Nuclear Chemistry Nuclear chemistry is the study of

- Slides: 18

Nuclear Chemistry Nuclear chemistry is the study of the structure of atomic nuclei and the changes they undergo. Ch 25 CVHS

The Discovery • Becquerel discovered invisible Xrays were by noticing that something cause photographic plates to darken (1895) • Marie Curie and her husband Pierre took Becquerel’s mineral sample (called pitchblende) and isolated the components emitting the rays. – They determined the plates were darkened by rays emitted from the mineral. – Process is called radioactivity – The rays & particles were named radiation

Why do atoms emit radiation? • The nucleus is unstable due in part to the ratio of protons and neutrons • The atoms that are unstable & emit radiation are called radioisotopes • Atoms that emit radiation &lose energy to attain a more stable configuration

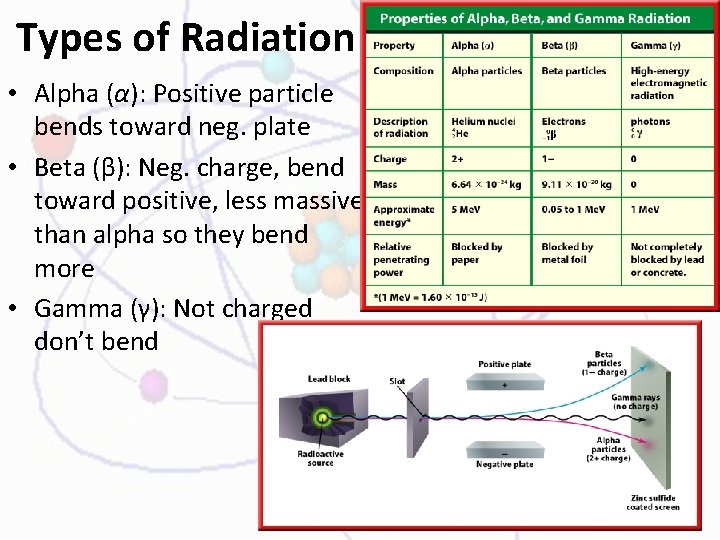
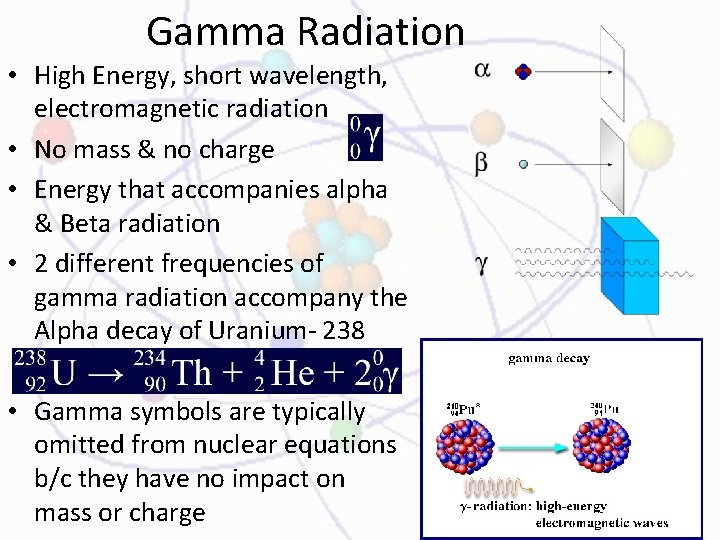
Types of Radiation • Alpha (α): Positive particle bends toward neg. plate • Beta (β): Neg. charge, bend toward positive, less massive than alpha so they bend more • Gamma (γ): Not charged don’t bend

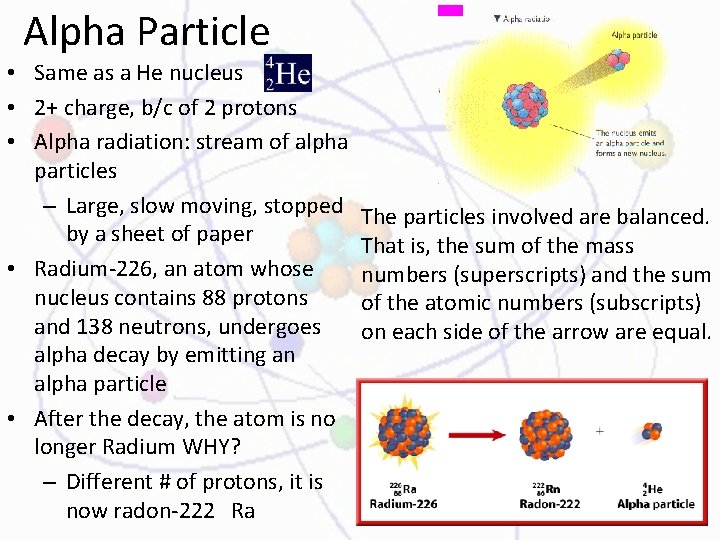
Alpha Particle • Same as a He nucleus • 2+ charge, b/c of 2 protons • Alpha radiation: stream of alpha particles – Large, slow moving, stopped by a sheet of paper • Radium-226, an atom whose nucleus contains 88 protons and 138 neutrons, undergoes alpha decay by emitting an alpha particle • After the decay, the atom is no longer Radium WHY? – Different # of protons, it is now radon-222 Ra The particles involved are balanced. That is, the sum of the mass numbers (superscripts) and the sum of the atomic numbers (subscripts) on each side of the arrow are equal.

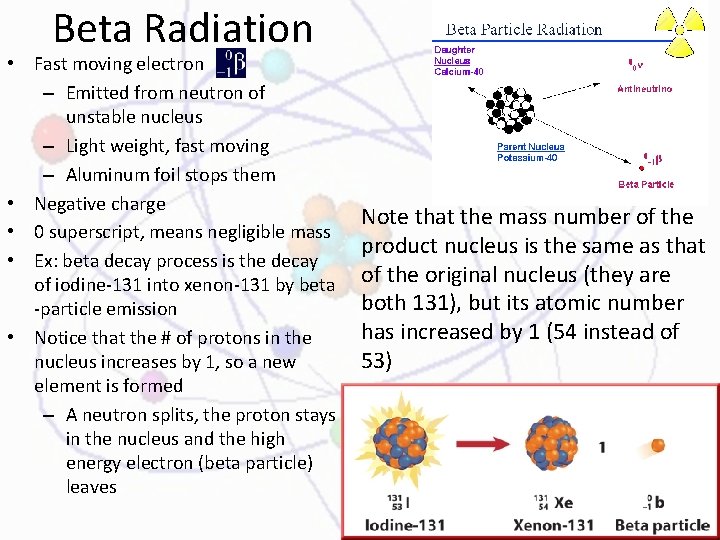
Beta Radiation • Fast moving electron – Emitted from neutron of unstable nucleus – Light weight, fast moving – Aluminum foil stops them • Negative charge • 0 superscript, means negligible mass • Ex: beta decay process is the decay of iodine-131 into xenon-131 by beta -particle emission • Notice that the # of protons in the nucleus increases by 1, so a new element is formed – A neutron splits, the proton stays in the nucleus and the high energy electron (beta particle) leaves Note that the mass number of the product nucleus is the same as that of the original nucleus (they are both 131), but its atomic number has increased by 1 (54 instead of 53)

Gamma Radiation • High Energy, short wavelength, electromagnetic radiation • No mass & no charge • Energy that accompanies alpha & Beta radiation • 2 different frequencies of gamma radiation accompany the Alpha decay of Uranium- 238 • Gamma symbols are typically omitted from nuclear equations b/c they have no impact on mass or charge

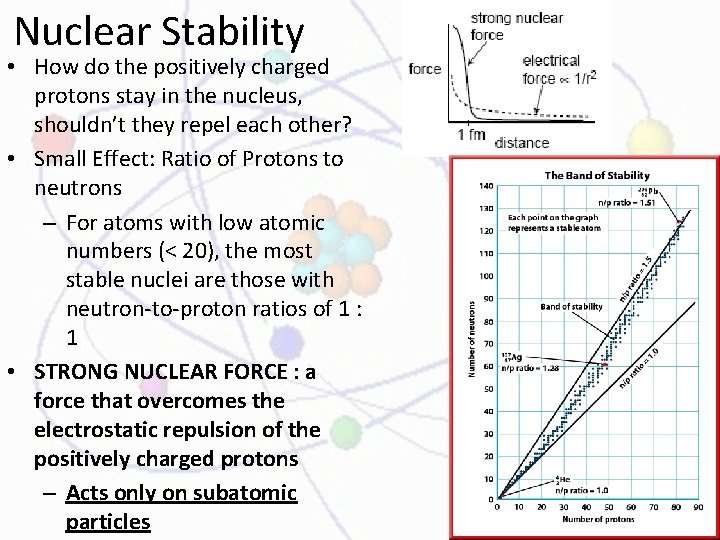
Nuclear Stability • How do the positively charged protons stay in the nucleus, shouldn’t they repel each other? • Small Effect: Ratio of Protons to neutrons – For atoms with low atomic numbers (< 20), the most stable nuclei are those with neutron-to-proton ratios of 1 : 1 • STRONG NUCLEAR FORCE : a force that overcomes the electrostatic repulsion of the positively charged protons – Acts only on subatomic particles

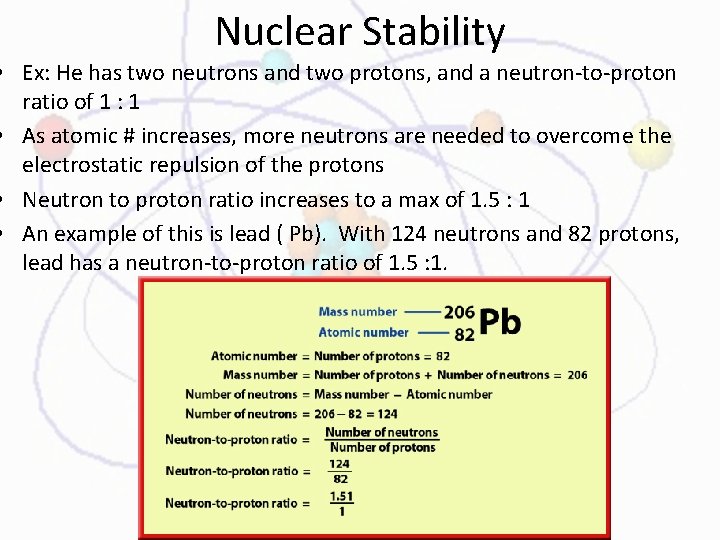
Nuclear Stability • Ex: He has two neutrons and two protons, and a neutron-to-proton ratio of 1 : 1 • As atomic # increases, more neutrons are needed to overcome the electrostatic repulsion of the protons • Neutron to proton ratio increases to a max of 1. 5 : 1 • An example of this is lead ( Pb). With 124 neutrons and 82 protons, lead has a neutron-to-proton ratio of 1. 5 : 1.

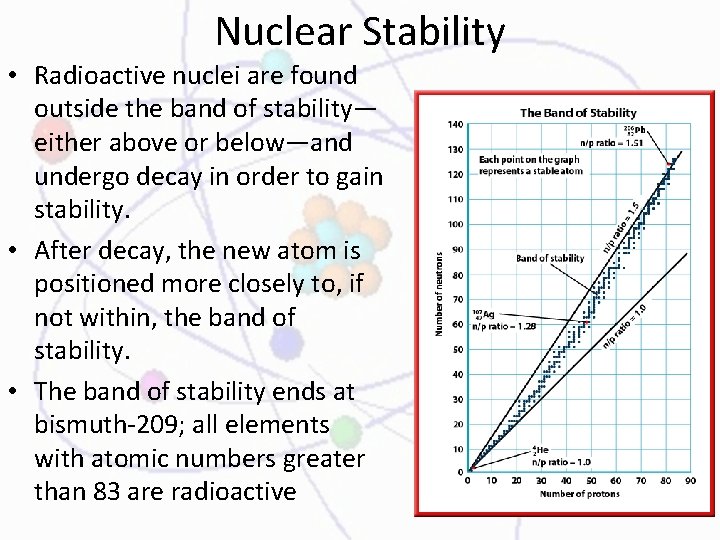
Nuclear Stability • Radioactive nuclei are found outside the band of stability— either above or below—and undergo decay in order to gain stability. • After decay, the new atom is positioned more closely to, if not within, the band of stability. • The band of stability ends at bismuth-209; all elements with atomic numbers greater than 83 are radioactive

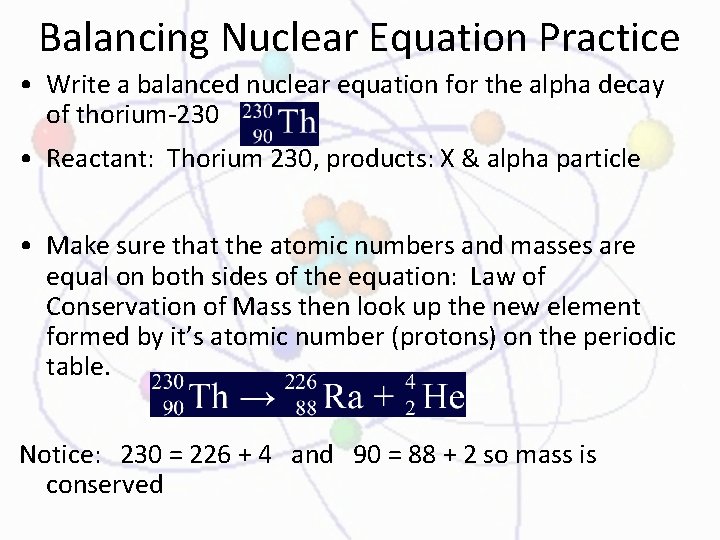
Balancing Nuclear Equation Practice • Write a balanced nuclear equation for the alpha decay of thorium-230 • Reactant: Thorium 230, products: X & alpha particle • Make sure that the atomic numbers and masses are equal on both sides of the equation: Law of Conservation of Mass then look up the new element formed by it’s atomic number (protons) on the periodic table. Notice: 230 = 226 + 4 and 90 = 88 + 2 so mass is conserved

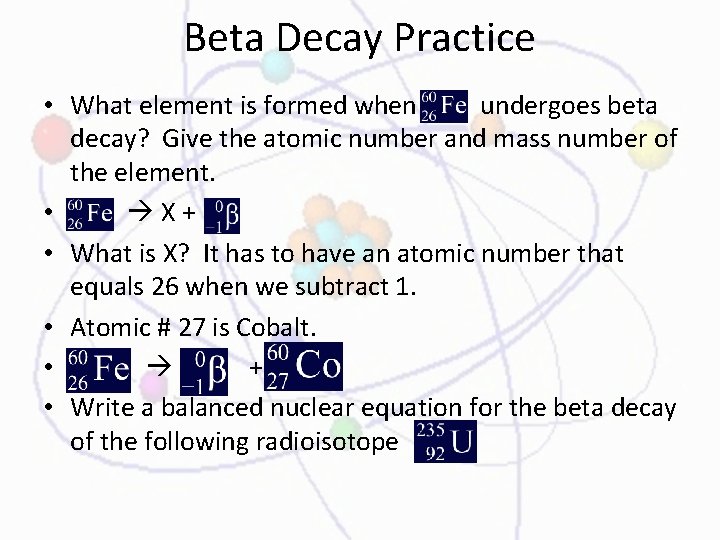
Beta Decay Practice • What element is formed when undergoes beta decay? Give the atomic number and mass number of the element. • X+ • What is X? It has to have an atomic number that equals 26 when we subtract 1. • Atomic # 27 is Cobalt. • + • Write a balanced nuclear equation for the beta decay of the following radioisotope

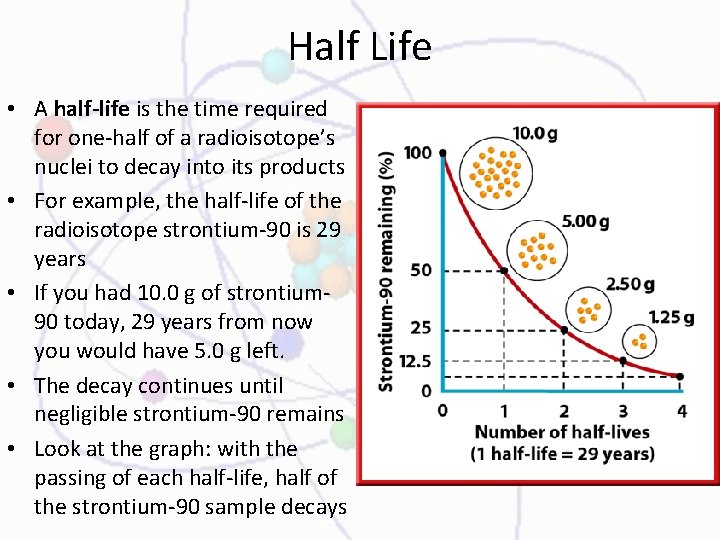
Half Life • A half-life is the time required for one-half of a radioisotope’s nuclei to decay into its products • For example, the half-life of the radioisotope strontium-90 is 29 years • If you had 10. 0 g of strontium 90 today, 29 years from now you would have 5. 0 g left. • The decay continues until negligible strontium-90 remains • Look at the graph: with the passing of each half-life, half of the strontium-90 sample decays

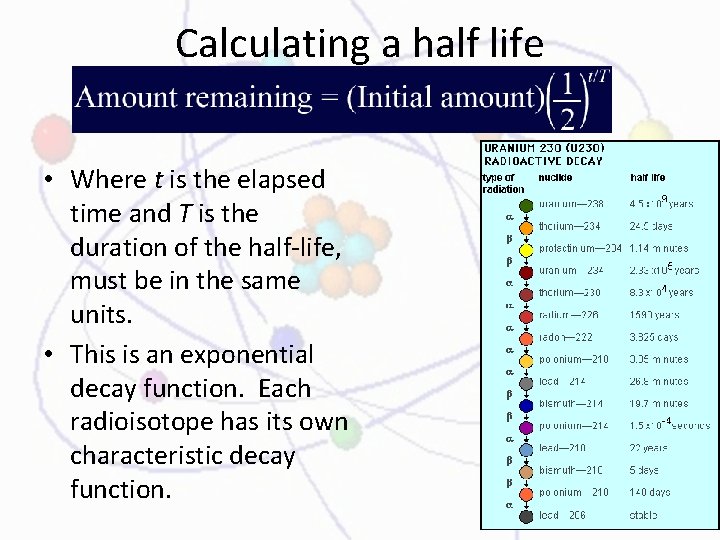
Calculating a half life • Where t is the elapsed time and T is the duration of the half-life, must be in the same units. • This is an exponential decay function. Each radioisotope has its own characteristic decay function.

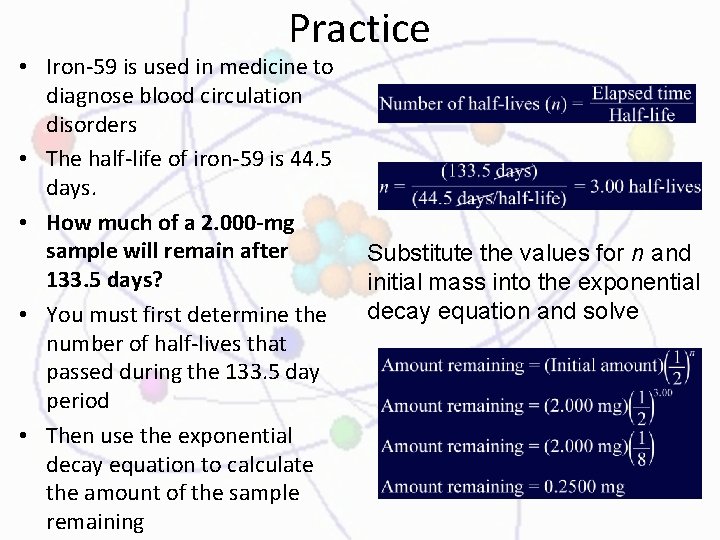
Practice • Iron-59 is used in medicine to diagnose blood circulation disorders • The half-life of iron-59 is 44. 5 days. • How much of a 2. 000 -mg sample will remain after 133. 5 days? • You must first determine the number of half-lives that passed during the 133. 5 day period • Then use the exponential decay equation to calculate the amount of the sample remaining Substitute the values for n and initial mass into the exponential decay equation and solve

C 14 Dating

Nuclear Fission • Splitting of nucleus into fragments – Large atoms – Releases large amt of energy – Critical mass creates a chain reaction • Nuclear power plants harness the energy released by fission reactions – Heat generates steam – Steam powers turbines to generate electricity – Cadmium and Boron rods keep the reaction under control by absorbing the atomic particles that are released

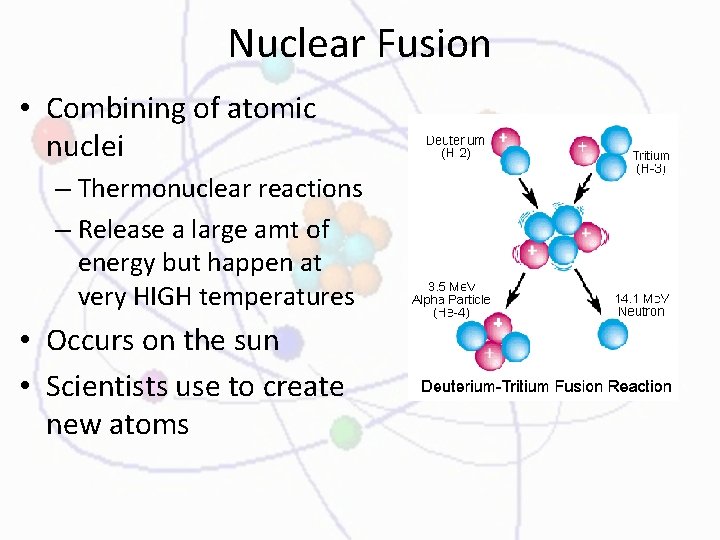
Nuclear Fusion • Combining of atomic nuclei – Thermonuclear reactions – Release a large amt of energy but happen at very HIGH temperatures • Occurs on the sun • Scientists use to create new atoms
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nuclear chemistry webquest
Nuclear chemistry webquest Chemistry
Chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry What is nuclear charge in chemistry
What is nuclear charge in chemistry Radioactive tracers in agriculture
Radioactive tracers in agriculture Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Nuclear chemistry review worksheet answer key
Nuclear chemistry review worksheet answer key Applications of nuclear chemistry
Applications of nuclear chemistry Nuclear chemistry
Nuclear chemistry Real life examples of fusion
Real life examples of fusion Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Copyright
Copyright Nuclear chemistry
Nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry