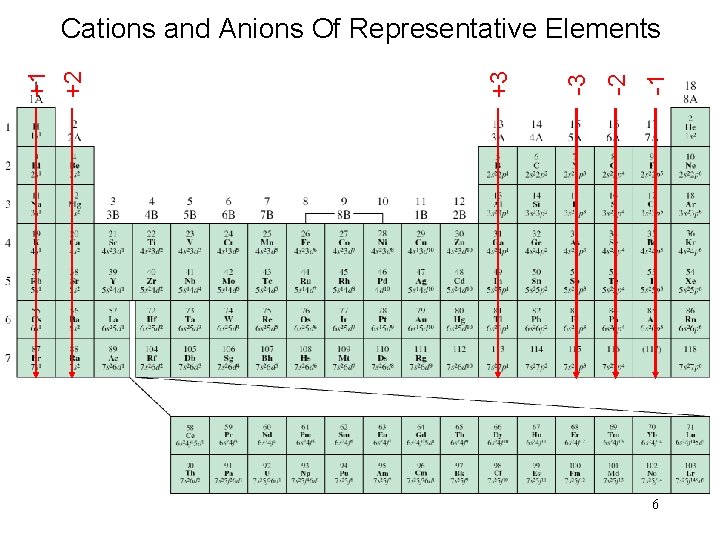
-1 -2 -3 +3 +1 +2 Cations and Anions Of Representative Elements 6

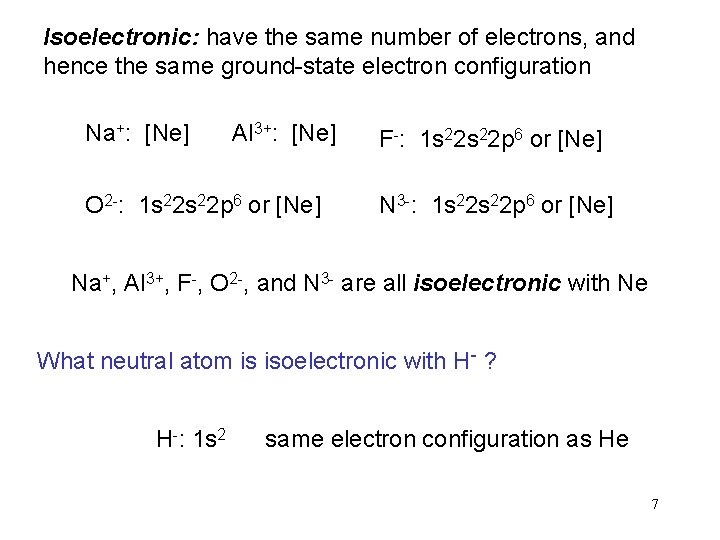
Isoelectronic: have the same number of electrons, and hence the same ground-state electron configuration Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or [Ne] F-: 1 s 22 p 6 or [Ne] N 3 -: 1 s 22 p 6 or [Ne] Na+, Al 3+, F-, O 2 -, and N 3 - are all isoelectronic with Ne What neutral atom is isoelectronic with H- ? H-: 1 s 2 same electron configuration as He 7

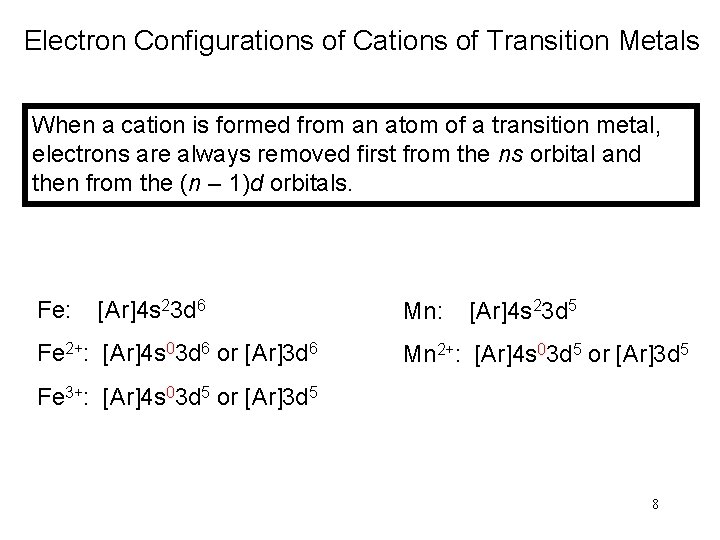
Electron Configurations of Cations of Transition Metals When a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n – 1)d orbitals. Fe: [Ar]4 s 23 d 6 Mn: [Ar]4 s 23 d 5 Fe 2+: [Ar]4 s 03 d 6 or [Ar]3 d 6 Mn 2+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Fe 3+: [Ar]4 s 03 d 5 or [Ar]3 d 5 8

EXAMPLE 8. 1 An atom of a certain element has 15 electrons. Without consulting a periodic table, answer the following questions: (a) What is the ground-state electron configuration of the element? (b) How should the element be classified? (c) Is the element diamagnetic or paramagnetic? Strategy (a) We refer to the building-up principle discussed in Section 7. 9 and start writing the electron configuration with principal quantum number n 5 1 and continuing upward until all the electrons are accounted for. (b) What are the electron configuration characteristics of representative elements? transition elements? noble gases? (c) Examine the pairing scheme of the electrons in the outermost shell. What determines whether an element is diamagnetic or paramagnetic? Solution (a) We know that for n 5 1 we have a 1 s orbital (2 electrons); for n 5 2 we have a 2 s orbital (2 electrons) and three 2 p orbitals (6 electrons); for n 5 3 we have a 3 s orbital (2 electrons). The number of electrons left is 15 2 12 5 3 and these three electrons are placed in the 3 p orbitals. The electron configuration is 1 s 2 2 p 6 3 s 2 3 p 3. (b) Because the 3 p subshell is not completely filled, this is a representative element. Based on the information given, we cannot say whether it is a metal, a nonmetal, or a metalloid. (c) According to Hund’s rule, the three electrons in the 3 p orbitals have parallel spins (three unpaired electrons). Therefore, the element is paramagnetic. Check For (b), note that a transition metal possesses an incompletely filled d subshell and a noble gas has a completely filled outer shell. For (c), recall that if the atoms of an element contain an odd number of electrons, then the element must be paramagnetic. Practice Exercise An atom of a certain element has 20 electrons. (a) Write the ground-state electron configuration of the element, (b) classify the element, (c) determine whether the element is diamagnetic or paramagnetic. 9

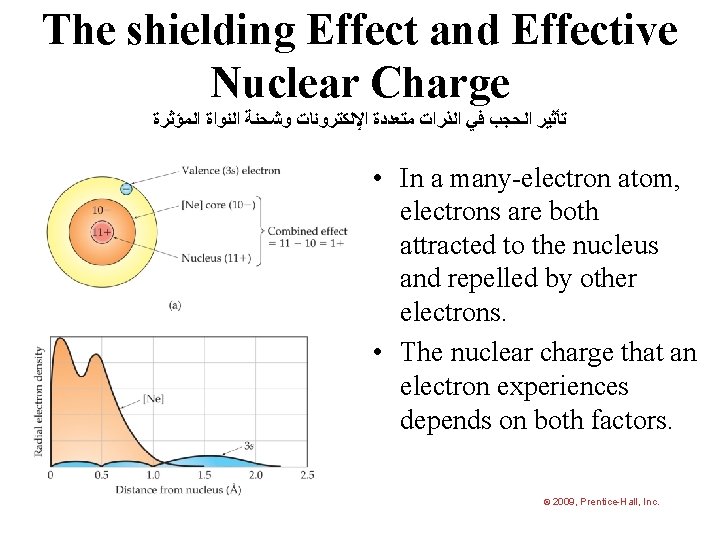
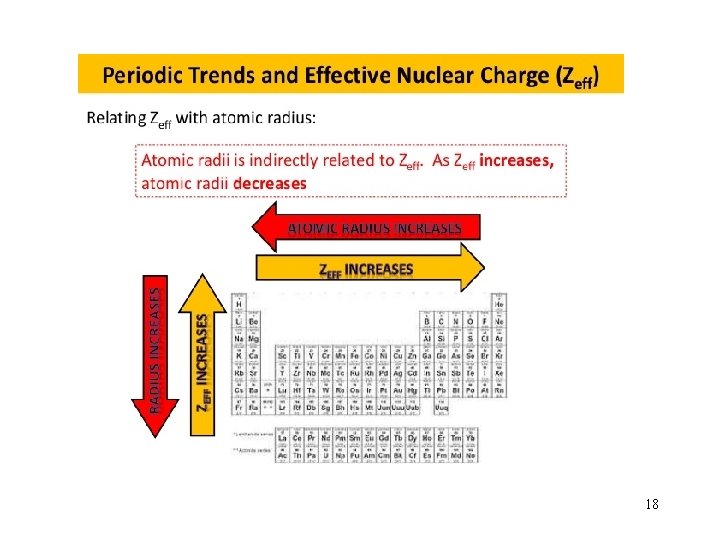
The shielding Effect and Effective Nuclear Charge ﺗﺄﺜﻴﺮ ﺍﻟﺤﺠﺐ ﻓﻲ ﺍﻟﺬﺭﺍﺕ ﻣﺘﻌﺪﺩﺓ ﺍﻹﻟﻜﺘﺮﻭﻧﺎﺕ ﻭﺷﺤﻨﺔ ﺍﻟﻨﻮﺍﺓ ﺍﻟﻤﺆﺜﺮﺓ • In a many-electron atom, electrons are both attracted to the nucleus and repelled by other electrons. • The nuclear charge that an electron experiences depends on both factors. © 2009, Prentice-Hall, Inc.

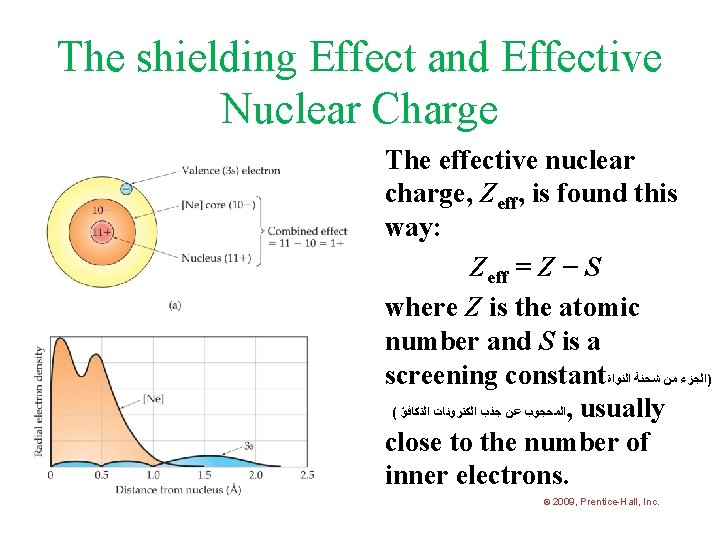
The shielding Effect and Effective Nuclear Charge The effective nuclear charge, Zeff, is found this way: Zeff = Z − S where Z is the atomic number and S is a screening constant )ﺍﻟﺠﺰﺀ ﻣﻦ ﺷﺤﻨﺔ ﺍﻟﻨﻮﺍﺓ ( ﺍﻟﻤﺤﺠﻮﺏ ﻋﻦ ﺟﺬﺏ ﺍﻟﻜﺘﺮﻭﻧﺎﺕ ﺍﻟﺘﻜﺎﻓﺆ , usually close to the number of inner electrons. © 2009, Prentice-Hall, Inc.

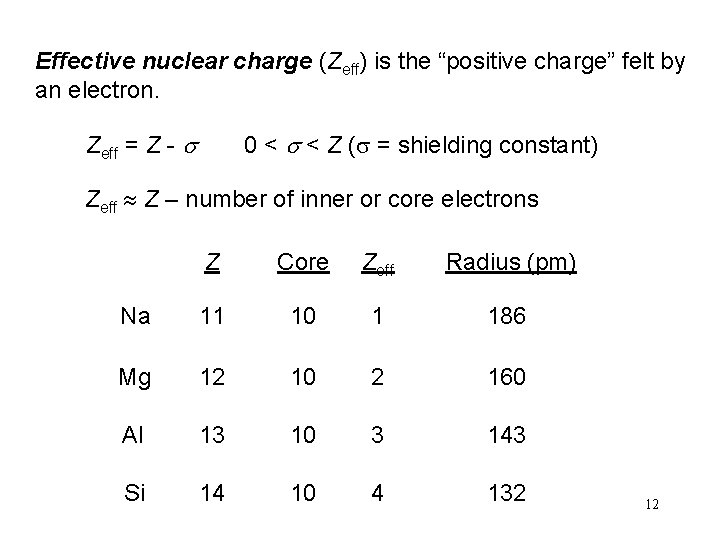
Effective nuclear charge (Zeff) is the “positive charge” felt by an electron. Zeff = Z - s 0 < s < Z (s = shielding constant) Zeff Z – number of inner or core electrons Z Core Zeff Radius (pm) Na 11 10 1 186 Mg 12 10 2 160 Al 13 10 3 143 Si 14 10 4 132 12

Effective Nuclear Charge (Zeff) 13

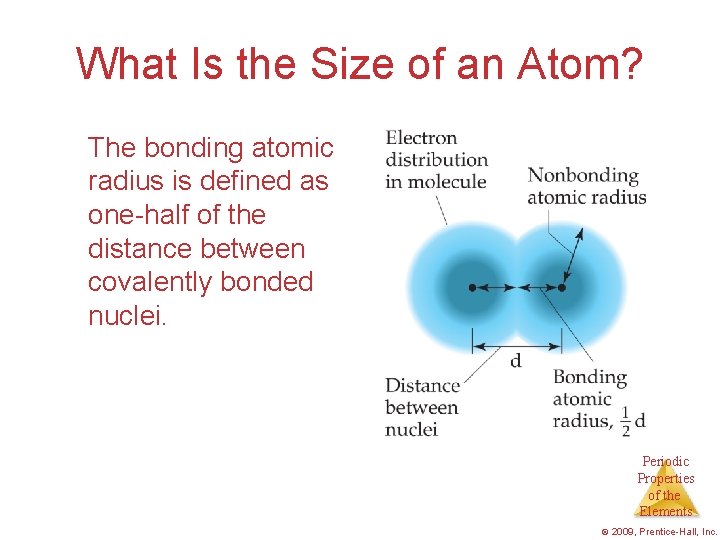
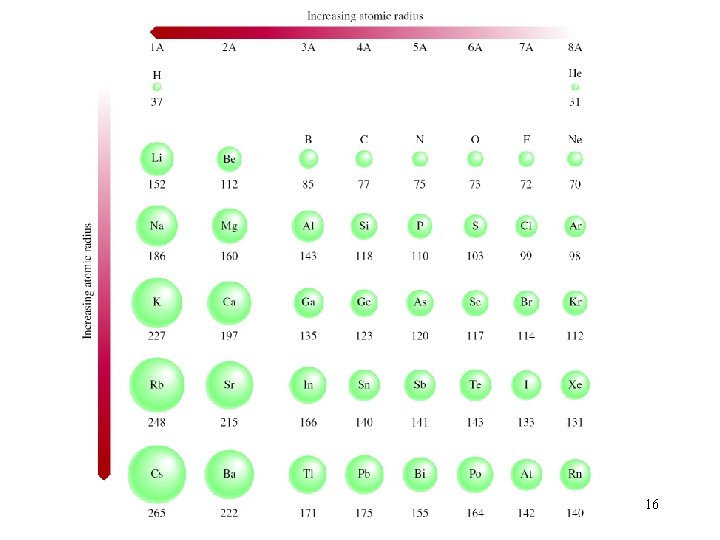
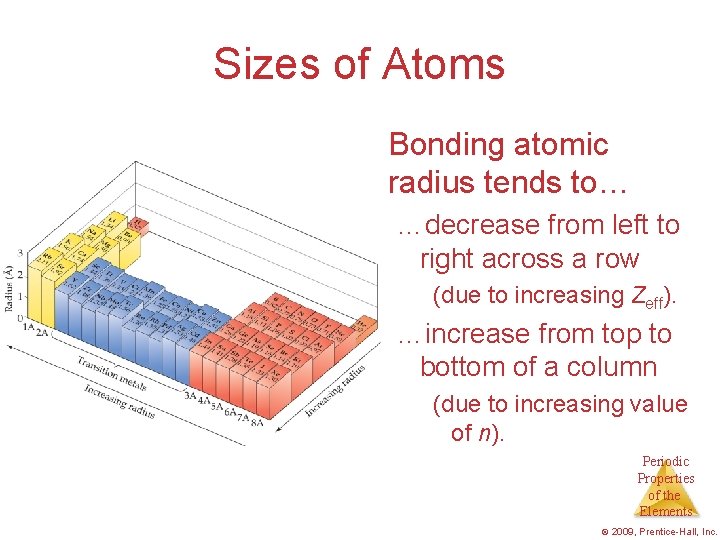
What Is the Size of an Atom? The bonding atomic radius is defined as one-half of the distance between covalently bonded nuclei. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

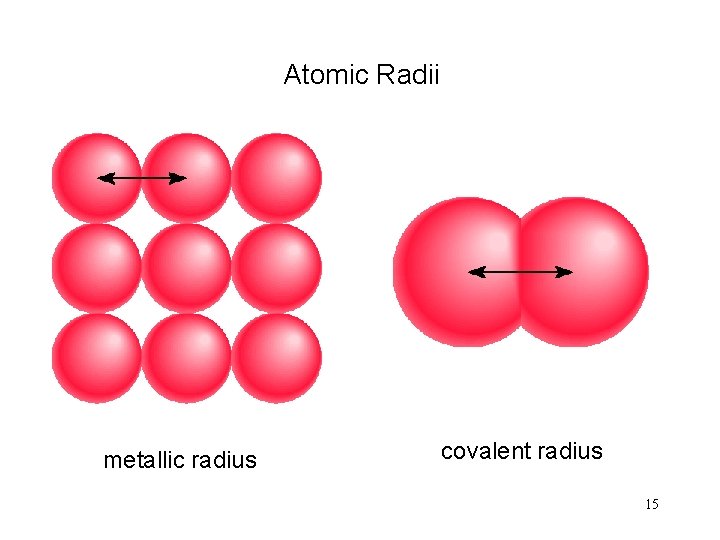
Atomic Radii metallic radius covalent radius 15

16

Sizes of Atoms Bonding atomic radius tends to… …decrease from left to right across a row (due to increasing Zeff). …increase from top to bottom of a column (due to increasing value of n). Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

18

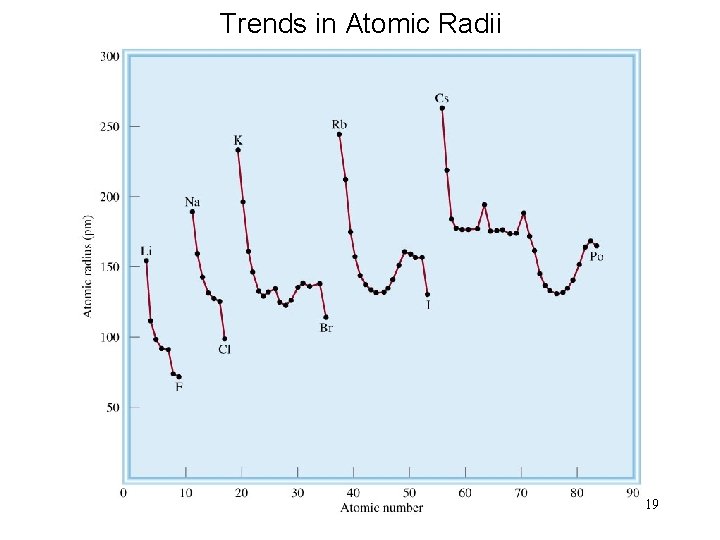
Trends in Atomic Radii 19

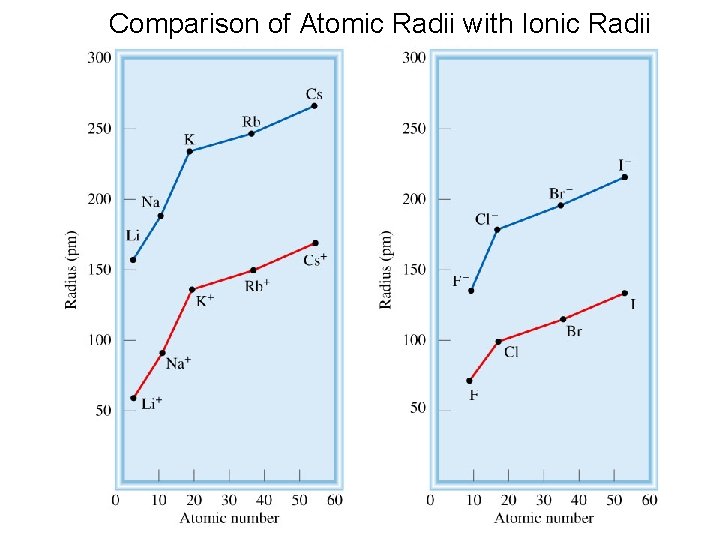
Comparison of Atomic Radii with Ionic Radii 20

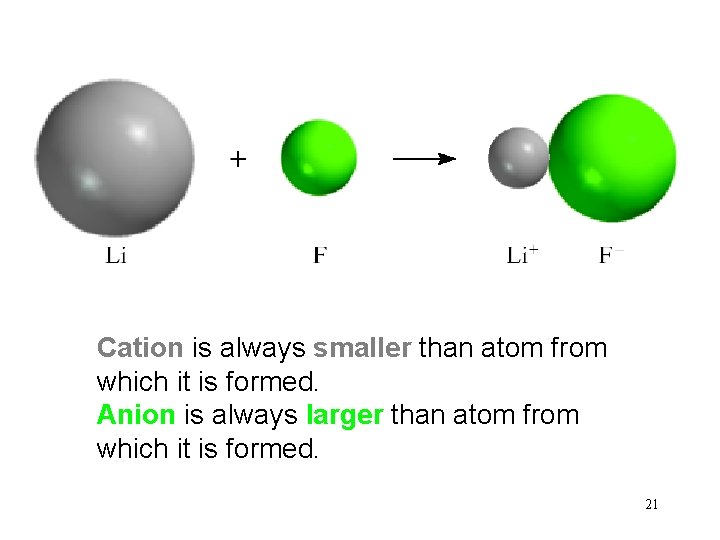
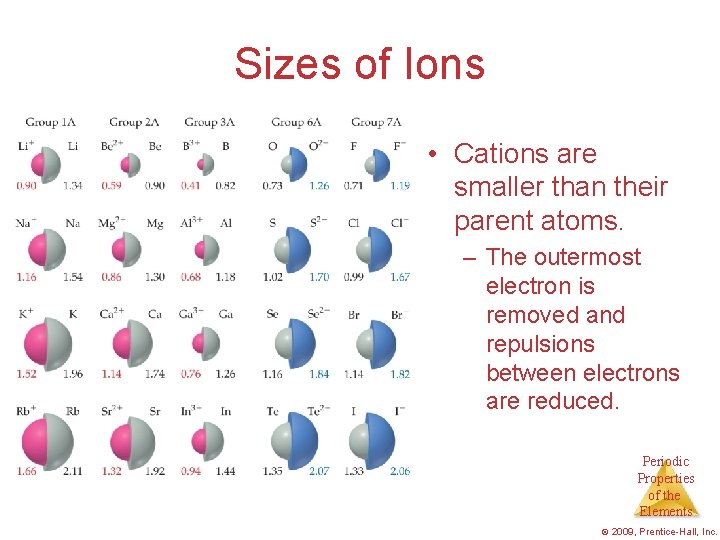
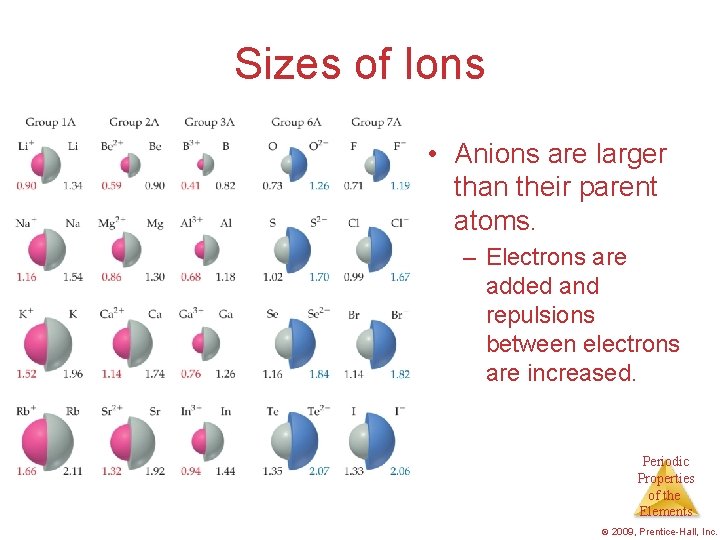
Cation is always smaller than atom from which it is formed. Anion is always larger than atom from which it is formed. 21

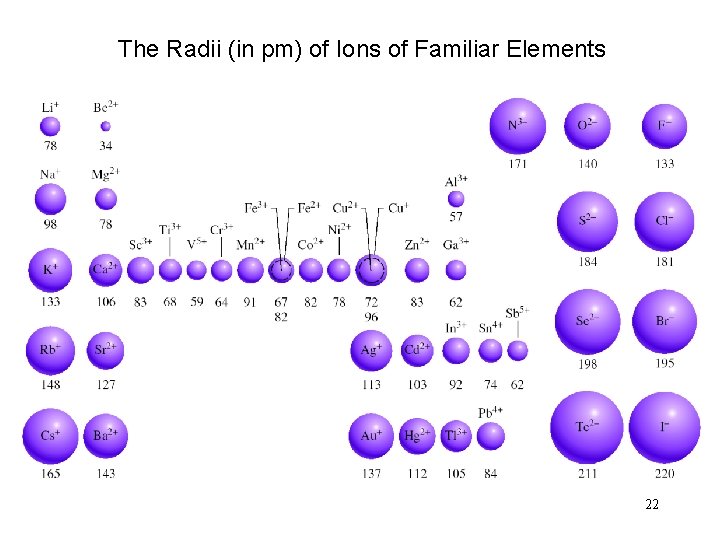
The Radii (in pm) of Ions of Familiar Elements 22

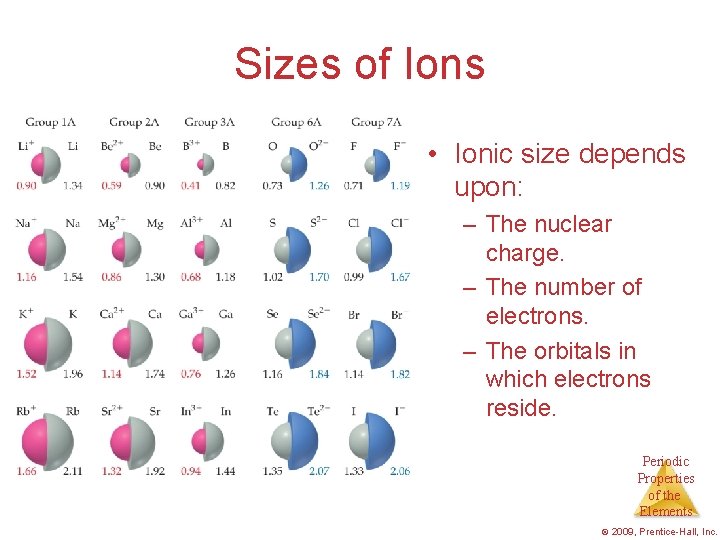
Sizes of Ions • Ionic size depends upon: – The nuclear charge. – The number of electrons. – The orbitals in which electrons reside. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

Sizes of Ions • Cations are smaller than their parent atoms. – The outermost electron is removed and repulsions between electrons are reduced. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

Sizes of Ions • Anions are larger than their parent atoms. – Electrons are added and repulsions between electrons are increased. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

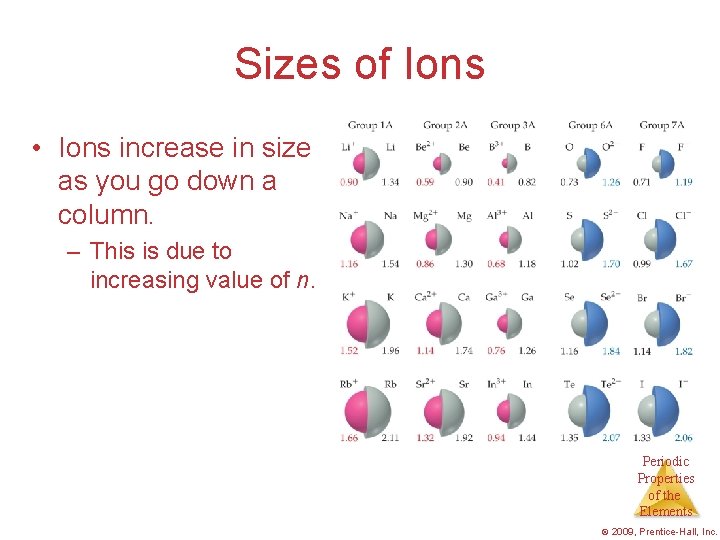
Sizes of Ions • Ions increase in size as you go down a column. – This is due to increasing value of n. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

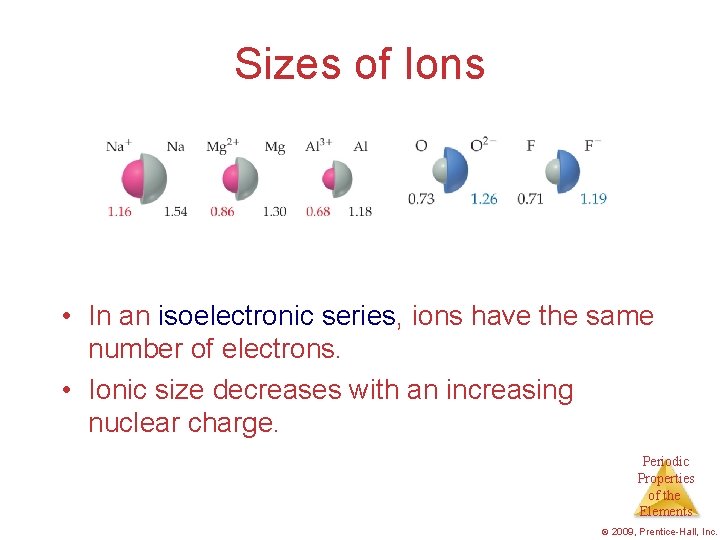
Sizes of Ions • In an isoelectronic series, ions have the same number of electrons. • Ionic size decreases with an increasing nuclear charge. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

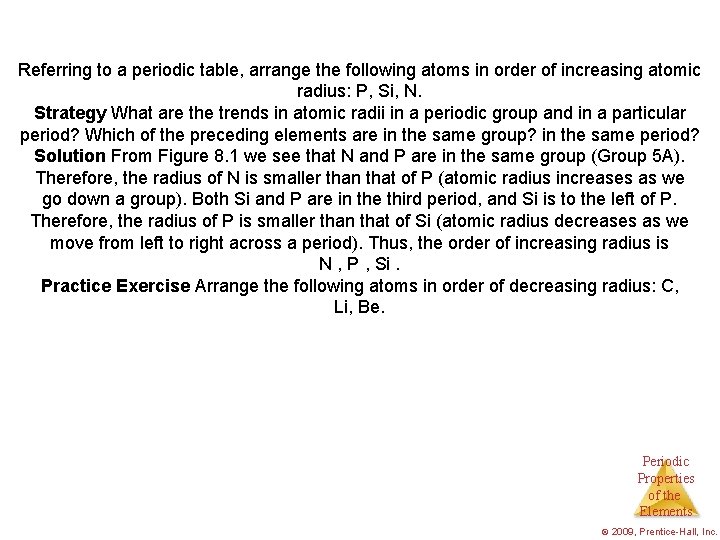
EXAMPLE 8. 2 Referring to a periodic table, arrange the following atoms in order of increasing atomic radius: P, Si, N. Strategy What are the trends in atomic radii in a periodic group and in a particular period? Which of the preceding elements are in the same group? in the same period? Solution From Figure 8. 1 we see that N and P are in the same group (Group 5 A). Therefore, the radius of N is smaller than that of P (atomic radius increases as we go down a group). Both Si and P are in the third period, and Si is to the left of P. Therefore, the radius of P is smaller than that of Si (atomic radius decreases as we move from left to right across a period). Thus, the order of increasing radius is N , P , Si. Practice Exercise Arrange the following atoms in order of decreasing radius: C, Li, Be. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

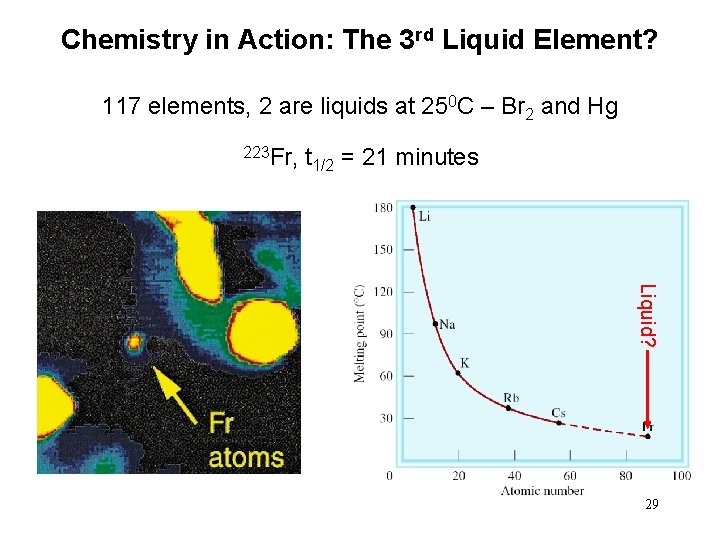
Chemistry in Action: The 3 rd Liquid Element? 117 elements, 2 are liquids at 250 C – Br 2 and Hg 223 Fr, t 1/2 = 21 minutes Liquid? 29

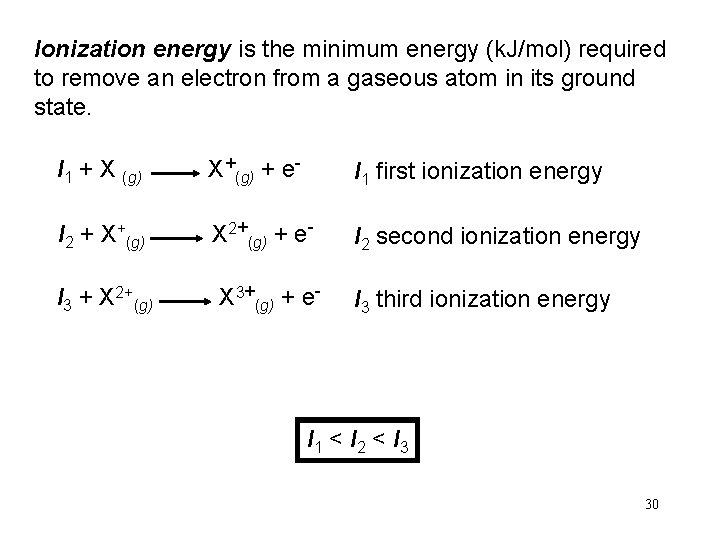
Ionization energy is the minimum energy (k. J/mol) required to remove an electron from a gaseous atom in its ground state. I 1 + X (g) X+(g) + e- I 1 first ionization energy I 2 + X+(g) X 2+(g) + e- I 2 second ionization energy I 3 + X 2+(g) X 3+(g) + e- I 3 third ionization energy I 1 < I 2 < I 3 30

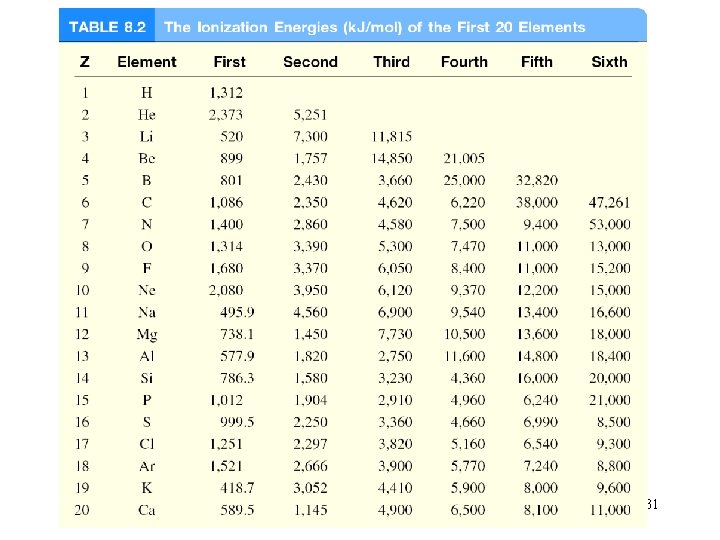
31

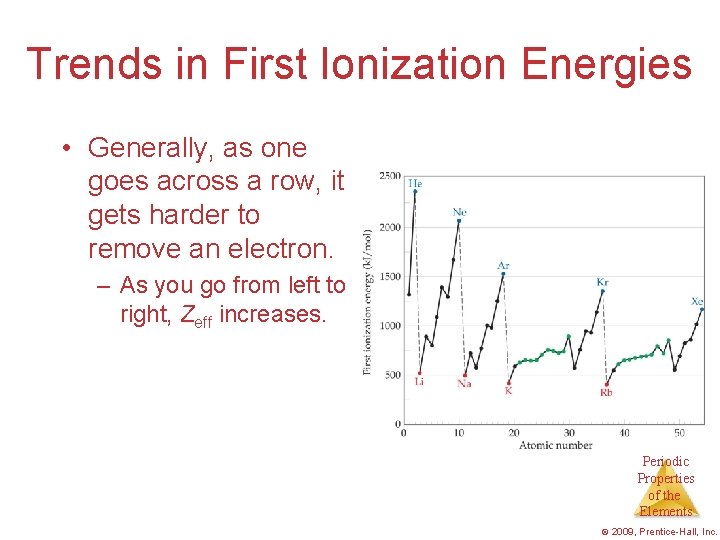
Trends in First Ionization Energies • Generally, as one goes across a row, it gets harder to remove an electron. – As you go from left to right, Zeff increases. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

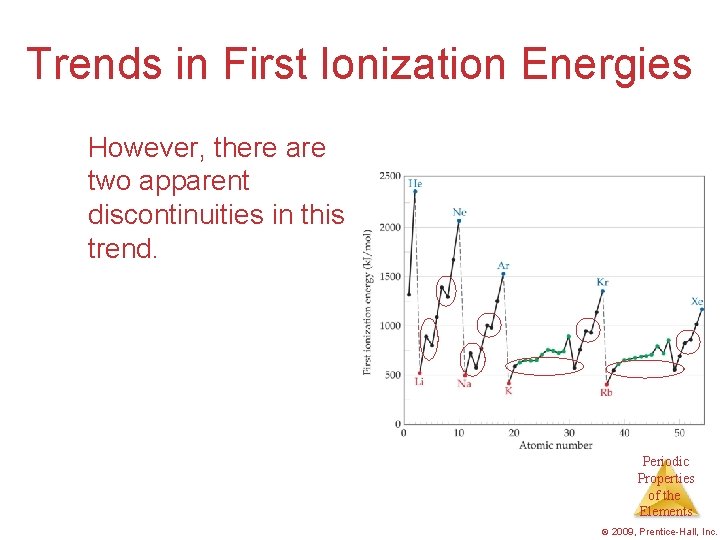
Trends in First Ionization Energies However, there are two apparent discontinuities in this trend. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

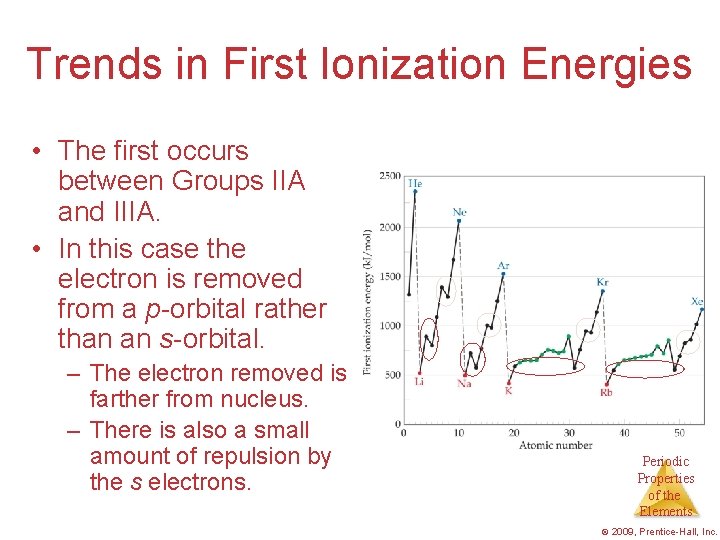
Trends in First Ionization Energies • The first occurs between Groups IIA and IIIA. • In this case the electron is removed from a p-orbital rather than an s-orbital. – The electron removed is farther from nucleus. – There is also a small amount of repulsion by the s electrons. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

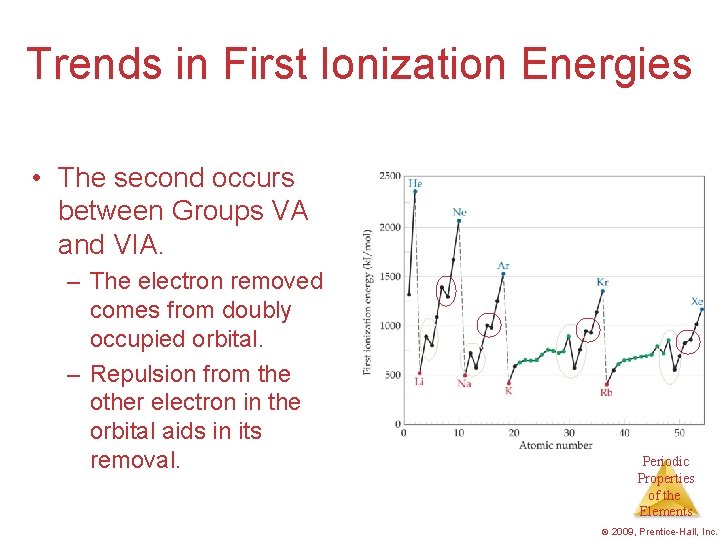
Trends in First Ionization Energies • The second occurs between Groups VA and VIA. – The electron removed comes from doubly occupied orbital. – Repulsion from the other electron in the orbital aids in its removal. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

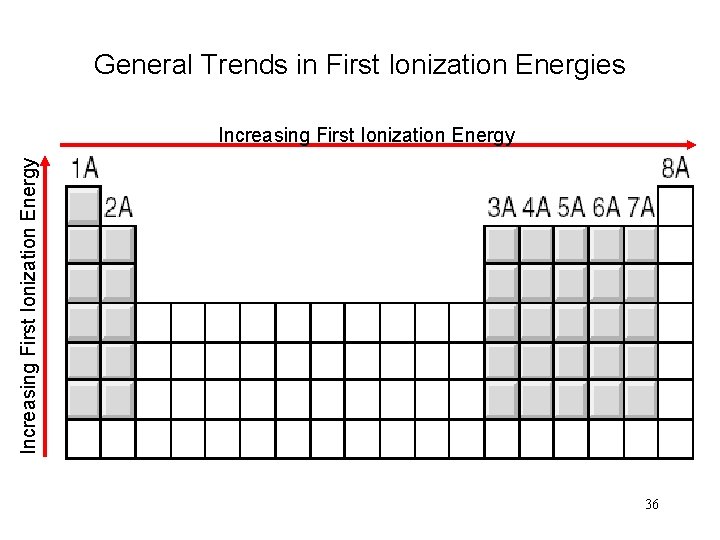
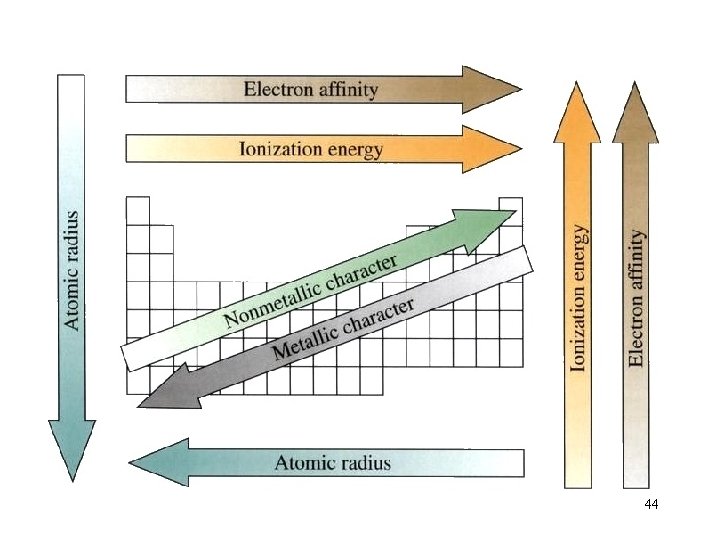
General Trends in First Ionization Energies Increasing First Ionization Energy 36

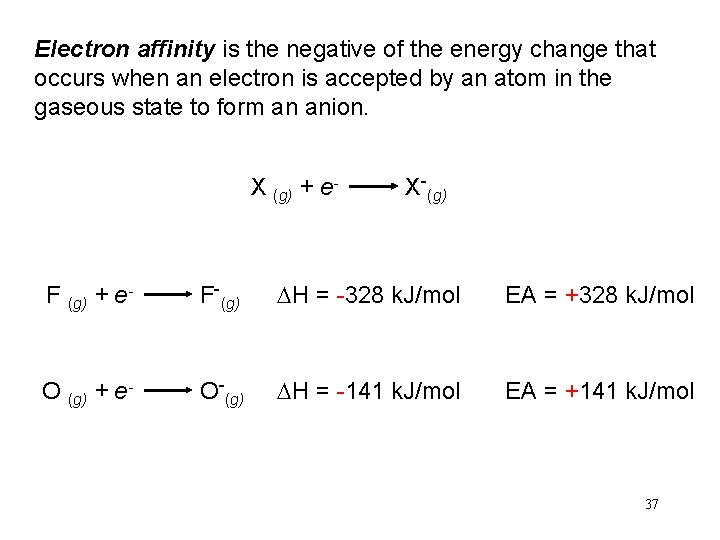
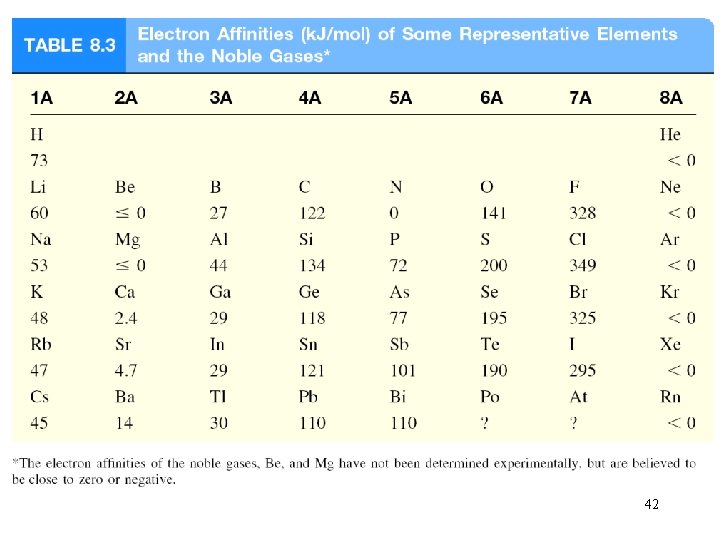
Electron affinity is the negative of the energy change that occurs when an electron is accepted by an atom in the gaseous state to form an anion. X (g) + e- X-(g) F (g) + e- F-(g) DH = -328 k. J/mol EA = +328 k. J/mol O (g) + e- O-(g) DH = -141 k. J/mol EA = +141 k. J/mol 37

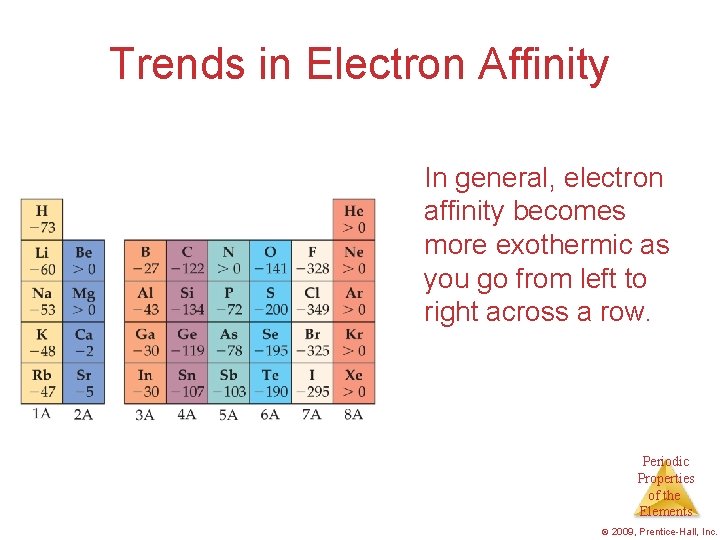
Trends in Electron Affinity In general, electron affinity becomes more exothermic as you go from left to right across a row. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

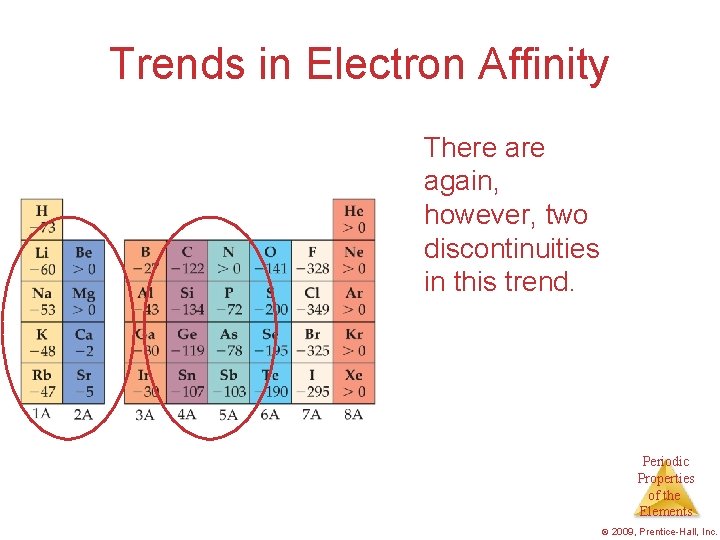
Trends in Electron Affinity There again, however, two discontinuities in this trend. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

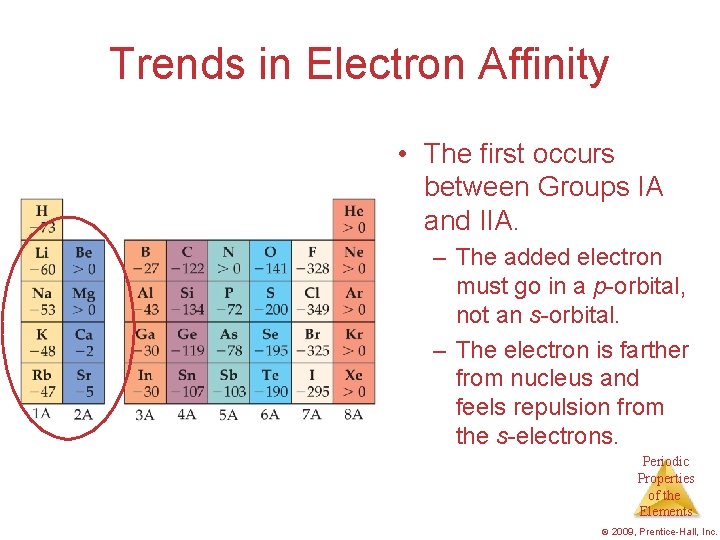
Trends in Electron Affinity • The first occurs between Groups IA and IIA. – The added electron must go in a p-orbital, not an s-orbital. – The electron is farther from nucleus and feels repulsion from the s-electrons. Periodic Properties of the Elements © 2009, Prentice-Hall, Inc.

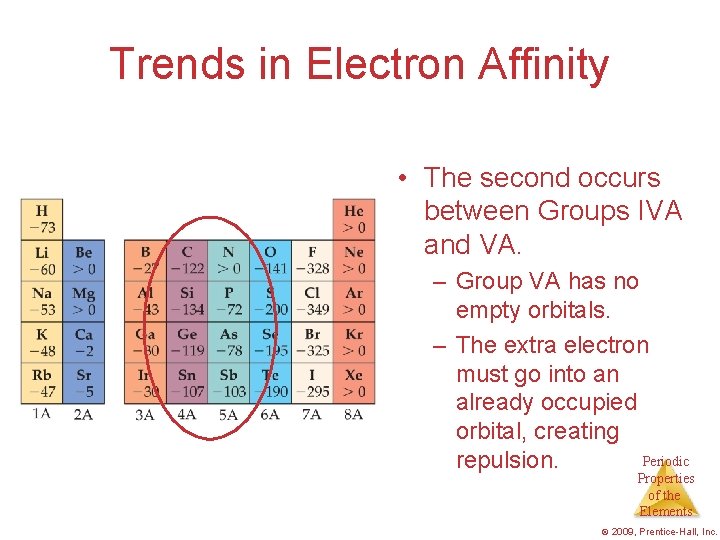
Trends in Electron Affinity • The second occurs between Groups IVA and VA. – Group VA has no empty orbitals. – The extra electron must go into an already occupied orbital, creating Periodic repulsion. Properties of the Elements © 2009, Prentice-Hall, Inc.

42

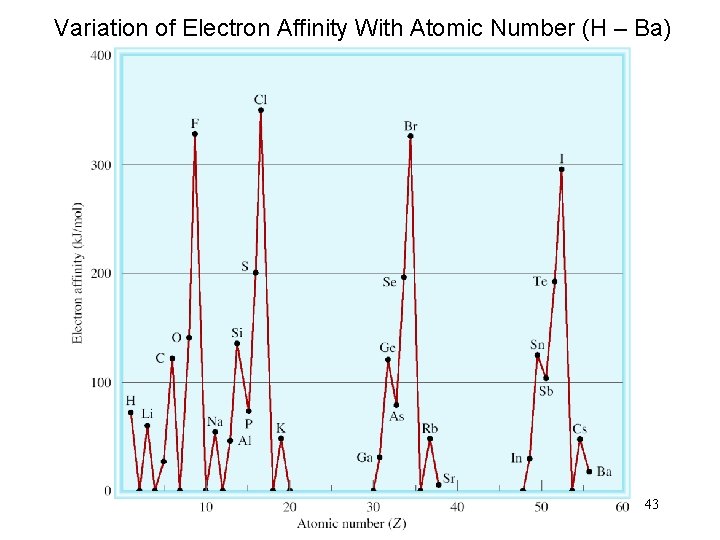
Variation of Electron Affinity With Atomic Number (H – Ba) 43

44

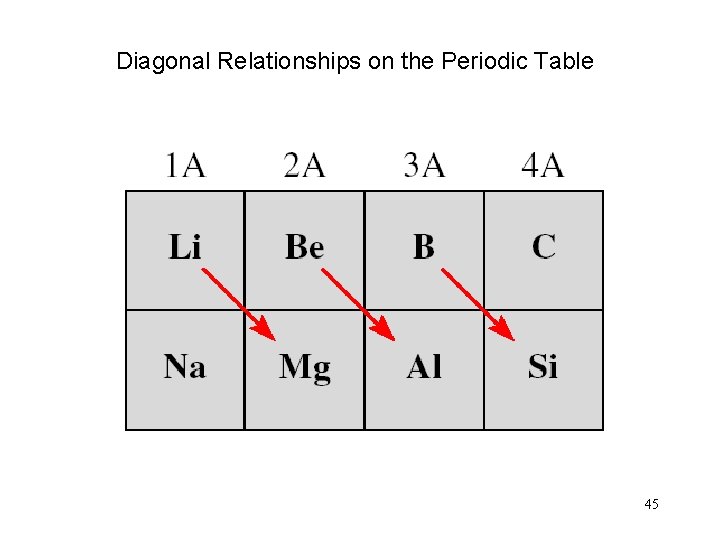
Diagonal Relationships on the Periodic Table 45

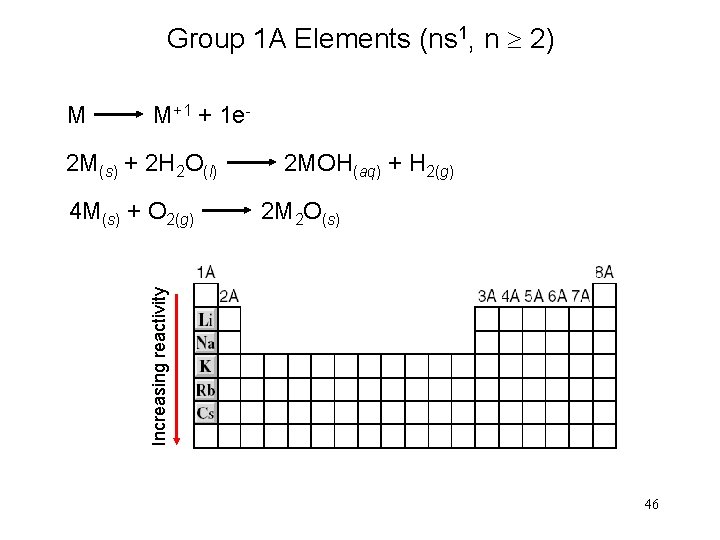
Group 1 A Elements (ns 1, n 2) M M+1 + 1 e 2 M(s) + 2 H 2 O(l) 2 MOH(aq) + H 2(g) Increasing reactivity 4 M(s) + O 2(g) 2 M 2 O(s) 46

Group 1 A Elements (ns 1, n 2) 47

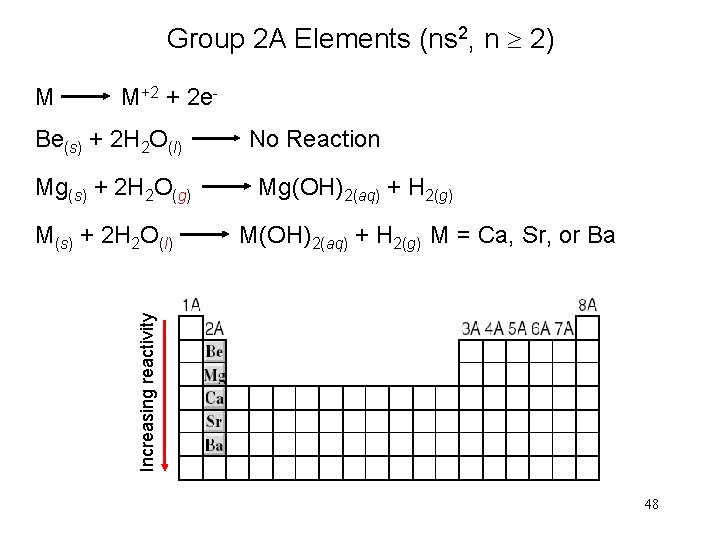
Group 2 A Elements (ns 2, n 2) M M+2 + 2 e. Be(s) + 2 H 2 O(l) No Reaction Mg(s) + 2 H 2 O(g) Mg(OH)2(aq) + H 2(g) Increasing reactivity M(s) + 2 H 2 O(l) M(OH)2(aq) + H 2(g) M = Ca, Sr, or Ba 48

Group 2 A Elements (ns 2, n 2) 49

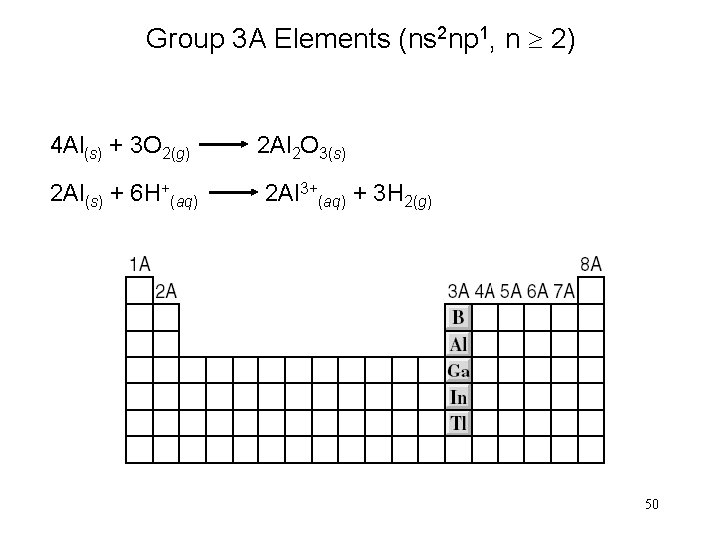
Group 3 A Elements (ns 2 np 1, n 2) 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) 2 Al(s) + 6 H+(aq) 2 Al 3+(aq) + 3 H 2(g) 50

Group 3 A Elements (ns 2 np 1, n 2) 51

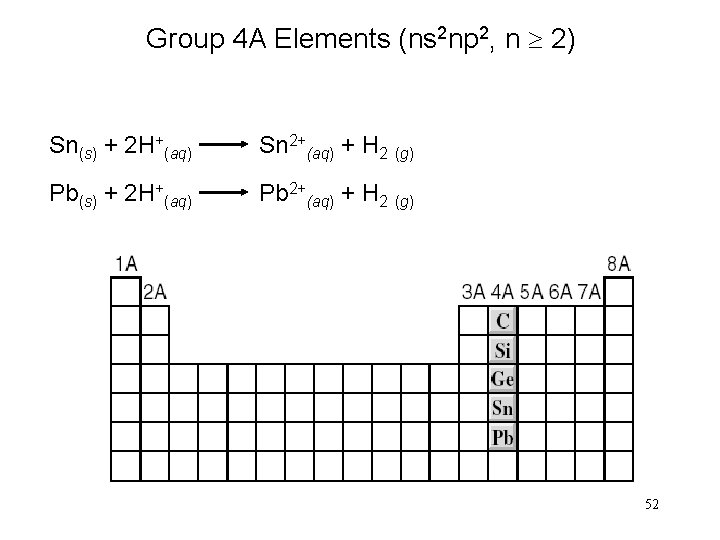
Group 4 A Elements (ns 2 np 2, n 2) Sn(s) + 2 H+(aq) Sn 2+(aq) + H 2 (g) Pb(s) + 2 H+(aq) Pb 2+(aq) + H 2 (g) 52

Group 4 A Elements (ns 2 np 2, n 2) 53

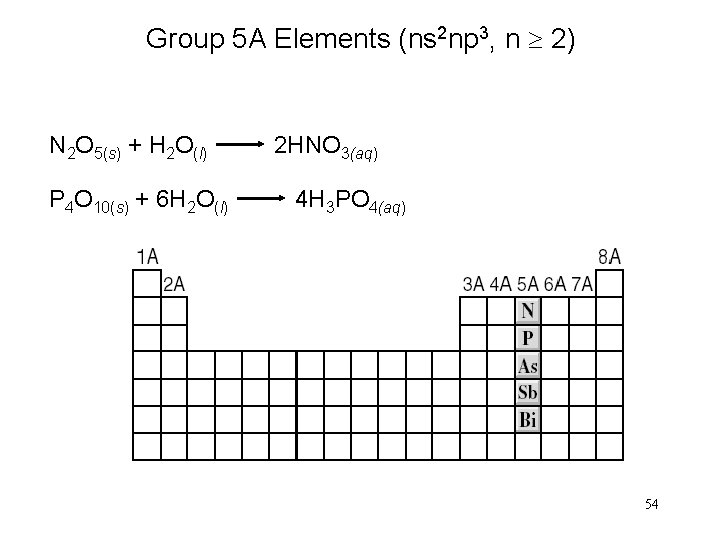
Group 5 A Elements (ns 2 np 3, n 2) N 2 O 5(s) + H 2 O(l) 2 HNO 3(aq) P 4 O 10(s) + 6 H 2 O(l) 4 H 3 PO 4(aq) 54

Group 5 A Elements (ns 2 np 3, n 2) 55

Group 6 A Elements (ns 2 np 4, n 2) SO 3(g) + H 2 O(l) H 2 SO 4(aq) 56

Group 6 A Elements (ns 2 np 4, n 2) 57

Group 7 A Elements (ns 2 np 5, n 2) X + 1 e- X-1 Increasing reactivity X 2(g) + H 2(g) 2 HX(g) 58

Group 7 A Elements (ns 2 np 5, n 2) 59

Group 8 A Elements (ns 2 np 6, n 2) Completely filled ns and np subshells. Highest ionization energy of all elements. No tendency to accept extra electrons. 60

Compounds of the Noble Gases A number of xenon compounds Xe. F 4, Xe. O 3, Xe. O 4, Xe. OF 4 exist. A few krypton compounds (Kr. F 2, for example) have been prepared. 61

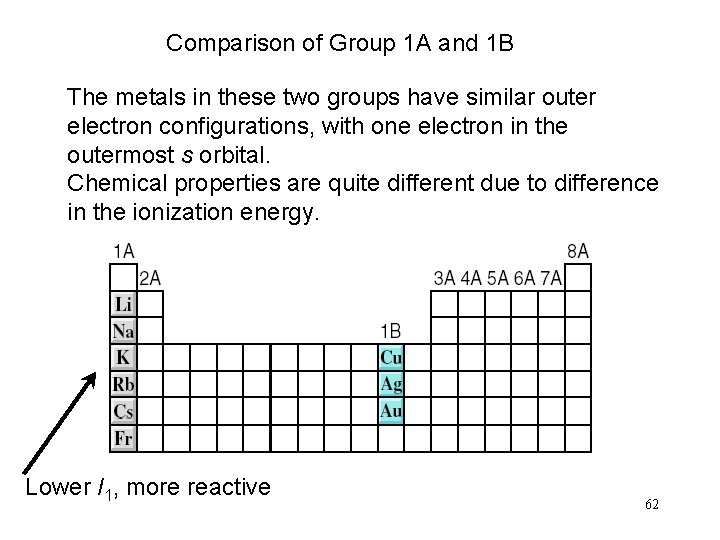
Comparison of Group 1 A and 1 B The metals in these two groups have similar outer electron configurations, with one electron in the outermost s orbital. Chemical properties are quite different due to difference in the ionization energy. Lower I 1, more reactive 62

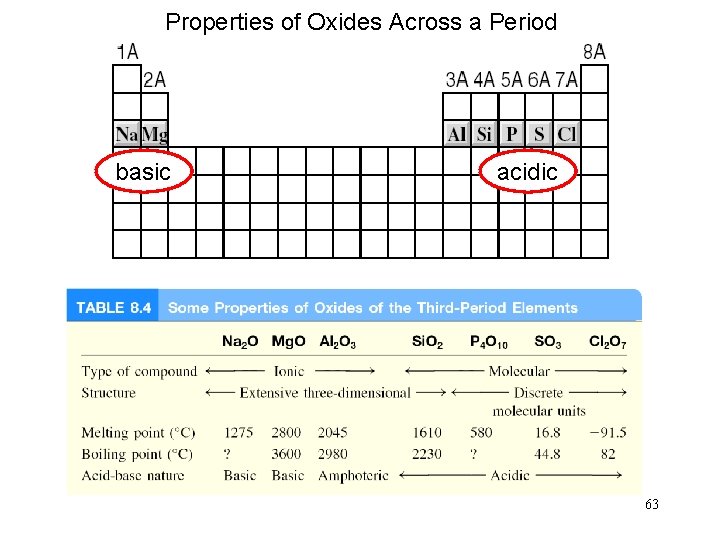
Properties of Oxides Across a Period basic acidic 63

Chemistry in Action: Discovery of the Noble Gases 64 Sir William Ramsay