Module 3 Nuclear Chemistry the chemistry of protons














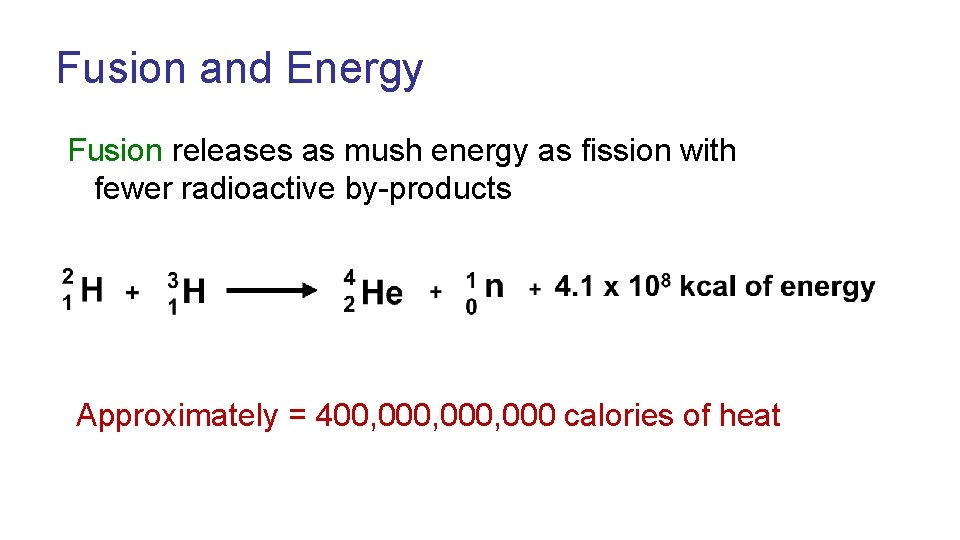
- Slides: 39

Module 3 Nuclear Chemistry- the chemistry of protons and neutrons

Key Questions? • When was radioactivity discovered? • What is a nuclear change? • Why do some atoms undergo radioactive decay and others don’t? • What are the products of this decay? • Why are some radioactive isotopes more harmful than others? • What are some useful applications of this process?

Discovery Henri Becquerel (1896) experimented with phosphorescence of certain minerals (uranium) It was later found that the radiation from these elements was alpha, beta particles and gamma rays.

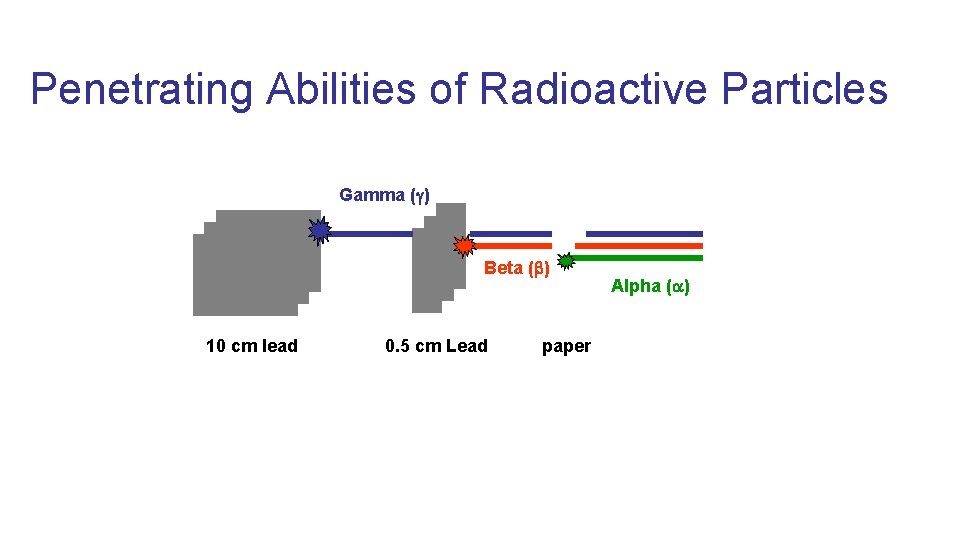
Discovery Earnest Rutherford (1899)- found that alpha rays could be stopped by thin pieces of paper. Whereas beta rays were only stopped by at least 0. 5 cm of lead. Paul Villard (1900)- discovered the high energy, extremely penetrating gamma ray having characteristics of light waves. Very damaging to human tissue

Penetrating Abilities of Radioactive Particles Gamma ( ) Beta ( ) 10 cm lead 0. 5 cm Lead paper Alpha ( )

Madame (Marie) Curie (1859 -1906) Won the noble prize along with Henri Becquerel for their work on radioactivity. She discovered that some elements are more radioactive than others.

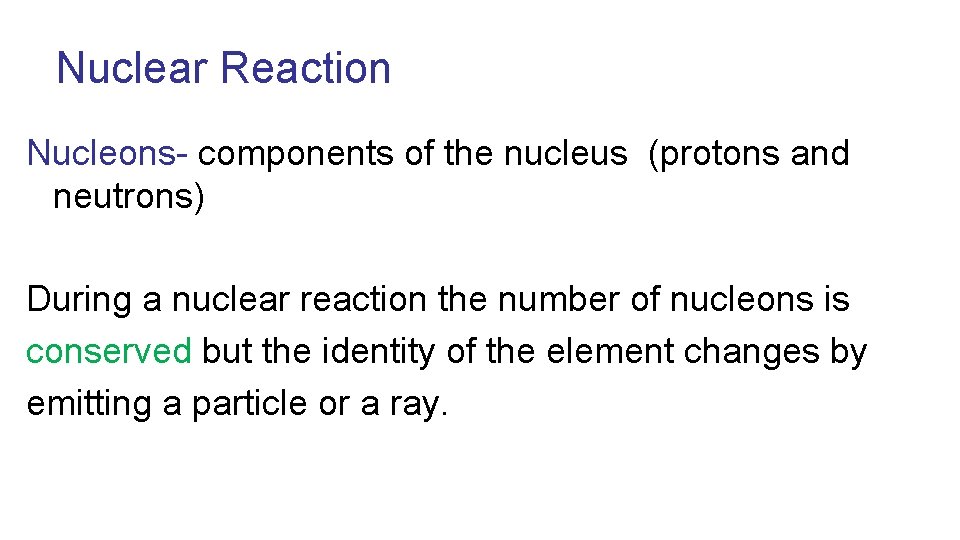
Nuclear Reactions Radioactivity- the result of a natural change of an isotope of one element into an isotope of a different element resulting in a nuclear reaction

Nuclear Reaction Nucleons- components of the nucleus (protons and neutrons) During a nuclear reaction the number of nucleons is conserved but the identity of the element changes by emitting a particle or a ray.

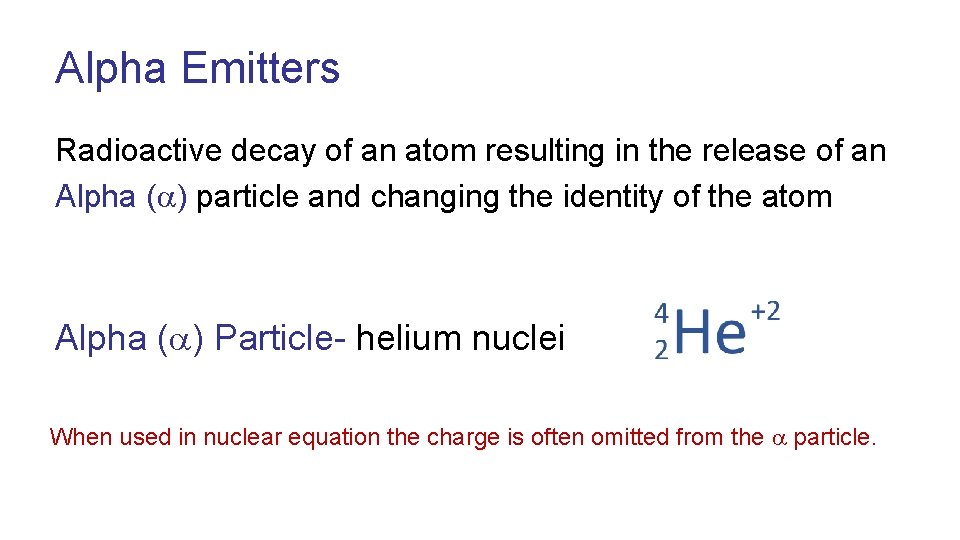
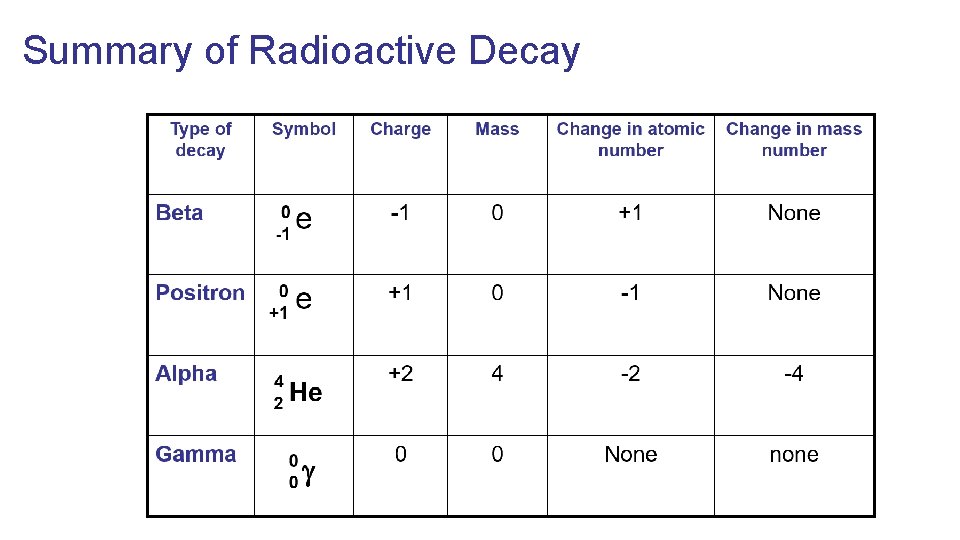
Alpha Emitters Radioactive decay of an atom resulting in the release of an Alpha ( ) particle and changing the identity of the atom Alpha ( ) Particle- helium nuclei When used in nuclear equation the charge is often omitted from the particle.

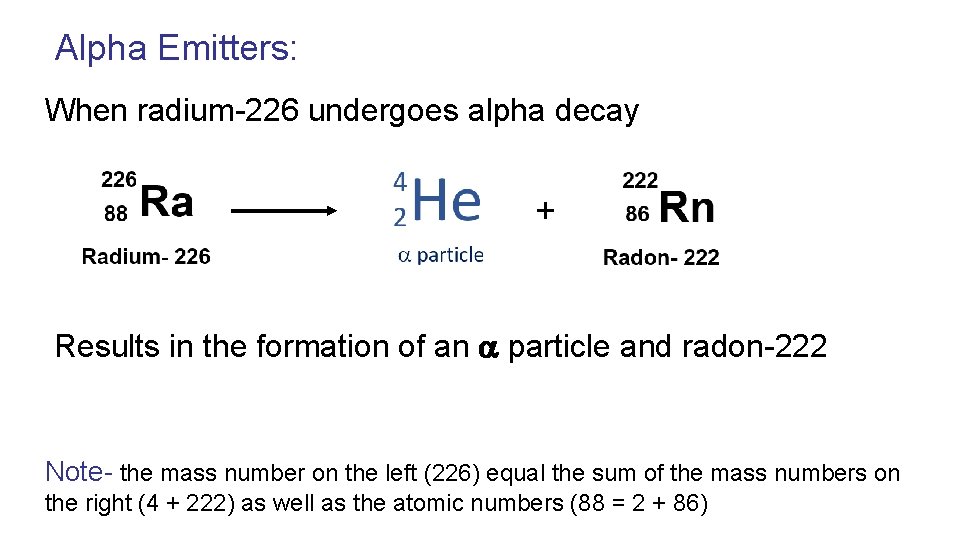
Alpha Emitters: When radium-226 undergoes alpha decay + Results in the formation of an particle and radon-222 Note- the mass number on the left (226) equal the sum of the mass numbers on the right (4 + 222) as well as the atomic numbers (88 = 2 + 86)

Beta Emitters Radioactive decay of an atom resulting in the nucleus releasing a Beta ( ) particle (which is an electron) and as a consequence changes the identity of the atom Beta ( ) particle- an electron

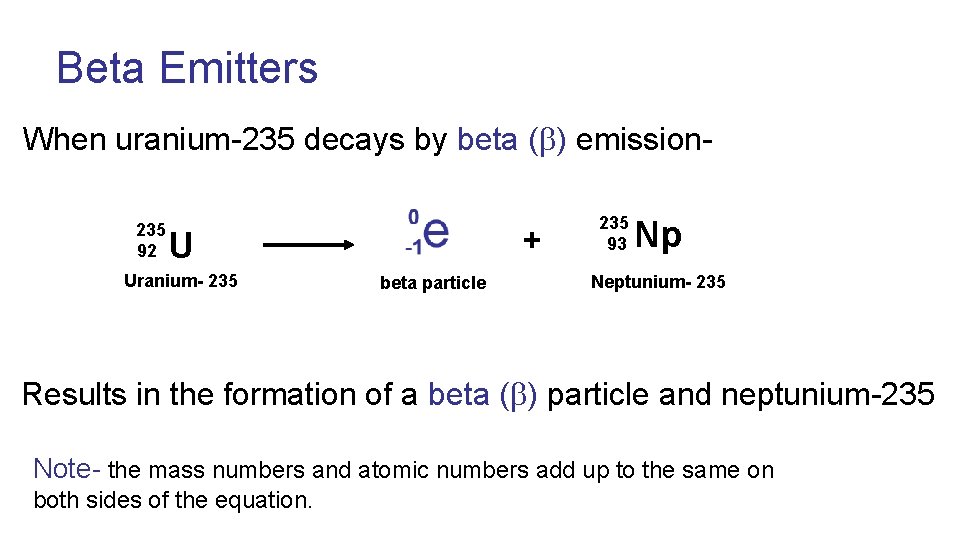
Beta Emitters When uranium-235 decays by beta ( ) emission 235 92 + U Uranium- 235 beta particle 235 93 Np Neptunium- 235 Results in the formation of a beta ( ) particle and neptunium-235 Note- the mass numbers and atomic numbers add up to the same on both sides of the equation.

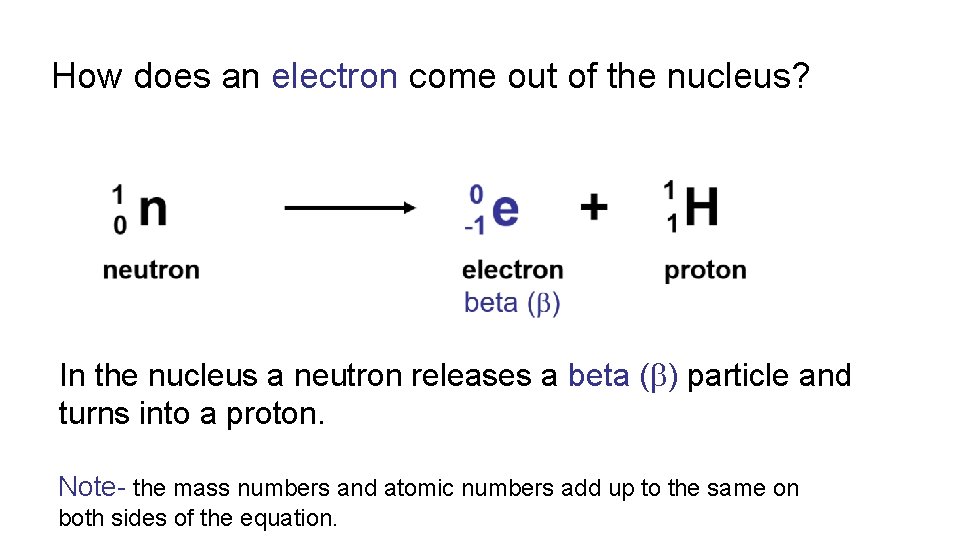
How does an electron come out of the nucleus? In the nucleus a neutron releases a beta ( ) particle and turns into a proton. Note- the mass numbers and atomic numbers add up to the same on both sides of the equation.

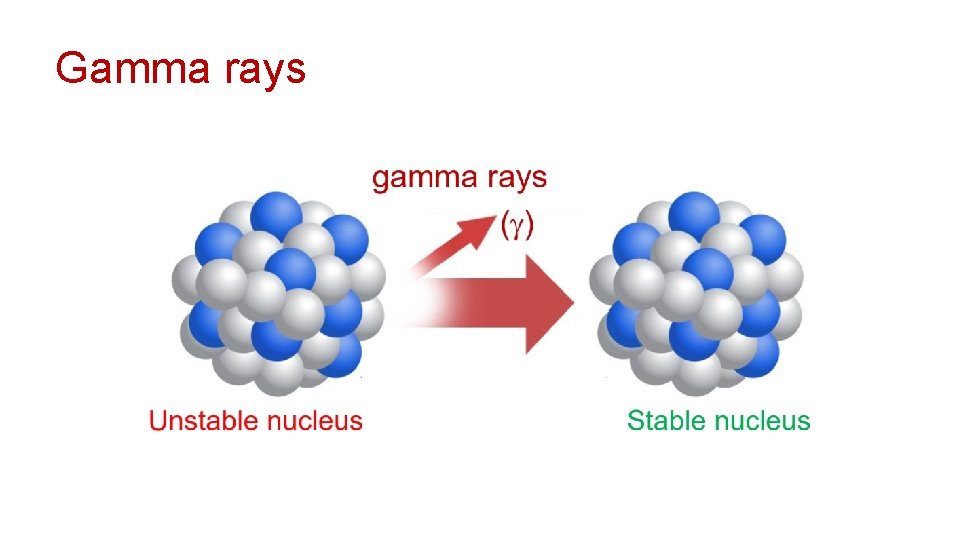
Gamma rays ( ) After a nucleus emits an alpha or beta particle, it can still contain excess energy. This energy can be released in the form of highly energetic photons called gamma rays ( ).

Gamma rays

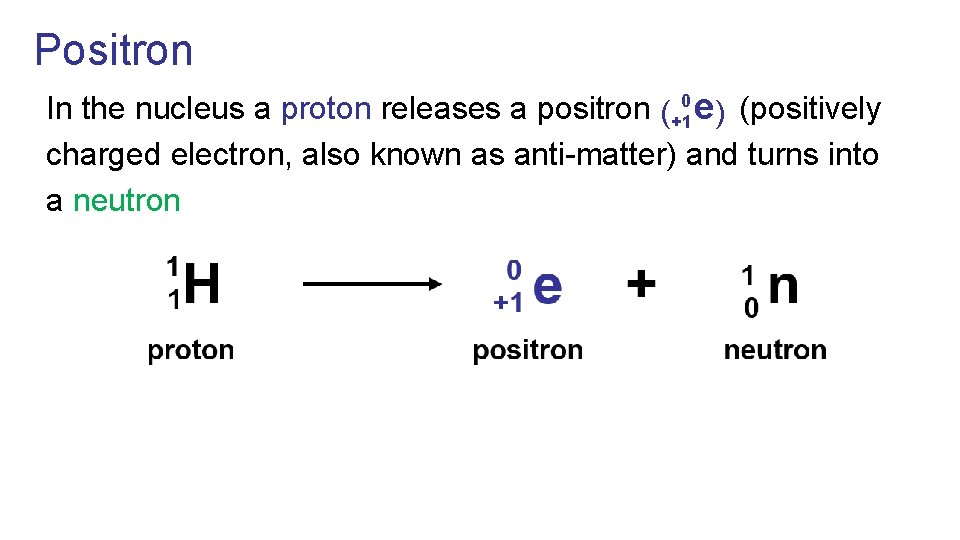
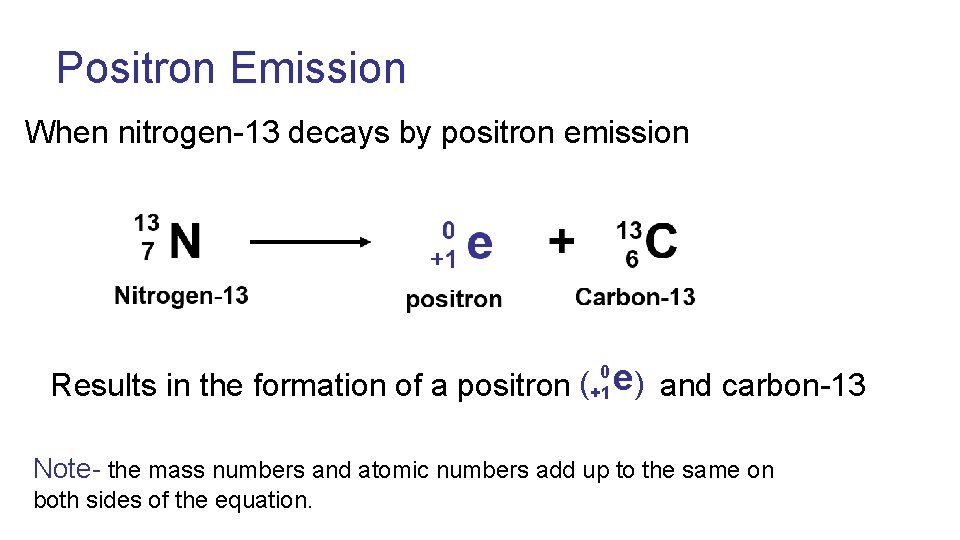
Positron 0 In the nucleus a proton releases a positron (positively e ( ) +1 charged electron, also known as anti-matter) and turns into a neutron

Positron Emission When nitrogen-13 decays by positron emission e ( ) Results in the formation of a positron and carbon-13 0 +1 Note- the mass numbers and atomic numbers add up to the same on both sides of the equation.

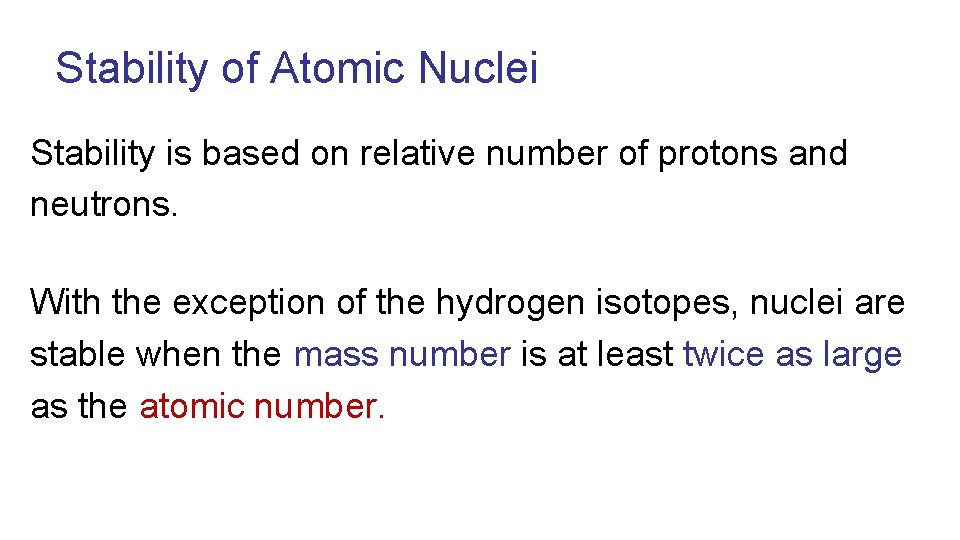
Stability of Atomic Nuclei Stability is based on relative number of protons and neutrons. With the exception of the hydrogen isotopes, nuclei are stable when the mass number is at least twice as large as the atomic number.

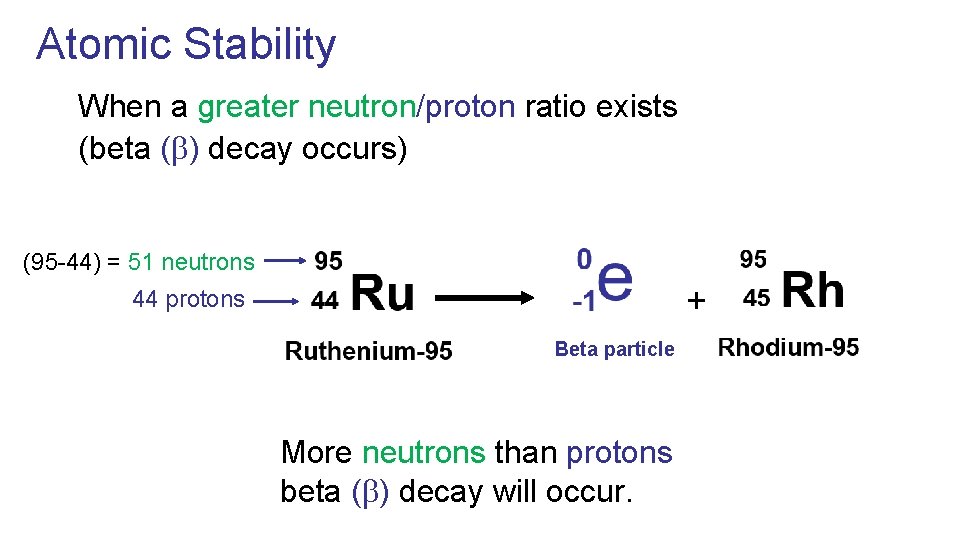
Atomic Stability When a greater neutron/proton ratio exists (beta ( ) decay occurs) (95 -44) = 51 neutrons + 44 protons Beta particle More neutrons than protons beta ( ) decay will occur.

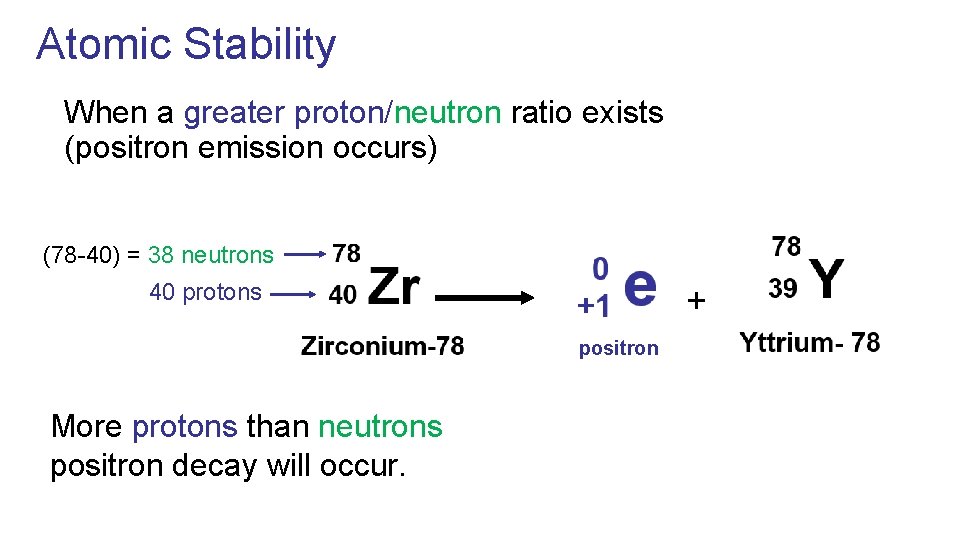
Atomic Stability When a greater proton/neutron ratio exists (positron emission occurs) (78 -40) = 38 neutrons 40 protons + positron More protons than neutrons positron decay will occur.

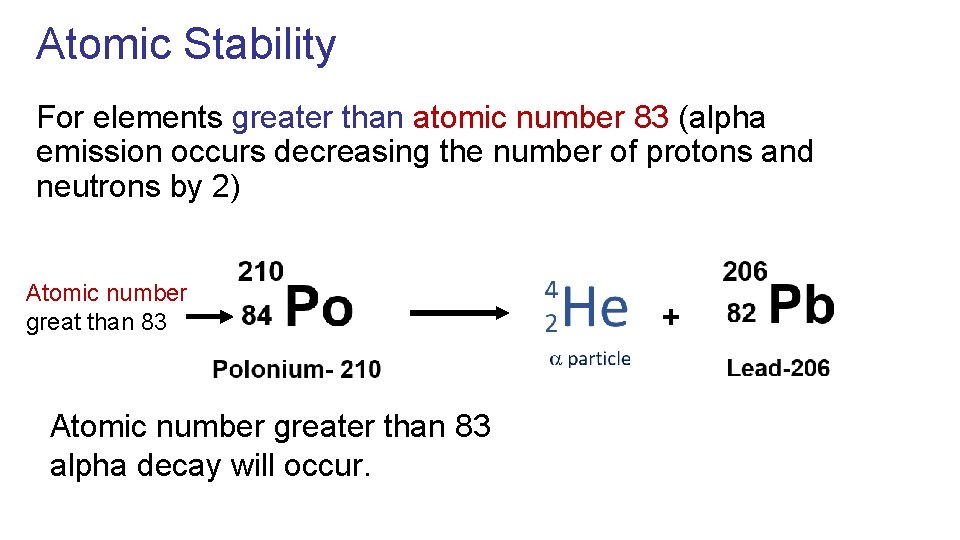
Atomic Stability For elements greater than atomic number 83 (alpha emission occurs decreasing the number of protons and neutrons by 2) Atomic number great than 83 Atomic number greater than 83 alpha decay will occur. +

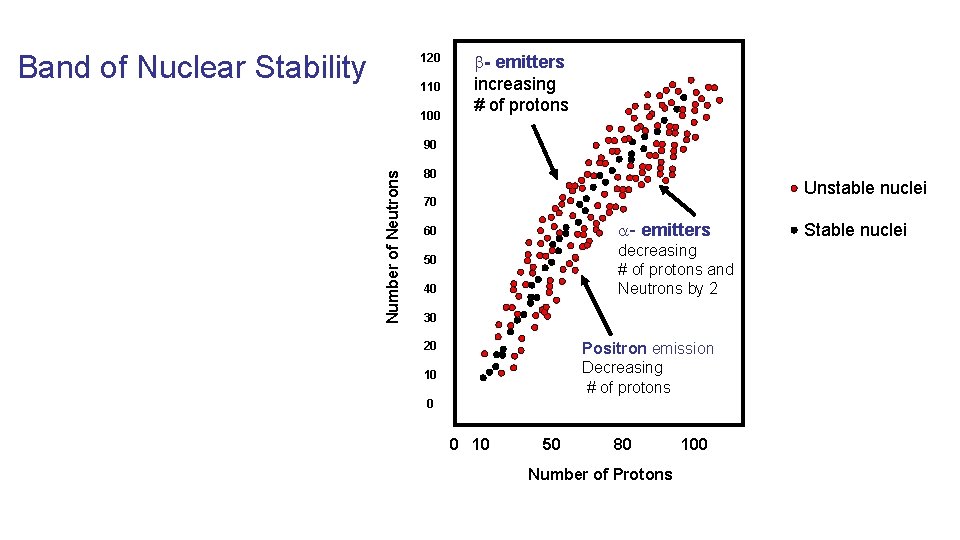
Band of Nuclear Stability 120 110 100 - emitters increasing # of protons Number of Neutrons 90 80 Unstable nuclei 70 - emitters 60 decreasing # of protons and Neutrons by 2 50 40 30 20 Positron emission Decreasing # of protons 10 0 0 10 50 80 Number of Protons 100 Stable nuclei

Summary of Radioactive Decay

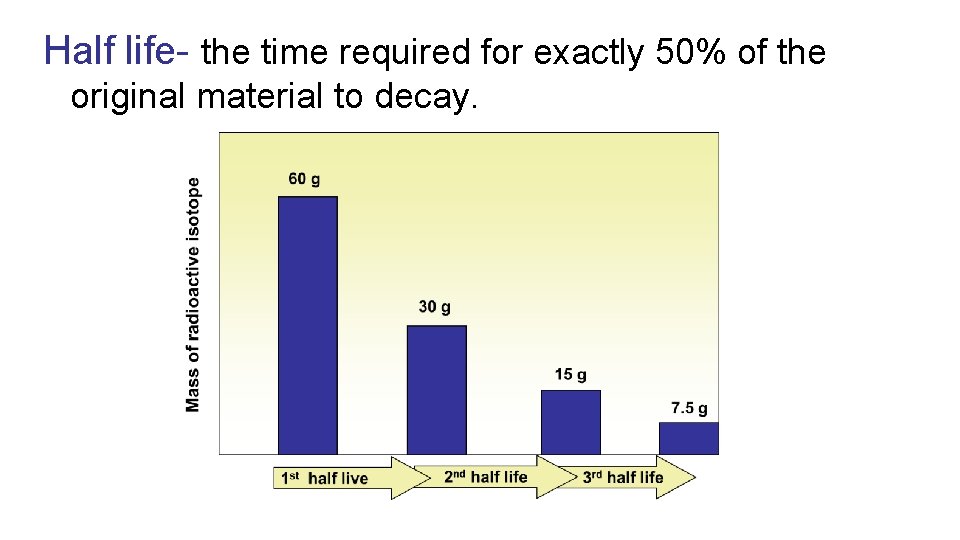
Half-life The rate at which a radioactive isotope decays is measured in half-life. The term half-life is defined as the time it takes for one-half of the atoms of a radioactive material to disintegrate. Half-lives for various radioisotopes can range from a few microseconds to billions of years.

Half life- the time required for exactly 50% of the original material to decay.

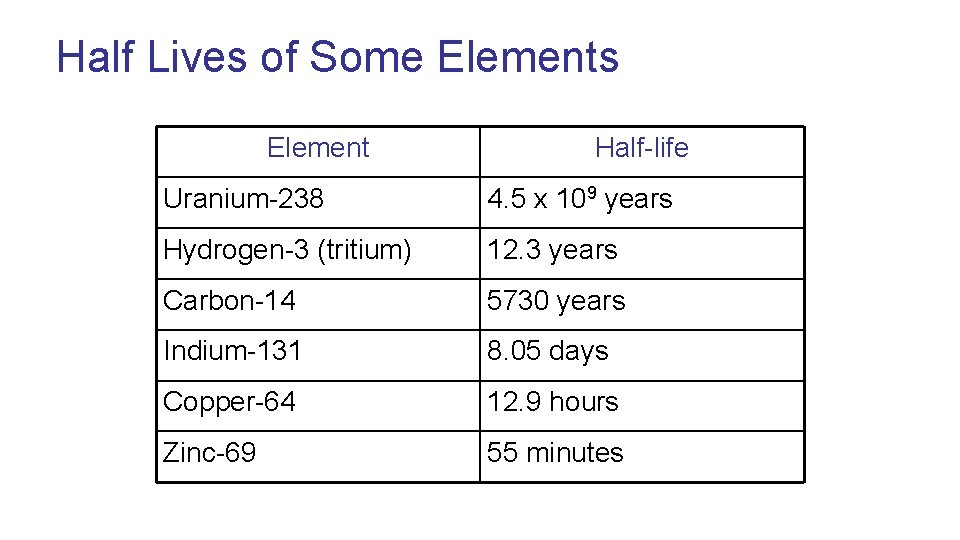
Half Lives of Some Elements Element Half-life Uranium-238 4. 5 x 109 years Hydrogen-3 (tritium) 12. 3 years Carbon-14 5730 years Indium-131 8. 05 days Copper-64 12. 9 hours Zinc-69 55 minutes

Half-Life Calculations We can calculate the fraction of the original isotope that remains after a given number of half-lives from the following relationship: Fraction remaining = Where n is the number of half-lives passed

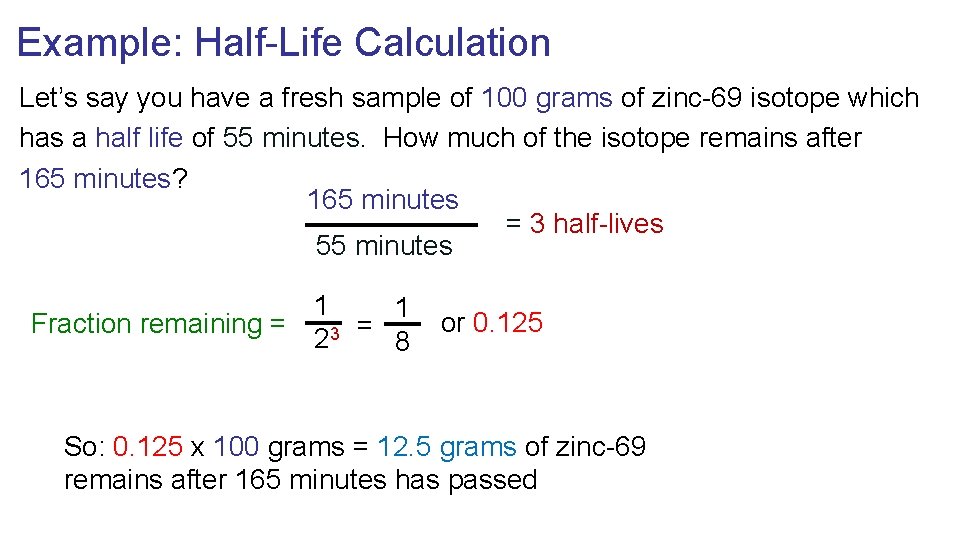
Example: Half-Life Calculation Let’s say you have a fresh sample of 100 grams of zinc-69 isotope which has a half life of 55 minutes. How much of the isotope remains after 165 minutes? 165 minutes = 3 half-lives 55 minutes 1 1 Fraction remaining = 3 = 2 8 or 0. 125 So: 0. 125 x 100 grams = 12. 5 grams of zinc-69 remains after 165 minutes has passed

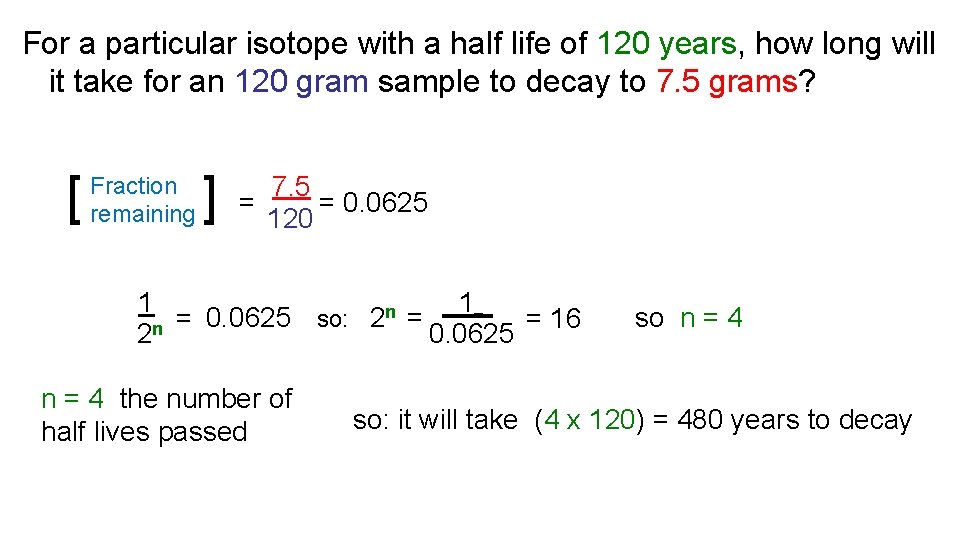
For a particular isotope with a half life of 120 years, how long will it take for an 120 gram sample to decay to 7. 5 grams? 7. 5 x 120 = 7. 5 [ ] = [ ] 120 = 0. 0625 Fraction remaining 1 1 n = 16 = 0. 0625 so: 2 = n 0. 0625 2 n = 4 the number of half lives passed so n = 4 so: it will take (4 x 120) = 480 years to decay

Applications of Radioactivity Radio carbon dating- determining the age of a sample using the carbon-14 isotope Gamma rays- from cobalt-60 and cesium-137 are used to irradiate food Food radiation- retards the growth of organisms such as molds, bacteria, and yeasts. .

Medicine Radioactive isotopes are used in two distinct ways- diagnosis and therapy. Diagnosis- radioisotopes are inserted into the patients body allowing an image to be produced of the problem area.

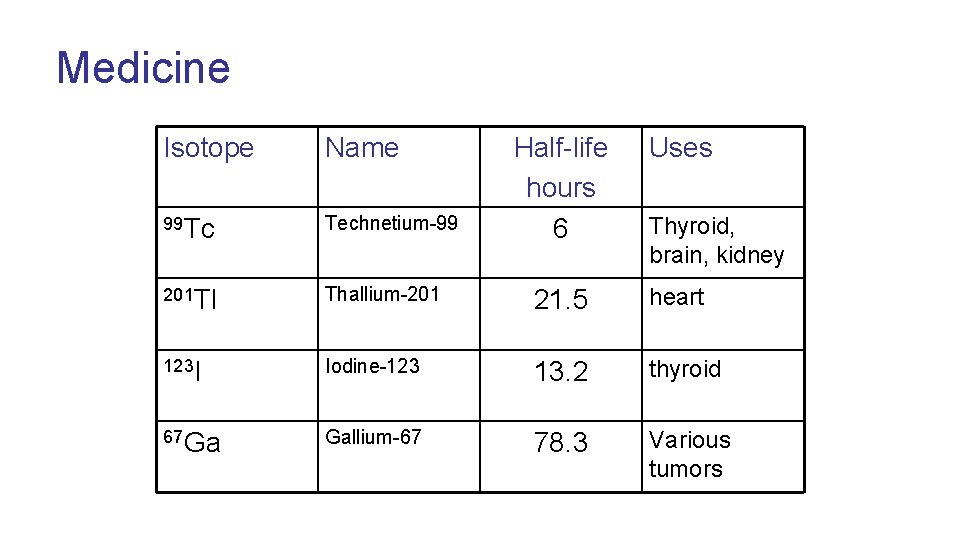
Medicine Isotope Name Half-life hours 6 Uses 99 Tc Technetium-99 201 Tl Thallium-201 21. 5 heart 123 I Iodine-123 13. 2 thyroid 67 Ga Gallium-67 78. 3 Various tumors Thyroid, brain, kidney

Energy- Nuclear Reactions Fission- large amounts of energy are released when heavy atomic nuclei split Fusion- large amounts of energy are released when small atomic nuclei are combined.

Fission and Energy About 25 million times more energy is released when a particular mass of uranium is used in a fission reaction compared to the same mass of natural gas during conventional combustion.

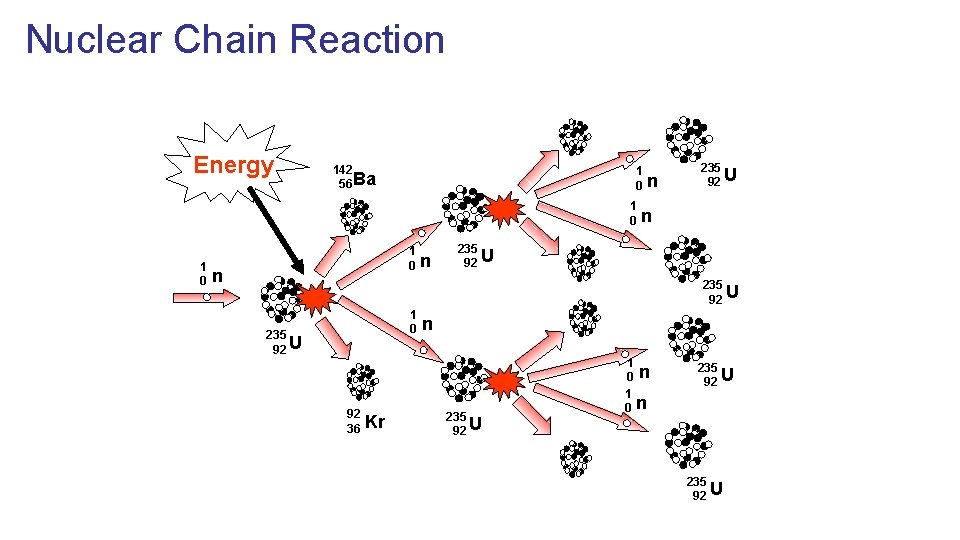
Nuclear Chain Reaction Energy 142 56 Ba 1 0 1 0 n n n 235 92 U 1 0 235 92 U 92 36 Kr n 235 92 U 1 0 n 235 92 U

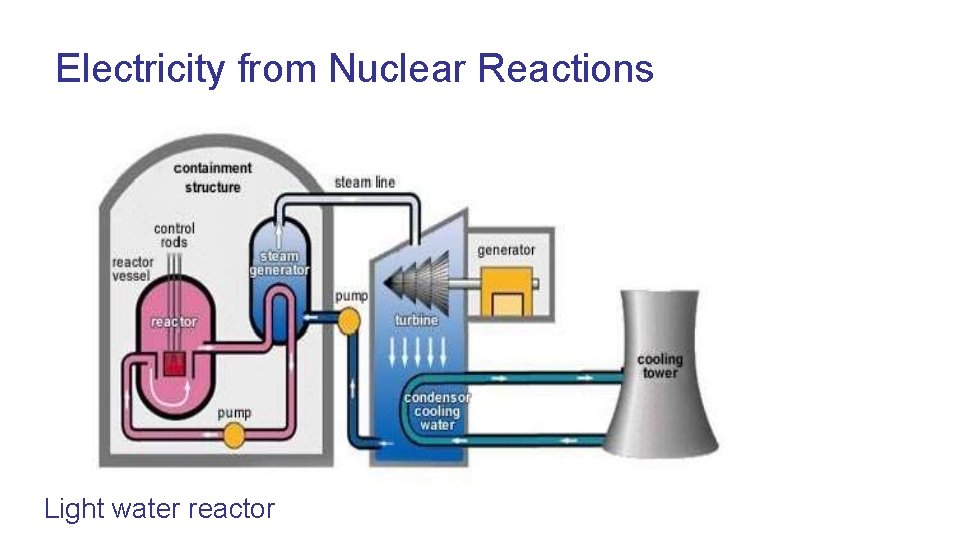
Electricity from Nuclear Reactions Light water reactor

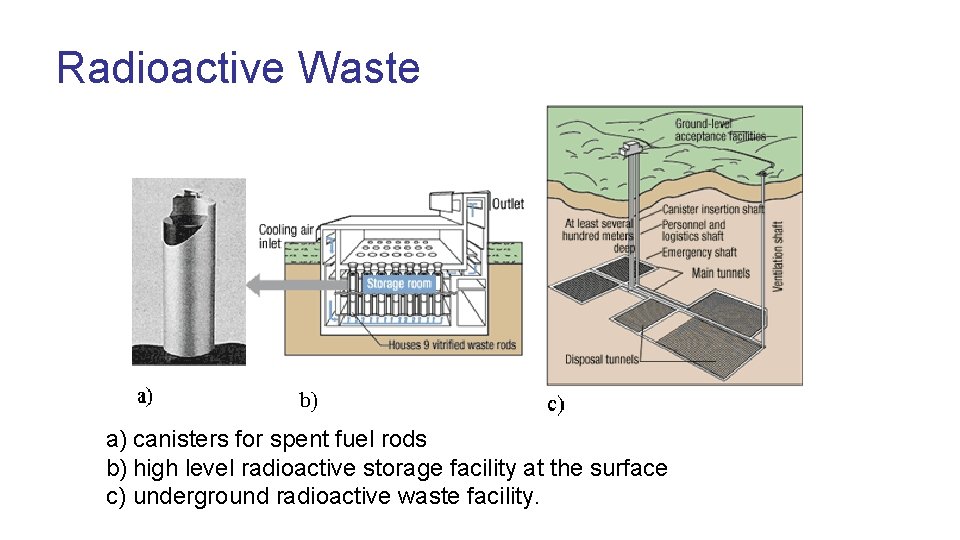
Radioactive Waste A major problem with fission energy is the amount of radioactive waste that must be stored. Some of which like plutonium is dangerously radioactive for thousands of years.

Radioactive Waste a) canisters for spent fuel rods b) high level radioactive storage facility at the surface c) underground radioactive waste facility.
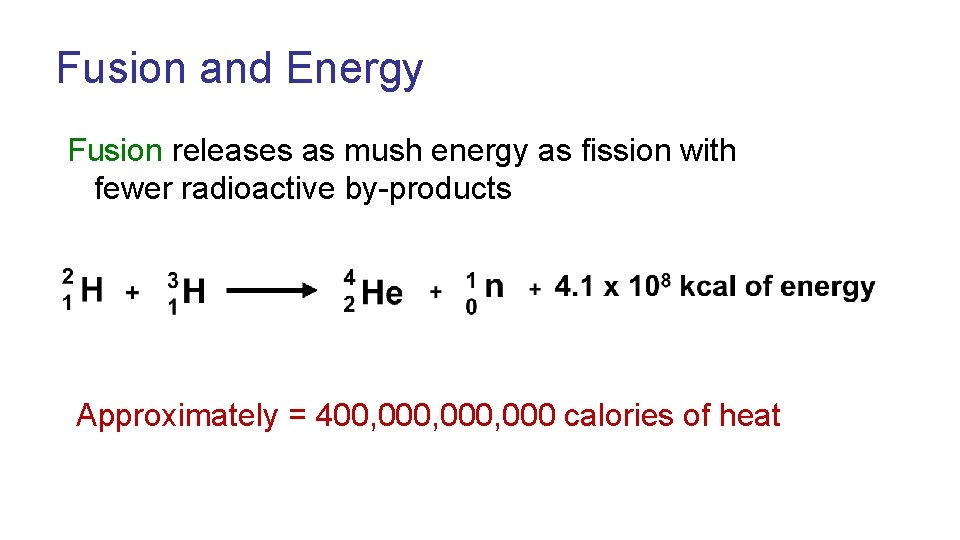
Fusion and Energy Fusion releases as mush energy as fission with fewer radioactive by-products Approximately = 400, 000, 000 calories of heat
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nuclear chemistry webquest answer key
Nuclear chemistry webquest answer key Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Effective nuclear charge trend
Effective nuclear charge trend Chapter 24 nuclear chemistry answer key
Chapter 24 nuclear chemistry answer key Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Nuclear chemistry review worksheet answer key
Nuclear chemistry review worksheet answer key Applications of nuclear chemistry
Applications of nuclear chemistry T half life formula
T half life formula Nuclear fusion in real life
Nuclear fusion in real life Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear chemistry
Nuclear chemistry Nuclear chemistry
Nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry C device module module 1
C device module module 1 Teach lab
Teach lab Atomic number of ytterbium
Atomic number of ytterbium Gas 48 neutrons
Gas 48 neutrons How many protons is in lithium
How many protons is in lithium Particles table
Particles table Which equation represents sublimation?
Which equation represents sublimation? Protons and neutrons size
Protons and neutrons size Mass of protons neutrons electrons
Mass of protons neutrons electrons Magnitude electric field
Magnitude electric field What element am i
What element am i Can an atom have more neutrons than protons
Can an atom have more neutrons than protons Enantiotopic protons
Enantiotopic protons Where do you find the number of protons
Where do you find the number of protons How does a positive ion form
How does a positive ion form How many protons are in hydrogen
How many protons are in hydrogen Number of neutrons
Number of neutrons Http //education.jlab.org/ga/pen number.html
Http //education.jlab.org/ga/pen number.html Lithium protons neutrons electrons
Lithium protons neutrons electrons